Abstract
Background: Neuropathic pain is an increasingly prevalent condition and has a major impact on health and quality of life. However, the risk factors for the development and maintenance of neuropathic pain are poorly understood. Clinical, genetic and psychosocial factors all contribute to chronic pain, but their interactions have not been studied in large cohorts. The DOLORisk study aims to study these factors.
Protocol: Multicentre cross-sectional and longitudinal cohorts covering the main causes leading to neuropathic pain (e.g. diabetes, surgery, chemotherapy, traumatic injury), as well as rare conditions, follow a common protocol for phenotyping of the participants. This core protocol correlates answers given by the participants on a set of questionnaires with the results of their genetic analyses. A smaller number of participants undergo deeper phenotyping procedures, including neurological examination, nerve conduction studies, threshold tracking, quantitative sensory testing, conditioned pain modulation and electroencephalography.
Ethics and dissemination: All studies have been approved by their regional ethics committees as required by national law. Results are disseminated through the DOLORisk website, scientific meetings, open-access publications, and in partnership with patient organisations.
Strengths and limitations:
Large cohorts covering many possible triggers for neuropathic pain
Multi-disciplinary approach to study the interaction of clinical, psychosocial and genetic risk factors
High comparability of the data across centres thanks to harmonised protocols
One limitation is that the length of the questionnaires might reduce the response rate and quality of responses of participants
Keywords: pain, neuropathy, neuropathic pain, diabetes, nerve injury, risk factors, protocol
Introduction
Neuropathic pain affects 7–10% of the general population 1 and has a major impact on physical health, psychological health and quality of life 2. The response to analgesic treatment is often inadequate with only 40–60% of patients achieving partial relief, often at the cost of adverse effects 3. The prevalence of neuropathic pain will increase due to the increasing prevalence of predisposing conditions, such as diabetes mellitus, and ageing, which is associated with neuropathic pain 1. There is an urgent clinical need to translate an increased preclinical level of understanding of neuropathic into clinical practice. In particular we need to understand the pathophysiology of neuropathic pain in clinical cohorts.
Neuropathic pain arises as a consequence of a disease or lesion in the somatosensory nervous system 4. However, not all patients with such a lesion develop neuropathic pain. We do not understand why only a sub-group of patients with the same disease or neurological lesion develop neuropathic pain. The severity and impact of neuropathic pain vary between individuals with similar conditions 5 and are unpredictable. A plausible explanation for the variation in neuropathic pain prevalence and severity is a complex interaction between genetic, psychosocial, and clinical risk factors in a vulnerable individual 6– 8.
A recent and significant advance in neuropathic pain research has been the development of clinical tools, such as standardised questionnaires and quantitative sensory testing for sensory phenotyping, that differentiate and stratify neuropathic pain 9– 13. We have entered an era whereby patients can be phenotyped in unprecedented detail in terms of sensory profile, psychological factors and physiological measures such as nerve excitability testing. We have the opportunity to combine major advances in phenotyping with genomics to improve our understanding of neuropathic pain.
Aims and objectives
DOLORisk is a multi-centre observational study that aims to understand the risk factors and determinants for neuropathic pain.
Primary objectives
The primary objectives of DOLORisk are (1) to identify the influence of demographic, environmental, psychological and clinical factors on the risk of developing and maintenance of neuropathic pain, and (2) to study the association of genetic factors with the risk of developing and maintaining neuropathic pain.
Secondary objectives
DOLORisk also aims to determine if patient stratification using physiological and psychological factors can predict neuropathic pain risk and progression. Based on the analysis of these risk factors, the study will lead to the development of a risk model for neuropathic pain, combining measurable genetic and environmental factors.
Methods
Study design
The first step was to develop a protocol that would be used by all participating centres to identify and characterise people with neuropathic pain. The instruments chosen to phenotype DOLORisk participants were the object of a consensus meeting between the recruitment centres in October 2015. This was based on a recent international consensus on phenotyping neuropathic pain (NeuroPPIC), led by the Special Interest Group on Neuropathic Pain (NeuPSIG), of the International Association for the Study of Pain 14. The respective merits and reported accuracy of available scales, questionnaires and self-reported measures were discussed and the following were included in the final DOLORisk protocol ( Table 1). The DOLORisk protocol has been aligned across all recruitment centres to make data integration possible. The “core” protocol consists of questionnaires only. All participants recruited complete the core protocol and are classified according to the presence and extent of any neuropathic pain. This information will be used to look for genetic, environmental and basic clinical risk factors using the methods outlined below. The “extended” protocol consists of more detailed phenotyping and uses multiple tools. The tools used for any subject depend on the recruitment centre to which he or she is recruited ( Table 2). A sub-group of participants will be recruited through the extended protocol.
Table 1. Questionnaires of the DOLORisk protocol.
| Category | Questionnaire | Core | Extended | Reference |
|---|---|---|---|---|
| Demographics | Age, gender, years in education,
working status, weight, height |
X | X | |
|
Characterisation of
pain |
Presence and duration of pain | X | X | |
| Family history | Family history of chronic pain | X | ||
| Pain medication | Currently taking pain medication | X | X | |
| Brief Pain Inventory – Usefulness of
medication |
X | Cleeland and Ryan 15 | ||
| Adherence to medication | X | |||
| Pain severity | Chronic Pain Grade | X | X | Von Korff, et al. 16 |
| Brief Pain Inventory – Pain Severity | X | Cleeland and Ryan 15 | ||
| Pain quality | DN4 Questionnaire | X | X | Bouhassira, et al. 9 |
| DN4 Examination | X | |||
| Neuropathic Pain Symptom Inventory | X | Bouhassira, et al. 17 | ||
| PainDETECT | X | Freynhagen, et al. 10 | ||
| Pain location | List of locations | X | X | |
| Body map | X | |||
| Pain interference | PROMIS Pain Interference | X | Cella, et al. 19 | |
| Pain catastrophizing | Pain Catastrophizing Scale | X | X | Sullivan, et al. 20 |
|
Health status and
quality of life |
EQ-5D-5L | X | X | Herdman, et al. 21 |
| PROMIS Depression | 4a | 8a | Cella, et al. 19 | |
| PROMIS Anxiety | 4a | 8a | ||
| PROMIS Sleep Disturbance | 4a | 8a | ||
| PROMIS Fatigue | X | |||
| Trauma | X | X | ||
|
Disease specific
(diabetic neuropathy) |
Michigan Neuropathy Screening
Instrument |
X | Feldman, et al. 18 | |
| Personality | Ten Item Personality Inventory | X | X | Gosling, et al. 22 |
| International Personality Item Pool
(Emotional Stability) |
X | Goldberg 23 | ||
| Lifestyle | Smoking | X | X | Campbell, et al. 24 |
| Alcohol | X | X | ||
| International Physical Activity
Questionnaire |
X | Craig, et al. 25 |
Table 2. Summary of tests performed during the DOLORisk protocol.
