Abstract
Objectives
Obstructive sleep apnea (OSA) is a prevalent disease with significant health impacts. While first line therapy is CPAP, long-term compliance is low and device misuse is common, highlighting the need for alternative therapies. Upper airway surgery is one alternative, but substantial side effects hamper efficacy. A new alternative is an implantable hypoglossal nerve stimulator (HNS). These devices utilize neuromodulation to dilate/reinforce the airway and reduce side effects associated with traditional surgery. Several recent trials investigated the efficacy of these devices. The purpose of this study was to perform meta-analysis of available HNS studies investigating treatment of OSA to analyze objective and subjective outcomes and side effects.
Methods
A comprehensive literature search of PubMed and Scopus was performed. Two independent reviewers examined clinical trials investigating HNS in treatment of sleep apnea in adults. Studies with objective and subjective endpoints in sleep were included for analysis. Adverse events from trials were also recorded.
Results
Across 16 studies, 381 patients were analyzed. At 6 months (p = 0.008), mean SAQLI improved by 3.1 (95%CI, 2.6–3.7). At 12 months (p < 0.0001), mean AHI was reduced by 21.1 (95%CI, 16.9–25.3), mean ODI was reduced by 15.0 (95%CI, 12.7–17.4), mean ESS was reduced by 5.0 (95%CI, 4.2–5.8), mean FOSQ improved by 3.1 (95%CI, 2.6–3.4). Pain (6.2%:0.7–16.6), tongue abrasion (11.0%:1.2–28.7), and internal (3.0%:0.3–8.4)/external device (5.8%:0.3–17.4) malfunction were common adverse events.
Conclusions
HNS is a safe and effective treatment for CPAP refractory OSA. Further study comparing HNS to other therapies is required.
Keywords: Surgical treatment of obstructive sleep apnea, Sleep medicine, Obstructive sleep apnea
Introduction
Obstructive sleep apnea (OSA) is a prevalent disease that significantly impacts quality of life. An apnea-hypopnea index (AHI) of 15 or greater increases risk for insulin resistance, dyslipidemia, vascular disease, and death1 While first line therapy for OSA is CPAP, long-term patient compliance is as low as 40%–60%.2, 3 Studies also highlight a majority of patients do not use CPAP optimally.4 Low compliance and suboptimal use highlight the need for alternative therapies.
Surgical therapies for OSA are one alternative for those who fail CPAP. The impetus for these surgeries is to expand the airway by removing redundant tissue and prevent airway collapse at specific anatomic sites.1 Use of these methods remains controversial due to the lack of controlled studies assessing success, substantial side effects and the debate over measuring surgical success.5 These limitations highlight the need for a better secondary therapy.
One such therapy for those who have failed CPAP is direct stimulation of the hypoglossal nerve to increase pharyngeal muscle tone to prevent airway collapse.6 Several recent studies have examined the role of hypoglossal nerve stimulation (HNS) as a secondary therapy for OSA.7, 8, 9 HNS targets pharyngeal tone and stimulates genioglossus protrusion to maintain airway patency.6 These devices also reduce the risk for severe dysphagia and throat pain.10 Other key advantages include targeting airway collapse at multiple anatomic levels and the ability to titrate the device during sleep studies.10, 11 Several recent clinical trials have looked at the efficacy of these devices.1, 9, 12, 13 The purpose of this study was to perform a meta-analysis of available HNS studies to determine the role of HNS in treating OSA.
