Abstract
Context
Congenital generalized lipodystrophy, type 4 (CGL4) is a rare autosomal recessive disorder caused by mutations in caveolae-associated protein 1. Patients with CGL4 also have myopathy and cardiomyopathy with a predisposition for sudden death due to ventricular arrhythmias. However, the underlying pathology for these morbidities remains unknown. Therefore, we report on an autopsy of a Hispanic boy with CGL4.
Case Description
Our patient had early-onset generalized lipodystrophy, feeding difficulties, myopathy, atlanto-axial dislocation, and learning disabilities. He was diagnosed with catecholaminergic polymorphic ventricular tachycardia (CPVT) at age 8 years, had poor compliance with medications, and died suddenly at age 15.3 years. Autopsy showed marked loss of subcutaneous and omental fat with no inflammatory cells in adipose tissue and normal adipocytes in the parathyroid glands. There were adipocytes interdigitating cardiac muscle fibers, with fibro-fatty infiltration in the right ventricle, near coronary sinus, and atrioventricular node. There was no evidence of coronary heart disease. The quadriceps femoris muscle did not show adipocyte infiltration, inflammation, or fibrosis. The muscularis mucosa layer was thickened in the esophagus and at the gastro-duodenal junction, and the esophagus had prominent, large nerves in the subserosa. The liver weighed 3000 g, with minimal chronic inflammation and steatosis in 40% of parenchyma, primarily in zones 2 and 3. There was no spermatogenesis in the spermatic tubules.
Conclusions
Our data suggest that fibro-fatty infiltration of the right ventricle may contribute to CPVT in patients with CGL4. Thick muscularis mucosa and large nerves in the esophagus likely contributed to dysphagia and dysmotility. A lack of spermatids suggests infertility in affected male patients.
We present an autopsy description of congenital generalized lipodystrophy, type 4. Fat cells interdigitating cardiac muscle fibers and fibro-fatty infiltration could explain life-threatening arrhythmias.
Congenital generalized lipodystrophies are rare heterogeneous autosomal recessive disorders presenting with near total loss of body fat at birth or shortly thereafter and predisposition for diabetes mellitus, hypertriglyceridemia, and hepatic steatosis (1). Four genetically distinct subtypes of congenital generalized lipodystrophy have been reported to date (1). Congenital generalized lipodystrophy, type 4 (CGL4), caused by mutations in caveolae-associated protein 1 (CAVIN1), has been reported in only 27 patients (2, 3). Besides generalized lipodystrophy, patients with CGL4 have congenital myopathy and cardiomyopathy and a predisposition for sudden death due to ventricular arrhythmias during their teens (3, 4). Other morbidities include pyloric stenosis, dysphagia, ileus, and atlanto-axial instability. The underlying pathology for these morbidities, especially cardiomyopathy, remains unknown. Therefore, we report the results of an autopsy of a young Hispanic male patient with CGL4.
Clinical Report
The clinical features of this 15-year-old male patient, with compound heterozygous null mutations c.518-521delAAGA and c.471+1G>T in CAVIN1, have been reported previously (3, 5). Briefly, the patient had feeding difficulty and gastroesophageal reflux with aspiration requiring tube feeding in early childhood. He had muscle weakness with elevated serum creatine kinase levels and atlanto-axial dislocation requiring posterior cervical spine fusion at 4 years of age. He had gross motor and speech delays early in life and learning disabilities in later years. At 8 years of age, he was diagnosed with catecholaminergic polymorphic ventricular tachycardia (CPVT) upon exercise testing and was started on nadolol 40 mg daily. His serum leptin level was 0.27 ng/mL (normal range, 0.6 to 16.8 ng/mL), and his serum adiponectin level was <2 ng/mL (normal range, 4 to 26 ng/mL). At 9 years of age, he was started on metreleptin (0.25 mg twice daily and increased to 0.45 mg twice daily in 1 month) and fish oil therapy, although he remained poorly compliant with the therapy. At 12 years, he was diagnosed with mild hypertension and was started on lisinopril 5 mg daily. At 13.75 years of age, he admitted noncompliance with all medications. He had axillary acanthosis, pectus excavatum, clinodactyly of third toe bilaterally, and mild joint contractures in the ankles and knees. His testes volume was 6 to 8 mL bilaterally, with Tanner III pubic hair. Transthoracic echocardiogram done at 14 years of age showed normal atrial and ventricular dimensions, with normal left ventricular ejection fraction of 59.9%. At 15.3 years of age, while playing, he developed sudden dyspnea and cyanosis, leading to sudden death.
