Abstract
Outer hair cells (OHCs) damage is a general phenomenon in clinical disorders such as noise-induced hearing loss and drug-induced hearing loss. In order to elucidate the mechanism underlying these disorders, OHCs – its diseased region needs to be deeply investigated. However, OHCs array on the basilar membrane which contains massive cells with different types. Therefore, to isolate OHCs from this huge population is significant for revealing its pathological and molecular changes during disease processing. In the present study, we tried to isolate OHCs from the commonly used animal model –Sprague-Dawley (SD) rats. By separating outer hair cells from SD rats with different day ages, we found that 9 days after birth was a suitable time for the separation of the OHCs. At this time, the number of OHCs isolated from rats was large, and the cell morphology was typical of cylindrical shape. OHCs isolated using this method are histologically suitable and quantitatively adequate for molecular biological and electrophysiological analyses.
Keywords: Outer hair cell, Isolation technique, Morphology, Sprague–Dawley rat
Introduction
Cochlear hair cells are terminal nerve cells of auditory sensation, including inner hair cells (IHCs) and outer hair cells (OHCs).1 In patients with sensorineural hearing loss, OHCs are more susceptible to injury compared to IHCs.2 Researches on animal models with drug-induced deafness or noise-induced deafness also showed that OHCs are more fragile.3, 4, 5 To better protect OHCs, it's important that we have a better understanding of the underlying mechanisms for the vulnerability of OHCs, and to isolate OHCs from animals was a critical way to study hair cells.
In 1953, Katsuki and Covell firstly isolated OHCs from guinea pig, it hence became important model for histological and pathological investigation.6 By studying the isolated OHCs of guinea pig, researchers discovered motor protein named prestin, and explored its critical role in OHCs electromotility, demonstrating that prestin-based electromotility is essential for cochlear amplification.7, 8, 9 The isolated OHCs also serve as a good model for studying the ionic channels of cochlea hair cells.10, 11, 12 For example, by investigating isolated OHCs, Meech et al13 found that the OHCs resting potential is determined by a Ca2+-activated K+ conductance at the base of the cell. OHCs in guinea pig were easy to isolate and the yield was large.14, 15 Guinea pig was used mostly as animal model for hair cells isolation, while Sprague–Dawley (SD) rat model was rarely used. However, in biological studies, the rat is a more widely used model animal, and the types of antibody of rat is far more abundant than that of the guinea pig. Cell count of isolation from adult rats was low by using collagenase digestion, the yield and quality of OHCs were unpredictable, which limit its application in scientific researches. Consequently, it's important that a more efficient method for OHCs isolation from SD rats be developed. In this article, by separating OHCs from SD rats of different days of age, we found that postnatal 9 days was a suitable time for the separation of OHCs. During this period, the number of OHCs isolated was large, and the cell morphology was typical of cylindrical shape. The morphology of OHCs isolated from postnatal 9 days rats is close to that of adult OHCs and can be used for subsequent pathological and electrophysiology researches.
Materials and methods
Postnatal 1 day, 3 days, 7 days, 9 days, 11 days, 14 days, and 28 days SD rats, regardless of sex, were purchased from the laboratory animal center of the Fourth Military Medical University. Collagenase IV and Leibovitz L-15 tissue culture media were purchased from Sigma. Hank's balanced salt solution (HBSS) was purchased from Solarbio.
P1 and P3 rats were decapitated. P7 rats and older rats were injected with 10% chloral hydrate (3 ml/kg i.p.). Cochlea were dissected out and rinsed in ice cold saline and then transported into ice cold HBBS. The isolation procedure was essentially the same as described by Zenner et al.16, 17 Basilar membrane together with the organ of Corti were dissociated without ligamentum spirale ductus cochlearista. The organ of Corti was incubated with collagenase IV (1 mg/ml, diluted in L-15 medium) for 10 min at room temperature. Enzyme was then removed using a sterilized pipette and 1 ml L-15 culture medium was added to the petri dish. The organ of Corti was repeatedly gently triturated using a microsyringe for isolation of OHCs and was then placed at room temperature for 10 min for OHCs to adhere to the bottom of the dish. The petri dish containing isolated OHCs was then mounted onto the stage of an inverted phase contrast microscope for observation and analysis. After sitting in room temperature for 10 min, single OHC can be recognized under microscope. Identification of OHCs was mainly based on their morphology according to the widely acknowledged criteria.14 The detailed morphology of OHCs observed was described in the Results and Discussion sections below. The procedures involving animals in this study were approved by the Animal Care and Use Committee at the Fourth Military Medical University.