Methods
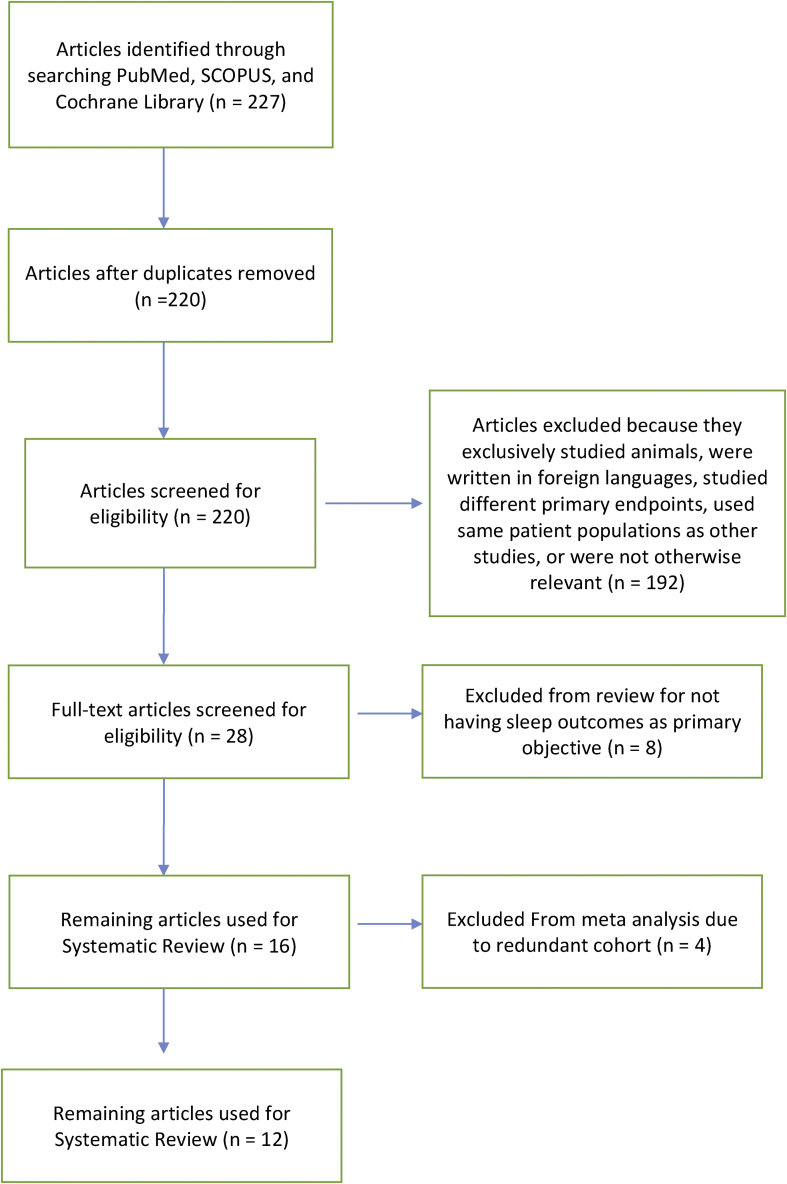
A comprehensive literature search was performed with the assistance of a research librarian starting on August 14, 2017. Articles were identified in PubMed, Cochrane Database, and Scopus using search terms “obstructive sleep apnea and (implantable nerve stimulator or hypoglossal or Inspire implant or “STAR trial” or ImThera implant)” (Fig. 1).
Fig. 1.
PRISMA Diagram illustrating how studies were selected.
Selection criteria
Only studies with the primary objective of examining the role of hypoglossal nerve stimulation in treatment of sleep apnea in adults were included. Abstracts were independently reviewed by two reviewers (ARK and JSN), and case reports, review articles, and nonhuman studies were excluded. Full texts of included articles were reviewed for all clinical trials and case series. Articles without primary endpoints of Apnea Hypopnea Index (AHI), Oxygen Desaturation Index (ODI), and Epworth Sleepiness Scale (ESS) were excluded. Several studies also included Functional Outcomes of Sleep Questionnaire (FOSQ) and Sleep Apnea Quality of Life Index (SAQLI) and these outcomes were recorded. Adverse events from trials were also recorded for further analysis as well. Multiple studies included of the same cohort from the STAR trial. In an effort to only analyze this cohort once we used the largest cohort with the most complete follow up data available.
