Abstract
Introduction:
The prognosis for severe burns has improved significantly over the past 50 years. Meanwhile, burns have become an affliction mainly affecting the less well-developed regions of the world. Early excision and skin grafting has led to major improvements in therapeutic outcomes.
Areas covered:
The purpose of this article is to survey the use of pharmacotherapy to treat different pathophysiological complications of burn injury. The author, herein, discusses the use of drug treatments for a number of systemic metabolic disturbances including hyperglycemia, elevated catabolism, and gluconeogenesis.
Expert opinion:
Advancements in personalized and molecular medicine will make an impact on burn therapy. Similarities between severe burns and other critically ill patients will lead to cross-fertilization between different medical specialties. Furthermore, advances in stem cells and tissue regeneration will lead to improved healing and less lifelong disability. Indeed, research in new drug therapy for burns is actively progressing for many different complications.
Keywords: Metabolic disturbances, burn epidemiology, burn progression, infection, smoke inhalation, stem cells, reduced scarring
1. History of burn treatment
Different way of treating burns have been described since ancient times, and depiction of burn treatments can even be seen in cave paintings more than 3500 years old [1]. In the Ebers papyrus from Ancient Egypt (1550 BC) a five-day regimen for burn treatment is described using a preparation made from cattle dung, beeswax, ground ram-horn, and barley porridge, all soaked together in resin, that was topically applied to burns [2]. In 600 BC, the Chinese described burn treatment using an extract brewed from tea leaves. Nearly 100 years later, Hippocrates described the use of pig skin impregnated with bitumen resin to produce a type of dressing that was applied to the burn, which could be regularly removed allowing irrigation with an infusion of oak bark in warm vinegar, and then reapplied. Celsus writing in the first century AD, recommended the application of a lotion made from wine and myrrh. In 300 AD, Hong Ge described a topical ointment containing ground-up calcarea sponges (sponges containing calcium carbonate) blended with pig fat cooked with willow bark [3]. In the middle of the 16th century, Ambroise Paré (a famous battlefield surgeon) treated burns with onions, and was one of the first to describe the need for the early excision of burn wounds [4]. In the early 17th century the Swiss surgeon, Guilhelmus Fabricius Hildanus published the first comprehensive textbook describing burn treatment [5]. He described the cause, diagnosis, treatment, and complications of burn injuries. In addition to topical treatment with ointments containing onions and camphor, surgical procedures like early excision, eschar removal and release of syndactly (contractures) in the hands were described for the first time.
In 1797, Edward Kentish wrote another early treatise on burn therapy ‘An Essay on Burns, Principally Upon Those which Happen to Workmen in Mines from the Explosions of Inflammable Air, (or Hydrogen Gas.)’ [6]. Soon afterwards, Nodes Dickinson replied with ‘Remarks on burns and scalds, chiefly in reference to the principle of treatment at the time of their infliction. Suggested by a perusal of the last edition of “An essay on burns”, by Edward Kentish, M.D.’ [7]. In 1832, Baron Guillaume Dupuytren first classified burns according to their depth and divided thermal injuries into six ascending degrees of severity [8]. He was also the first to recognize gastric and duodenal ulceration as a serious complication of severe burns, a concept described in more detail by Curling in 1842 [9]. In the 1800s, Jaques Reverdin introduced the concept of skin grafting to cover burns that affected large areas of the body, and improved the diagnostic and surgical understanding of burns [10]. During and after World War I, a consensus was reached that the best treatment for acute, deep, or extensive burn injuries should include excision, skin grafting, and pain management. However, many patients still died of shock and infection because the fundamental pathophysiology of burn wounds was not completely understood, particularly the need for rapid fluid replacement [11].
Research inspired by fire disasters such as the Rialto fire in 1921 and the Coconut Grove nightclub fire in 1942 resulted in better understanding of the pathophysiology of burns [12]. It is quite clear that our ability to treat severe burn injuries has improved dramatically since World War II. It used to be accepted that 3rd degree burns covering 40% of total body surface area (TBSA) would result in fatality for roughly half the victims in the United States [13]. At present this number has increased to approximately 80% TBSA [14]. According to a recent study, even a 98% TBSA injury now has a 50% survival rate in children [15].
Modern treatment of severe burns is a complex and lengthy process, involving a multidisciplinary team with many separate components. In recent years there has been a tendency toward early excision (4–7 days) and skin grafting as opposed to delayed excision (1–4 weeks) [16]. Recently, ‘ultra-early’ excision at 2–3 days was shown to be even better than early excision [17].
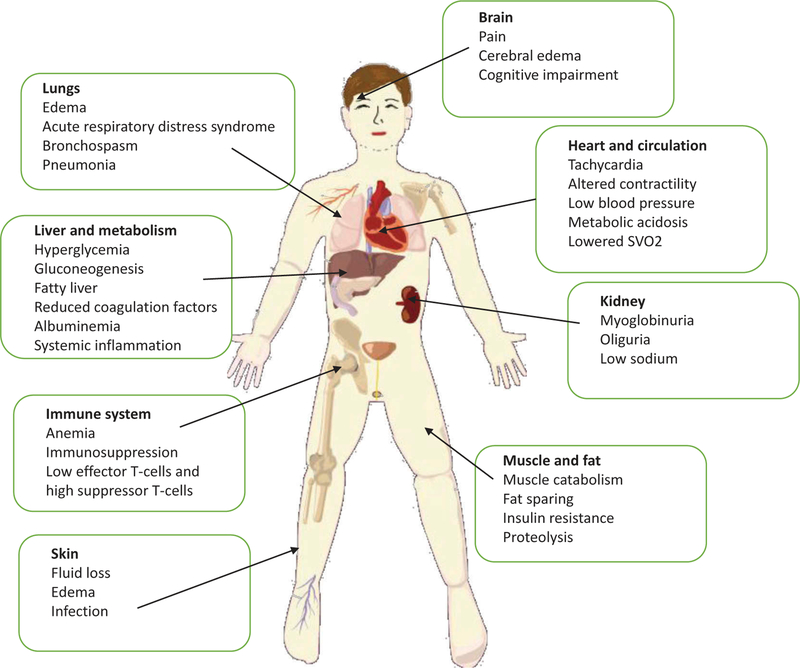
The present review will survey recent advances concentrating on pharmacotherapy for burns in the following areas: metabolic disturbances; smoke inhalation, burn wound progression, burn dressings; treatment of infection; tissue engineering and regeneration; scar prevention [18]. Figure 1 shows a graphical illustration of all the pathophysiological consequences of severe burns.
Figure 1. A survey of the pathological effects of severe burns affecting nearly all body systems.
While local damage is primarily to the skin, underlying tissue, and lungs, regional and systemic effects can affect the liver and metabolism, fat and muscle, heart and circulation, kidneys, brain and suppress the immune system.
2. Burn epidemiology
Overall there has been a worldwide downwards trend in burn incidence, burn severity, length of hospital stay, and in mortality rates [19]. Nevertheless, there has at the same time been a remarkable change in the worldwide distribution of burn injuries in the 21st century compared to past centuries. Nowadays approximately 90% of burns and 95% of deaths occur in low-to middle-income countries, regions that generally lack the necessary infrastructure to reduce the incidence and severity of burns [20]. The rate of childhood injury or death from fire and scalds is nearly 11 times higher in lowincome countries (LIC) than in high-income countries (HIC). Even in HIC, burns still disproportionately afflict racial and ethnic minorities characterized by relatively low socioeconomic status. In 2004, nearly 11 million people throughout the world were afflicted by burns severe enough to receive medical attention [21]. In the US, the average hospital charges for care of a patient with extensive third-degree burns requiring skin grafting is estimated to be more than US$ 95,000 [22].
3. Chemical, electrical, and radiation burns
While thermal injury is by far the most common cause of burns, it should not be forgotten that burns can also be caused by chemicals, electrical contact, and radiation. These types of burns have some particular features that may affect the prognosis and treatment [23]. According to the 2015 report of the American Burn Association, chemical injuries represented 3.4% of patients admitted to participating hospitals during the 2004 to 2015 period. Acids, alkalis, hydrofluoric acid, white phosphorous, and phenol are the commonest chemical agents, and burns caused by some of these materials may require special measures. Electrical injuries comprise 4% of all reported burns. Although electrical injuries cause mainly external injuries, they can also cause internal burns caused by the current passing through bones and muscles inside the body. Radiation burns nowadays are usually associated with cancer treatment, and are complicated by the hematologic consequences of radiation exposure.
4. Animal models of burns and burn infection
The development of animal models of burn wounds is necessary to study the course and treatment of various infections, and to study the healing processes of established wounds [24]. Over the last few decades, several animal burn wound models have been developed. The animals used in these models have included different species: rodents, rabbits, and pigs. The severity of burns is commonly classified according to the depth of the injury which varies depending on the duration of exposure [25] and several other factors. These include: animal species (which affects skin structure and thickness); location on the body (in larger animals, such as pigs, skin thickness varies in different body sites); method of burn (e.g., scald, contact, flame, radiant heat); temperature of instrument or water; and pressure used for contact. The size of the wound is also an important factor for classifying burns and burn models. Additionally, if an infection is established in the wound, the number of inoculated bacteria, the method of inoculation and the virulence of those strains also affect the severity. In the models covered in this review, the sizes of the burn wounds range from 5% to 50% of total body surface area (TBSA) and the wounds have different depths.
In 1968, Mason and Walker developed the first burn model in rats that used boiling water to inflict a scald injury [26]. This model has been widely used for studying burn infections, Pseudomonas aeruginosa infection and potential treatment [27], bacterial translocation and intestinal atrophy after thermal injury [28], burn sepsis [29,30], candidiasis after thermal injury [31], and gene therapy [32,33]. Bjornson et al. [34] developed a similar model using guinea pigs to study burn wounds infected with Staphylococcus aureus, P. aeruginosa, and Candida albicans. The model of Orenstein et al. [35] also used a guinea pig where the animal is subjected to a 15second metal plate application, which was pre-heated to 150°C. A thermal injury burn model was established by Stieritz and Holder [36] using mice. The mice in the model are shaved and ethanol is applied on the shaved dorsum. Following the ethanol application, the substance is ignited and allowed to burn for 10 seconds, which creates a wound comprising about 30% of TBSA. Katakura et al. [37] created a novel burn wound model that induces thermal injury by exposing the shaved back of the mouse to a gas flame for nine seconds. Several burn models have been established which create the wound by applying the heat directly with preheated objects. The model by Stevens et al. uses two brass blocks that are pre-heated to 92°C-95°C and applies them on either side of mice toward the elevated skin folds subsequently causing a burn wound that is approximately 5% of TBSA. Manafi et al. [38] introduced a novel burn model, which uses a heated metal block that is applied on the dorsal side of mice to produce burns that are 10% of TBSA and subcutaneously injected P. aeruginosa. Kumari [39] incorporated a similar model but infected the wound by topically inoculating bacteria. Gurfinkel et al. [40] used a radiant heater set at 400°C and exposed the shaved skin of pigs and rats to the heater for 20 seconds to establish wounds that comprise 30–50% of TBSA [41].
5. Metabolism and nutrition
Severe burns are associated with a profound metabolic and catabolic response which persists long after the initial insult [42]. This response is characterized by elevated metabolic rates, hyperdynamic circulation, muscle and bone catabolism, and insulin resistance [43]. Immediately after the injury, there is a temporary decrease in metabolism and tissue perfusion, the so-called ‘ebb’ phase, which is quickly followed by a period of increased metabolic rate and hyperdynamic circulation, called the ‘flow’ phase [44]. The flow phase is characterized by elevated glycolysis, lipolysis, proteolysis, insulin resistance, liver dysfunction, and decreases in total body mass. A 10% loss in total body mass was claimed to lead to immune dysfunction; 20% leads to decreased wound healing; 30% leads to severe infections; and a 40% loss leads to death [45].
Inflammatory cytokine levels, serum hormones, protein production, and protein secretion are altered in severe burn injuries. Muscle protein is degraded much faster than it is synthesized, and protein catabolism is directly related to increases in the metabolic rate [42]. Elevated circulating levels of catecholamines, glucagon, cortisol, and gluconeogenic hormones lead to inefficient production of glucose in the liver [46]. Glycolytic-gluconeogenic cycling leads to hyperglycemia and impaired insulin sensitivity [47]. Lactate is recycled to the liver to produce more glucose via the gluconeogenic pathway. While in classical starvation, fat provides the major source of calories, in severe burns the body fails to utilize fat as an energy source, leading instead to protein catabolism to provide an energy source. Treatment of these metabolic disturbances occurring in severe burn patients, involves both nutritional support and pharmacological intervention.