| Cohort | Protocol | Neurological
examination |
TCSS | TNSn | Skin
biopsy |
QST | NCS | EEG | Threshold
tracking |
CPM |
|---|---|---|---|---|---|---|---|---|---|---|
| Population | Core | |||||||||
| Diabetes | Extended | X | X | X | X | X | X | X | X | |
| Traumatic nerve
injury |
Extended | X | X | X | X | |||||
| Surgery | Extended | X | X | X | X | |||||
| Chemotherapy | Extended | X | X | X | X | X | ||||
| Extreme
phenotypes |
Extended | X | X | X | X | X |
TCSS- Toronto clinical scoring system; TNSn- Total Neuropathy Score – Nurse; QST- Quantitative sensory testing; EEG - Electroencephalography; CPM- Conditioned pain modulation.
Tools for phenotyping
Questionnaires
Demographics
Demographic information captured includes age, gender, weight, height, years in education, and working status.
Characterisation of pain
The presence and duration of pain (and also dysaesthesia) are assessed. Family history of chronic pain is recorded. Pain medication, analgesic relief obtained and adherence to medication are recorded according to the Brief Pain Inventory (BPI) 15.
Pain intensity
Intensity of the pain is assessed with two questionnaires: the Chronic Pain Grade (CPG) 16 over the past three months, and the BPI’s subscale for assessment of average pain severity over 24 hours (which uses an 11 point numerical rating scale). One additional item asks about average pain over the past seven days.
Pain quality
Neuropathic descriptors of the pain are characterised with three tools: the DN4 ( Douleur Neuropathique en 4 questions) 9, the Neuropathic Pain Symptom Inventory (NPSI) 17, and the painDETECT 10. The Michigan Neuropathy Screening Instrument (MNSI) 18 is used specifically for diabetic neuropathy.
Pain location
The participants are asked to indicate in which body site they feel pain. This is assessed in two ways: a list of body sites and a body map. The participants are asked to identify all the body locations in which they experienced pain over the previous three months, and to mark the pain that bothers them the most. The body sites include: Back pain; Neck or shoulder pain; Facial or dental pain; Headache; Stomach ache or abdominal pain; Pain in the arms; Pain in the hands; Chest pain; Pain in the hips; Pain in the legs or knees; Pain in the feet; Pain throughout the body (widespread pain); Other pain.
The core and the extended protocols take a different approach to identify the location in which the participant should be asked to rate pain. The rationale for this is that the recommendation for grading neuropathic pain is based upon pain and clinical signs in a neuroanatomically plausible distribution 26. The core protocol is designed for the assessment of neuropathic pain of diverse aetiologies at population level, and there is no prior expectation as to the neuroanatomically plausible distribution. Then, participants are asked to specify body regions in which they experience pain, and choose one body region in which the pain bothers them most. In the core protocol, participants are asked to answer the questions that relates to pain intensity, quality and interference in respect to the body region in which pain bothers them most. The approach in the extended protocol is different because in these cohorts the likely aetiology of neuropathic pain is known and therefore the neuroanatomically plausible distribution is pre-determined. For instance in diabetic neuropathy or chemotherapy induced neuropathy the neuroanatomically plausible distribution is the feet, whereas following post-traumatic nerve injury the neuroanatomically plausible distribution is the innervation territory of the affected nerve. Participants are explicitly asked by the investigator to focus on the neuroanatomically plausible distribution when answering the questions on pain intensity, quality and interference. To capture information on other types of pain we then ask about pain in other body regions.
Pain interference, quality of life and psychological variables
The Patient-Reported Outcomes Measurement Information System (PROMIS) 19 questionnaires are used to assess various psychological and psychosocial variables. They include depression, anxiety, sleep disturbance, fatigue and pain interference. Two bespoke questions adapted from the existing population data ask about traumatic life experiences. The EQ-5D-5L 21 measures quality of life with a visual analogue scale and five items evaluating the impact of pain on the ability of the participant to perform everyday tasks.
Two questionnaires assessing personality and in particular neuroticism are included in the DOLORisk protocol. The Ten-Item Personality Inventory (TIPI) 22 evaluates extraversion, agreeableness, conscientiousness, neuroticism, and openness to experience. The 10-item International Personality Item Pool’s (IPIP) 23 representation of the Goldberg 27 markers for Emotional Stability offers a more precise characterisation of neuroticism. Pain catastrophizing behaviours are recorded through the Pain Catastrophizing Scale (PCS) 20.
Lifestyle
Smoking and alcohol are recorded according to Campbell, et al. 24 The short form of the International Physical Activity Questionnaire (IPAQ) 25 is included in the lifestyle variables to account for physical activity.
Clinical assessment and specialised investigations
Neurological examination
A comprehensive structured upper and lower limb neurological examination is performed to detect clinical signs of a neurological lesion such as a peripheral neuropathy 5, 28– 30. The examination includes assessment of temperature (using Somedic RollTemp, Somedic AB, Sweden), light touch (using 10g monofilament) and pinprick sensation (using ‘Neurotip’), joint position sense (proprioception), vibration perception using a 128Hz tuning fork, deep-tendon reflexes (using a Queen square tendon hammer and recorded as present as normal, present with reinforcement, absent or brisk), muscle bulk, and motor power. The clinical findings for a length-dependent neuropathy are quantified with the Toronto Clinical Scoring System (TCSS) 31. The Total Neuropathy Score – Nurse (TNSn) 32 is used for chemotherapy-induced neuropathy. For other causes of neuropathic pain the spatial extent of sensory deficits and sensory hypersensitivity is recorded on a body map.