Results
Yield and morphology of OHCs isolated from rats with different day age
OHCs isolation method used in this study is described in the above method section. According to the results, OHCs with cylindrical features can barely be separated from P1, P3 rats. Under the same conditions, OHCs could be harvested from P7 rats with 200–250 OHCs separated from each cochlea. The figures of the cells were irregular cylindrical with the site of the nucleus larger in diameter and top of the cells slightly narrower (Fig. 1A). About 140–190 OHCs can be isolated from each cochlea of P9 rats, and the cells were in typical cylindrical shape (Fig. 1B, C). Since P11, the lamina spiralis ossea ossification was completed, and the number of isolated OHCs decreased markedly, with approximately 30–40 OHCs per cochlea. The outer hair cells were cylindrical in shape, with cell length further extending (Fig. 1D). The isolated OHCs from P14 rats are similar to cells isolated from P11 rats, also presenting cylindrical shape (Fig. 1E), and the number of OHCs that can be separated from each cochlea decreased to 20–30. The cochlea of P28 rats was mature and the number of isolated cells further reduced to about 10–20 OHCs each cochlea (Fig. 1F). The number of separated OHCs is closely related to the degree of cochlear ossification. Rat lamina spiralis ossea ossification started from basal turn at day 9 after birth, and completed at day 11 or day 12. After ossification, the number of outer hair cells separated from the basal membrane dropped dramatically.
Fig. 1.
Morphology of outer hair cells (OHCs) isolated from Sprague–Dawley (SD) rats of different day age. The common features of the cells are as follows: the cells are in cylindrical shape, the nuclei are located at the bottom of the cells, and the cilia are visible on the cuticular plate. A: OHC isolated from P7 rat. The cell is in irregular cylindrical shape and the cytoplasm is not quite transparent. B: OHC in the apex turn isolated from P9 rat. C: OHC in the basal turn isolated from P9 rat. The cell in the basal turn is shorter than that in the apex turn and the cytoplasm is less transparent. D: OHC isolated from P11 rat. E: OHC isolated from P14 rat. F: OHC isolated from P28 rat is in typical cylindrical shape and the cytoplasm is transparent.
Characteristics of OHCs isolated from P9 rats
Morphology observation of the P9 rats OHCs under the microscope showed that P9 OHCs are long and shaped in standard cylindrical, which are close to OHCs isolated from mature rats. These unique morphological features are easily identified under microscope and provide evidence for us to distinguish hair cells. The nucleus of OHC locates at the bottom of the cell body. Kinocilium of apex turn OHCs were long and obvious, with less transparent cytoplasm. Kinocilium of basal turn OHCs were not so obvious with transparent cytoplasm.
Comparison of OHCs isolated from P9 rats and adult rats
The shape of OHCs from P9 rats was cylindrical with slightly shorter length compared with cells from P28 rats. The average length of P9 rats OHCs was (23.2 ± 5.0) μm, which is shorter than the cells with (32.3 ± 4.3) μm in adult rats. The average diameter of P9 rats OHCs was (8.6 ± 0.8) μm, which was similar to cells from the (8.3 ± 0.9) μm of adult rats. In general, the OHCs isolated from P9 rats resembled OHCs isolated from P28 rats, namely the young adult rats.