5.1. Nutrition
Despite the obvious need for additional nutritional support in catabolic burn patients, at the present time there are no standardized guidelines for nutritional support [48]. If excess nutrition is supplied in the form of carbohydrates and fat, it could result in hyperglycemia [49] and fatty infiltration of organs such as the liver [50]. Current guidelines consist of high carbohydrate and glucose, high protein and amino acids, and a low-fat diet with emphasis on unsaturated fatty acids [48], with the addition of supplements such as glutamine, arginine, and essential fatty acids [51]. Exercise training has been shown to increase lean body mass and strength, without any exacerbation of postburn hypermetabolism [52].
5.2. Growth hormone and IGF
Recombinant human growth hormone (rhGH) is a peptide hormone that stimulates growth, cell division, and tissue regeneration. rhGH has been tested to treat the metabolic disturbances in severe burns. In children with 40% TBSA burns, daily administration of rhGH significantly improved weight gain, growth, lean body mass, bone mineral content and cardiac function, while at the same time it reduced hypermetabolism and produced hypoglycemia [53] The beneficial effects of rhGH were mediated by insulin like growth factor (IGF-1), and patients demonstrated a doubling in the serum levels of IGF-1 and IGFBP-3 (IGF binding protein 3).
Infusion of equimolar doses of recombinant human IGF-1 and IGFBP-3 improved protein metabolism in burn patients (both pediatric and adult) and caused significantly less hypoglycemia than rhGH itself [54]. This treatment attenuated muscle catabolism, and improved mucosal integrity of the gut in children with serious burns. Immune function was improved along with attenuation of the hepatic acute phase response, and reduction of the hypercatabolic loss of body protein mass [55].
5.3. Insulin
Insulin decreases blood glucose because it mediates peripheral glucose uptake into skeletal muscle and adipose tissue, and also suppresses hepatic gluconeogenesis [56]. Insulin increases protein synthesis (via control of amino acid uptake), increases fatty acid synthesis, and decreases proteinolysis. Insulin given during hospitalization for major acute conditions has been shown to improve muscle protein synthesis, accelerate the healing time of skin graft donor sites, and attenuate the loss of lean body mass, while reducing the acute phase response. Many published papers have studied the effect of insulin therapy in ICU patients, and in patients with sepsis, rather than in severe burns per se [57,58]. However, maintaining a continuous hyperinsulinemic-euglycemic clamp in burn patients is challenging since these patients are often continuously fed high caloric loads via enteral feeding tubes in an attempt to maintain body weight.
5.4. Oxandrolone
Anabolic steroids such as oxandrolone (a testosterone analog which possesses only 5% of the undesirable androgenic effects of testosterone) are beneficial for muscle protein catabolism because they enhance protein synthesis, reduce weight loss, and improve the healing of skin graft donor sites [59]. In a prospective randomized study, administration of 10 mg of oxandrolone every 12 h decreased the hospital stay. However, it is important to monitor hepatic transaminases to avoid liver toxicity [60]. Oxandrolone reduced hypermetabolism after burns and significantly increased lean body mass at 6, 9, and 12 months post-burn, and bone mineral content at 12 months [61]. Patients treated with oxandrolone showed fewer complications compared to treatment with rhGH.
5.5. Metformin
The antidiabetic biguanide drug, metformin (Glucophage) is used to inhibit gluconeogenesis in the liver, and increase peripheral insulin sensitivity by stimulating glucose transporters [62]. In 10 patients with >60% TBSA burns, metformin attenuated hyperglycemia, and led to a significantly lower rate of endogenous glucose production and glucose oxidation [63]. Both insulin, metformin, and the combination thereof, increased the rate of muscle protein synthesis in adults with >40% TBSA burns [64]. Care must be taken to avoid metformin-associated lactic acidosis [65].
5.6. Propanolol
Adrenergic blockade (the administration of beta blockers) has effects on metabolism besides the well-known effects on blood pressure and heart rate [66]. In 406 children with >30% TBSA burns propranolol treatment reduced thermogenesis, heart rate, and resting energy expenditure [67]. Propranolol also increased lean body mass and decreased skeletal muscle catabolism. The combination of propanolol and oxandrolone (called ‘Oxprop’) was tested together with exercise therapy, in 42 children with 30% TBSA burns [68]. Patients were randomized to placebo or to Oxprop and began drug administration within 96 h of admission. Muscle strength and power, lean body mass, and VO2 peak increased in both groups, but the increase in strength and power was significantly greater with Oxprop versus control, resulting in improved protein net balance.
5.7. Glucagon-like-peptide 1 analogues
Glucagon like peptide 1 (GLP1) is an incretin, which is secreted into the hepatic portal system (by the intestinal L-cells) after a meal, thus enhancing the secretion of insulin [69]. An infusion of GLP1 by osmotic pump was tested in rats with 40% TBSA burns [70]. GLP-1 reduced protein breakdown and total energy expenditure. The increased expression of caspase 3 and decreased expression of bcl-2 in islet cells were reversed. Because GLP1 is rapidly degraded by dipeptidyl peptidase-4, long lasting analogues have been developed for pharmaceutical purposes [71]. Exenatide or exendin-4 is one such GLP1 analogue that has been widely studied in diabetes treatment [72] In a recent study, exendin 4 treatment in a rat model of 50% TBSA scald injury improved both insulin secretion and intracellular insulin reserves under different glucose stimulation conditions. Expression of insulin mRNA in the islet cells was restored by exendin-4 [73]
5.8. Szeto-schiller peptide
The Szeto-Schiller peptide, SS31 is a tetrapeptide (D-Arg-Dmt-Lys-Phe-NH2) with cell penetrating properties that accumulates in mitochondria and acts as a targeted anti-oxidant [74]. It is under investigation for the treatment of Alzheimer’s disease and numerous other diseases [75]. Carter et al studied the effects of SS31 on burn-induced insulin resistance in mice [76]. SS31 administration ameliorated burn-induced insulin resistance and reversed the increased 18FDG uptake by brown adipose tissue (BAT) in burned but not in cold- stressed animals.
6. Smoke inhalation
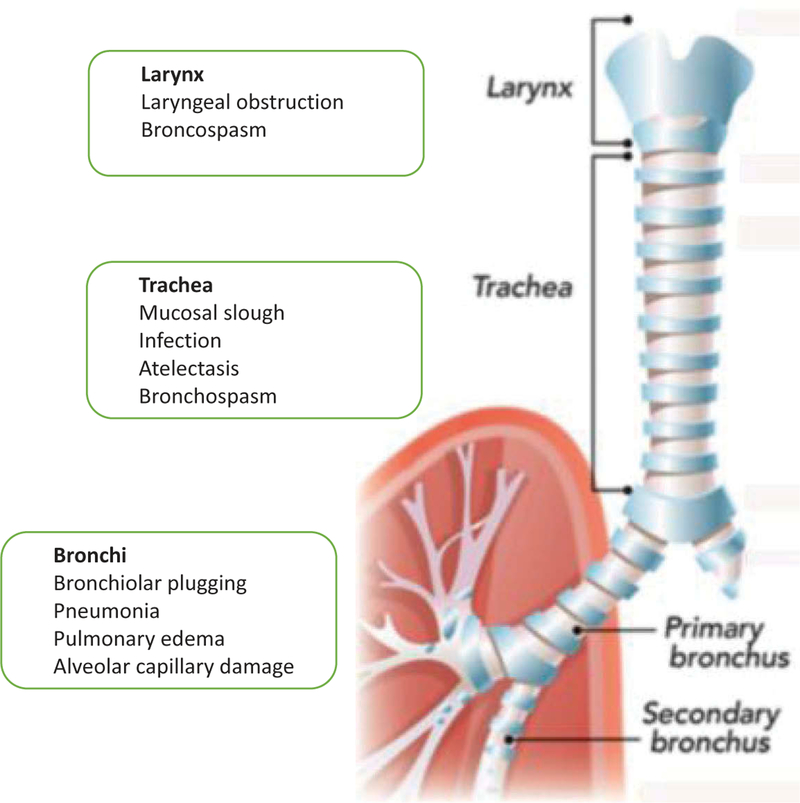
Smoke inhalation injury is a major cause of morbidity and mortality in fire victims. It is a complex multifaceted injury initially affecting the airways but can become a complex life-threatening systemic disease affecting every organ in the body. The major pathophysiological phenomenon is development of edema in the respiratory tract. The tracheobronchial tree is injured by hot air, steam, and toxic chemicals, leading to bronchoconstriction. The lung parenchyma is damaged by the release of proteolytic elastase enzymes, leading to release of inflammatory mediators, an increase in extravasation of fluid, and development of pulmonary edema and atelectasis. Decreased levels of surfactant and pro-inflammatory cytokines such as interleukins and tumor-necrosis-factor-α exacerbate the injury [77]. The main toxic compounds contained in smoke are carbon monoxide, nitrogen oxides, hydrogen vcyanide, phosgene, ammonia, sulfur dioxide, hydrogen sulfide, formaldehyde, and acrylonitrile [78]. Figure 2 shows a summary of the pathophysiological changes caused in the respiratory system by smoke inhalation and burns.
Figure 2. Pathophysiological changes caused in the respiratory system by smoke inhalation and burns.
Damage can be caused to the larynx, the trachea and to the bronchi.
Respiratory therapy consists of airway management, endotracheal intubation, mechanical ventilation, bronchoalveolar lavage, and bronchial hygiene therapy [77].
Pharmacological treatments for smoke inhalation are designed to relieve bronchospasm, reduce mucosal/submucosal edema, liquefy secretions, and improve oxygenation. Many of these agents have been administered by inhalation of nebulized formulations [79]. Beta-2 adrenergic receptor agonists (salbutamol or albuterol) are bronchodilators relaxing bronchial smooth via increasing intracellular cyclic adenosine monophosphate, and are commonly used in asthma [80]. They also exert an anti-inflammatory action, reducing inflammatory mediators, such as histamine, leukotrienes, and TNF-α. Palmieri et al showed that continuous nebulized albuterol attenuated acute lung injury in a sheep model of combined burn and smoke inhalation [81]. The parasympathetic pathway stimulates secretion of acetylcholine, which interacts with M1 and M3 muscarinic receptors, to produce airway smooth muscle constriction and submucosal gland secretion [82]. Jonkam et al. showed that low dose inhaled nebulized tiotropium bromide (M1 and M3 muscarinic receptor antagonist) reduced ventilatory pressure, and attenuated pulmonary dysfunction, and upper airway obstruction in a sheep model of smoke inhalation [83]. Epinephrine has a vasoconstrictive action, thus attenuating mucosal/submucosal edema in the airways. Lopez et al. showed that nebulized epinephrine reversed the hyperperme ability to water and protein in the pulmonary vasculature in a sheep model of burn and smoke inhalation injury [84]. In a recent pilot clinical trial, Foncerrada et al. found that inhaled epinephrine was safe in children suffering from >30% TBSA burns and smoke inhalation [85]. The antioxidant N-acetylcysteine (NAC) can reverse oxidative stress which is a significant component in smoke-induced lung injury. Csontos et al. found that NAC could lower oxidative stress, reduce interleukin levels, and lower the requirement for vasopressors [86]. Smoke inhalation injury has a procoagulant activity leading to fibrin deposition in the airways, therefore, aerosolized administration of anticoagulants can have beneficial effects [87]. Nebulized heparin, either combined with recombinant human antithrombin [88], or combined with NAC and albuterol [89] has shown beneficial effects.
