Abstract
A supramolecular peptide vaccine system was designed in which epitope-bearing peptides self-assemble into elongated nanofibers composed almost entirely of alpha-helical structure. The nanofibers were readily internalized by antigen presenting cells and produced robust antibody, CD4+ T-cell, and CD8+ T-cell responses without supplemental adjuvants in mice. Epitopes studied included a cancer B-cell epitope from the epidermal growth factor receptor class III variant (EGFRvIII), the universal CD4+ T-cell epitope PADRE, and the model CD8+ T-cell epitope SIINFEKL, each of which could be incorporated into supramolecular multi-epitope nanofibers in a modular fashion.
Keywords: nanofiber, self-assembly, coiled-coil, vaccine, self-adjuvanting
Graphical Abstract

Peptides that non-covalently self-assemble into nanofibers have garnered interest towards a broad range of biomedical applications including the delivery of cells and drugs,1–3 scaffolds for regenerative medicine,4–7 and immunotherapies.8–11 In particular, self-assembling peptide nanofibers have been promising for immunotherapies owing to their ability to elicit strong immune responses without the use of adjuvants.12–16 Fiber-forming peptides investigated to date in this regard have included peptides such as Q1115–19, KFE820, 21, RADA1622–24, and peptide amphiphiles that form cylindrical micelles25–27. Each of these, when appended with appropriate T-cell or B-cell epitopes, can function as a self-adjuvanting vaccine platform. In these systems, multiple epitope-bearing peptides can be co-assembled into integrated nanofibers in a modular fashion,8 and they raise immune responses without significant inflammation14, making them attractive candidates for development towards a wide range of diseases and conditions including cancer2, 28, malaria9, influenza29, drug addiction30, and bacterial infections31.
Previous immunogenic self-assembled peptide nanofibers have been constructed using β-sheet fibrillization.14–16 Although this mode of assembly has been useful for incorporating multiple different epitope-bearing peptides together into nanofibers,13, 17 it has remained an open question whether the β-sheet fibrillar structure is necessary for the observed self-adjuvanting properties. Moreover, β-sheet fibers possess some shortcomings: Their kinetics of assembly and disassembly are difficult to control, they lack structural precision in the topology of individual β-strands, their lengths are polydisperse, and it is difficult to control lateral interactions between nanofibers.32–34
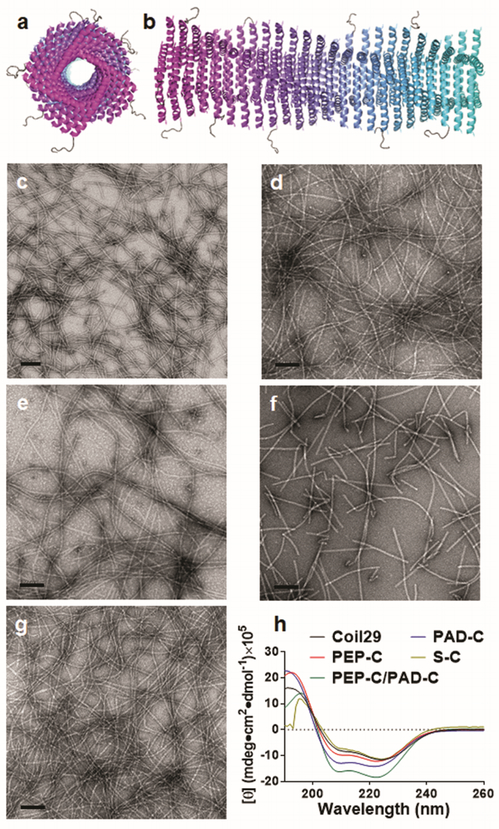
To develop a new fiber-forming system that could address some of these issues, we designed self-adjuvanting peptide nanofibers having almost entirely α-helical structure. Helical peptide nanofibers have been designed previously, notably by the Woolfson35–37 and Conticello38, 39 groups, but it has not been known whether they possess self-adjuvanting properties. Here we focused on the peptide QARILEADAEILR-AYARILEAHAEILRAQ (Coil29) originally reported by Egelman, Conticello, and coworkers as the “Form I” peptide, PDB ID 3J8938. Previous electron cryomicroscopy studies showed that Coil29 forms long α-helical nanofibers, where the helical peptides run perpendicularly to the axis of the fiber (Figure 1a–b). In each layer of the stack of helices, four peptides form a square, with the C-termini clustered near the axis of the nanofiber and the N-termini extending outward towards the surface of the nanofiber.38 This availability of the N-terminus for conjugation to epitopes, along with the all-helix structure, made for an intriguing candidate as a vaccine platform.