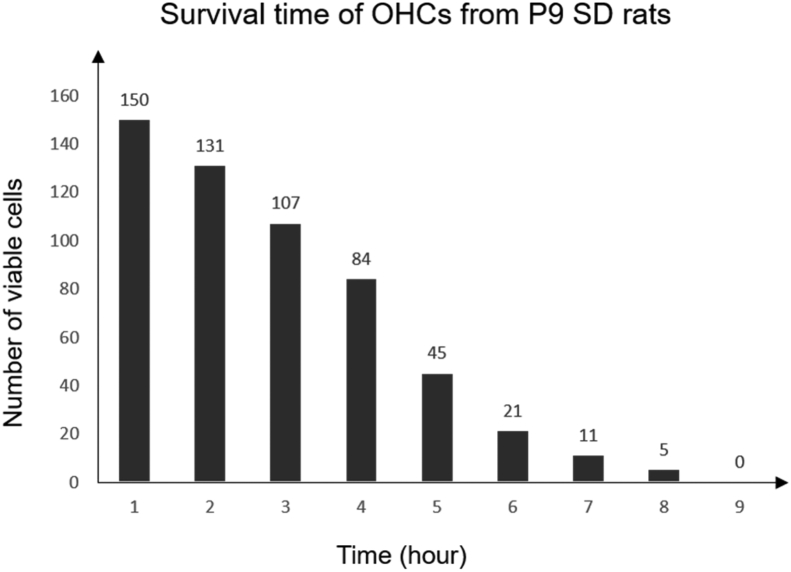
Survival of OHCs in vitro
The survival of a total of 150 single OHCs from P9 rats was observed over time (Fig. 2). After resting at room temperature for 10 min, most vital OHCs became attached to the bottom of the petri dish. According to our experiment, the longer OHCs from apex turn could survive up to 8 hours in vitro while the shorter OHCs from basal turn could only survive for approximately 3 hours. Six hours after isolation, only 21 cells in the total 150 cells survived, and all of them were long outer hair cells. Five hours after isolation, more than half of the cells were dead. No cells survived longer than 9 hours. Generally, the longer OHCs survived longer time than shorter ones.
Fig. 2.
The survival condition of OHCs in vitro were observed over time. Five hours after isolation, more than half of the cells were dead. No cells survived longer than 9 hours.
Degeneration of OHCs over time
Morphological changes during cell degeneration were also observed in this study. After 1–3 h, the OHCs began to degenerate. In the process of degeneration of the OHCs, there appeared cell edema, nuclear displacement, cell membrane shrinkage, particles in Brownian motion in cytoplasm and disappearance of birefringence. Degeneration of most cells was accompanied by edema (Fig. 3A). In normal state, IHCs look like flasks, while OHCs are in cylindrical shape, and they can be easily distinguished from each other visually. However, when OHCs degenerated and became swollen, their appearance tended to become similar to that of IHCs, thus, it can be hard to tell these two cell types apart. Also, we discovered that in the process of degeneration of some OHCs, there showed the upward shift of the nucleus, but with no obvious cell swelling (Fig. 3B). Eventually, the cells died, with the outlines of the cells remained.
Fig. 3.
In the degeneration of OHCs, there appeared swelling at the bottom of the cell (A) and upward displacement of nucleus (B).
Discussion
In the present study, we attempted to isolate OHCs from SD rats. The results indicated that the yield and morphology of isolated OHCs were closely related with the developmental stages of the rats. The ossification of basement membrane is an important factor affecting the yield of OHCs. The higher the ossification degree, the lower the yield. Hardly can OHCs in typical cylindrical shape be separated from cochlear in P1—P3 rats. In P7 days, the yield of OHCs reached the highest, even up to 1.5 times of the yield of P9 days, but during this period the OHCs morphology is different from a typical cylindrical shape. Since P9, the appearance of OHCs gradually developed into standard cylinder and 140–190 OHCs can be obtained from each cochlea. The cell count of OHCs isolated from P14 rats were 20–30, and in adult rats, the number was 10–20. Since P9, lamina spiralis ossea became ossified from basal turn to apical turn, and the ossification completed at P11. With the completion of ossification, the yield of isolated OHCs dramatically decreased, which may be caused by the more compact connection between cells.