7. Agents to treat burn progression
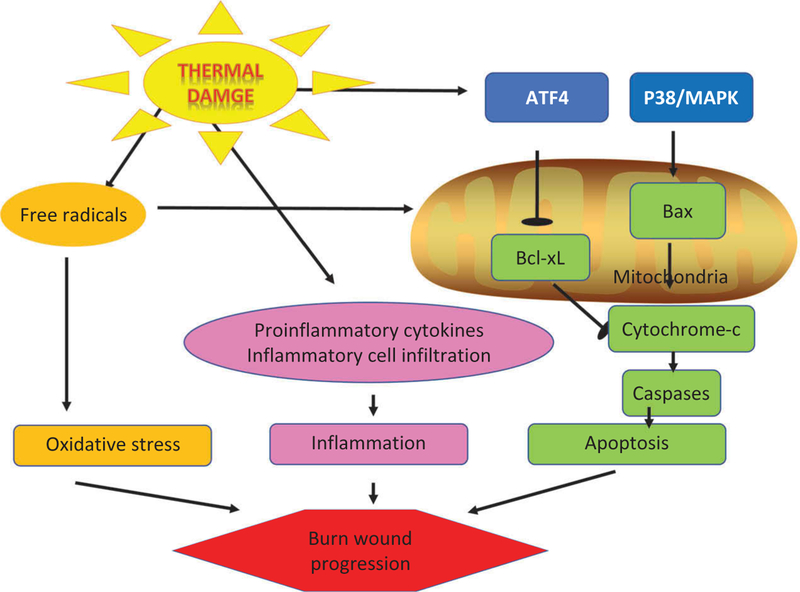
Burns have some aspects in common with ischemic injuries of the heart and brain [90]. The core zone in these infarcts is irreversibly damaged beyond repair, whereas the tissue in the hyperemic zone usually recovers without any long-term damage. However the intermediate area called the penumbra in the case of ischemic stroke, or the zone of stasis in the case of burns can progress either way and is therefore critical. Although these areas are functionally impaired tissue, they can still be theoretically viable. However, this zone is likely to expand into the core necrotic zone if left untreated [91]. This process is called ‘burn conversion’ or secondary progression. The zone of stasis is characterized by blood coagulation, ischemia, and inflammation. Coagulation of the tissue interrupts microcirculation due to the direct effect of thermal injury. Microvessels become plugged by clot formation due to the hypercoagulation that occurs 2 to 3 h after a burn. Edema formation further aggravates the local lack of perfusion, reducing oxygenation and energy supply to the tissues. The zone of irreversible injury therefore increases, unless adequate perfusion can be reestablished and inflammation brought under control. Due to the seriousness of this problem many attempts have been made to find pharmaceutical agents that can slow or even reverse this process [90]. Figure 3 shows some of the signaling pathways that contribute to burn wound progression. To model this particular aspect of burns, investigators usually use a ‘comb burn’ in rats. This model employs a heated brass probe with four metal areas (10 × 20 mm) and three interspaces (5 × 20 mm). The interspace between the actual burns mimics the zone of stasis around a naturally inflicted burn.
Figure 3. Signaling pathways resulting in burn wound progression.
Generation of free radical in the burned tissue produces oxidative stress. Triggering of release of pro-inflammatory cytokines leads to infiltration by immune cells. The transcription ATF4 is activated leading to an apoptotic cascade mediated via the mitochondrial pathway. Abbreviations: ATF4 – activating transcription factor 4; P38/MAPK – p38/mitogen activated protein kinase; Bcl-2 – B-cell lymphoma 2; Bax – Bcl-2-associated X protein.
7.1. Agents that increase perfusion
Erythropoietin (EPO) is a 30kDa glycoprotein hormone produced in the kidney that stimulates erythropoiesis in the bone marrow. Accumulating evidence suggests that EPO is a multifunctional, cytoprotective cytokine with antiapoptotic, anti-inflammatory, and immunomodulatory properties [92]. Tobalem et al. [93] reported that administration of EPO (500 units/kg once a day for 5 days starting 45 min after burn injury) decreased the burn depth, burn area, and shortened the healing time. This improvement was correlated with re-establishment of perfusion on day 4. In another study [94], the same group showed that EPO when combined with local water cooling, not only produced better healing, but also a significant increase in inducible nitric oxide synthase expression with decreased inflammation, and a significant increase in the hematocrit at day 4.
Endothelins are vasoconstrictor peptides (produced by vascular endothelial cells) that regulate the systemic blood circulation. Plasma levels of endothelins are increased by burn injury [95], leading to progressive ischemia and necrosis. Battal et al. tested whether a nonselective endothelin receptor antagonist, TAK-044 could restore blood flow and reduce secondary tissue damage [96]. Rats received three burns and received 0.01, 0.1, 1, or 10 mg/kg of TAK-044 IV immediately after the burn. Skin blood-flow improved and the development of edema and the area of necrotic tissue were reduced. The same group tested Beraprost sodium (a chemically stable prostaglandin I2 analogue with antiplatelet and vasodilator activity) administered at a dose of 0.015 mg of beraprost sodium intraperitoneally immediately after burn injury, in the same rat model [97]. Beraprost also increased blood flow, and reduced both edema and the burn area. Poloxamer-188 (P-188) is a block-copolymer surfactant that exerts an anticoagulatory effect and can prevent endothelial cell injury and reduce leukocyte adhesion. Its mechanism of action relies on non-specific inhibition of hydrophobic adhesion, which occurs when damaged membranes develop defects that expose underlying hydrophobic structures [98]. Baskaran et al showed that P-188 (200 mg/kg) = 5), prevented a decrease in red cell speed around the burn. Twenty-four hours after the burn, the ‘zero red blood cell speed zone,’ was reduced in P-188-treated rats [99]. Yuhua et al [100] showed that i.v. administration of P-188 (200mg/kg every 24 h) significantly reduced burn depth progression at 72 h in rats. Improved tissue survival correlated with increased Na-KATPase, decreased malondialdehyde/oxidative damage, and reduced inflammation in the zone of stasis.
7.2. Anticoagulants
Işik et al [101] evaluated recombinant tissue–type plasminogen activator (r-tPA) in rats subjected to a comb-burn. r-tPA was administered i.v. at a dosage of 1mg/kg 2 h after the burn. After 7 days, the perfusion in the zones of stasis was significantly improved (r-tPA 87.8% vs control 31.8%). Meyerholz et al [102] tested activated protein C (APC, Drotrecogin alfa), an anticoagulatory and anti-inflammatory agent. Rats received lactated Ringer’s solution (LRS) plus 24 μg APC/kg/h i.v. immediately after burn injury for 5 h. Unfortunately, the addition of APC led to decreased perfusion, deeper burns, and increased inflammation compared to LRS alone-treated animals. Statin analogues have pleiotropic effects on the vessel walls and the coagulation and fibrinolytic systems [103]. Uygur et al [104] tested the effect of simvastatin, a commonly used statinanalogue with non-lipid related pleiotropic effects, on the progression of burn wounds in rats. Simvastatin was administered IP at 5mg/kg 30 min after the burn, and continued on a daily basis for 7 days. A significant decrease in inflammation, coagulation, and intravascular fibrin-collection was observed at day 7 within the zone of stasis, resulting in significantly improved tissue survival both at the surface and at depth.
7.3. Anti-inflammatory agents
Excessive inflammation may cause both local and systemic complications in severe burns, eventually endangering tissue survival within the zone of stasis. TNF-alpha, IL1-beta, IL6, IL2, substance P, histamine, complement and xanthine oxidase have all been implicated in the inflammatory cascade that contributes to secondary burn wound progression [105–107]. Severe burns can cause a systemic inflammatory response syndrome, due to the systemic effects of tumor necrosis factor-α (TNF-α) and other cytokines [105,108]. Activation of complement and neutrophils aggravate the inflammatory response within the zone of stasis [109].
Singer et al [110] tested Semapimod (an inhibitor of p38 MAP kinase and macrophage activation) that reduces secretion of TNFα and lowers IL1 levels. The authors compared i.v. administration of 1 mg/kg Semapimod with untreated controls in a swine model with 2nd degree burns. Additionally, all groups received daily dressings with silver-sulfadiazine cream. Semapimod significantly reduced the number of thrombosed microvessels, decreased the burn depth progression, and led to faster reepithelialization. Sun et al [111] tested topical application of antibodies (Ab) against TNF-α or IL6 conjugated to hyaluronic acid (HA) in partialthickness burn wounds in rats and compared wound healing to untreated controls. Anti-TNF-α-HA conjugated Ab significantly reduced burn depth progression on day 7 correlating with decreased macrophage infiltration and lower IL1b levels in the tissue. The anti-IL6 Ab did not prevent burn wound progression. Bucky et al [112] investigated IV administration of a monoclonal antibody (Mab 60.3) against the leukocyte adherence glycoprotein cluster of differentiation (CD)18 in rabbits with 2nd degree burns. Treatment with 2mg/kg MAb 60.3 was initiated 30 min after the burn. Burn contraction, thickness of the eschar and edemaformation were significantly reduced in the treatment group compared to controls after 8 days. Histology revealed a significant increase of live hair follicles and a significant reduction in burn surface progression in MAb 60.3 treated animals. Choi et al used a different MAb (M2) to block leukocyte adherence (CD11b/CD18) in rats [113]. Immediately after burn induction, MAb M2 was administered IV at doses between 25 μL and 150 μL/kg. At 72 h, both MAbs showed an increase in perfusion and a significant reduction of burn wound progression and depth. Mileski et al [114] tested two different MAbs, one MAb R15.7 directed against the leukocyte-CD18-adhesion complex (1mg/kg) and the other MAb R6.5 against its endothelial ligand, intercellular-adhesionmolecule-1 (ICAM-1, CD54: 2mg/kg) administered IV 30 min after burn. At 72 h, perfusion in the zone of stasis was equal or above baseline in all the antibody-treated groups, while perfusion in the control animals was only 34% of baseline.
Agonists of the peroxisome proliferator-activated receptor gamma (PPARγ) such as rosiglitazone, can act as anti-inflammatory agents via a phosphatase and tensin homolog(PTEN)-dependent pathway. Rosiglitazone significantly inhibited lipopolysaccharide-(LPS)-induced nitric oxide (NO) release, prostaglandin E2 (PGE2) production and activation of Akt in macrophages [115].
Taira et al [116] investigated whether rosiglitazone could inhibit burn progression in a rat model. The authors administered 4 mg/kg rosiglitazone orally at 30 min, as well as 24 and 48 h after the burn. After 7 days, rosiglitazone significantly reduced the local burn progression in terms of surface area and depth. Decreased burn progression was associated with improved perfusion and anti-inflammatory effects.
Although corticosteroids are the classical anti-inflammatory agents, their use is controversial in burn wound healing [103]. Singer et al [103] showed that the topical application of a high potency topical steroid cream (clobetasol propionate) to pigs with 2nd degree burns was not able to prevent secondary burn wound progression.
7.4. Anti-oxidants
Progressive tissue damage in burns is accompanied by the generation of abnormally high levels of reactive-oxygen-species (ROS) [91]. Therefore antioxidants are one approach to neutralize ROS and possibly reduce burn progression [25]. Singer et al. [117] investigated the effect of curcumin, a constituent of the oriental spice turmeric that has antioxidant, anti-inflammatory, and anti-apoptotic effects [118] on burn wound progression in rats. Crude or purified curcumin was administered IV at different dosages (1–100 μg/kg purified or 4–40 mg/kg crude) 1 and 24 h after burn induction. Both crude and purified curcumin were able to significantly reduce burn wound progression both at the surface and at depth in a dose-dependent manner. There were no toxic effects at higher doses or with pure curcumin. Lazaroids are 21-aminosteroids that act as inhibitors of lipid peroxidation in membranes, and can also act as scavengers of ROS and free radicals [119]. Choi et al. [120] tested the lazaroid compound (U75412E) administered intramuscularly in rats at a dosage of 2 mg/kg either 0, 1 or 2 h after a burn. At 24 h, microvascular patency and tissue perfusion were significantly increased with lazaroid-administration either immediately or at 1 h. At day 5, the lazaroid significantly reduced burn wound progression at the surface and at depth. Deniz et al [121] administered N-acetylcysteine (NAC), 1 h after burn induction daily for 10 days either orally (490 mg/kg) or IP (100 mg/kg). There was significantly less progression of the burn wound (27% viable interspaces with IP NAC; 20% with oral NAC; vs 4% with control). Wang et al tested the topical application of the metal chelator EDTA [122]. EDTA was contained in Livionex LF lotion, a preparation that contains disodium EDTA and methyl sulfonyl methane (MSM) as a permeability enhancer. LF lotion-treated burn sites and interspaces showed morphological improvement compared to untreated burn sites. There was less immunostaining for lipid aldehyde-protein adducts with HNE, MDA and ACR, and the expression of aldehyde dehydrogenase isozymes in the unburned interspaces was restored.
Human-recombinant copper-zinc-superoxide dismutase (Hr-CuZnSOD) is an enzyme that destroys superoxide radicals and can be topically applied to burns, either injected directly into the lesions, spread as an enzyme-containing gel onto the burned tissue, or encapsulated into liposomes consisting of 1,2 dipalmitoy-sn-glycero-3-phosphocholine, cholesterol and stearylamine [123]. The gel formulation reduced edema, led to smaller wound sizes, and less tissue necrosis compared to the controls, resulting in significantly faster reepithelialization at 3 weeks. However, in two studies Shalom et al [124,125] evaluated the IV administration of Hr-CuZnSOD at a dosage of 20 mg/kg before or after burn injury in rats. This agent did not show any effect in preventing local burn injury progression.