Figure 1.
Coil29 self-assembled into α-helical nanofibers when appended with different epitopes. Axial view (a) and side view (b) schematics of Coil29 fibers displaying PEPvIII epitopes, drawn using PDB structures for Coil29 (PDB ID 3J89) and PEPvIII (PDB ID 1I8I). By TEM, nanofibers were formed by Coil29 (c), PEP-C alone (d), PEP-C and PAD-C co-assembled at 20:1 (e), PAD-C alone (f), and S-C alone (g). Alpha-helical secondary structures were preserved in all fiber formulations as evidenced by circular dichroism spectra (h). All scale bars: 100 nm.
We synthesized peptides with the Coil29 sequence at the C-terminus and various epitopes at the N-terminus (Table S1). These epitopes included the pan-DR CD4+ T-cell epitope PADRE, aKXVAAWTLKAa, where “X” is cyclohexylalanine, and “a” is D-alanine; the CD8+ OT-I peptide epitope SIINFEKL; and the B-cell epitope PEPvIII, LEEKKGNYVVTDH, from the epidermal growth factor receptor class III variant (EGFRvIII).40–42 EGFRvIII is a tumor-specific receptor present in a significant proportion of glioblastomas and other human cancers. The SIINFEKL-Coil29 peptide was additionally modified with a proteasome-cleavable linker (AAYGG)43, to facilitate processing of this epitope within APCs. Epitope-bearing peptides were named PEP-C (PEPvIII-Coil29), PAD-C (PADRE-Coil29), and S-C (SIINFEKL-AAYGG-Coil29).
The peptides were produced using solid phase peptide synthesis, confirmed using MALDI, and purified via HPLC (Table S1 and Figure S1). To examine whether Coil29 would still assemble when appended to epitopes, transmission electron microscopy (TEM) was employed (Figure 1). In our hands, unmodified Coil29 self-assembled into nanofibers in phosphate buffered saline (PBS), with diameters identical to that reported previously38 (~6 nm, Figure 1c). PEP-C and S-C formed regular nanofibers, with diameters of about 10 nm, slightly wider than unmodified Coil29 (Figure 1d and 1g). Moreover, circular dichroism spectropolarimetry confirmed that the α-helical secondary structure was preserved in epitope-bearing fibers, as evidenced by the two molar ellipticity minima at 208 nm and 222 nm (Figure 1h).
PAD-C formed shorter nanofibers, possibly due to the hydrophobicity of the PADRE sequence interfering with the self-assembly process, although the secondary structure of PAD-C fibers remained helical (Figure 1f and 1h). This disruption was overcome by mixing PEP-C and PAD-C at a molar ratio of 20:1 before assembly in PBS, which produced nanofibers (Figure 1e) with lengths similar to those of unmodified Coil29 (Figure 1c).