Statistical analysis
The meta-analysis utilized pre-procedure (baseline) to post-procedure measures, with all subjects serving as their own controls. Meta-analysis of selected studies with a continuous measure (comparison of means and standard deviations between pre-procedure and post-procedure groups) was performed with Cochrane Review Manager (RevMan) version 5.3 (Nordic Cochrane Centre, Cochrane Collaboration, 2011, Copenhagen, Denmark). Both the fixed effects model and the random effects model were used in this study. Under the fixed effects model, it is assumed that all studies come from a common population, and that the effect size (standardized mean difference) is not significantly different among the different trials. This assumption is tested by the “heterogeneity test.” If this test yields a low probability value (p < 0.05), then the fixed effects model may be invalid. In this case, the random effects model may be more appropriate, in which both the random variation within the studies and the variation between the different studies are incorporated. Under the random effects model, the true effects in the studies are assumed to vary between studies, and the summary effect is the weighted average of the effects reported in the different studies.14 The random effects model provides a more conservative estimate (i.e., with a wider confidence interval [CI]), but the results from the 2 models usually agree when there is no heterogeneity. When heterogeneity was present, the random effects model was the preferred model. Additionally, the Sterne and Egger tests were performed for further assessment of risk of publication bias.15, 16 For this study, the null hypothesis was that there was no difference between pre-procedure and post-procedure with respect to AHI, ODI, ESS, and FOSQ. Data are presented as mean ± standard deviation (95% CI) in this text and as mean difference (MD) in the figures. The total MD with 95% CI is given for both the fixed effects model and the random effects model. If the value 0 is not inside the 95% CI, then the MD is statistically significant at the 5% level (p < 0.05).