7.5. Anti-apoptotic and pro-survival agents
Autophagy has been reported to be protective against apoptosis, ischemic injury and inflammatory diseases. Rapamycin induces autophagy by interacting with the mechanistic target of rapamycin (mTOR) which is a master regulator of lysosomal biogenesis and autophagic processes. Cells utilize autophagy to recycle damaged or unwanted organelles and macromolecules and in so doing, generate energy and recover precursor building blocks necessary for normal growth [126]. Xiao et al v[127] administered 1 mg/kg rapamycin IP in rats immediately after burn. Rapamycin improved perfusion, decreased inflammation, and apoptotic cell death, resulting in decreased burn depth and more residual hair follicles and less collagen denaturation. Complete re-epithelialization occurred 2 days earlier in rapamycin treated rats.
Methylene blue (MB) is a ‘repurposed drug’ that displays mitochondrial protective, anti-oxidant and nitric oxide blocking properties [128]. MB inhibits glycogen synthase kinase3-beta (GSK3beta) in a AMPK-dependent manner. MB facilitated PKA-mediated GSK3beta serine phosphorylation independently of AMPK [129]. MB increased the NAD(+)/NADH ratio in hepatocytes and up-regulated SIRT1, thereby decreasing PGC-1α acetylation [130]. Rosique et al [131] administered a single dose of IP MB (2 mg/kg), one or six hours after injury in rats with a comb burn. MB at 1 h gave only (13.3%) necrosis in the interspaces while MB at 6 h gave 44.7% and control had 64.8%. Re-epithelialization skin areas were higher in both MB groups (39.94% for 1 h and 31.89% for 6 h) than control (14.63%).
Piracetam is a nootropic drug (2-oxo-1-pyrrolidineacetamide, a cyclic derivative of GABA) that has been widely tested to improve cognitive impairment [132]. Germonpre et al [133] administered piracetam IM to rats twice daily for 3 days, starting 4 h after the burn injury. Piracetam gave less destruction of the basal membrane, although subepidermal leukocyteinfiltration and destruction of the skin appendages were not significantly decreased compared to the controls. Sari et al [134] administered piracetam either IV or topically for 14 days to rabbits with 3rd degree burns. After 21 days, both formulations significantly enhanced healing with the topical piracetam somewhat superior.
7.6. Physical agents
The application of negative pressure (NP, 125mm Hg vacuum) is widely used in the therapy of chronic wounds [135]. Morykwas et al [136] applied NP to burn wounds in pigs for 6 h within 12 h of burn decreased the burn depth progression and inflammatory response compared to the controls. Hyperbaric oxygen therapy (HBOT) is used to stimulate wound healing in chronic wounds and complicated acute wounds [137]. Germonpré et al used 100% oxygen pressurized at 203 kPa for 60 min in a rat model to reduce dermal leukocyte infiltration, inhibit destruction of the basal membrane, and reduce the progression of the burn [133]. Türkaslan et al [138] applied oxygen at 2.5 atmosphere for 90 min twice daily starting 30 min after burn injury. Reduced progression of surface burn depth necrosis was seen at 5 days in treated. Photobiomodulation, also known as ‘low level laser therapy’ is the use of relatively low power red or near-infrared light to reduce inflammation, protect tissue from dying and stimulate healing [139]. Rathnakar [140], Yadav [141],
8. Burn dressings
The application of dressings began in ancient times, and as time progressed dressings began to have defined goals such as prevention of infection, promotion of reepithelialization, avoidance of loss of moisture and heat, keeping the wound moist, and decreasing pain [142]. A variety of biological, hybrid biological, and other dressings have been used to cover burn wounds, to aid epithelialization, and to protect the wounds from desiccation, infection and mechanical trauma [143]. Biological dressings, such as allograft skin [144], xenograft skin (e.g., porcine) [145], and human amniotic membrane [146], have been used to cover the wound until reepithelialization occurs. The biological dressings have drawbacks, including limited availability, need for tissue collection, storage, risk of disease transmission, and high cost [147]. Therefore, conventional dressings such as Vaseline gauze or silicone sheets (i.e., Mepitel) and synthetic dressings such as Mepilex, DuoDERM, Omniderm, Tegaderm, and hydrocolloids may be preferred to cover the wound. A number of silver containing dressings are currently used for antimicrobial protection in burn care including ACTICOAT*, Mepilex Ag and Aquacel Ag products [148]. Burn dressings have been extensively reviewed, and to conserve space I will refer the readers to some of these excellent reviews [148–151].
9. Infection
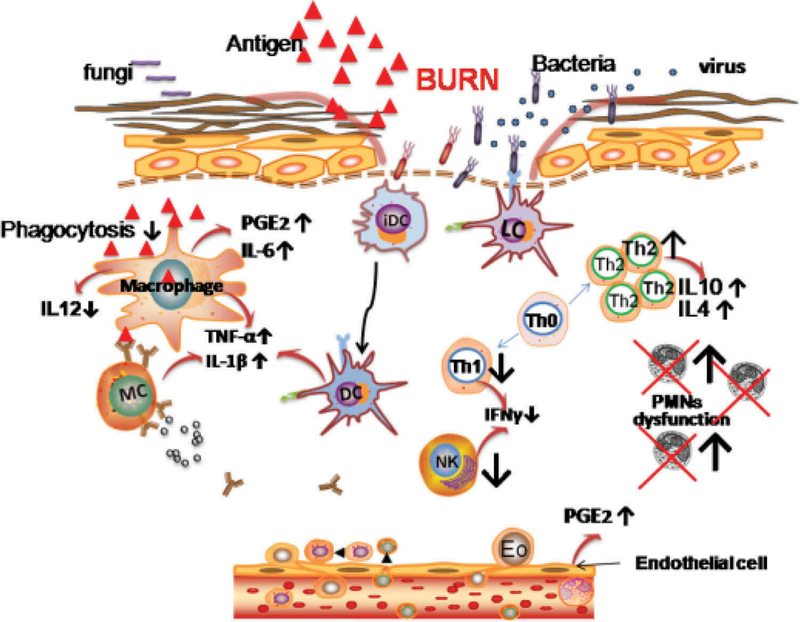
Skin is the first line of defense acting as a physical barrier against microbial invasion. The disruption of the epidermal barrier combined with denaturation of proteins and lipids provides a fertile environment for microbial growth [152]. Furthermore, a complex cascade of biochemical events leads to a ‘systemic apoptotic response’ and the onset of both systemic and local immunosuppression that abrogates the normal self-defense mechanisms that would fight against infection [153]. The area of the body that is burned is the most important factor in deciding outcomes in patients, with burns over 50% of the body sometimes proving fatal [154]. The difficulties faced by systemically administered antibiotics in reaching the damaged tissue with its compromised circulation, has encouraged the use of topically applied antimicrobial products [155,156]. Figure 4 illustrates some of the biological pathways occurring in burns that predispose toward infection.
Figure 4. Signaling pathways predisposing burn wounds to infection.
Abbreviations: PGE2 – prostaglandin E2; IFNƔ ¯ interferon gamma; Th1/2 – T-helper cells type ½; PMNs – polymorphonuclear cells.
Silver still represents one of the major topical agents used in prevention and treatment of burn infections [157]. Silver sulfadiazine 1% (SSD) has been regarded as the gold standard for more than 40 years, and many advanced silver delivery systems have been compared with SSD. Despite the fact that many dressings showed superior healing properties compared to SSD, however no dressing was so far able to show a clear benefit over SSD regarding infection [158]. Fewer dressing changes, less pain and greater patient satisfaction were found with the newer dressings, especially with solid and biological dressings.
Many high-tech methods have been developed to combat burn wound infections in animal models. In the interests of space, we will only summarize the use of two approaches here. Smart targeted therapies have been devised that includevarious nanostructures that can be activated in response to physical and biochemical stimuli [159]. Grützner et al. [160] studied the effect of enzyme-sensitive antimicrobial nanoplatforms loaded with polyhexanide biguanide (cationic polymer) and octenidine (cationic surfactant) for prevention of burn infection. Temperature-sensitive poly-N-isopropylacrylamide polymeric nanoparticles, showed triggered release of bacteriophages targeted against specific bacteria in infected burns [161]. In another study N-acetyl chitosan was hydrolyzed by lysozyme in burn infection to give N-acetylated chito oligosaccharides that served as substrates for added cellobiose dehydrogenase producing hydrogen peroxide that could kill S. aureus and E. coli [162].
Light is attractive as an antimicrobial approach to treat burn infections that tend to be superficial in nature and to not respond to antibiotics due to resistance of poorly perfused tissue. Due to its broad target specificity light cannot cause resistance to develop against itself [163]. Antimicrobial photodynamic therapy uses the combination of topically applied photosensitizing dyes excited by visible light to generate ROS and kill microbial cells [150]. The addition of common non-toxic salts such as potassium iodide can dramatically potentiate the microbial killing [164]. Blue light alone (without the addition of any photosensitizer) can also kill bacteria and fungi and has been used to treat infected burns [165]. Ultraviolet C light (200–280 nm) is highly antimicrobial and although it can cause damage to mammalian cells, the damage is rapidly repaired by DNA repair systems. UVC can be used to treat superficial burn infections [166,167].
10. Regeneration
Miscellaneous types of stem cells have been utilized for skin regeneration and healing in burns and other wounds, such as embryonic SCs [168], adult SCs [169], induced pluripotent SCs (iPSCs) [170], mesenchymal SCs (MSCs) [171], adipose derived SCs (ADSCs) [172], human umbilical cord-derived SCs [173] and melanocyte stem cells [174]. Applying MSCs into the wound area increased re-epithelization and increased angiogenesis [175]. Embedded ADSCs within an acellular dermal matrix applied onto the wound led to increased wound healing and better vascularization [172]. Human induced melanocyte stem cells produce hair follicles and epidermal pigment [173]. Skin-derived SCs were utilized for regenerating the neural cells in the skin that could be damaged in burn wounds [176]. A complete discussion of the role of stem cells in burns and burn wound healing is beyond the scope of this review [177].
11. Prevention and treatment of scars
Hypertrophic scars are mild forms, and keloids are severe forms of excessive scarring. Keloids extend beyond the original wound margins. Scars are particularly troublesome for burns survivors. The symptoms include pruritus, pain, burning, stiffness, and disabling contractures [178]. Conventional treatments that have been tried, include laser therapy, silicone gel sheets, compression garments, silicone combined with pressure, massage therapy, topical emollients, adhesive tape support, intralesional corticosteroids, radiotherapy, immunotherapy, antimetabolites, botulinum toxin A, and surgical treatment [179].
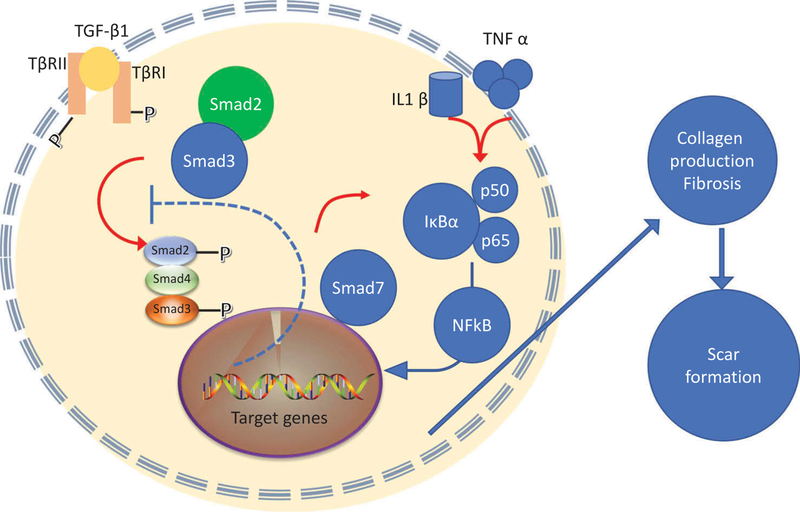
The discovery of the transforming growth factor-beta (TGFß) superfamily, with its complex signaling crosstalk and interactions with other cytokines and pathways led to the understanding of why fetal wounds heal without any scarring [180,181]. This discovery led to several attempts to interfere with scar formation by modulating various components of the TGF-ß pathway. Figure 5 shows signaling pathways resulting from the activation of TGF-ß pathway.