To examine whether Coil29 nanofibers promoted epitope uptake by antigen presenting cells (APCs) in vivo, mice were immunized intraperitoneally with fluorescently labelled PEP-C nanofibers or soluble PEPvIII peptide. Conjugation of TAMRA fluorophore to PEP-C did not alter nanofiber morphology (Figure S2). The peritoneal lavage fluid was collected 20 h after injections and the percentage of TAMRA positive dendritic cells (DCs, CD11c+ MHCII+ F4/80−) and macrophages (CD11c− F4/80+) were determined using flow cytometry (Figure S3). Whereas the uptake of soluble PEPvIII by DCs and macrophages was statistically indistinguishable from the negative control group (PBS), about 98% of macrophages and 65% of dendritic cells acquired labeled PEP-C fibers (Figure 2), indicating that the nanofiber significantly enhanced uptake relative to the soluble epitope. It is worth noting that we employed intraperitoneal injections rather than subcutaneous injections in these experiments to gain access to large numbers of APCs. However, the observed trends should be translatable to subcutaneous injections. Previous studies by our group indicated that epitope-bearing beta-sheet nanofibers elicit comparable antibody titers via intraperitoneal and subcutaneous delivery.14
Figure 2.
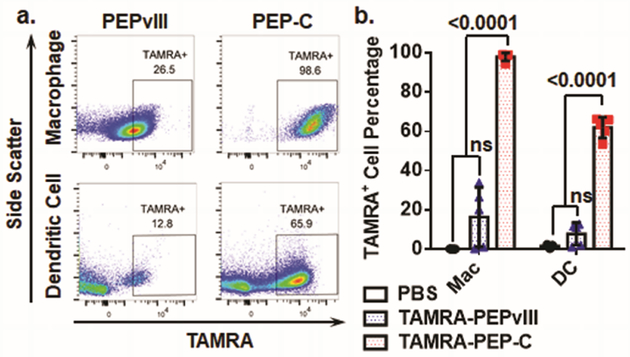
PEP-C nanofibers were efficiently internalized by antigen presenting cells (APCs) in vivo. (a) Representative flow cytometry plots showing TAMRA-positive dendritic cells (DCs) and macrophages 20 h after i.p. injections of TAMRA-PEPvIII or TAMRA-PEP-C. (b) Quantified uptake by APCs. A significantly larger fraction of both DCs and macrophages took up nanofibers compared to labeled soluble epitopes. (N=5 mice per group, p-values shown were determined by Student’s t-test.)
We next investigated the ability of the Coil29 peptide platform to raise antibody responses against the PEPvIII epitope. Mice were immunized subcutaneously with PEPvIII peptide alone, PEPvIII emulsified with complete Freund’s adjuvant (CFA), as PEP-C nanofibers, or as coassembled nanofibers of PEP-C and PAD-C (Figure 3). Consistent with its lack of uptake by APCs, PEPvIII peptide alone failed to elicit an antibody response. PEP-C nanofibers likewise were not immunogenic, as they lacked T cell epitopes. However, PEP-C/PAD-C coassemblies were remarkably immunogenic, raising antibody titers higher than PEPvIII in CFA after one prime and two boosts, and these titers persisted for the duration of the study (18 weeks, Figure 3a). This strong self-adjuvanting capacity and T-cell dependency of the Coil29 nanofibers is similar to the behavior of β-sheet fibrillizing peptides.13 For example, Q11 peptide nanofibers bearing OVA323–339 epitopes or B-cell epitopes from S. Aureus elicited log10 antibody titers of around 4 without adjuvant, similar to Coil29.14, 15 Further, Q11 nanofibers similarly do not raise antibody responses without the incorporation of T cell epitopes, whether the T cell epitope overlaps with the B cell epitope as in OVA323–339 or if it is co-assembled, for example by using PADRE-Q11.13 More importantly, our present study indicates that β-sheet structure is not necessary for the adjuvant activity of fibrillar peptide assemblies. This finding presents the possibility that a wider range of fibrillar peptide materials may be appropriate for immunotherapy development than previously believed.
Figure 3.