Nerve conduction studies
Nerve conduction tests, to confirm the presence of a length dependent neuropathy, are performed in line with those recommended by the American Academy of Neurology and American Association of Electrodiagnostic Medicine 33, 34. Sural sensory and peroneal motor nerve conduction studies are performed in one lower extremity. If both studies are normal 33 no further tests are performed. If either test is abnormal additional nerve conduction studies are performed that include: ipsilateral tibial motor nerve; contralateral sural sensory nerve, peroneal motor or tibial motor nerves; or ulnar sensory, median sensory, and ulnar motor nerves in one upper extremity. The minimum case definition criterion for electrodiagnostic confirmation of a length dependant neuropathy is an abnormality of any attribute of nerve conduction studies in two separate nerves, one of which is the sural nerve. Variables such as skin temperature, age, height, gender, and weight are measured and accounted for when interpreting nerve conduction tests. Nerve conduction tests are not repeated if study participants have previous results.
Electroencephalography
Electroencephalography (EEG) reflects the summated activity of synchronised arrays of brain neurons. Establishing EEG as an appropriate biomarker for pain perception relies on its accuracy to correctly classify subjects as belonging to the pain or no-pain conditions. In order to achieve this goal we follow the standard statistical steps of multivariate pattern analysis. A range of classifiers that distinguish the painful from the non-painful brain include measures of peak activity within the various EEG frequency bands per electrode, point to point connectivity between each of 64 electrodes, as well as identification of brain networks. This is expected to allow new understanding about the neurophysiological aspects of pain processing in the painful brain. The classification method finally employed will be the one with the highest classification accuracy on a test set after being trained on a separate training set.
Threshold tracking
Threshold tracking is an electrophysiological tool that assesses nerve excitability 35. Nerve excitability measures are determined by the biophysical properties of myelinated axons and the axon membrane potential. The information obtained about nerve properties is complementary to conventional nerve conduction studies 35. In DOLORisk several measures of axonal excitability, such as refractoriness, supernormality, strength-duration time constant and threshold electrotonus, are assessed. The excitability measures are recorded from the motor and sensory divisions of the median nerve in line with published recommendations 36. Training will be provided to clinicians performing threshold tracking measurements to ensure the reliability of the data and harmonisation of nerve excitability protocols in all centres.
Conditioned pain modulation
Conditioned pain modulation (CPM) provides insight into an individual’s endogenous analgesic mechanisms 37, 38. It can be assessed in a non-invasive manner and may be a key vulnerability factor for chronic pain and has also been shown to be predictive of treatment response. The protocol for CPM testing is in keeping with published recommendations 39, 40.
Skin biopsy for intra-epidermal nerve fibre assessment
Intra-epidermal nerve fibre density (IENFD) is a validated tool for the assessment of small fibre pathology 41. In DOLORisk IENFD is determined from skin biopsy samples taken in accordance with published guidelines provided by the European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the utilisation of skin biopsy samples in the diagnosis of peripheral neuropathies 41. The skin biopsies are taken at the end of the clinical assessment once all relevant investigations are completed. Participants do not under undergo a skin biopsy if they are on warfarin or found to have other contraindications.
Quantitative sensory testing
Quantitative sensory testing (QST) is a measure of sensory perception in response to a defined sensory stimulus. This test can show abnormalities in sensory function and be used to generate a sensory profile in respect to different sensory modalities assessing both gain and loss of function. For bilateral neuropathic pain disorders such as peripheral neuropathy QST is performed unilaterally on the dorsum of the most affected foot. For unilateral neuropathic pain disorders QST is performed bilaterally in the affected area and the contralateral equivalent body region (which acts as a helpful comparator). QST is performed according to a modification of the previously published protocol of the German Research Network on Neuropathic Pain (DFNS) 42. These modifications were made in order to improve efficiency when performed in a restricted timescale. The wind up ratio (WUR) is not performed unless the patient is having CPM in which case it will be helpful to have a measure of central sensitisation. WUR is performed on the forearm instead of the dorsum of the hand in order to minimise the influence of peripheral sensory loss on detection of central processes. Thermal sensory limen is performed in those patients with peripheral neuropathy but not in peripheral nerve injury (a situation where it is less informative). The assessment of mechanical pain sensitivity is shortened and two rounds of tests are performed instead of the five rounds included in the full DFNS protocol. All other tests are identical to the DFNS protocol and all study sites will be trained and certificated in the DFNS protocol to promote standardisation. QST data is entered into the data analysis system, Equista (version 1.2.2., CASQUAR GmbH), which was developed by the DFNS. Equista transforms the raw QST data into z-scores thus normalising for age, gender, and the body location of testing 43, 44. A z-score of zero is equal to the mean of the population. A score of greater or less than two standard deviations from the mean indicates gain of function or loss of function, respectively.
Genetics
DNA is extracted from a whole blood sample collected at recruitment. The analysis will follow three complementary approaches: genome-wide association studies (GWAS); whole exome sequencing to identify rare, high-impact coding variants; and targeted sequencing of selected candidate genes.
Definition of neuropathy
The participants who undergo the extended protocol are assessed for neuropathy (when this is considered a relevant possibility by the investigator e.g. a patient with diabetes) and in all cases are also graded for neuropathic pain. To diagnose peripheral neuropathy, we use the criteria outlined by Tesfaye, et al. 45 that classify neuropathy as possible, probable or confirmed:
Possible peripheral neuropathy is defined as the presence either of sensory symptoms, i.e. decreased sensation (e.g. “asleep, numbness”), positive neuropathic sensory symptoms (e.g. prickling or stabbing, burning or aching pain) predominantly in the toes, feet, or legs; or of sensory signs, i.e. symmetric decrease of distal sensation or unequivocally decreased or absent ankle reflexes.