Figure 5. Signaling pathways resulting in scar formation.
Abbreviations: TGFβ – transforming growth factor beta; TβRI – TGFβ receptor; smad – similar to ‘mothers against decapentaplegic’; TNFα – tumor necrosis factor alpha; IL1β – interleukin 1beta; NFkB – nuclear factor kappa B; IkBα – inhibitor of NFkB alpha.
Mannose-6-phosphate (M6P) inhibits the activation of TGF-β1 and TGF-β2 [182] and local application of recombinant M6P was studied to treat scars (clinicalTrials.gov: NCT00984516 and NCT00984854) by the company Renovo, under the brand name Juvidex. However, the trials failed to meet the primary endpoint (https://scrip.pharmaintelligence.informa.com/SC000865/Renovos-Juvidex-fails-to-accelerate-healing-of-skingraft-wounds-in-Phase-II).
Dipeptidyl peptidase IV (DPPIV) is a protease that promotes cell invasion and tumor growth, as well as the activity of fibroblast activation protein α (FAP-α). Keloid fibroblasts are characterized by increased expression of FAP-α, and normal adult tissues are generally FAP-α-negative. Inhibition of DPPIV using the irreversible inhibitor H2N-Gly-Pro-diphenylphosphonate suppressed TGF-β1, and could be a treatment option to prevent keloid progression [183].
Phosphorylation of Smad3 and not Smad2 primarily mediates the pro-fibrotic effect of TGF-ß [184]. This is because Smad2 and Smad 3 show non-overlapping target gene binding specificity and differential transcriptional activity [185]. Samd3 is more likely to regulate genes implicated in tissue fibrosis [186]. Furthermore, it has been reported that RNAi-mediated knockdown of Smad3 expression decreased collagen production in keloid fibroblasts [187]. A range of different inhibitors could be candidates for this goal including TRAP-1-like protein (TLP), which in turn co-activates Smad2, halofuginone, quercetin, P144, trichostatin A (TSA) and paclitaxel, among others [188]. Halofuginone is a selective inhibitor of type-1 collagen synthesis and blocks TGF-ß-mediated Smad3 activation in fibroblasts, suppressing dermal fibrosis in vivo [189]. Halofuginone reduced fibrosis in a murine model of sclerodermatous graft versus host disease [190].
Quercetin (3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one dihydrate) is a dietary flavonoid glycone found in onions, apples, red wine and gingko biloba, that has been studied for inhibition of burn-related scarring [191]. It inhibits not only Smad3, but also Smad2, Smad4, TGF-ß, the coactivator proteins p300 and Creb binding protein (CBP), phosphatidylinositol 3-kinase (PI3-K) and insulin-like-growth factor1 (IGF-1), eventually blocking fibrosis [192]. The peptide P144 is derived from the human TGFβ type III receptor (betaglycan) and inhibits both Smad3 and Smad2 as well as TGFβ1. P144 was shown to reverse bleomycin-induced skin fibrosis when topically applied to mice [193] and reduced periprosthetic capsular contracture in pigs [194].
Asiaticoside is a phytochemical extracted from the leaves of the Indian pennywort (Centella asiatica) that activates Smad7 in normal and hypertrophic scar fibroblasts, reducing expression of both TGF-ßRI and TGF-ßRII, and inhibiting fibrosis [195]. Another herbal medicine which up-regulates Smad7 and blocks Smad2/3 is ‘Boui’ or tetandrine which is a bis-benzylisoquinoline alkaloid [196]. Interferon-γ (IFN-γ) is known to induce endogenous Smad7 and therefore antagonize TGF-β signals [83]. Two small clinical trials have suggested the possibility of using intralesional IFN-γ to treat abnormal dermal scarring [197,198].
12. Conclusion
Research into pharmaceutical therapy for severe burns can be regarded as having some overlap with treatment options for patients with sepsis and other critical illnesses. Agents that modulate glucose metabolism, insulin, and circulatory parameters are being studied in both types of conditions, despite superficial differences. Severely burned patients are also likely to have smoke inhalation injury, and again there is overlap in the investigational agents for both conditions, for instance antioxidants such as NAC.
One area of pharmacological research that is thriving, however, is the search for agents that can prevent or slow down the local worsening of burn injuries, known as burn conversion. Again this pathology has aspects in common with the progression is ischemic injuries to heart or brain. Due to the inexorable rise in multi-antibiotic resistance amongst common bacteria, and the general inability of systemically administered antibiotics to penetrate to burn wounds, research into novel non-antibiotic ways of killing bacteria and fungi in burns has proliferated, and only limits of space has limited further coverage of this intriguing topic. The reader is referred to some excellent reviews in this area [150,199,200]. Some of the worst scars suffered after any type of trauma, are those typical of severe burn injuries, and attempts to discover new pharmacological agents which can prevent or slow down scar development by interfering with the TGF-ß pathway are underway in many laboratories.
13. Expert opinion
As discussed in Section 2, severe burns are now largely a problem in the less-developed regions of the world. This consideration may explain the relative lack of pharmacotherapy research by large pharmaceutical companies which are generally based in developed Western countries. In the US the number of specialized burn centers has been dropping, to the extent that a 2007 story by Bill Poovey in The Washington Post stated that ‘U.S. hospitals are increasingly shutting down their burn centers in a trend experts say could leave the nation unable to handle widespread burn casualties from a fiery terrorist attack or other major disaster.’ [201]. Another reason that has limited research into new treatments for burns by pharmaceutical companies, is that a burn is an acute traumatic event which requires only short-term treatment, and big pharma has found that it is more profitable to develop drugs for chronic complaints that can require regular treatment for many years.
Nevertheless, researchers have been searching for new drugs and treatments that can ameliorate some of the pathologies associated with severe burns. Many of the pathophysiological challenges faced by severe burn patients have aspects in common with severely ill patients in intensive care units with sepsis or polytrauma. Burns interfere with metabolism in a similar fashion to type 2 diabetes, by producing changes in glucose metabolism and impaired insulin sensitivity. Adding to the complexity is the fact that an increasing proportion of the population will have diabetes even before these individuals suffer burns. Approximately 30.3 million Americans have diabetes and another 84.1 million have prediabetes, amounting in total to 30% of the population [202]. Burns patients with diabetes have a worse prognosis with regard to infection, wound healing, and overall survival [203]. Therefore, pharmaceutical interventions designed to correct the metabolic disturbances encountered in burn patients will have applications that could range more widely than just in burns.
In a similar vein, the pathophysiological aspects of smoke inhalation injury have aspects in common with acute respiratory distress syndrome (ARDS) that is found in patients with pneumonia and sepsis. Excessive inflammation and disturbances in coagulation can in principle be treated with known pharmaceutical agents, and some success has been shown in animal models. The difficulty arises when it comes to testing these agents in controlled clinical trials. The history of failure in many different clinical trials for sepsis underlines this difficulty. One example is the controversy surrounding the use of low dose corticosteroids in sepsis and critically ill patients [204, 205]. Although corticosteroids may eventually be useful it will almost certainly be in combination with other agents.
Agents designed to prevent the progression of burn injury have shown success in many animal models, however as yet there have not been many controlled clinical trials. Perhaps this is because of the adoption of early excision and skin grafting makes it less critical to follow and intervene in the zone of stasis to prevent tissue necrosis from enlarging. However the investigation into new agents designed to halt the progression of tissue necrosis have applications far beyond the issue of burn conversion. Myocardial infarction, traumatic brain injury, stroke, and other ischemic injuries are widespread conditions where tissue protective strategies could have significant benefits. One example is the use of anti-oxidants that has had mixed results in many clinical trials. The modern viewpoint is that oxidative stress can be both good and bad at the same time. Many signaling pathways are triggered by reactive oxygen pathways, and blocking all oxidative stress may indeed be counter-productive. A similar conclusion can be made about inflammation. Although excessive inflammation is clearly bad in many circumstances, blocking all inflammation, again can be counter-productive.
The use of mesenchymal stem cells is under intensive investigation for prevention and repair of multiple types of tissue injuries, and burns is no exception [206]. Debate still continues about how much of the benefit of stem cell therapy is due to the long-term engraftment of the stem cells into the actual site of injury, and how much is due to the anti-inflammatory effects that stem cells exert on the host response [207].
The prevention of scar formation is also under intensive investigation, since scars can be life-changing sequelae for burns victims. The discovery of the TGFβ pathway has led to studies investigating pharmaceutical interventions to modulate various biochemical steps that lead to formation of hypertrophic scars and keloids.
The modern era of molecular and personalized medicine is expected to lead to the introduction of pharmaceuticals that will play a part in treatment of victims of serious burns as well as other critically ill patients.
Article highlights.
The prognosis for severe burns has improved in the last 50 years but research in pharmacotherapy continues.
Agents that ameliorate the systemic disturbances in metabolism and hyperglycemia.
Agents to combat smoke inhalation and prevent local burn progression.
New antimicrobial dressings and approaches based on light and nanotechology to fight infection.
Stem cell based approaches to help wound healing and TGFβ interventions to prevent scarring.
This box summarizes key points contained in the article
Acknowledgments
Funding
MR Hamblin was supported by US National Institutes of Health/National Institute of Allergy and Infectious Diseases Grants R01AI050875 and R21AI121700.
Footnotes
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
References
- 1.Artz CP. Historical aspects of burn management. Surg Clin North Am 1970;50:1193–1200. [DOI] [PubMed] [Google Scholar]
- 2.Liu HF, Zhang F, Lineaweaver WC. History and advancement of burn treatments. Ann Plast Surg 2017;78:S2–S8. [DOI] [PubMed] [Google Scholar]
- 3.Majno G The healing hand: man and wound in the ancient world MA: HarvardUniversity Press Cambridge; 1975. [Google Scholar]
- 4.Markatos K, Tzivra A, Tsoutsos S, et al. Ambroise pare (1510–1590) and his innovative work on the treatment of war injuries. Surg Innov 2018;25:183–186. [DOI] [PubMed] [Google Scholar]
- 5.Kirkpatrick JJ, Curtis B, Fitzgerald AM, et al. A modern translation and interpretation of the treatise on burns of Fabricius Hildanus (1560–1634). Br J Plast Surg 1995;48:460–470. [DOI] [PubMed] [Google Scholar]
- 6.Kentish E An essay on burns, principally upon those which happpen [sic] to workmen in mines from the explosions of inflammable air, (or hydrogen gas.) Newcastle,UK: : G.G. and J. Robinson; 1797. [Google Scholar]
- 7.Dickinson N Remarks on burns and scalds, chiefly in reference to the principle of treatment at the time of their infliction. Suggested by a perusal of the last edition of ‘An essay on burns’, by Edward Kentish MDJ UK: London; 1818. [Google Scholar]
- 8.Causes Dupuytren G., different degrees, complications, anatomical characters and treatment of burns. Clin Lect Surg 1832;18:229–280. [Google Scholar]
- 9.Curling TB. On acute ulceration of the duodenum in cases of burn. Med Chir Trans 42:30–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reverdin J De la greffe epidermique (Classic reprint) London¸UK: FB&C Ltd; 2017. (1872). [Google Scholar]
- 11.Shirani KZ, Vaughan GM, Mason AD Jr., et al. Update on current therapeutic approaches in burns. Shock 1996;5:4–16. [PubMed] [Google Scholar]
- 12.Jeschke MG, Kamolz L-P, Sjöberg F, et al. Handbook of burns volume 1: acute burn care New York, NY: Springer Science & Business Media; 2012. [Google Scholar]
- 13.Bull JP, Fisher AJ. A study of mortality in a burns unit: a revised estimate. Ann Surg 1954;139:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pruitt BA Jr., Goodwin CW, Mason ADJ. Epidemiological, emographic, and outcome characteristics of burn injury. In: Herndon DN, Saunders WB, editors. Total burn care Philadelphia, PA: WB Saunders; 2002. p. 16–30. [Google Scholar]
- 15.Barrow RE, Spies M, Barrow LN, et al. Influence of demographics and inhalation injury on burn mortality in children. Burns 2004;30:72–77. [DOI] [PubMed] [Google Scholar]
- 16.Saaiq M, Zaib S, Ahmad S. Early excision and grafting versus delayed excision and grafting of deep thermal burns up to 40% total body surface area: a comparison of outcome. Ann Burns Fire Disasters 2012;25:143–147. [PMC free article] [PubMed] [Google Scholar]
- 17.Keshavarzi A, Ayaz M, Dehghankhalili M. Ultra-early versus early excision and grafting for thermal burns up to 60% total body surface area; a historical cohort study. Bull Emerg Trauma 2016;4:197–201. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Beekman J, Hew J, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev 2018;123:3–17. [DOI] [PubMed] [Google Scholar]
- 19.Smolle C, Cambiaso-Daniel J, Forbes AA, et al. Recent trends in burn epidemiology worldwide: a systematic review. Burns 2017;43:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peck M, Pressman MA. The correlation between burn mortality rates from fire and flame and economic status of countries. Burns 2013;39:1054–1059. [DOI] [PubMed] [Google Scholar]
- 21.World_Health_Organization. The global burden of disease: 2004 update. The Global Burden of Disease: 2004 Update 2008.