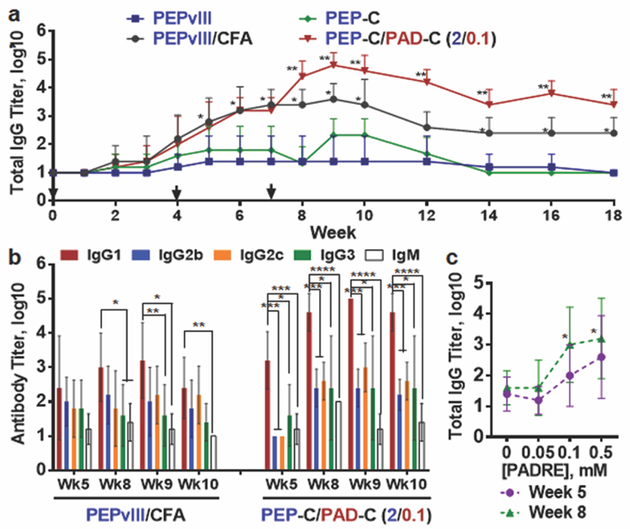
The Coil29 platform elicited strong antibody responses against PEPvIII. (a) Mice were immunized (2 mM of PEPvIII, 100 μL per mouse) on week 0, followed by two booster injections (2 mM of PEPvIII, 50 μL per mouse) on weeks 4 and 7 (N=5 mice per group, analyzed by Student’s t-test. *p<0.01 compared with both PEPvIII and P-C groups; **p<0.01 compared with all other groups). (b) Distribution of PEPvIII-specific antibody isotypes in mice immunized by PEPvIII peptide with CFA adjuvant (left grouping), and PEP-C/PAD-C co-assembled peptide fibers (right grouping). Shown are mean values ± standard deviations. (n=5 mice per group, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, analyzed by two-way ANOVA for multiple comparison.). (c) Increasing the T-cell epitope concentration within Coil29 nanofibers increased the resulting total IgG antibody response on week 5 and week 8 (*p<0.05 by Student’s t-test comparing with 0 mM formulaton).
Antibody isotype analysis revealed that immunization with PEP-C/PAD-C nanofibers produced a different immune phenotype compared with PEPvIII in CFA (Figure 3b). At all measured time points, PEP-C/PAD-C immunization promoted higher titers of IgG1 compared with all other tested isotypes, while PEPvIII in CFA exhibited only a slight bias toward IgG1 at some time points. This polarization towards IgG1 suggested that the PEP-C/PAD-C nanofibers promoted a Th2-polarized response.44 This bias towards IgG1 could have therapeutic benefit, as IgG1 monoclonal antibodies have been found to be more potent than other isotypes in mediating tumor cell killing in humans via the mechanisms of antibody–dependent cellular cytotoxicity and complement-dependent cytotoxicity.45, 46 Interestingly, mice in PEP-C and PEP-C/PAD-C groups also exhibited humoral responses against the linker-Coil29 sequence (SGSG-Coil29, Table S1), but this antibody response gradually diminished to a negligible level after the total IgG titers peaked at week 9 (Figure S4).
We further analyzed how dosing the PADRE epitope within Coil29 nanofibers could tune the humoral response (Figure 3c, and Figure S5). Mice were immunized with Coil29 nanofibers formulated with four different PADRE epitope concentrations ranging from 0 to 0.5 mM in final concentration, and the PEPvIII-specific total IgG titers were monitored over 17 weeks (Figure S5a). Consistent with our previous results, PEP-C nanofibers alone or those with low levels of PADRE (0.05 mM) elicited negligible levels of IgG. However, when the PADRE dose was increased to 0.1 mM or 0.5 mM, antibody titers were increased significantly throughout the experimental period (Figure 3c). This observation differed from previous findings with the beta-sheet Q11 platform, where the antibody response at different PADRE dosing regimens exhibited a bell-shaped curve with the peak response at 0.05–0.1 mM PADRE.13 Several factors could possibly explain this difference, including the use of a different B cell epitope, differences in epitope availability or spatial arrangement, or differences in the mechanical properties of the fibers, all interesting subjects of future investigation.
T cell responses induced by PEP-C/PAD-C fibers were specific to PADRE (Figure S5b), as measured by ELISPOT with the splenocytes of immunized mice. Mice immunized with PEP-C, conversely, responded to neither PADRE peptide nor PEPvIII peptide. Higher doses of PADRE in the immunizing nanofibers produced correspondingly higher numbers of IFNγ and IL4-secreting cells, and stimulation with PEPvIII peptides within the ELISPOT assays elicited only very low levels of cytokine secretion. These results underscored the essential role of T cells in the antibody response against the PEPvIII epitope, and formulations producing maximal T cell responses corresponded with the formulations that produced the highest antibody titers.