In addition, a meta-analysis of proportions was done for surgical outcomes. The program MedCalc 17.9.7 (MedCalcSoftware, Oostende, Belgium) lists the proportions (expressed as a percentage), with their 95% CIs, found in the individual studies included in the meta-analysis. The pooled proportion with 95% CI is given both for the fixed effects model and the random effects model. Each technique was weighted according to the number of patients treated. Analysis of pooled proportions was performed where appropriate. MedCalc used a Freeman-Tukey transformation to calculate the weighted summary proportion under the fixed and random effects models.17, 18 Data were presented as weighted proportions with corresponding 95% CIs. Both the fixed effects model and the random effects model were used in this study.
Results
Through a comprehensive literature review 16 papers were identified for the systematic review.1, 8, 9, 12, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Meta-analysis of 12 studies encompassing 381 patients was also performed. According to the Oxford Centre for Evidence-Based Medicine grading system, the methodological quality of the studies was assessed as level of evidence 4, since they were case series. Sterne and Egger testing suggested a relationship between the sample size of these studies and their effect sizes indicating a high likelihood of publication bias. These data were significantly heterogeneous (I2 = 64%, p < 0.00001).
Objective and subjective outcomes of HNS
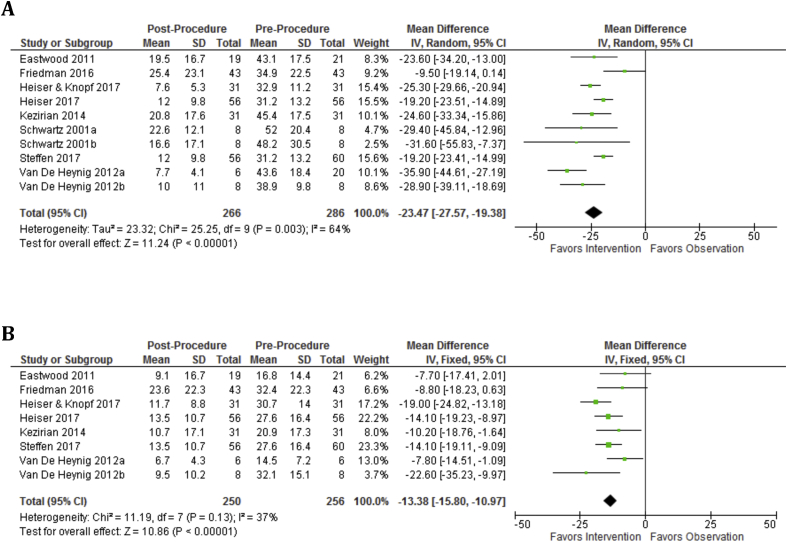
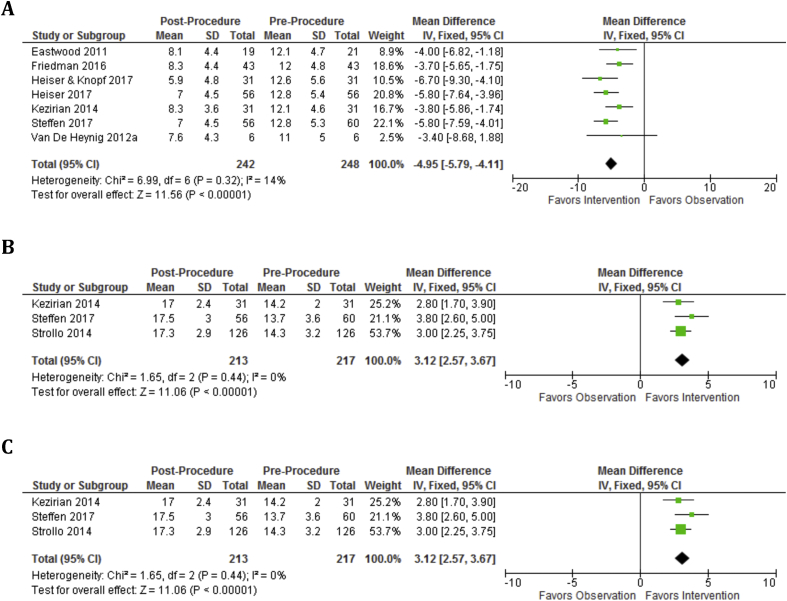
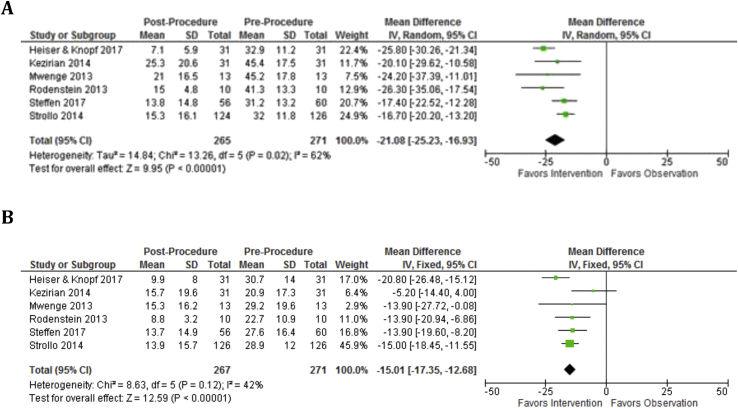
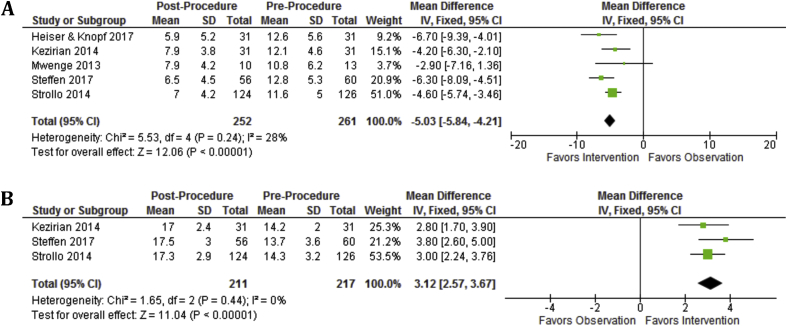
At 6 months, mean AHI was reduced by 23.5 (p < 0.00001, 95%CI, 19.4–27.6) and mean ODI was reduced by 13.4 (p < 0.00001, 95%CI, 11.0 to 15.8) (Fig. 2). At 6 months, mean ESS was reduced by 5.0 (95%CI, 4.1–5.8), mean FOSQ improved by 3.1 (p < 0.00001, 95%CI, 2.6–3.8), and mean SAQLI improved by 3.1 (p = 0.008, 95%CI, 2.6–3.8) (Fig. 3). At 12 months, mean AHI was reduced by 21.1 (p < 0.00001, 95%CI, 16.9–25.2) and mean ODI was reduced by 15.0 (p < 0.00001, 95%CI, 13.3–16.7) (Fig. 4). At 12 months, mean ESS remained reduced by 4.8 (p < 0.00001, 95%CI, 4.2–5.4) and mean FOSQ improved by 3.1 (p < 0.00001, 95%CI, 2.6–3.7) (Fig. 5).