- 22.American_Burn_Association. National Burn Repository (2012 report) National Burn Repository (2012 report). 2012.
- 23.Friedstat J, Brown DA, Levi B. Chemical, electrical, and radiation injuries. Clin Plast Surg 2017;44:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci 2014;71:3241–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shupp JW, Nasabzadeh TJ, Rosenthal DS, et al. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res 2010;31:849–873. [DOI] [PubMed] [Google Scholar]
- 26.Walker HL, Mason AD Jr. A standard animal burn. J Trauma 1968;8:1049–1051. [DOI] [PubMed] [Google Scholar]
- 27.Steinstraesser L, Trust G, Rittig A et al. Colistin-loaded silk membranes against wound infection with Pseudomonas aeruginosa. Plast Reconstr Surg 2011;127:1838–1846. [DOI] [PubMed] [Google Scholar]
- 28.Jones WG 2nd, Minei JP, Barber AE, et al. Bacterial translocation and intestinal atrophy after thermal injury and burn wound sepsis. Ann Surg 1990;211:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakae H, Inaba H, Endo S. Usefulness of procalcitonin in Pseudomonas burn wound sepsis model. Tohoku J Exp Med 1999;188:271–273. [DOI] [PubMed] [Google Scholar]
- 30.Barnea Y, Carmeli Y, Kuzmenko B, et al. The establishment of a Pseudomonas aeruginosa-infected burn-wound sepsis model and the effect of imipenem treatment. Ann Plast Surg 2006; 56:674–679. [DOI] [PubMed] [Google Scholar]
- 31.Fader RC, Nunez D, Unbehagen J, et al. Experimental candidiasis after thermal injury. Infect Immun 1985;49:780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinstraesser L, Tack BF, Waring AJ et al. Activity of novispirin G10 against Pseudomonas aeruginosa in vitro and in infected burns. Antimicrob Agents Chemother 2002;46:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobsen F, Mittler D, Hirsch T, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther 2005;12:1494–1502. [DOI] [PubMed] [Google Scholar]
- 34.Bjornson AB, Bjornson HS, Lincoln NA, et al. Relative roles of burn injury, wound colonization, and wound infection in induction of alterations of complement function in a guinea pig model of burn injury. J Trauma 1984;24:106–115. [DOI] [PubMed] [Google Scholar]
- 35.Orenstein A, Klein D, Kopolovic J, et al. The use of porphyrins for eradication of Staphylococcus aureus in burn wound infections. FEMS Immunol Med Microbiol 1997;19:307–314. [DOI] [PubMed] [Google Scholar]
- 36.Stieritz DD, Holder IA. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis 1975;131:688–691. [DOI] [PubMed] [Google Scholar]
- 37.Katakura T, Yoshida T, Kobayashi M, et al. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin Exp Immunol 2005;142:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manafi A, Kohanteb J, Mehrabani D, et al. Active immunization using exotoxin A confers protection against Pseudomonas aerugi–nosa infection in a mouse burn model. BMC Microbiol 2009;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumari S, Harjai K, Chhibber S. Topical treatment of Klebsiella pneumoniae B5055 induced burn wound infection in mice using natural products. J Infect Dev Ctries 2010;4:367–377. [PubMed] [Google Scholar]
- 40.Gurfinkel R, Singer AJ, Cagnano E, et al. Development of a novel animal burn model using radiant heat in rats and swine. Acad Emerg Med 2010;17:514–520. [DOI] [PubMed] [Google Scholar]
- 41.Summer GJ, Puntillo KA, Miaskowski C, et al. TrkA and PKC-epsilon in thermal burn-induced mechanical hyperalgesia in the rat. J Pain 2006;7:884–891. [DOI] [PubMed] [Google Scholar]
- 42.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery 2000;128:312–319. [DOI] [PubMed] [Google Scholar]
- 43.Williams FN, Herndon DN. Metabolic and endocrine considerations after burn injury. Clin Plast Surg 2017;44:541–553. [DOI] [PubMed] [Google Scholar]
- 44.Jeschke MG, Chinkes DL, Finnerty CC, et al. Pathophysiologic response to severe burn injury. Ann Surg 2008;248:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams FN, Herndon DN, Hawkins HK, et al. The leading causes of death after burn injury in a single pediatric burn center. Crit Care 2009;13:R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gauglitz GG, Williams FN, Herndon DN, et al. Burns: where are we standing with propranolol, oxandrolone, recombinant human growth hormone, and the new incretin analogs? Curr Opin Clin Nutr Metab Care 2011;14:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gauglitz GG, Herndon DN, Kulp GA, et al. Abnormal insulin sensitivity persists up to three years in pediatric patients post-burn. J Clin Endocrinol Metab 2009;94:1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdullahi A, Jeschke MG. Nutrition and anabolic pharmacotherapies in the care of burn patients. Nutr Clin Pract 2014;29:621–630. [DOI] [PubMed] [Google Scholar]
- 49.Kulp GA, Tilton RG, Herndon DN, et al. Hyperglycemia exacerbates burn-induced liver inflammation via noncanonical nuclear factor-kappaB pathway activation. Mol Med 2012;18:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma 2001;51:736–739. [DOI] [PubMed] [Google Scholar]
- 51.Prelack K, Dylewski M, Sheridan RL. Practical guidelines for nutritional management of burn injury and recovery. Burns 2007;33:14–24.•• Authoritative review on the metabolic and nutritional effects of serious burns.
- 52.Al-Mousawi AM, Williams FN, Mlcak RP, et al. al. Effects of exercise training on resting energy expenditure and lean mass during pediatric burn rehabilitation. J Burn Care Res 2010;31:400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Branski LK, Herndon DN, Barrow RE, et al. Randomized controlled trial to determine the efficacy of long-term growth hormone treatment in severely burned children. Ann Surg 2009;250:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeschke MG, Herndon DN, Barrow RE. Insulin-like growth factor I in combination with insulin-like growth factor binding protein 3 affects the hepatic acute phase response and hepatic morphology in thermally injured rats. Ann Surg 2000;231:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spies M, Wolf SE, Barrow RE, et al. Modulation of types I and II acute phase reactants with insulin-like growth factor-1/binding protein-3 complex in severely burned children. Crit Care Med 2002;30:83–88. [DOI] [PubMed] [Google Scholar]
- 56.Pidcoke HF, Wade CE, Wolf SE. Insulin and the burned patient. Crit Care Med 2007;35:S524–S530.• Stresses the importance and glucose control in patients with severe burns.
- 57.Finney SJ, Zekveld C, Elia A, et al. Glucose control and mortality in critically ill patients. JAMA 2003;290:2041–2047. [DOI] [PubMed] [Google Scholar]
- 58.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–139. [DOI] [PubMed] [Google Scholar]
- 59.Jeschke MG, Finnerty CC, Suman OE, et al. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg 2007;246: 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolf SE, Edelman LS, Kemalyan N, et al. Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res 2006;27: 131–139. [DOI] [PubMed] [Google Scholar]
- 61.Przkora R, Herndon DN, Suman OE. The effects of oxandrolone and exercise on muscle mass and function in children with severe burns. Pediatrics 2007;119:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sirtori CR, Pasik C. Re-evaluation of a biguanide, metformin: mechanism of action and tolerability. Pharmacol Res 1994; 30:187–228. [DOI] [PubMed] [Google Scholar]
- 63.Gore DC, Wolf SE, Herndon DN, et al. Metformin blunts stress-induced hyperglycemia after thermal injury. J Trauma 2003;54:555–561. [DOI] [PubMed] [Google Scholar]
- 64.Gore DC, Herndon DN, Wolfe RR. Comparison of peripheral metabolic effects of insulin and metformin following severe burn injury. J Trauma 2005;59: 316–322. [DOI] [PubMed] [Google Scholar]
- 65.Angioi A, Cabiddu G, Conti M, et al. Metformin associated lactic acidosis: a case series of 28 patients treated with sustained low-efficiency dialysis (SLED) and long-term follow-up. BMC Nephrol 2018;19:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Flores O, Stockton K, Roberts JA, et al. The efficacy and safety of adrenergic blockade after burn injury: a systematic review and meta-analysis. J Trauma Acute Care Surg 2016;80:146–155.• Suggests that adrenergic bloackade with propanolol may help with the hypermetabolic state after severe burns.