Probable peripheral neuropathy corresponds to any two or more of the following: sensory symptoms (as above), decreased distal sensation, or unequivocally decreased or absent ankle reflexes.
Confirmed peripheral neuropathy is defined as the presence of an abnormality of nerve conduction studies and a sensory symptoms OR signs of neuropathy. If nerve conduction studies are normal, a validated measure of small fibre neuropathy (abnormal thermal thresholds on QST or reduced intra-epidermal nerve fibre density) may be used 45.
Definition of neuropathic pain
The Neuropathic Pain Special Interest Group (NeuPSIG) of the International Association for the Study of Pain (IASP)’s grading for neuropathic pain 46 is used to grade neuropathic pain for all study participants recruited. Each study participant’s pain is assessed using these published criteria as below. Possible neuropathic pain must fulfil criteria 1 and 2. Probable neuropathic pain must fulfil criteria 1, 2 and 3. Definite neuropathic pain must fulfil all 4 criteria.
-
1.
Pain with a distinct neuroanatomically plausible distribution, e.g. pain symmetrically distributed in the extremities – completion of body map and clinical history.
-
2.
A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system – e.g. diagnosis of diabetes mellitus and a history of neuropathy symptoms including decreased sensation, positive sensory symptoms, e.g. burning, aching pain mainly in the toes, feet or legs.
-
3.
Demonstration of distinct neuroanatomically plausible distribution of neuropathic pain – e.g. presence of clinical signs of peripheral neuropathy, i.e. decreased distal sensation or decreased/absent ankle reflexes.
-
4.
Demonstration of the relevant lesion or disease by at least one confirmatory test – e.g. abnormality on either the nerve conduction tests or IENFD.
In the large, population-based cohorts, the core protocol permits the ‘entry level’ approximation to a classification of “possible neuropathic pain”, based on the NeuroPPIC phenotyping consensus 14. This includes positive responses to the DN4 screening questionnaire, and relevant site and severity of pain as outlined above. Additional information on diagnosis of any pain conditions will be available.
Cohorts
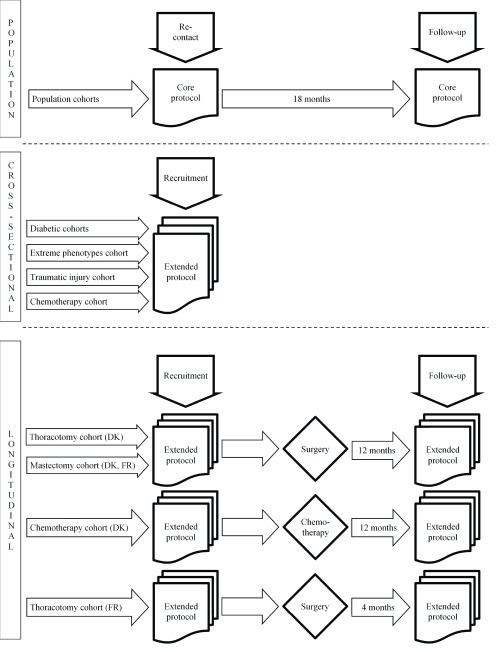
DOLORisk is a multi-centre cross-sectional and longitudinal observational study. Multiple cohorts with neuropathic pain from different causes will be included. Each cohort has its own specific inclusion and exclusion criteria, and follows a specific recruitment flow ( Figure 1; Table 3– Table 5).
Figure 1. DOLORisk Recruitment flow.
DK = Denmark, FR = France.
Table 3. Inclusion and exclusion criteria for invitation to the population cohort for the DOLORisk protocol.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population cohort | • Previous participation with GoDARTS or GS:SFHS.
• Existing consent to be re-contacted. • Identified as being currently alive. • Currently has a postal address on file. • ≥ 18 years. |
• Unable to give consent.
• No current postal address available. • Identified as having died. |
Table 4. Inclusion and exclusion criteria for the cross-sectional cohorts for the DOLORisk protocol.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
|
Peripheral
neuropathic pain |
• ≥18 years with a diagnosis of peripheral neuropathy
based on a prior clinical assessment combined with supportive clinical investigations such as abnormal nerve conduction studies, reduced intraepidermal nerve or abnormal findings on quantitative sensory testing. • Symptoms highly suggestive of neuropathy that in the judgement of the clinical researcher are suitable for the study even if they do not fulfil other inclusion criteria. • Patients who do not fulfil any of the exclusion criteria. • Diabetes cohorts: Type 1 or Type 2 diabetes |
• Pregnant.
• Incapacity to give consent or to complete the study questionnaires due to insufficient language command or mental deficiencies. • Concurrent severe psychological or psychiatric disorders. • Moderate to severe pain from other causes that may confound assessment or reporting of pain (e.g. spinal canal stenosis). • Central nervous lesions, which may complicate somatosensory testing. • Patients who are in the opinion of the investigator unsuitable for participation in the study. |
|
Extreme
phenotypes |
• ≥16 years with a set of symptoms that resemble those
seen on Paroxysmal Extreme Pain Disorder, Familial Episodic Pain Syndrome or Erythromelalgia. • Existing diagnosis of Paroxysmal Extreme Pain Disorder or Familial Episodic Pain Syndrome or Erythromelalgia. • Reduced pain sensibility. • First degree relatives of patients who meet diagnostic criteria for Paroxysmal Extreme Pain Disorder, Familial Episodic Pain Syndrome, Erythromelalgia or inability to experience pain. • Patients who do not fulfil any of the exclusion criteria. |
• Pregnant.