Finally, we compared the Coil29 platform’s ability to stimulate responses against CD8+ T cell epitopes with the Q11 platform, which has been shown previously to elicit CD8+ T cell responses against the model epitope SIINFEKL47. We N-terminally linked Coil29 and Q11 to SIINFEKL via a proteasome-cleavable linker (AAYGG). Coil29 nanofibers promoted more efficient presentation of SIINFEKL than Q11 nanofibers, with about 4.1% of DCs presenting the epitope 20 h after i.p. immunization, compared with 1.8% of DCs with SIINFEKL-Q11, as measured with an antibody recognizing the epitope/MHC-I complex (Figure 4a, 4b, see methods in Supplementary Information). The efficiency of MHC-I restricted epitope presentation by DCs (4.1%) was lower than the overall efficiency of fiber internalization observed with PEP-C nanofibers containing a B cell epitope (65.9%, Figure 2), but it should be noted that the former measurement represents epitopes that have been fully processed and displayed in MHC-I, while the latter is simply a measure of internalization. To determine the level of cellular immune response raised, IFNγ ELISPOTs were performed on splenocytes harvested from mice three weeks after primary injections. When restimulated with SIINFEKL peptides, splenocytes immunized with both platforms produced significant IFNγ responses, similar to that of mice immunized with SIINFEKL in CFA (Figure 4c).
Figure 4.
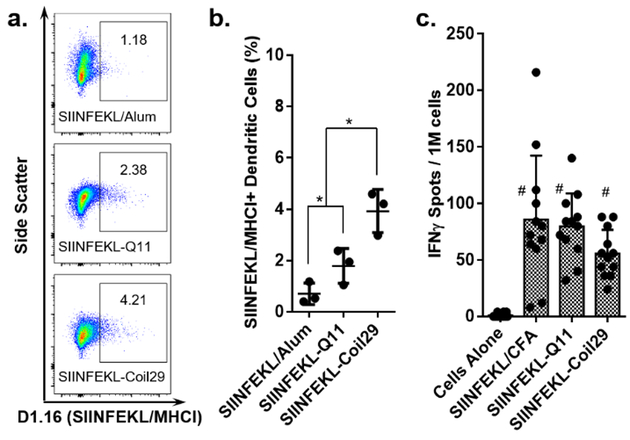
Peptide nanofibers efficiently delivered CD8+ T cell epitopes to APCs and stimulated CD8+ T cell responses. (a) Representative flow cytometry data showing SIINFEKL/MHC-I positive dendritic cells 20 h after i.p. injections of SIINFEKL/Alum, SIINFEKL-Coil29, or SIINFEKL-Q11 (b) Quantification of SIINFEKL presentation in MHC-I by DCs. SIINFEKL-Coil29 immunization led to a significantly larger proportion of SIINFEKL-presenting DCs compared with SIINFEKL-Q11 and SIINFEKL/Alum (N=3 mice per group, p<0.05, analyzed by two-way ANOVA for multiple comparison.) (c) Splenocytes harvested from mice immunized with SIINFEKL/CFA, SIINFEKL-Coil29, or SIINFEKL-Q11 exhibited comparable IFNγ responses when restimulated with SIINFEKL peptide. (# statistically insignificant, N=12 mice per group, analyzed by two-way ANOVA for multiple comparison.)