P9 rats are still in developmental stage, and their cochlea have not been complete ossified. However, morphologically, the OHCs in P9 are mature enough. Besides, compared to adult rats, the yield of OHCs was much larger in P9 rats. The isolation of OHCs from P9 rats could ensure cell yield large enough for subsequent researches and guarantee that OHCs that were close to mature state at the same time. In 1986, Zajic and Schacht14 brought up with the following standards of good morphological quality of OHCs: the cell must be in cylindrical shape with no swelling or distortion of the cell membrane, the nucleus must locate near the bottom of the cell and no extrusion of cytosol or Brownian particles should be observed. The OHCs we isolated from P9 rats complied to all above requirements and display similar morphology with OHCs from adult rats.
In this study, microsyringe was used to significantly increase the yield of isolated OHCs and can ensure a relatively stable production of OHCs. Enzymatic digestion was also a critical step. There are several types of enzymes that can be used to digest the organ, such as collagenase, trypsin and papain.1, 18, 19, 20, 21 Previous study has reported that trypsin was harmful to the cells, and isolated OHCs’ survival period is comparatively shorter whereas collagenase is almost non-harmful to the cells.22 In addition, the yield of OHCs was closely related with experiment operation. To obtain OHCs with high quality and strong vitality, basal membrane has to be separated quickly and completely, and then be cautiously transferred to culture medium L-15 with gentle trituration.
We introduced an optimized method for isolating OHCs from SD rats in this study, nevertheless, there are some limitations in this research. For instance, the present study simply compared the maturity of cells from morphological perspective, and not from aspects such as protein expression and electrophysiological characteristics. In the future, we would identify hair cells in a more comprehensive way.
Conclusions
The present study is performed on rats, but this method could also be used for the isolation of OHCs in other species. There is a period of easy separation of OHCs in the early stage of animals, that is, before the basement membrane is ossified. The large yield as well as the mature morphology were the distinct advantages of isolating OHCs during this period.
Conflicts of interest
None.
Acknowledgement
The work was funded by the National Natural Science Foundation of China (NSFC 81120108008).
Edited by Xin Jin
Footnotes
Peer review under responsibility of Chinese Medical Association.
Contributor Information
Ding-Jun Zha, Email: zhadjun@fmmu.edu.cn.
Jian-Hua Qiu, Email: qiujh@fmmu.edu.cn.
References
- 1.Liu H., Pecka J.L., Zhang Q., Soukup G.A., Beisel K.W., He D.Z. Characterization of transcriptomes of cochlear inner and outer hair cells. J Neurosci. 2014;34:11085–11095. doi: 10.1523/JNEUROSCI.1690-14.2014. http://www.ncbi.nlm.nih.gov/pubmed/25122905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan H., Wang X., Hill K. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid Redox Signal. 2015;22:1308–1324. doi: 10.1089/ars.2014.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X., Sun S., Qi J. Bmi1 Regulates the proliferation of cochlear supporting cells via the canonical wnt signaling pathway. Mol Neurobiol. 2017;54:1326–1339. doi: 10.1007/s12035-016-9686-8. http://www.ncbi.nlm.nih.gov/pubmed/26843109 [DOI] [PubMed] [Google Scholar]