• Incapacity to give consent or to complete the study questionnaires due to insufficient language command or mental deficiencies. • Concurrent severe psychological or psychiatric disorders, especially severe claustrophobia. • Moderate to severe pain arising as a consequence of other disorders causing pain but that are not associated with those mentioned before as channelopathies. • Central nervous system diseased that may complicate the somatosensory testing. • Patients who are in the opinion of the investigator unsuitable for participation in the study. • Treatment or topical capsaicin cream/ ointment or Lidocaine patch within 30 days prior to Day 1 on the skin area that will be tested. • Presence of oedema or any skin condition at the ankle level that may interfere with the microneurography procedure. |
Table 5. Inclusion and exclusion criteria for the longitudinal cohorts for the DOLORisk protocol.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Chemotherapy | • ≥18 years.
• Diagnosed with high-risk colorectal cancer. • Planned adjuvant treatment with oxaliplatin and flourouracil (5-FU) or capecitabine (Pro 5-FU). |
• Known metastatic cancer.
• Previous treatment with chemotherapy. • Receiving another treatment than oxaliplatin and flourouracil (5-FU) or capecitabine (Pro 5-FU). • Significant mental illness. • Alcohol abuse. • Known diabetes. • Significant neuropathic diseases. • Spinal cord stenosis. • Peripheral vascular diseases (Fontaine >2). • Chronic pain with a pain intensity on a 0-10 numeric rating scale >5. • Patients who do not speak, read or understand Danish. |
| Thoracotomy | • ≥ 18 years.
• Scheduled for lung cancer resection performed via thoracoscopy and/or thoracotomy, including lobectomy, bilobectomy, pneumonectomy, resection of the tracheobronchial bifurcation, wedge resection, sleeve resection and combinations hereof. • Willingness and ability to comply with study procedures as judged by the site investigator/ manager. • Expected availability for follow-up throughout the study. |
• Mental incapacity or language barriers precluding
adequate understanding of study procedures. • Current alcohol or substance abuse according to the site investigator’s medical judgement. • Unsuitability for participation in the study for any other reason, e.g. due to a significant serious underlying condition (e.g. other cancer or AIDS), as determined by the site investigator/manager. ADDITIONALLY in FR: • Previous surgery on the same area. • Surgery targeting only the pleura or mediastinum. • Peripheral neurological pathology or central (brain damage, multiple sclerosis) susceptible to interfere with the evaluation of the post-operative pain. • History of significant mental illness: psychosis, severe depression having motivated a hospitalisation, suicide attempt. • Current major depressive episode at the time of the evaluation. • Abuse of drug or psychoactive substance during the last six months. • Patients participating in another protocol of biomedical research. |
| Mastectomy | • Women ≥ 18 years.
• Scheduled for breast cancer resection performed via lumpectomy (partial or segmental mastectomy) or mastectomy with or without sentinel lymph node biopsy and axillary lymph node dissection, and any combinations hereof. • Affiliated to a social security scheme. • Danish/French language (read, written and spoken). • Willingness and ability to comply with study procedures as judged by the site investigator. • Expected availability for follow-up throughout the study. |
• Other cancer or AIDS.
• Scheduled for bilateral mastectomy. • Presence of chronic pain before the breast cancer surgery. • Workplace accident, litigation or search for compensation. • Previous surgery on the same area. • Peripheral neurological pathology or central (brain damage, multiple sclerosis) susceptible to interfere with the evaluation of the post-operative pain. • History of significant mental illness: psychosis, severe depression having motivated a hospitalisation, suicide attempt. • Current major depressive episode at the time of assessment. • Abuse of drug or psychoactive substance during the last six months. • Cognitive or psychological disorders incompatible with the respect and/or the understanding of the protocol. • Participating in another protocol of biomedical research. • Current alcohol or substance abuse according to the site investigator's medical judgement. • Unsuitability for participation in the study for any other reason, e.g. due to a significant serious underlying condition (e.g. other cancer or AIDS), as determined by the site investigator. |
Population cohort
Generation Scotland: the Scottish Family Health Study (GS:SFHS) 47 and Genetics of Diabetes Audit and Research Tayside (GoDARTS) 48 are population-based genetic epidemiology studies. DNA, socio-demographic and clinical data are available for 24,000 GS:SFHS participants and 20,000 (9,000 with diabetes) GoDARTS participants across Scotland. Participants will be contacted by post and invited to complete the DOLORisk core protocol. After 18 months, enrolled participants will be invited to complete the same questionnaire to assess development, progression or remission of any pain. For the population cohorts it is estimated that between 7% (GS:SFHS) and 25% (GoDARTS) of those with chronic pain will have neuropathic pain 49. Therefore, 1,500 participants with neuropathic pain and 3,000 controls are anticipated from GS:SFHS and 2,000 participants with neuropathic pain and 4,000 controls are anticipated from GoDARTS.
Cross-sectional cohorts assessed with the extended protocol
Patients with peripheral neuropathic pain, e.g. diabetic neuropathy, chemotherapy-induced neuropathy, and traumatic nerve injury will be recruited by the University of Oxford, Imperial College London, Kiel University, Technion – Israel Institute of Technology, Neuroscience Technologies, and Aarhus University, from both primary and secondary care. Patients with extreme pain phenotypes, such as insensitivity to pain, will also be recruited. The study participants will be assessed as per the DOLORisk extended protocol.
Longitudinal cohorts assessed with the extended protocol
Patients undergoing mastectomy, thoracotomy or receiving chemotherapy will be recruited by INSERM (French National Institute for Health and Medical Research) and Aarhus University. The surgical cohort of study participants will be recruited among patients scheduled for lung surgery or breast cancer surgery. The study participants receiving chemotherapy will be recruited from patients diagnosed with colorectal cancer. All study participants in this cohort will undergo the extended protocol before surgery or receiving chemotherapy. Thereafter, at different times ranging from 4 to 12 months participants will be re-assessed, using the extended protocol, to determine the development of neuropathic pain ( Figure 1). We expect to include 50 patients scheduled to undergo chemotherapy and 590 patients scheduled for lung or breast surgery.
Data analysis
Sample size calculation
The sample size for the protocol is largely based on the primary outcome, which is the number of participants to explore the genetic risk factors of neuropathic pain. The main comparison will be between those study participants diagnosed with neuropathic pain and those are diagnosed with no pain or pain of non-neuropathic nature. We will also be exploring physiological and psychosocial risk factors and these outcomes will require smaller sample sizes.