In summary, we designed a vaccine delivery platform based on a self-assembling α-helical coiled-coil peptide fiber, which was amenable to inclusion of several different epitopes. We expect that this system will be useful for the design of biomaterials-based vaccines and immunotherapies.8,34,48 It was established that Coil29 nanofibers could be efficiently internalized by APCs. Peptide nanofibers containing a CD4+ T cell epitope, PADRE, were capable of eliciting durable epitope-specific antibody responses against PEPvIII that were even higher than a CFA-adjuvanted formulation, while the B cell epitope-bearing fiber alone failed to raise any humoral responses. PEP-C/PAD-C nanofibers raised higher IgG1 titers when compared with the CFA-adjuvanted group, indicating that the nanofibers produced a relatively Th2-polarized response. Additionally, increasing the PADRE epitope dosing improved the magnitude of both antibody and T cell responses. The ability of the Coil29 platform to elicit CD8+ T cell responses was also shown to be comparable to CFA emulsion. This work represents the first demonstration of a self-adjuvanting vaccine delivery platform based on α-helical peptide nanofibers, indicating that a variety of fibrillar peptide architectures, both α-helical and β-sheet, can possess self-adjuvanting properties.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Jianjun Chen for assistance with flow cytometry.
Funding Sources
This work was supported by the US National Institutes of Health (NIBIB 7R01EB009701; NIAID 5R01AI118182). The contents are solely the responsibility of the authors and do not necessarily represent the official views of these agencies.
Footnotes
Supporting Information.
Experimental methods, a peptide molecular weight summary table, MALDI spectra, antibody response curves, and flow cytometry gating processes are included. The material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Sato K; Ji W; Palmer LC; Weber B; Barz M; Stupp SI, Programmable assembly of peptide amphiphile via noncovalent-to-covalent bond conversion. J Am Chem Soc 2017, 139, (26), 8995–9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang P; Cheetham AG; Lin Y.-a.; Cui H, Self-assembled Tat nanofibers as effective drug carrier and transporter. ACS Nano 2013, 7, (7), 5965–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li IC; Moore AN; Hartgerink JD, “Missing tooth” multidomain peptide nanofibers for delivery of small molecule drugs. Biomacromolecules 2016, 17, (6), 2087–2095. [DOI] [PubMed] [Google Scholar]
- 4.Choe S; Bond CW; Harrington DA; Stupp SI; McVary KT; Podlasek CA, Peptide amphiphile nanofiber hydrogel delivery of sonic hedgehog protein to the cavernous nerve to promote regeneration and prevent erectile dysfunction. Nanomedicine 2017, 13, (1), 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capulli AK; MacQueen LA; Sheehy SP; Parker KK, Fibrous scaffolds for building hearts and heart parts. Adv Drug Deliv Rev 2016, 96, 83–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arslan E; Garip IC; Gulseren G; Tekinay AB; Guler MO, Bioactive supramolecular peptide nanofibers for regenerative medicine. Adv Healthc Mater 2014, 3, (9), 1357–1376. [DOI] [PubMed] [Google Scholar]
- 7.Moore AN; Hartgerink JD, Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc Chem Res 2017, 50, (4), 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webber MJ; Appel EA; Meijer EW; Langer R, Supramolecular biomaterials. Nat Mater 2016, 15, (1), 13–26. [DOI] [PubMed] [Google Scholar]