- 67.Williams FN, Herndon DN, Kulp GA, et al. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery 2011;149:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chao T, Porter C, Herndon DN, et al. Propranolol and oxandrolone therapy accelerated muscle recovery in burned children. Med Sci Sports Exerc 2018. March;50(3):427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen A, Lund A, Knop FK, et al. Glucagon-like peptide 1 in health and disease. Nat Rev Endocrinol 2018. July;14(7):390–403. [DOI] [PubMed] [Google Scholar]
- 70.Shen CA, Fagan S, Fischman AJ, et al. Effects of glucagon-like peptide 1 on glycemia control and its metabolic consequence after severe thermal injury–studies in an animal model. Surgery 2011;149:635–644.• Shows that glucagon like peptide can help with
- 71.Gupta V Glucagon-like peptide-1 analogues: an overview. Indian J Endocrinol Metab 2013;17:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19:524–536. [DOI] [PubMed] [Google Scholar]
- 73.Zhao D, Ma L, Shen C, et al. Long-lasting glucagon-like peptide 1 analogue exendin-4 ameliorates the secretory and synthetic function of islets isolated from severely scalded rats. J Burn Care Res 2018. June 13;39(4):545–554. [DOI] [PubMed] [Google Scholar]
- 74.Jia YL, Sun SJ, Chen JH, et al. SS31, a small molecule antioxidant peptide, attenuates beta-amyloid elevation, mitochondrial/synaptic deterioration and cognitive deficit in SAMP8 mice. Curr Alzheimer Res 2016;13:297–306. [DOI] [PubMed] [Google Scholar]
- 75.Calkins MJ, Manczak M, Reddy PH. Mitochondria-targeted antioxidant SS31 prevents amyloid beta-induced mitochondrial abnormalities and synaptic degeneration in alzheimer’s disease. Pharmaceuticals (Basel) 2012;5:1103–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carter EA, Bonab AA, Goverman J, et al. Evaluation of the antioxidant peptide SS31 for treatment of burn-induced insulin resistance. Int J Mol Med 2011;28:589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta K, Mehrotra M, Kumar P, et al. Smoke inhalation injury: etiopathogenesis, diagnosis, and management. Indian J Crit Care Med 2018;22:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Einhorn IN. Physiological and toxicological aspects of smoke produced during the combustion of polymeric materials. Environ Health Perspect 1975;11:163–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palmieri TL, Enkhbaatar P, Sheridan R, et al. Studies of inhaled agents in inhalation injury. J Burn Care Res 2009;30:169–171. [DOI] [PubMed] [Google Scholar]
- 80.Yim RP, Koumbourlis AC. Tolerance & resistance to beta(2)-agonist bronchodilators. Paediatr Respir Rev 2013;14:195–198. [DOI] [PubMed] [Google Scholar]
- 81.Palmieri TL, Enkhbaatar P, Bayliss R, et al. Continuous nebulized albuterol attenuates acute lung injury in an ovine model of combined burn and smoke inhalation. Crit Care Med 2006; 34:1719–1724. [DOI] [PubMed] [Google Scholar]
- 82.Roux E, Molimard M, Savineau JP, et al. Muscarinic stimulation of airway smooth muscle cells. Gen Pharmacol 1998;31:349–356. [DOI] [PubMed] [Google Scholar]
- 83.Jonkam C, Zhu Y, Jacob S, et al. Muscarinic receptor antagonist therapy improves acute pulmonary dysfunction after smoke inhalation injury in sheep. Crit Care Med 2010;38:2339–2344. [DOI] [PubMed] [Google Scholar]
- 84.Lopez E, Fujiwara O, Lima-Lopez F, et al. Nebulized epinephrine limits pulmonary vascular hyperpermeability to water and protein in ovine with burn and smoke inhalation injury. Crit Care Med 2016;44:e89–e96. [DOI] [PubMed] [Google Scholar]
- 85.Foncerrada G, Lima F, Clayton RP, et al. Safety of nebulized epinephrine in smoke inhalation injury. J Burn Care Res 2017;38:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Csontos C, Rezman B, Foldi V, et al. Effect of N-acetylcysteine treatment on oxidative stress and inflammation after severe burn. Burns 2012;38:428–437. [DOI] [PubMed] [Google Scholar]
- 87.Miller AC, Elamin EM, Suffredini AF. Inhaled anticoagulation regimens for the treatment of smoke inhalation-associated acute lung injury: a systematic review. Crit Care Med 2014;42:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Enkhbaatar P, Cox RA, Traber LD, et al. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med 2007;35:2805–2810. [DOI] [PubMed] [Google Scholar]
- 89.Mcginn KA, Weigartz K, Lintner A, et al. Nebulized heparin with N-Acetylcysteine and albuterol reduces duration of mechanical ventilation in patients with inhalation injury. J Pharm Pract 2017. January 1:897190017747143. doi: 10.1177/0897190017747143. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 90.Schmauss D, Rezaeian F, Finck T, et al. Treatment of secondary burn wound progression in contact burns-a systematic review of experimental approaches. J Burn Care Res 2015;36:e176–e189. [DOI] [PubMed] [Google Scholar]
- 91.Salibian AA, Rosario ATD, Severo L, et al. Current concepts on burn wound conversion-A review of recent advances in understanding the secondary progressions of burns. Burns 2016;42:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rocha J, Eduardo-Figueira M, Barateiro A, et al. Erythropoietin reduces acute lung injury and multiple organ failure/dysfunction associated to a scald-burn inflammatory injury in the rat. Inflammation 2015;38:312–326. [DOI] [PubMed] [Google Scholar]
- 93.Tobalem M, Harder Y, Schuster T, et al. Erythropoietin in the prevention of experimental burn progression. Br J Surg 2012;99: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 94.Tobalem M, Harder Y, Rezaeian F, et al. Secondary burn progression decreased by erythropoietin. Crit Care Med 2013;41:963–971. [DOI] [PubMed] [Google Scholar]
- 95.Mcmillen MA. Endothelin plasma levels in burn patients. Arch Surg 2001;136:1084. [DOI] [PubMed] [Google Scholar]
- 96.Battal MN, Hata Y, Matsuka K, et al. Reduction of progressive burn injury by using a new nonselective endothelin-A and endothelin-B receptor antagonist, TAK-044: an experimental study in rats. Plast Reconstr Surg 1997;99:1610–1619. [PubMed] [Google Scholar]
- 97.Battal MN, Hata Y, Matsuka K, et al. Reduction of progressive burn injury by a stable prostaglandin I2 analogue, beraprost sodium (Procylin): an experimental study in rats. Burns 1996;22:531–538. [DOI] [PubMed] [Google Scholar]
- 98.Hunter RL, Luo AZ, Zhang R, et al. Poloxamer 188 inhibition of ischemia/reperfusion injury: evidence for a novel anti-adhesive mechanism. Ann Clin Lab Sci 2010;40:115–125. [PubMed] [Google Scholar]
- 99.Baskaran H, Toner M, Yarmush ML, et al. Poloxamer-188 improves capillary blood flow and tissue viability in a cutaneous burn wound. J Surg Res 2001;101:56–61. [DOI] [PubMed] [Google Scholar]
- 100.Yuhua S, Ligen L, Jiake C, et al. Effect of Poloxamer 188 on deepening of deep second-degree burn wounds in the early stage. Burns 2012;38:95–101. [DOI] [PubMed] [Google Scholar]
- 101.Isik S, Sahin U, Ilgan S, et al. Saving the zone of stasis in burns with recombinant tissue-type plasminogen activator (r-tPA): an experimental study in rats. Burns 1998;24:217–223. [DOI] [PubMed] [Google Scholar]
- 102.Meyerholz DK, Piester TL, Mcnamara AR, et al. Pharmacologic modification to resuscitation fluid after thermal injury–is drotrecogin alfa the answer to arrest burn depth progression? J Trauma 2009;67:996–1003. [DOI] [PubMed] [Google Scholar]
- 103.Undas A, Brummel-Ziedins KE, Mann KG. Anticoagulant effects of statins and their clinical implications. Thromb Haemost 2014;111:392–400. [DOI] [PubMed] [Google Scholar]
- 104.Uygur F, Evinc R, Urhan M, et al. Salvaging the zone of stasis by simvastatin: an experimental study in rats. J Burn Care Res 2009;30:872–879. [DOI] [PubMed] [Google Scholar]
- 105.Dahiya P Burns as a model of SIRS. Front Biosci (Landmark Ed) 2009;14:4962–4967. [DOI] [PubMed] [Google Scholar]
- 106.Friedl HP, Till GO, Trentz O, et al. Roles of histamine, complement and xanthine oxidase in thermal injury of skin. Am J Pathol 1989;135:203–217. [PMC free article] [PubMed] [Google Scholar]
- 107.Ward PA, Till GO. Pathophysiologic events related to thermal injury of skin. J Trauma 1990;30:S75–S79. [DOI] [PubMed] [Google Scholar]
- 108.Endo S, Inada K, Yamada Y, et al. Plasma tumour necrosis factor-alpha (TNF-alpha) levels in patients with burns. Burns 1993;19:124–127. [DOI] [PubMed] [Google Scholar]
- 109.Moore FD Jr., Davis C, Rodrick M, et al. Neutrophil activation in thermal injury as assessed by increased expression of complement receptors. N Engl J Med 1986;314:948–953. [DOI] [PubMed] [Google Scholar]
- 110.Singer AJ, Mcclain SA, Hacht G, et al. Semapimod reduces the depth of injury resulting in enhanced re-epithelialization of partial-thickness burns in swine. J Burn Care Res 2006;27:40–49. [DOI] [PubMed] [Google Scholar]
- 111.Sun LT, Friedrich E, Heuslein JL, et al. Reduction of burn progression with topical delivery of (antitumor necrosis factor-alpha) hyaluronic acid conjugates. Wound Repair Regen 2012;20:563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bucky LP, Vedder NB, Hong HZ, et al. Reduction of burn injury by inhibiting CD18-mediated leukocyte adherence in rabbits. Plast Reconstr Surg 1994;93:1473–1480. [DOI] [PubMed] [Google Scholar]
- 113.Choi M, Rabb H, Arnaout MA, et al. Preventing the infiltration of leukocytes by monoclonal antibody blocks the development of progressive ischemia in rat burns. Plast Reconstr Surg 1995;96: 1177–1185. [PubMed] [Google Scholar]
- 114.Mileski W, Borgstrom D, Lightfoot E, et al. Inhibition of leukocyte-endothelial adherence following thermal injury. J Surg Res 1992;52:334–339. [DOI] [PubMed] [Google Scholar]
- 115.Lin CF, Young KC, Bai CH, et al. Rosiglitazone regulates anti-inflammation and growth inhibition via PTEN. Biomed Res Int 2014;787924:2014. [DOI] [PMC free article] [PubMed]
- 116.Taira BR, Singer AJ, Mcclain SA, et al. Rosiglitazone, a PPAR-gamma ligand, reduces burn progression in rats. J Burn Care Res 2009;30:499–504. [DOI] [PubMed] [Google Scholar]
- 117.Singer AJ, Taira BR, Lin F, et al. Curcumin reduces injury progression in a rat comb burn model. J Burn Care Res 2011;32:135–142. [DOI] [PubMed] [Google Scholar]
- 118.Jha A, Mohapatra PP, Alharbi SA, et al. Curcumin: not so spicy after all. Mini Rev Med Chem 2017;17:1425–1434. [DOI] [PubMed] [Google Scholar]
- 119.Clark WM, Hazel JS, Coull BM. Lazaroids. CNS pharmacology and current research. Drugs 1995;50:971–983. [DOI] [PubMed] [Google Scholar]
- 120.Choi M, Ehrlich HP. U75412E, a lazaroid, prevents progressive burn ischemia in a rat burn model. Am J Pathol 1993;142:519–528. [PMC free article] [PubMed] [Google Scholar]
- 121.Deniz M, Borman H, Seyhan T, et al. An effective antioxidant drug on prevention of the necrosis of zone of stasis: N-acetylcysteine. Burns 2013;39:320–325. [DOI] [PubMed] [Google Scholar]
- 122.Wang CZ, Ayadi AE, Goswamy J, et al. Topically applied metal chelator reduces thermal injury progression in a rat model of brass comb burn. Burns 2015;41:1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vorauer-Uhl K, Furnschlief E, Wagner A, et al. Reepithelialization of experimental scalds effected by topically applied superoxide dismutase: controlled animal studies. Wound Repair Regen 2002;10:366–371. [DOI] [PubMed] [Google Scholar]
- 124.Shalom A, Kramer E, Westreich M. Protective effect of human recombinant copper-zinc superoxide dismutase (hr-cuznsod) on intermediate burn survival in rats. Ann Burns Fire Disasters 2008;21:16–19. [PMC free article] [PubMed] [Google Scholar]
- 125.Shalom A, Kramer E, Westreich M. Protective effect of human recombinant copper-zinc superoxide dismutase on zone of stasis survival in burns in rats. Ann Plast Surg 2011;66:607–609. [DOI] [PubMed] [Google Scholar]
- 126.Dunlop EA, Tee AR. mTOR and autophagy: a dynamic relationship governed by nutrients and energy. Semin Cell Dev Biol 2014;36:121–129. [DOI] [PubMed] [Google Scholar]
- 127.Xiao M, Li L, Hu Q, et al. Rapamycin reduces burn wound progression by enhancing autophagy in deep second-degree burn in rats. Wound Repair Regen 2013;21:852–859. [DOI] [PubMed] [Google Scholar]
- 128.Appleby BS, Nacopoulos D, Milano N, et al. A review: treatment of Alzheimer’s disease discovered in repurposed agents. Dement Geriatr Cogn Disord 2013;35:1–22. [DOI] [PubMed] [Google Scholar]
- 129.Wu HM, Lee CG, Hwang SJ, et al. Mitigation of carbon tetrachloride-induced hepatic injury by methylene blue, a repurposed drug, is mediated by dual inhibition of GSK3beta downstream of PKA. Br J Pharmacol 2014;171:2790–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shin JY, Kim SJ, Kim DK, et al. Fundamental characteristics of deep-UV light-emitting diodes and their application to control foodborne pathogens. Appl Environ Microbiol 2016;82:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rosique MJ, Rosique RG, Faria FM, et al. Methylene blue reduces progression of burn and increases skin survival in an experimental rat model. Burns 2017;43:1702–1708. [DOI] [PubMed] [Google Scholar]
- 132.Malykh AG, Sadaie MR. Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders. Drugs 2010;70:287–312. [DOI] [PubMed] [Google Scholar]
- 133.Germonpre P, Reper P, Vanderkelen A. Hyperbaric oxygen therapy and piracetam decrease the early extension of deep partial-thickness burns. Burns 1996;22:468–473. [DOI] [PubMed] [Google Scholar]
- 134.Sari E, Dincel GC. Effect of piracetam and nimodipine on full-thickness skin burns in rabbits. Int Wound J 2016;13:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morykwas MJ, Simpson J, Punger K, et al. Vacuum-assisted closure: state of basic research and physiologic foundation. Plast Reconstr Surg 2006;117:121S–126S. [DOI] [PubMed] [Google Scholar]