For example, based on the CaTS power calculator 50, we will have 80% power in an additive model with p=10 -8, prevalence of neuropathic pain in the general population of 8%, with a disease allele frequency of 0.30 (GS:SFHS) or 0.38 (GoDARTS), and therefore a genotype relative risk of 1.34. Based on the CaTS GWAS power calculator 50, with 1,500 cases and 3,000 controls (as in the GS:SFHS cohort), we will have 82.7% power to identify SNP associations with a significance level of 5×10 −8, assuming an additive model, a minor disease allele frequency of 0.3, a genotypic relative risk of 1.35, and a prevalence of the diabetic neuropathic pain in the general population of 10% 1.
For the extended phenotyping of painful versus painless diabetic neuropathy (estimating 1000 subjects in each group) we will have 80% power to detect an allelic odds ratio of 1.7 at genome wide significance level (p<5×10 -8). We will also be able to cross-validate between these cohorts. We have identified a further cohort of diabetic neuropathy individuals in Sweden who will be available for replication genotyping. In collaboration with the SUMMIT consortium, we would also like to combine data across diverse diabetic complications in order to enhance the power to detect genetic determinants of the microvascular complications of diabetes.
Further sample size calculations have been performed depending on the individual outcome measures being measured.
QST
Sample size was determined according to the warm detection threshold data for patients with diabetes 5. This calculation revealed a minimum sample size of 34 was required per group for a power of >0.8 (difference in means 2.0; standard deviation 4.3; a = 0.05).
CPM
A cohort of 53 subjects gives an 80% power in between group differences of >0.25 standard deviations equivalent to 1.0 to 1.6 range on the 0-10 pain numerical rating scale using a typical QST parameter such as conditional pain modulation.
Data management
The University of Dundee’s Health Informatics Centre (HIC) Services acts as a hub for data management. HIC Services develops bespoke software to support secure data collection, provides recruitment support for clinical studies and manages a data entry service. All services provided by HIC are delivered within a secure Safe Haven environment to ensure data are managed safely and in compliance with Data Protection legislation. All HIC processes are governed by approved Standard Operating Procedures.
GoDARTS and GS:SFHS datasets are already hosted on secure HIC servers. Participants’ identities will be shielded at all times from the research team, according to the secure SOPs.
External datasets generated by DOLORisk will be sent to HIC in anonymised format. When ready, these updated datasets will be transferred to the analytics platform held on a separate server and network from the HIC data management function within a remote-access Safe Haven for research projects. It has full analytical functionality including software (e.g. R and SAS) and is supported by powerful processing. Remote access to the Safe Haven analytics platform is available to approved project researchers, after they have signed appropriate agreements. No individual-level data can be removed from the Safe Haven, but summary outputs of analysis are released, after prompt screening by HIC to ensure that no potentially identifiable information is included to reduce the risk of accidental disclosure. Clinical phenotype data will be linked in anonymised format to genomic outputs.
Ethics and dissemination
Ethic approvals were obtained at the national level. Details can be found in Table 6. Participants are included in the protocol only after having given their written informed consent. Their decision whether to take part, or withdrawal during the course of the study, in no way alters their normal medical care. The signed informed consent is obtained by the clinician in charge of the patient or the healthy volunteer.
Table 6. DOLORisk cohorts approvals.
NT: Neuroscience Technologies. INSERM : Institut National de la Santé Et de la Recherche Médicale. CS: cross-sectional. Pro: prospective. REC: Research Ethics Committee. ANSM: Agence nationale de sécurité du médicament et des produits de santé (national agency for medicines and health products safety). CPP : Comité de protection des personnes (ethical research committee). CCTIRS: Comité consultatif sur le traitement de l'information en matière de recherche dans le domaine de la santé (advisory committee on data processing in health research). CNIL: Commission nationale de l’informatique et des libertés (data protection authority).
| Centre | CS or
Pro |
Aetiology | Anticipated
sample size |
Ethics
committee |
Ethics
reference |
Registration link | Reference | End date |
|---|---|---|---|---|---|---|---|---|
| Dundee | Pro | Mixed | 5500 | Tayside
Committee on Medical Research Ethics |
05/S1401/89 |
https://www.hra.nhs.uk/planning-
and-improving-research/ application-summaries/research- summaries/dolorisk-dundee/ |
Smith, et al. 47 | April 2018 |
| Yorkshire & The
Humber - South Yorkshire REC |
15/YH/0285 | |||||||
| Dundee | Pro | Diabetes | 3000 | Tayside
Committee on Medical Research Ethics |
053/04 |
https://clinicaltrials.gov/ct2/show/
NCT02783469 |
Hebert, et al. 48 | |
| Yorkshire & The
Humber - South Yorkshire REC |
15/YH/0285 | |||||||
| Oxford | CS | Extreme
phenotypes |
100 | NRES
Committee London - Riverside |
12/LO/0017 |
https://clinicaltrials.gov/ct2/show/
NCT02696746 |
January
2019 |
|
| CS | Diabetes | 300 | West London
REC 3 |
10/H0707/35 |
https://clinicaltrials.gov/ct2/show/
NCT02672059 |
Themistocleous,
et al. 5 |
June 2019 | |
| Imperial | CS | Diabetes | 200 | London -
Bromley REC |
16/LO/1470 | |||
| Kiel | CS | Mixed | 200 | Ethics
Committee of the Faculty of Medicine of Kiel University |
D454/16 |
https://clinicaltrials.gov/ct2/show
NCT02666456 |
March
2019 |
|
| Technion | CS | Diabetes | 200 | Helsinki
Committee of Rambam Health Care Campus |
0052-15-RNB |
https://clinicaltrials.gov/ct2/show/
NCT02402361 |
July 2018 | |
| NT | CS | Diabetes | 100 | Clinical
Research Ethics Committee (CREC) of idcsalud in Catalonia |
2016/43-NEU-
MC Mutual |
https://clinicaltrials.gov/ct2/show/
NCT02985294 |
March
2019 |
|
| CS | Traumatic | 100 | ||||||