Fig. 2.
Objective outcomes are significantly improved at 6 months as demonstrated by AHI and ODI. A: AHI Baseline vs. 6 month and AHI is significantly decreased over baseline. B: ODI Baseline vs. 6 months and ODI is significantly decreased over baseline.
Fig. 3.
Subjective outcomes are significantly improved from baseline to 6 months. A: ESS Baseline vs. 6 months shows significant improvement at 6 months. B: FOSQ Baseline vs. 6 months shows significant improvement. C: SAQLI Baseline vs. 6 months.
Fig. 4.
Objective outcomes remain improved at 12 months as measured by AHI and ODI. A: AHI Baseline vs. 12 months and AHI is significantly decreased over baseline. B: ODI Baseline vs. 12 months and ODI is significantly decreased over baseline.
Fig. 5.
Patient reported subjective outcomes remain improved over baseline at 12 months. A: ESS Baseline vs. 12 months shows significant improvement at 12 months. B: FOSQ Baseline vs. 12months shows significant improvement at 12 months.
Safety of HNS
Several adverse events (Table 1) were relatively common among patients. 6.2% of patients experienced pain (p < 0.0001, 95%CI, 0.7%–16.%). 11.0% of patients had a tongue abrasion with or without lesions (p < 0.0001, 95%CI. 1.2%–28.7%). 3.0% of patients experienced an internal device malfunction (p = 0.0001, 95%CI, 0.3%–8.4%) and 5.8% of patients experienced an external device malfunction (p < 0.0001, 95%CI, 0.3%–17.4%). 7.0% of patients experienced some other adverse event (p < 0.0001, 95%CI, 0.6%–19.2%).
Table 1.
Safety of Hypoglossal Nerve Stimulation.
| Items | Percent of patients | 95% CI | p-value |
|---|---|---|---|
| Pain | 6.2% | 0.7%–16.6% | p < 0.0001 |
| Tongue abrasion | 11.0% | 1.2%–28.7% | p < 0.0001 |
| Internal device malfunction | 3.0% | 0.3%–8.4% | p = 0.0001 |
| External device malfunction | 5.8% | 0.3%–17.4% | p < 0.0001 |
| Other | 7.0% | 0.6%–19.2% | p < 0.0001 |
Discussion
OSA imposes significant cardiovascular risk and daytime sleepiness increases risk of automobile accidents.30, 31 CPAP is currently the first line therapy, but alternative therapies, such as HNS, are necessary due to the improper use of and poor compliance with CPAP. This meta-analysis and systematic review examined 16 clinical trials that reported objective and subjective outcomes for CPAP refractory OSA treated with HNS to understand its clinical utility. Across all trials, patients that receive HNS have significantly improved AHI, ODI, and FOSQ at 6 and 12 months (Fig. 2, Fig. 3, Fig. 4, Fig. 5).
