To the Editor:
Pregnancy has rarely been attempted following successful islet transplantation because of concerns over a possible detrimental effect of the increased insulin demands of pregnancy on the islet graft with subsequent postgestation hyperglycemia,1,2 and for allogeneic islets, concern over a possible detrimental effect of the required immunosuppression on fetal outcomes.2 In a recent online publication, Assalino et al report a 29-year-old woman with type 1 diabetes who became insulin independent after receiving 2 islet infusions and 2 years later successfully carried a pregnancy to term that resulted in a healthy male child without requiring gestational insulin therapy.3 During the first postgestational year the patient developed fasting hyperglycemia and subsequently returned to insulin therapy,3 suggesting that meeting the increased metabolic demands of pregnancy may have exhausted the functional capacity of the islet graft.
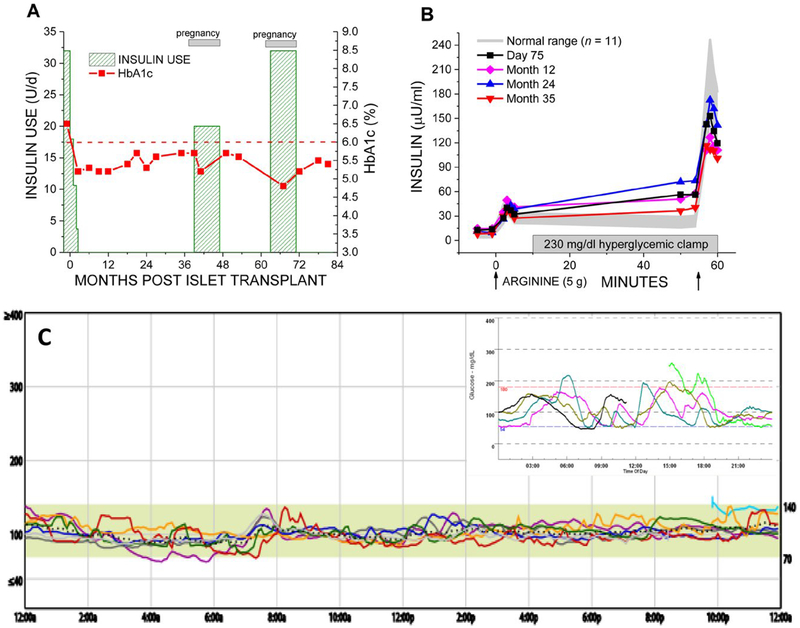
Pregnancy increases metabolic demands for insulin through the development of maternal insulin resistance and requirement for increased nutritional intake to support maternal and fetal growth. We report a 27-year-old woman with a 12-year history of type 1 diabetes complicated by hypoglycemia unawareness and recurrent severe hypoglycemia events despite sensor-augmented insulin pump therapy who received an intraportal infusion of 411 023 islet equivalents (for 6285 IE/kg recipient body weight) isolated from a single donor pancreas according to the CIT07 protocol4 and after 2 months discontinued exogenous insulin while maintaining normoglycemia (Figure 1A). While the patient received maintenance immunosuppression with low-dose tacrolimus (trough target 3–6 μg/L) and sirolimus (trough target 5–8 μg/L), the β-cell secretory capacity, a measure of functional β-cell mass, remained stable during 3 years of per protocol assessment at ~ 50% of normal (Figure 1B). Pregnancy was then desired. Following multidisciplinary consultation, the sirolimus was tapered off while the tacrolimus dosing increased to target 5–7 μg/L, and 3 months after stopping sirolimus conception occurred. With confirmation of pregnancy, long-acting insulin was started preemptively with detemir to maintain fasting glucose < 95 mg/dL and protect the islet graft from the increased metabolic demands of pregnancy; postprandial glucose remained < 140 mg/dL without requiring short-acting insulin.5 At 38 weeks gestation a healthy female child, 21”, 7 lb 8 oz, was born by scheduled cesarean section; insulin was stopped at delivery, and our patient nursed for over 6 months while continuing tacrolimus therapy alone for immunosuppression. A little more than a year later, another planned conception occurred and with confirmation of pregnancy long-acting insulin was again started empirically with detemir to meet gestational glycemic targets. At 39 weeks gestation, another healthy female child, 21”, 7 lb8 oz, was born by scheduled C-section; insulin was again stopped at delivery, and our patient again nursed for over 6 months while continuing tacrolimus.
FIGURE 1.
Metabolic control and posttransplant islet β-cell secretory capacity over 7 years following islet transplantation including 2 successful pregnancies. (A) Normoglycemia (glycosylated hemoglobin, HbA1c < 6.0% [42 mmol/mol]) was established after islet transplantation with withdrawal of insulin therapy except where reinstituted preemptively during pregnancy. (B) The incremental acute insulin response to arginine under hyperglycemic clamp conditions gives the β-cell secretory capacity as a measure of functional β-cell mass that was stable during 3 years of per protocol assessment at 74 ± 5 μU/mL, which is ~ 50% of the normal 143 ± 15 μU/mL. (C) One year following the second pregnancy, 7 years after islet transplantation and remaining off insulin therapy, continuous glucose monitoring demonstrated minimal glucose variability (glucose SD 12 mg/dL [0.7 mmol/L]) with 99% of time spent with on-target glycemia (70–140 mg/dL [3.9–7.8 mmol/L]) and essentially no (1%) time spent with hypoglycemia (< 70 mg/dL [3.9 mmol/l]). The inset shows the pretransplant continuous glucose monitoring assessment with markedly increased glucose variability (glucose SD 42 mg/dL [2.3 mmol/L]) and 22% of time spent with hypoglycemia when previously on sensor-augmented insulin pump therapy
One year following the second delivery, 7 years following islet transplantation, our patient remains normoglycemic (Figure 1C). Assessment for alloantibodies has remained negative. Establishing a sufficient β-cell secretory capacity (at ~ 50% of normal) following islet transplantation,4 and minimizing increased demand for insulin secretion during pregnancy with preemptive insulin therapy, both likely contributed to our patient’s ability to maintain long-term normoglycemia without requiring postgestation insulin therapy.
ACKNOWLEDGMENTS
Supported by Public Health Services Research Grants U01 DK070430 (to AN), R01 DK091331 (to MRR), UL1 TR000003 (Penn Clinical & Translational Research Center), P30 DK19525 (Penn Diabetes Research Center), by the W.W. Smith Charitable Trust, and by the Schiffrin Award in Autoimmune Research (to MRR).
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Wahoff DC, Leone JP, Farney AC, Teuscher AU, Sutherland DE. Pregnancy after total pancreatectomy and autologous islet transplantation. Surgery. 1995;117(3):353–354. [DOI] [PubMed] [Google Scholar]
- 2.Schive SW, Scholz H, Sahraoui A, et al. Graft function 1 year after pregnancy in an islet-transplanted patient. Transpl Int. 2015;28(10):1235–1239. [DOI] [PubMed] [Google Scholar]
- 3.Assalino M, Podetta M, Demuylder-Mischler S, et al. Successful pregnancy and delivery after simultaneous islet-kidney transplantation. Am J Transplant. 2018. 10.1111/ajt.14884 [DOI] [PubMed] [Google Scholar]
- 4.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in beta-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes. 2013;62(8):2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(suppl 1):S137–S143. [DOI] [PubMed] [Google Scholar]