- 9.Rudra JS; Mishra S; Chong AS; Mitchell RA; Nardin EH; Nussenzweig V; Collier JH, Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials 2012, 33, (27), 6476–6484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudalla GA; Modica JA; Tian YF; Rudra JS; Chong AS; Sun T; Mrksich M; Collier JH, A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv Healthc Mater 2013, 2, (8), 1114–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black M; Trent A; Kostenko Y; Lee JS; Olive C; Tirrell M, Self-assembled peptide amphiphile micelles containing a cytotoxic T-cell epitope promote a protective immune response in vivo. Adv Mater 2012, 24, (28), 3845–3849. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y; Waltman A; Han H; Collier JH, Switching the immunogenicity of peptide assemblies using surface properties. ACS Nano 2016, 10, (10), 9274–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pompano RR; Chen J; Verbus EA; Han H; Fridman A; McNeely T; Collier JH; Chong AS, Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv Healthc Mater 2014, 3, (11), 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J; Pompano RR; Santiago FW; Maillat L; Sciammas R; Sun T; Han H; Topham DJ; Chong AS; Collier JH, The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials 2013, 34, (34), 8776–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudra JS; Sun T; Bird KC; Daniels MD; Gasiorowski JZ; Chong AS; Collier JH, Modulating adaptive immune responses to peptide self-assemblies. ACS Nano 2012, 6, (2), 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudra JS; Tian YF; Jung JP; Collier JH, A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A 2010, 107, (2), 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JP; Nagaraj AK; Fox EK; Rudra JS; Devgun JM; Collier JH, Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 2009, 30, (12), 2400–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian YF; Hudalla GA; Han H; Collier JH, Controllably degradable β-sheet nanofibers and gels from self-assembling depsipeptides. Biomater Sci 2013, 1, (10), 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon TS; Antonietta R; Anthony DS; Kevin RK; Stephen JH; Gregory AH, Co-assembly tags based on charge complementarity (CATCH) for installing functional protein ligands into supramolecular biomaterials. Cell Mol Bioeng 2016, 9, (3), 335–350. [Google Scholar]
- 20.Marini DM; Hwang W; Lauffenburger DA; Zhang SG; Kamm RD, Left-handed helical ribbon intermediates in the self-assembly of a beta-sheet peptide. Nano Lett 2002, 2, (4), 295–299. [Google Scholar]
- 21.Sieminski AL; Semino CE; Gong H; Kamm RD, Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J Biomed Mater Res A 2008, 87A, (2), 494–504. [DOI] [PubMed] [Google Scholar]
- 22.Yokoi H; Kinoshita T; Zhang S, Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci U S A 2005, 102, (24), 8414–8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho H; Balaji S; Sheikh AQ; Hurley JR; Tian YF; Collier JH; Crombleholme TM; Narmoneva DA, Regulation of endothelial cell activation and angiogenesis by injectable peptide nanofibers. Acta Biomater. 2012, 8, (1), 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormier AR; Pang X; Zimmerman MI; Zhou H-X; Paravastu AK, Molecular structure of RADA16-I designer self-assembling peptide nanofibers. ACS Nano 2013, 7, (9), 7562–7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartgerink JD; Beniash E; Stupp SI, Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, (5547), 1684–1688. [DOI] [PubMed] [Google Scholar]
- 26.Webber MJ; Berns EJ; Stupp SI, Supramolecular nanofibers of peptide amphiphiles for medicine. Isr J Chem 2013, 53, (8), 530–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartgerink JD; Beniash E; Stupp SI, Peptide-amphiphile nanofibers: A versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci U S A 2002, 99, (8), 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung EJ; Cheng Y; Morshed R; Nord K; Han Y; Wegscheid ML; Auffinger B; Wainwright DA; Lesniak MS; Tirrell MV, Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma. Biomaterials 2014, 35, (4), 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zope H; Quer CB; Bomans PHH; Sommerdijk N; Kros A; Jiskoot W, Peptide amphiphile nanoparticles enhance the immune response against a CpG-adjuvanted influenza antigen. Adv Healthc Mater 2014, 3, (3), 343–348. [DOI] [PubMed] [Google Scholar]