| Aarhus | CS | Diabetes | 350 | Central
Denmark Region Committees on Health Research Ethics |
Diabetic
neuropathy, 1- 10-72-130-16 |
https://clinicaltrials.gov/ct2/show/
NCT02947828 |
May 2018 | |
| CS | Chemotherapy | 70 | Central
Denmark Region Committees on Health Research Ethics |
Chronic
neuropathy following chemotherapy, 20110158 |
https://clinicaltrials.gov/ct2/show/
NCT02654691 |
April 2017 | ||
| Pro | Chemotherapy | 50 | Central
Denmark Region Committees on Health Research Ethics |
Acute and
chronic neuropathy after oxaliplatin, 1-10- 72-154-16 |
||||
| Aarhus | Pro | Post-surgical | 250 | Central
Denmark Region Committees on Health Research Ethics |
Understanding
risk factors and determinants for neuropathic pain after lung or breast surgery, 1-10-72-254-16 and 1-10-72- 23-17 |
https://clinicaltrials.gov/ct2/show/
NCT03124511 https://clinicaltrials.gov/ct2/show/ NCT02960971 |
November
2019 |
|
| INSERM | Pro | Post-surgical | 340 | ANSM | 160106B-32,
160287B-32 |
https://clinicaltrials.gov/ct2/show/
NCT02944721 |
November
2020 |
|
| CPP | CPP/2-16, 16
03 18 |
|||||||
| CCTIRS | 16-331bis, 16-
330bis |
|||||||
| CNIL | 2007306 v 0,
1251929 v 0 |
Where possible, datasets will be made publicly available once the study is completed. Gene variants associated with neuropathic pain risk will be entered into the existing PainNetworks database 51 that undergoes longstanding curation by the London Pain Consortium. Transcriptional profiling data will be entered into painnetworks.org and ArrayExpress. We will enrich this with anonymised normative data on sensory profiling and physiological variables. It will be possible to download clinical screening tools from the DOLORisk website.
Findings will be communicated to the scientific community via peer-reviewed publications (open access), and presentations at conferences. DOLORisk has partnered with patient organisations supporting people with pain and neuropathy-related disorders such as Pain Association Scotland, the InDependent Diabetes Trust, and Fibromyalgia Action UK. The results of the study will be sent to the organisations periodically.
Current study status
Recruitment started in 2016 and is ongoing in all centres. As of December 2017, 1,915 participants in GoDARTS and 7,240 participants in Generation Scotland have returned the questionnaires of the core protocol. 1,062 participants have been recruited throughout the rest of the centres according to the extended protocol. All recruitment and follow-up activities are expected to be completed by mid-2019.
Data availability
No data are associated with this article.
Funding Statement
This work was supported by the European Union’s Horizon 2020 research and innovation programme under grant agreement No 633491 (DOLORisk), and the International Diabetic Neuropathy Consortium (IDNC) research programme, which is supported by a Novo Nordisk Foundation Challenge programme grant (Grant number NNF14SA0006). D.L.B. is a Wellcome senior clinical scientist [202747].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 1 approved, 1 approved with reservations]
References
- 1. van Hecke O, Austin SK, Khan RA, et al. : Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155(4):654–62. 10.1016/j.pain.2013.11.013 [DOI] [PubMed] [Google Scholar]
- 2. Attal N, Lanteri-Minet M, Laurent B, et al. : The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152(12):2836–43. 10.1016/j.pain.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 3. Finnerup NB, Sindrup SH, Jensen TS: The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150(3):573–81. 10.1016/j.pain.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 4. Treede RD, Jensen TS, Campbell JN, et al. : Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–5. 10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 5. Themistocleous AC, Ramirez JD, Shillo PR, et al. : The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. 2016;157(5):1132–45. 10.1097/j.pain.0000000000000491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Hehn CA, Baron R, Woolf CJ: Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron. 2012;73(4):638–52. 10.1016/j.neuron.2012.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denk F, McMahon SB, Tracey I: Pain vulnerability: a neurobiological perspective. Nat Neurosci. 2014;17(2):192–200. 10.1038/nn.3628 [DOI] [PubMed] [Google Scholar]
- 8. Denk F, McMahon SB: Neurobiological basis for pain vulnerability: why me? Pain. 2017;158(Suppl 1):S108–S14. 10.1097/j.pain.0000000000000858 [DOI] [PubMed] [Google Scholar]
- 9. Bouhassira D, Attal N, Alchaar H, et al. : Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. 10.1016/j.pain.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 10. Freynhagen R, Baron R, Gockel U, et al. : pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20. 10.1185/030079906X132488 [DOI] [PubMed] [Google Scholar]
- 11. Rolke R, Baron R, Maier C, et al. : Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–43. 10.1016/j.pain.2006.01.041 [DOI] [PubMed] [Google Scholar]
- 12. Bennett MI, Smith BH, Torrance N, et al. : The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–58. 10.1016/j.jpain.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 13. Haanpää M, Attal N, Backonja M, et al. : NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152(1):14–27. 10.1016/j.pain.2010.07.031 [DOI] [PubMed] [Google Scholar]
- 14. van Hecke O, Kamerman PR, Attal N, et al. : Neuropathic pain phenotyping by international consensus (NeuroPPIC) for genetic studies: a NeuPSIG systematic review, Delphi survey, and expert panel recommendations. Pain. 2015;156(11):2337–53. 10.1097/j.pain.0000000000000335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cleeland CS, Ryan KM: Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 16. Von Korff M, Ormel J, Keefe FJ, et al. : Grading the severity of chronic pain. Pain. 1992;50(2):133–49. 10.1016/0304-3959(92)90154-4 [DOI] [PubMed] [Google Scholar]
- 17. Bouhassira D, Attal N, Fermanian J, et al. : Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108(3):248–57. 10.1016/j.pain.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 18. Feldman EL, Stevens MJ, Thomas PK, et al. : A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–9. 10.2337/diacare.17.11.1281 [DOI] [PubMed] [Google Scholar]