- 4.Park J.S., Jou I., Park S.M. Attenuation of noise-induced hearing loss using methylene blue. Cell Death Dis. 2014;5:e1200. doi: 10.1038/cddis.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fetoni A.R., De Bartolo P., Eramo S.L. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: cochlear and cortical responses after an increase in antioxidant defense. J Neurosci. 2013;33:4011–4023. doi: 10.1523/JNEUROSCI.2282-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsuki Y., Covell W.P. The organ of Corti by phase contrast microscopy. Laryngoscope. 1953;63:1–17. doi: 10.1288/00005537-195301000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Zheng J., Shen W., He D.Z., Long K.B., Madison L.D., Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 8.He D.Z., Jia S., Dallos P. Prestin and the dynamic stiffness of cochlear outer hair cells. J Neurosci. 2003;23:9089–9096. doi: 10.1523/JNEUROSCI.23-27-09089.2003. http://www.ncbi.nlm.nih.gov/pubmed/14534242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dallos P., Wu X., Cheatham M.A. Prestin-based outer hair cell motility is necessary for mammalian cochlear amplification. Neuron. 2008;58:333–339. doi: 10.1016/j.neuron.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park C., Kalinec F. PKCα-mediated signals regulate the motile responses of cochlear outer hair cells. Biophys J. 2015;108:2171–2180. doi: 10.1016/j.bpj.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song L., Santos-Sacchi J. An electrical inspection of the subsurface cisternae of the outer hair cell. Biophys J. 2015;108:568–577. doi: 10.1016/j.bpj.2014.12.010. http://www.ncbi.nlm.nih.gov/pubmed/25650924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H.Y., Gao W.Y., Wen W., Zhang Y.M. Taurine modulates calcium influx through L-type voltage-gated calcium channels in isolated cochlear outer hair cells in Guinea pigs. Neurosci Lett. 2006;399:23–26. doi: 10.1016/j.neulet.2006.01.070. http://www.ncbi.nlm.nih.gov/pubmed/16513269 [DOI] [PubMed] [Google Scholar]
- 13.Ashmore J.F., Meech R.W. Ionic basis of membrane potential in outer hair cells of Guinea pig cochlea. Nature. 1986;322:368–371. doi: 10.1038/322368a0. [DOI] [PubMed] [Google Scholar]
- 14.Zajic G., Schacht J. Comparison of isolated outer hair cells from five mammalian species. Hear Res. 1987;26:249–256. doi: 10.1016/0378-5955(87)90061-x. http://www.ncbi.nlm.nih.gov/pubmed/3583926 [DOI] [PubMed] [Google Scholar]
- 15.Guo Y., Su Z., Yang W., Jiang S. Isolation of outer hair cells from varying turns of the Guinea-pig cochlea. Clin Otorhinolaryngol Head Neck Surg(China) 2001;15:26–27. http://www.ncbi.nlm.nih.gov/pubmed/12541880 [PubMed] [Google Scholar]
- 16.Zenner H.P., Gitter A., Zimmermann U., Schmitt U., Frömter E. The isolated living hair cell. A new model for the study of hearing function. Laryngol Rhinol Otol (Stuttg) 1985;64:642–648. http://www.ncbi.nlm.nih.gov/pubmed/4087997 [PubMed] [Google Scholar]
- 17.Zenner H.P., Zimmermann U., Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985;18:127–133. doi: 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]
- 18.He D.Z., Zheng J., Edge R., Dallos P. Isolation of cochlear inner hair cells. Hear Res. 2000;145:156–160. doi: 10.1016/s0378-5955(00)00084-8. http://www.ncbi.nlm.nih.gov/pubmed/10867288 [DOI] [PubMed] [Google Scholar]
- 19.Burns J.C., Kelly M.C., Hoa M., Morell R.J., Kelley M.W. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun. 2015;6:8557. doi: 10.1038/ncomms9557. http://www.ncbi.nlm.nih.gov/pubmed/26469390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimitsuki T., Ohashi M., Wada Y., Fukudome S., Komune S. Dissociation enzyme effects on the potassium currents of inner hair cells isolated from Guinea-pig cochlea. Hear Res. 2005;199:135–139. doi: 10.1016/j.heares.2004.08.020. http://www.ncbi.nlm.nih.gov/pubmed/15574308 [DOI] [PubMed] [Google Scholar]
- 21.Spreadbury I.C., Kros C.J., Meech R.W. Effects of trypsin on large-conductance Ca(2+)-activated K(+) channels of Guinea-pig outer hair cells. Hear Res. 2004;190:115–127. doi: 10.1016/S0378-5955(03)00376-9. http://www.ncbi.nlm.nih.gov/pubmed/15051134 [DOI] [PubMed] [Google Scholar]
- 22.Zenner H.P., Zimmermann R., Gitter A.H. Active movements of the cuticular plate induce sensory hair motion in mammalian outer hair cells. Hear Res. 1988;34:233–239. doi: 10.1016/0378-5955(88)90003-2. http://www.ncbi.nlm.nih.gov/pubmed/3170366 [DOI] [PubMed] [Google Scholar]