- 30.Rudra JS; Ding Y; Neelakantan H; Ding C; Appavu R; Stutz S; Snook JD; Chen H; Cunningham KA; Zhou J, Suppression of cocaine-evoked hyperactivity by self-adjuvanting and multivalent peptide nanofiber vaccines. ACS Chem Neurosci 2016, 7, (5), 546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett JC; Ulery BD; Trent A; Liang S; David NA; Tirrell MV, Modular peptide amphiphile micelles improving an antibody-mediated immune response to group A streptococcus. ACS Biomater Sci Eng 2017, 3, (2), 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobson CM, Protein folding and misfolding. Nature 2003, 426, (6968), 884–890. [DOI] [PubMed] [Google Scholar]
- 33.Chiti F; Dobson CM, Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006, 75, (1), 333–366. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y; Collier JH, α-Helical coiled-coil peptide materials for biomedical applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burgess NC; Sharp TH; Thomas F; Wood CW; Thomson AR; Zaccai NR; Brady RL; Serpell LC; Woolfson DN, Modular design of self-assembling peptide-based nanotubes. J Am Chem Soc 2015, 137, (33), 10554–10562. [DOI] [PubMed] [Google Scholar]
- 36.Papapostolou D; Smith AM; Atkins ED; Oliver SJ; Ryadnov MG; Serpell LC; Woolfson DN, Engineering nanoscale order into a designed protein fiber. Proc Natl Acad Sci U S A 2007, 104, (26), 10853–10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryadnov MG; Woolfson DN, MaP peptides: programming the self-assembly of peptide-based mesoscopic matrices. J Am Chem Soc 2005, 127, (35), 12407–12415. [DOI] [PubMed] [Google Scholar]
- 38.Egelman EH; Xu C; DiMaio F; Magnotti E; Modlin C; Yu X; Wright E; Baker D; Conticello VP, Structural plasticity of helical nanotubes based on coiled-coil assemblies. Structure 2015, 23, (2), 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu C; Liu R; Mehta AK; Guerrero-Ferreira RC; Wright ER; Dunin-Horkawicz S; Morris K; Serpell LC; Zuo X; Wall JS; Conticello VP, Rational design of helical nanotubes from self-assembly of coiled-coil lock washers. J Am Chem Soc 2013, 135, (41), 15565–15578. [DOI] [PubMed] [Google Scholar]
- 40.Heimberger AB; Crotty LE; Archer GE; Hess KR; Wikstrand CJ; Friedman AH; Friedman HS; Bigner DD; Sampson JH, Epidermal growth factor receptor VIII peptide vaccination is efficacious against established intracerebral tumors. Clin Cancer Res 2003, 9, (11), 4247–4254. [PubMed] [Google Scholar]
- 41.Sampson JH; Archer GE; Mitchell DA; Heimberger AB; Herndon JE; Lally-Goss D; McGehee-Norman S; Paolino A; Reardon DA; Friedman AH; Friedman HS; Bigner DD, An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther 2009, 8, (10), 2773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi BD; Archer GE; Mitchell DA; Heimberger AB; McLendon RE; Bigner DD; Sampson JH, EGFRvIII-targeted vaccination therapy of malignant glioma. Brain Pathol 2009, 19, (4), 713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huebener N; Lange B; Lemmel C; Rammensee HG; Strandsby A; Wenkel J; Jikai J; Zeng Y; Gaedicke G; Lode HN, Vaccination with minigenes encoding for novel ‘self’ antigens are effective in DNA-vaccination against neuroblastoma. Cancer Lett 2003, 197, (1–2), 211–217. [DOI] [PubMed] [Google Scholar]
- 44.Snapper CM; Mond JJ, Towards a comprehensive view of immunoglobulin class switching. Immunol Today 1993, 14, (1), 15–17. [DOI] [PubMed] [Google Scholar]
- 45.Scott AM; Wolchok JD; Old LJ, Antibody therapy of cancer. Nat Rev Cancer 2012, 12, (4), 278–287. [DOI] [PubMed] [Google Scholar]
- 46.Monteverde M; Milano G; Strola G; Maffi M; Lattanzio L; Vivenza D; Tonissi F; Merlano M; Lo Nigro C, The relevance of ADCC for EGFR targeting: a review of the literature and a clinically-applicable method of assessment in patients. Crit Rev Oncol Hematol 2015, 95, (2), 179–190. [DOI] [PubMed] [Google Scholar]
- 47.Chesson CB; Huelsmann EJ; Lacek AT; Kohlhapp FJ; Webb MF; Nabatiyan A; Zloza A; Rudra JS, Antigenic peptide nanofibers elicit adjuvant-free CD8⁺ T cell responses. Vaccine 2014, 32, (10), 1174–1180. [DOI] [PubMed] [Google Scholar]
- 48.Mora-Solano C; Collier JH, Engaging adaptive immunity with biomaterials. J Mater Chem B Mater Biol Med 2014, 2, (17), 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.