- 19. Cella D, Riley W, Stone A, et al. : The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–94. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sullivan MJL, Bishop SR, Pivik J: The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7(4):524–32. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 21. Herdman M, Gudex C, Lloyd A, et al. : Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gosling SD, Rentfrow PJ, Swann WB: A very brief measure of the Big-Five personality domains. J Res Pers. 2003;37(6):504–28. 10.1016/S0092-6566(03)00046-1 [DOI] [Google Scholar]
- 23. Goldberg LR: A broad-bandwidth, public domain, personality inventory measuring the lower-level facets of several five-factor models. Reference Source [Google Scholar]
- 24. Campbell A, Kerr SM, Porteous DJ: Generation Scotland SFHS Data Dictionary, 2006-2011. University of Edinburgh. School of Molecular, Genetic and Population Health Sciences. Institute of Genetics and Molecular Medicine.,2018. 10.7488/ds/2277 [DOI] [Google Scholar]
- 25. Craig CL, Marshall AL, Sjöström M, et al. : International physical activity questionnaire: 12-country reliability and validity. Med Sci Sport Exer. 2003;35(8):1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 26. Finnerup NB, Haroutounian S, Kamerman P, et al. : Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–606. 10.1097/j.pain.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldberg LR: The development of markers for the Big-Five factor structure. Psychol Assess. 1992;4(1):26–42. 10.1037/1040-3590.4.1.26 [DOI] [Google Scholar]
- 28. Kleyweg RP, van der Meché FG, Schmitz PI: Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barré syndrome. Muscle Nerve. 1991;14(11):1103–09. 10.1002/mus.880141111 [DOI] [PubMed] [Google Scholar]
- 29. Medical Research Council - Nerve Injuries Research Committee: Aids to the examination of the peripheral nervous system. Fifth Edition ed: Saunders Elsevier on behalf of Guarantors of Brain. 2010. Reference Source [Google Scholar]
- 30. Phillips TJ, Brown M, Ramirez JD, et al. : Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: A cross-sectional deep profiling study. Pain. 2014;155(9):1846–60. 10.1016/j.pain.2014.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bril V, Perkins BA: Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care. 2002;25(11):2048–52. 10.2337/diacare.25.11.2048 [DOI] [PubMed] [Google Scholar]
- 32. Smith EM, Cohen JA, Pett MA, et al. : The reliability and validity of a modified total neuropathy score-reduced and neuropathic pain severity items when used to measure chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Cancer Nurs. 2010;33(3):173–83. 10.1097/NCC.0b013e3181c989a3 [DOI] [PubMed] [Google Scholar]
- 33. Buschbacher R, Orahlow N: Manual of nerve conduction studies. Second edition ed. New York, New York: Demos Medical Publishing.2006. Reference Source [Google Scholar]
- 34. England JD, Gronseth GS, Franklin G, et al. : Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199–207. 10.1212/01.WNL.0000149522.32823.EA [DOI] [PubMed] [Google Scholar]
- 35. Z'Graggen WJ, Bostock H: Nerve membrane excitability testing. Eur J Anaesthesiol Suppl. 2008;42:68–72. 10.1017/S0265021508003505 [DOI] [PubMed] [Google Scholar]
- 36. Kiernan MC, Burke D, Andersen KV, et al. : Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23(3):399–409. [DOI] [PubMed] [Google Scholar]
- 37. Yarnitsky D: Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23(5):611–5. 10.1097/ACO.0b013e32833c348b [DOI] [PubMed] [Google Scholar]
- 38. Yarnitsky D, Granot M, Nahman-Averbuch H, et al. : Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153(6):1193–8. 10.1016/j.pain.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 39. Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. : Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain. 2010;14(4):339. 10.1016/j.ejpain.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 40. Yarnitsky D, Bouhassira D, Drewes AM, et al. : Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. 2015;19(6):805–6. 10.1002/ejp.605 [DOI] [PubMed] [Google Scholar]
- 41. Lauria G, Hsieh ST, Johansson O, et al. : European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903–12, e44-9. 10.1111/j.1468-1331.2010.03023.x [DOI] [PubMed] [Google Scholar]
- 42. Rolke R, Baron R, Maier C, et al. : Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–43. 10.1016/j.pain.2006.01.041 [DOI] [PubMed] [Google Scholar]
- 43. Magerl W, Krumova EK, Baron R, et al. : Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151(3):598–605. 10.1016/j.pain.2010.07.026 [DOI] [PubMed] [Google Scholar]
- 44. Rolke R, Magerl W, Campbell KA, et al. : Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77–88. 10.1016/j.ejpain.2005.02.003 [DOI] [PubMed] [Google Scholar]
- 45. Tesfaye S, Boulton AJ, Dyck PJ, et al. : Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93. 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Finnerup NB, Haroutounian S, Kamerman P, et al. : Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–606. 10.1097/j.pain.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Smith BH, Campbell A, Linksted P, et al. : Cohort Profile: Generation Scotland: Scottish Family Health Study (GS:SFHS). The study, its participants and their potential for genetic research on health and illness. Int J Epidemiol. 2013;42(3):689–700. 10.1093/ije/dys084 [DOI] [PubMed] [Google Scholar]
- 48. Hébert HL, Shepherd B, Milburn K, et al. : Cohort Profile: Genetics of Diabetes Audit and Research in Tayside Scotland (GoDARTS). Int J Epidemiol. 2018;47(2):380–381j. 10.1093/ije/dyx140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Torrance N, Ferguson JA, Afolabi E, et al. : Neuropathic pain in the community: more under-treated than refractory? Pain. 2013;154(5):690–99. 10.1016/j.pain.2012.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Skol AD, Scott LJ, Abecasis GR, et al. : Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38(2):209–13. 10.1038/ng1706 [DOI] [PubMed] [Google Scholar]
- 51. Perkins JR, Lees J, Antunes-Martins A, et al. : PainNetworks: a web-based resource for the visualisation of pain-related genes in the context of their network associations. Pain. 2013;154(12):2586.e1–12. 10.1016/j.pain.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]