Autopsy Findings
The parents gave written informed consent for performing autopsy. The patient had generalized prominence of musculature with a paucity of subcutaneous fat and sparse facial hair. He had well-preserved mechanical adipose tissue in the palms, in the soles, and around joints. The histology of fat deposits was normal, with no inflammatory cells (Fig. 1A, 1B). The thyroid had lymphocytic infiltrates. The parathyroid contained a normal range of adipocytes, constituting ∼10% to 15% of parenchyma (Fig. 1H), and brown fat around the parathyroid gland.
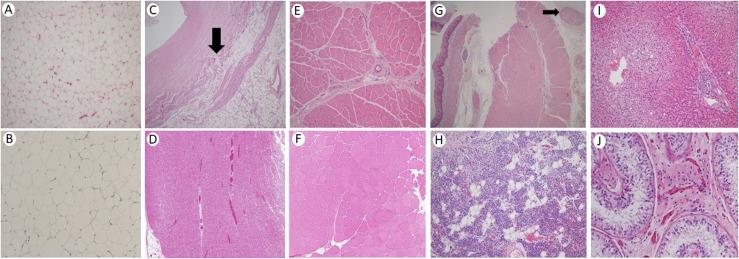
Figure 1.
Histopathology of various organs in our patient with CGL4. (A) Hematoxylin and eosin (H&E) staining of a section of adipose tissue showing normal-size adipocytes without inflammation or atrophy (magnification ×100). (B) CD68 immunostain of a section of adipose tissue showing an absence of histiocytic inflammation (magnification ×200). (C) H&E stain of right ventricular myocardium with fatty infiltration shown by an arrow in the middle of field and thick endocardium at upper left (magnification ×40). (D) H&E stain of cardiac muscle from an unaffected 37-year-old woman showing normal ventricular myocardium with no fatty infiltration (magnification ×40). (E). H&E stain of quadriceps femoris skeletal muscle shows mild variation in myocyte size without inflammation, atrophy, fatty infiltration, dystrophic, or regenerative changes (magnification ×100). (F) H&E stain of skeletal muscle in cross-section from an unaffected 54-year-old man showing mild variation in fiber size and shape and less perimysial fibrous tissue than our patient (magnification ×100). (G) H&E stain of esophagus showing thickened muscularis mucosa (left of field, under mucosa), with normal muscularis propria at the right of the field and large subserosal nerve in the upper right at the arrow (magnification ×40). (H) H&E stain of parathyroid with scattered clusters of adipocytes (magnification ×100). (I). H&E stain of liver with steatosis in central and midlobule (left half of field), sparing the periportal area (right half of field, magnification ×100). (J) H&E stain of testicular spermatic tubules with only Sertoli cells, absent spermatogenesis, and normal-appearing interstitial Leydig cells in the center of field (magnification ×200).
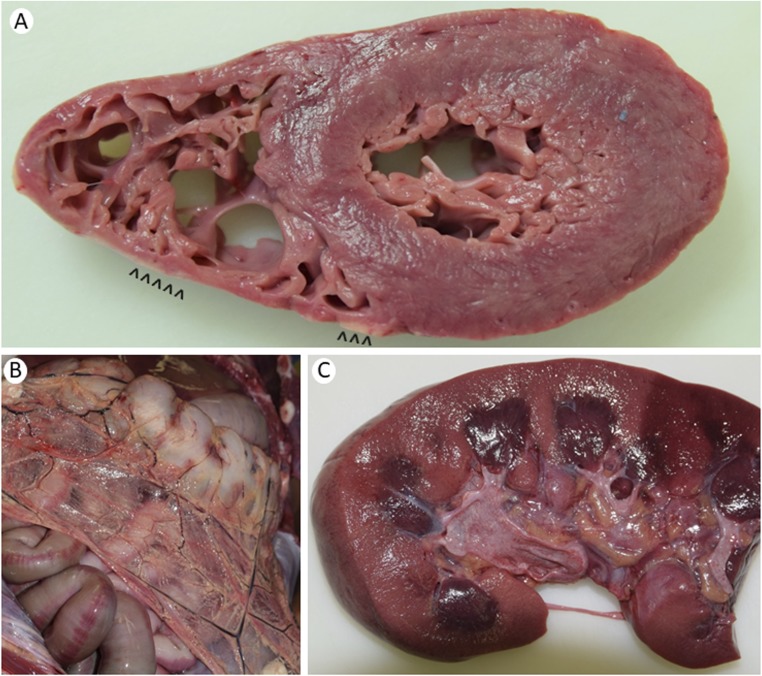
The heart weighed 350 g (normal, 300 g), with no structural anomaly but with anterior right ventricular wall fatty infiltration (Fig. 2A). There was minimal atherosclerosis in the aorta but none in the right dominant coronary circulation. The right ventricular myocardial wall was normal in thickness but showed fat cells interdigitating muscle fibers, fibro-fatty infiltration, and intimal thickening anteriorly (Fig. 1C) as compared with normal cardiac muscle histology (Fig. 1D). The left ventricular myocardium, atria, and valves had no necrosis, fatty infiltrates, or scars. There was near occlusion of sinoatrial nodal artery, with adipose tissue and strands of myocardium surrounding it. The atrioventricular node showed mild fibro-fatty infiltration near the coronary sinus area. There was mild fibrous thickening of intima, but there were no plaques in coronary arteries. Pericardial fat was normal. Endocardial fibrous tissue was thickened, but the collagen was histologically normal (Fig. 1C). Skeletal muscle showed mild variation in myocyte size, with no inflammation, atrophy, fatty infiltration, or dystrophic or regenerative changes as compared with normal histology (Fig. 1E, 1F).