- 136.Morykwas MJ, David LR, Schneider AM, et al. Use of subatmospheric pressure to prevent progression of partial-thickness burns in a swine model. J Burn Care Rehabil 1999;20:15–21. [DOI] [PubMed] [Google Scholar]
- 137.Dauwe PB, Pulikkottil BJ, Lavery L, et al. Does hyperbaric oxygen therapy work in facilitating acute wound healing: a systematic review. Plast Reconstr Surg 2014;133:208e–215e. [DOI] [PubMed] [Google Scholar]
- 138.Turkaslan T, Yogun N, Cimsit M, et al. Is HBOT treatment effective in recovering zone of stasis? An experimental immunohistochemical study. Burns 2010;36:539–544. [DOI] [PubMed] [Google Scholar]
- 139.De Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 2016;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rathnakar B, Rao BS, Prabhu V, et al. Photo-biomodulatory response of low-power laser irradiation on burn tissue repair in mice. Lasers Med Sci 2016;31:1741–1750. [DOI] [PubMed] [Google Scholar]
- 141.Yadav A, Gupta A, Keshri GK, et al. Photobiomodulatory effects of superpulsed 904nm laser therapy on bioenergetics status in burn wound healing. J Photochem Photobiol B 2016;162:77–85. [DOI] [PubMed] [Google Scholar]
- 142.Wasiak J, Cleland H. Burns: dressings. BMJ Clin Evid 2015. July 14;2015 pii: 1903 Review. [PMC free article] [PubMed]
- 143.Zarrintaj P, Moghaddam AS, Manouchehri S, et al. Can regenerative medicine and nanotechnology combine to heal wounds? The search for the ideal wound dressing. Nanomedicine 2017;12:2403–2422. [DOI] [PubMed] [Google Scholar]
- 144.Gaucher S, Duchange N, Jarraya M, et al. Severe adult burn survivors. What information about skin allografts? Cell Tissue Bank 2013;14:505–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hermans MH. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference? Burns 2014;40:408–415. [DOI] [PubMed] [Google Scholar]
- 146.Tenenhaus M The use of dehydrated human amnion/chorion membranes in the treatment of burns and complex wounds: current and future applications. Ann Plast Surg 2017;78:S11–S13. [DOI] [PubMed] [Google Scholar]
- 147.Gierek M, Kawecki M, Mikus K, et al. Biological dressings as a substitutes of the skin in the treatment of burn wounds. Pol Przegl Chir 2013;85:354–359. [DOI] [PubMed] [Google Scholar]
- 148.Wasiak J, Cleland H, Campbell F, et al. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev 2013. March 28; (3):CD002106. doi: 10.1002/14651858.CD002106.pub4. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carta T, Gawaziuk JP, Diaz-Abele J, et al. Properties of an ideal burn dressing: a survey of burn survivors and front-line burn healthcare providers. Burns 2018. October 13 pii: S0305–4179;(18)30843-X. doi: 10.1016/j.burns.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 150.Dai T, Huang YY, Sharma SK, et al. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov 2010; 5:124–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jahromi M, Zangabad PS, Basri SMM, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev 2018. January 1;123:33–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev 2006;19:403–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gravante G, Delogu D, Sconocchia G. “Systemic apoptotic response” after thermal burns. Apoptosis 2007;12:259–270. [DOI] [PubMed] [Google Scholar]
- 154.Zavlin D, Chegireddy V, Boukovalas S, et al. Multi-institutional analysis of independent predictors for burn mortality in the United States. Burns Trauma 2018;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Honari S Topical therapies and antimicrobials in the management of burn wounds. Crit Care Nurs Clin North Am 2004;16:1–11. [DOI] [PubMed] [Google Scholar]
- 156.Glasser JS, Guymon CH, Mende K, et al. Activity of topical antimicrobial agents against multidrug-resistant bacteria recovered from burn patients. Burns 2010;36:1172–1184. [DOI] [PubMed] [Google Scholar]
- 157.Marx DE, Barillo DJ. Silver in medicine: the basic science. Burns 2014;40(Suppl 1):S9–S18. [DOI] [PubMed] [Google Scholar]
- 158.Heyneman A, Hoeksema H, Vandekerckhove D, et al. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns 2016;42: 1377–1386. [DOI] [PubMed] [Google Scholar]
- 159.Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev 2018;123:33–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Grützner V, Unger RE, Baier G, et al. Enzyme-responsive nanocomposites for wound infection prophylaxis in burn management: in vitro evaluation of their compatibility with healing processes. Int J Nanomedicine 2015;10:4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hathaway H, Alves DR, Bean J, et al. Poly(N-isopropylacrylamide-coallylamine) (PNIPAM-co-ALA) nanospheres for the thermally triggered release of Bacteriophage K. Eur J Pharm Biopharm 2015;96:437–441. [DOI] [PubMed] [Google Scholar]
- 162.Öhlknecht C, Tegl G, Beer B, et al. Cellobiose dehydrogenase and chitosan-based lysozyme responsive materials for antimicrobial wound treatment. Biotechnol Bioeng 2017;114:416–422. [DOI] [PubMed] [Google Scholar]
- 163.Yin R, Dai T, Avci P, et al. Light based anti-infectives: ultraviolet C irradiation, photodynamic therapy, blue light, and beyond. Curr Opin Pharmacol 2013. October;13(5):731–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hamblin MR. Potentiation of antimicrobial photodynamic inactivation by inorganic salts. Expert Rev Anti Infect Ther 2017. November;15 (11):1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dai T, Gupta A, Huang YY, et al. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: efficacy, safety, and mechanism of action. Antimicrob Agents Chemother 2013. March;57(3):1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dai T, Kharkwal GB, Zhao J, et al. Ultraviolet-C light for treatment of Candida albicans burn infection in mice. Photochem Photobiol 2011;87:342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Dai T, Murray CK, Vrahas MS, et al. Ultraviolet C light for Acinetobacter baumannii wound infections in mice: potential use for battlefield wound decontamination? J Trauma Acute Care Surg 2012;73:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sennett R, Wang Z, Rezza A, et al. An integrated transcriptome atlas of embryonic hair follicle progenitors, their niche, and the developing skin. Dev Cell 2015;34:577–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Plikus MV, Van Spyk EN, Pham K, et al. The circadian clock in skin implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J Biol Rhythms 2015;30:163–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Streckfuss-Bömeke K, Wolf F, Azizian A, et al. Comparative study of human-induced pluripotent stem cells derived from bone marrow cells, hair keratinocytes, and skin fibroblasts. Eur Heart J 2013;34:2618–2629. [DOI] [PubMed] [Google Scholar]
- 171.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Condé-Green A, Marano AA, Lee ES, et al. Fat grafting and adipose-derived regenerative cells in burn wound healing and scarring: a systematic review of the literature. Plast Reconstr Surg 2016;137:302–312. [DOI] [PubMed] [Google Scholar]
- 173.Antonyshyn JA, Fitzpatrick LE. Stem cell and stem cell-derived molecular therapies to enhance dermal wound healing. In: Singh Ankur, Gaharwar Akhilesh K., editors. Microscale technologies for cell engineering Basel, Switzerland: Springer International Publishing; 2016. p. 113–141. [Google Scholar]
- 174.Kumar A, Mohanty S, Nandy SB, et al. Hair & skin derived progenitor cells: in search of a candidate cell for regenerative medicine. Indian J Med Res 2016;143:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blais M, Parenteau-Bareil R, Cadau S, et al. Concise review: tissue-engineered skin and nerve regeneration in burn treatment. Stem Cells Transl Med 2013;2:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Watt SM, Pleat JM. Stem cells, niches and scaffolds: applications to burns and wound care. Adv Drug Deliv Rev 2018;123:82–106. [DOI] [PubMed] [Google Scholar]
- 178.Friedstat JS, Hultman CS. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 2014;72:S198–S201. [DOI] [PubMed] [Google Scholar]
- 179.Rabello FB, Souza CD, Farina Junior JA. Update on hypertrophic scar treatment. Clinics (Sao Paulo) 2014;69:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Adzick NS, Lorenz HP. Cells, matrix, growth factors, and the surgeon. The biology of scarless fetal wound repair. Ann Surg 1994;220:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dang C, Ting K, Soo C, et al. Fetal wound healing current perspectives. Clin Plast Surg 2003;30:13–23. [DOI] [PubMed] [Google Scholar]
- 182.Gary-Bobo M, Nirde P, Jeanjean A, et al. Mannose 6-phosphate receptor targeting and its applications in human diseases. Curr Med Chem 2007;14:2945–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dienus K, Bayat A, Gilmore BF, et al. Increased expression of fibroblast activation protein-alpha in keloid fibroblasts: implications for development of a novel treatment option. Arch Dermatol Res 2010;302:725–731. [DOI] [PubMed] [Google Scholar]
- 184.Roberts AB, Russo A, Felici A, et al. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann N Y Acad Sci 2003;995:1–10. [DOI] [PubMed] [Google Scholar]
- 185.Liu L, Liu X, Ren X, et al. Smad2 and Smad3 have differential sensitivity in relaying TGFbeta signaling and inversely regulate early lineage specification. Sci Rep 2016;6:21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 2018;292:76–83. [DOI] [PubMed] [Google Scholar]
- 187.Wang Z, Gao Z, Shi Y, et al. Inhibition of Smad3 expression decreases collagen synthesis in keloid disease fibroblasts. J Plast Reconstr Aesthet Surg 2007;60:1193–1199. [DOI] [PubMed] [Google Scholar]
- 188.Arno AI, Gauglitz GG, Barret JP, et al. New molecular medicine-based scar management strategies. Burns 2014;40: 539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mcgaha TL, Phelps RG, Spiera H, et al. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol 2002;118:461–470. [DOI] [PubMed] [Google Scholar]
- 190.Mccormick LL, Zhang Y, Tootell E, et al. Anti-TGF-beta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus-host disease: a model for human scleroderma. J Immunol 1999;163:5693–5699. [PubMed] [Google Scholar]
- 191.Mehta M, Branford OA, Rolfe KJ. The evidence for natural therapeutics as potential anti-scarring agents in burn-related scarring. Burns Trauma 2016;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Horton JA, Li F, Chung EJ, et al. Quercetin inhibits radiation-induced skin fibrosis. Radiat Res 2013;180:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Santiago B, Gutierrez-Canas I, Dotor J, et al. Topical application of a peptide inhibitor of transforming growth factor-beta1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol 2005;125:450–455. [DOI] [PubMed] [Google Scholar]
- 194.San-Martin A, Dotor J, Martinez F, et al. Effect of the inhibitor peptide of the transforming growth factor beta (p144) in a new silicone pericapsular fibrotic model in pigs. Aesthetic Plast Surg 2010;34:430–437. [DOI] [PubMed] [Google Scholar]
- 195.Tang B, Zhu B, Liang Y, et al. Asiaticoside suppresses collagen expression and TGF-beta/Smad signaling through inducing Smad7 and inhibiting TGF-betaRI and TGF-betaRII in keloid fibroblasts. Arch Dermatol Res 2011;303:563–572. [DOI] [PubMed] [Google Scholar]
- 196.Zunwen L, Shizhen Z, Dewu L, et al. Effect of tetrandrine on the TGF-beta-induced smad signal transduction pathway in human hypertrophic scar fibroblasts in vitro. Burns 2012;38:404–413. [DOI] [PubMed] [Google Scholar]
- 197.Granstein RD, Rook A, Flotte TJ, et al. A controlled trial of intralesional recombinant interferon-gamma in the treatment of keloidal scarring. Clinical and histologic findings. Arch Dermatol 1990;126:1295–1302. [PubMed] [Google Scholar]
- 198.Larrabee WF Jr., East CA, Jaffe HS, et al. Intralesional interferon gamma treatment for keloids and hypertrophic scars. Arch Otolaryngol Head Neck Surg 1990;116:1159–1162. [DOI] [PubMed] [Google Scholar]
- 199.Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev 2017;30:827–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sevgi M, Toklu A, Vecchio D, et al. Topical antimicrobials for burn infections an update. Recent Pat Antiinfect Drug Discov 2013. December;8 (3):161–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Poovey B Hospitals are shutting down burn centers. The Washington Post 2007. Available from: http://www.washingtonpost.com/wp-dyn/content/article/2007/08/08/AR2007080800272.html?noredirect=on
- 202.New CDC report: more than 100 million Americans have diabetes or prediabetes. Centers for Disease Control and Prevention 2017Available from: https://www.cdc.gov/media/releases/2017/p0718-diabetes-report.html
- 203.Maghsoudi H, Aghamohammadzadeh N, Khalili N. Burns in diabetic patients. Int J Diabetes Dev Ctries 2008;28:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Briegel J, Bein T, Mohnle P. Update on low-dose corticosteroids. Curr Opin Anaesthesiol 2017;30:186–191. [DOI] [PubMed] [Google Scholar]
- 205.Batzofin BM, Weiss YG, Ledot SF. Do corticosteroids improve outcome for any critical illness? Curr Opin Anaesthesiol 2013;26:164–170. [DOI] [PubMed] [Google Scholar]
- 206.Li Z, Maitz P. Cell therapy for severe burn wound healing. Burns Trauma 2018;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rodgers K, Jadhav SS. The application of mesenchymal stem cells to treat thermal and radiation burns. Adv Drug Deliv Rev 2018;123:75–81. [DOI] [PubMed] [Google Scholar]