Figure 2.
Gross autopsy pathology of the heart, abdomen, and kidney in our patient with CGL4. (A) Cross section of the heart showing uniform left ventricular myocardium and normal right ventricular wall thickness, with anterior right ventricular wall fatty infiltration (arrowheads). (B) Anterior view of the abdomen showing a lack of omental fat, except for cuffing of blood vessels. (C) Longitudinal section of the kidney shows well-preserved pericalyceal and peripelvic fat, which serves as mechanical cushioning, with normal sharp demarcation between the cortex and the medulla.
The right and left lungs weighed 1150 and 975 g, respectively. The pulmonary vasculature had no thrombo-emboli or atherosclerosis. Microscopic examination showed congestion, edema, and alveolar macrophages.
The stomach mucosa had patchy petechiae. The stomach and intestines had smooth serosa, with minimal to no omental fat (Fig. 2B). Microscopically, the muscularis mucosa layer was thickened in the esophagus and in the gastro-duodenal junction, with normal muscularis propria. The esophagus had prominent/large nerves in the subserosa, with normal nerves in the stomach on light microscopy (Fig. 1G). The pancreas showed tan-brown, lobular parenchyma without nodules, cysts, fibrosis, or necrosis. Microscopically, islet cells looked normal, and there was no amyloidosis.
The liver weighed 3000 g (normal, 1500 g). Microscopic examination revealed steatosis in ∼40% of hepatocytes, more in zones 2 and 3 (around central vein) than in zone 1 (around portal tract). There was minimal chronic inflammation in portal tracts and focal hepatocyte necrosis as acidophil bodies with chronic inflammatory cells in lobules, without Mallory hyaline or bridging fibrosis (Fig. 1I). The spleen weighed 375 g with normal red pulp and some hemosiderin deposits. The right and left kidneys weighed 200 and 225 g, respectively, with pericalyceal fat (Fig. 2C). Ureters and the urinary bladder were unremarkable. The testes showed spermatic tubules with only Sertoli cells and an absence of spermatogenesis. Interstitial Leydig cells appeared normal (Fig. 1J).
Discussion
The reported patients with CGL4 have been 1 to 29 years old (2, 3). Most patients develop generalized lipodystrophy during infancy, sparing the mechanical and bone marrow fat (5), which was confirmed on autopsy in our patient. The lack of inflammatory cells in adipose tissue excludes inflammation as a cause of profound insulin resistance in patients with CGL4. Cavin1−/− mice have a generalized lack of caveolae, and the absence of caveolae in adipocytes can impair triglyceride storage there (6), resulting in ectopic triglyceride deposition in the liver, as seen in our patient. Our patient had not developed diabetes and had normal pancreatic islets on histology, unlike the severe islet amyloidosis previously reported by us in a 24-year-old patient with CGL1 and severe diabetes (7).
The major cause of mortality in patients with CGL4 is ventricular arrhythmias (3, 4). Although some patients have a prolonged QT interval (3, 4), others are also predisposed to CPVT and sudden death, as was our patient. The underlying mechanism of CPVT in patients with CGL4 is unclear. Our patient demonstrated novel cardiac pathology (i.e., adipocytes interdigitating muscle fibers in the anterior wall of right ventricle with near occlusion of sinoatrial nodal artery along with mild fibro-fatty infiltration near the coronary sinus area and the atrioventricular node, which may also be associated with the development of CPVT and other arrhythmias). Whether Cavin1−/− mice develop CPVT is not known, but they develop progressive cardiomyopathy, with left ventricular hypertrophy and interstitial/perivascular fibrosis around cardiomyocytes and by 24 weeks reveal low electrocardiographic voltages and wide QRS complexes (8). Other researchers have reported right ventricular hypertrophy secondary to elevated pulmonary arterial pressure in adult Cavin1−/− mice (9); however, our patient did not show such findings.
Previous investigators have reported dystrophic changes with variation in skeletal muscle fiber size in nine patients with CGL4 at 3 to 27 years of age (4, 5, 10). However, our patient and his affected sister at 7 years of age (5) did not show adipocyte infiltration, myositis, or fibrosis, indicating a variable spectrum of findings. Electron microscopy of his sister’s skeletal muscle showed a normal sarcomeric pattern, with very mild but unremarkable pleomorphic changes in the mitochondria (5). Hayashi et al. (10) have shown reduced caveolae formation on electron microscopy of skeletal muscle in a patient with CGL4. Although the exact pathogenesis of myopathy remains unclear, it is likely due to a lack of caveolae.
Thick muscularis mucosa in the esophagus and at the gastro-duodenal junction and prominent/large nerves in the esophagus may underlie the difficulty swallowing, esophageal dysmotility, infantile hypertrophic pyloric stenosis, and ileus reported in patients with CGL4 (4).
No previous male or female patient with CGL4 has been reported to have children. Although our patient had normal secondary sex characteristics, the lack of spermatids in his testes suggests that he may have been infertile. This finding adds to the multisystem pathology seen in patients with CGL4.
In summary, our autopsy findings reveal fibro-fatty infiltration of right ventricular myocardium, which may underlie CPVT in patients with CGL4. Thick muscularis mucosa and large nerves in the esophagus may cause dysphagia, and a similar pathology in the stomach and intestine may cause dysmotility. Finally, affected male patients may be infertile due to a lack of spermatids.
Acknowledgments
We thank the patient’s family for permission to perform the autopsy; Satish Chundru, Deputy Chief Medical Examiner, Travis County Medical Examiner’s Office for help in conducting the autopsy; Dennis K. Burns, Professor of Pathology, and Bret Evers, Assistant Professor of Pathology, UT Southwestern, for providing photomicrographs of normal cardiac and skeletal muscle; Claudia Quittner for help in evaluating the patient; and Jeongwoo Lee for help with figures.
Financial Support: This work was supported by National Institutes of Health Grant R01-DK105448 (to A.G.) and by the Southwestern Medical Foundation. N.P. is recipient of “Friends of the Center for Human Nutrition Fellow” award from UT Southwestern.
Author Contributions: N.P. conceptualized and designed the study, evaluated the patient, reviewed the pathology, drafted the initial manuscript, and approved the final manuscript as submitted. F.V. reviewed the pathology, reviewed and revised the manuscript, and approved the final manuscript as submitted. A.G. conceptualized and designed the study, evaluated the patient, was present during autopsy, reviewed the pathology, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- CAVIN1
caveolae-associated protein 1
- CGL4
congenital generalized lipodystrophy, type 4
- CPVT
catecholaminergic polymorphic ventricular tachycardia
References
- 1. Patni N, Garg A. Congenital generalized lipodystrophies: new insights into metabolic dysfunction. Nat Rev Endocrinol. 2015;11(9):522–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jelani M, Ahmed S, Almramhi MM, Mohamoud HS, Bakur K, Anshasi W, Wang J, Al-Aama JY. Novel nonsense mutation in the PTRF gene underlies congenital generalized lipodystrophy in a consanguineous Saudi family. Eur J Med Genet. 2015;58(4):216–221. [DOI] [PubMed] [Google Scholar]
- 3. Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK, Garg A. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet A. 2010;152A(9):2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, Schulze A, Lucke B, Lützkendorf S, Karbasiyan M, Bachmann S, Spuler S, Schuelke M. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6(3):e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simha V, Agarwal AK, Aronin PA, Iannaccone ST, Garg A. Novel subtype of congenital generalized lipodystrophy associated with muscular weakness and cervical spine instability. Am J Med Genet A. 2008;146A(18):2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu L, Brown D, McKee M, Lebrasseur NK, Yang D, Albrecht KH, Ravid K, Pilch PF. Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metab. 2008;8(4):310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg A, Chandalia M, Vuitch F. Severe islet amyloidosis in congenital generalized lipodystrophy. Diabetes Care. 1996;19(1):28–31. [DOI] [PubMed] [Google Scholar]
- 8. Taniguchi T, Maruyama N, Ogata T, Kasahara T, Nakanishi N, Miyagawa K, Naito D, Hamaoka T, Nishi M, Matoba S, Ueyama T. PTRF/Cavin-1 deficiency causes cardiac dysfunction accompanied by cardiomyocyte hypertrophy and cardiac fibrosis. PLoS One. 2016;11(9):e0162513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swärd K, Sadegh MK, Mori M, Erjefält JS, Rippe C. Elevated pulmonary arterial pressure and altered expression of Ddah1 and Arg1 in mice lacking cavin-1/PTRF. Physiol Rep. 2013;1(1):e00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi YK, Matsuda C, Ogawa M, Goto K, Tominaga K, Mitsuhashi S, Park YE, Nonaka I, Hino-Fukuyo N, Haginoya K, Sugano H, Nishino I. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119(9):2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]