While HNS may not be the definitive choice for second line therapy, it is a useful tool in managing OSA. Through several case series, a cohort of patients that would benefit most from HNS has been defined: patients with a BMI less than <35 kg/m2, an AHI between 20 and 50, and nonconcentric collapse of the retropalatal airway.1, 28, 32 Difficulties with compliance with CPAP may warrant HNS use as compliance was reported to be 86% at 12 months compared to 40%–60% with CPAP.1, 2, 3, 29 Interestingly, one study found that discontinuation of HNS did not immediately cause patients to revert to baseline AHI or ODI.22 This response highlights the potential for HNS to modify the disease course of OSA. However, long-term discontinuation results in reversion to baseline characteristics.33
Common adverse events across all studies included pain, tongue abrasion and device malfunction (Table 1). Pain, which was managed with analgesics, was characterized as a non-serious adverse event by most trials. However, one trial noted 3 patients with serious pain, which resolved for only 1 patient.19 Tongue abrasions were a common and expected side effect for HNS, as the device stimulates the genioglossus to protrude the tongue against teeth.1 These abrasions were remedied by reprogramming the device or using a tooth guard.1 Other adverse events mentioned by authors included abnormal sensations, paresthesias, change in salivary flow and lip weakness.1, 7, 9, 12, 19, 21, 26, 27, 28 A serious complication in previous studies was device migration, and further study to prevent this complication may be necessary. Of note, there was no mention of dysphagia in any trial, a common complaint in traditional upper airway surgeries.
One key limitation is the lack of long-term follow-up data for implanted patients. While most trials focused on time points within 1 year following implantation, the authors of the STAR trial have published follow up data for 18, 24, 36, and 48 months. They demonstrated the device maintains effectiveness.25, 27, 29, 34 A meta-analysis on the role of HNS on sleep outcomes was performed previously.35 However, our study performs further analysis of risks via a meta-analysis of proportions. Furthermore, several studies have been published since this that previous systematic review, including a large multi-institution post market study.7, 19, 20, 26, 27, 29
Further investigation is needed to compare traditional airway surgery to HNS. Currently, there is a shortage of evidence of what role traditional airway surgery plays compared to HNS. However, one case report presented a patient with an extensive history of unsuccessful upper airway surgery who underwent HNS improved drastically improved.36 Several trials included in our analysis mentioned patients in their cohorts that had previous surgeries, but no study has further investigated how this cohort faired compared to those without previous upper airway surgery.13, 33
Conclusions
HNS is a safe and effective treatment for CPAP refractory OSA. HNS is associated with high compliance and significantly improves subjective and objective outcomes of sleep. Complications are generally uncommon and benign. Further study comparing HNS to other therapies, such as airway surgery, is required.
Financial disclosures/funding
None.
Conflicts of interest
None.
Edited by Jing Li
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Strollo P.J., Jr., Soose R.J., Maurer J.T. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370:139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 2.Sawyer A.M., Gooneratne N.S., Marcus C.L., Ofer D., Richards K.C., Weaver T.E. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. http://www.ncbi.nlm.nih.gov/pubmed/21652236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotenberg B.W., Murariu D., Pang K.P. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45:43. doi: 10.1186/s40463-016-0156-0. http://www.ncbi.nlm.nih.gov/pubmed/27542595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravesloot M.J., de Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34:105–110. doi: 10.1093/sleep/34.1.105. http://www.ncbi.nlm.nih.gov/pubmed/21203364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caples S.M., Rowley J.A., Prinsell J.R. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. http://www.ncbi.nlm.nih.gov/pubmed/21061863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisele D.W., Smith P.L., Alam D.S., Schwartz A.R. Direct hypoglossal nerve stimulation in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1997;123:57–61. doi: 10.1001/archotol.1997.01900010067009. [DOI] [PubMed] [Google Scholar]
- 7.Kent D.T., Lee J.J., Strollo P.J., Jr., Soose R.J. Upper airway stimulation for OSA: early adherence and outcome results of one center. Otolaryngol Head Neck Surg. 2016;155:188–193. doi: 10.1177/0194599816636619. [DOI] [PubMed] [Google Scholar]
- 8.Kezirian E.J., Boudewyns A., Eisele D.W. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14:299–305. doi: 10.1016/j.smrv.2009.10.009. http://www.ncbi.nlm.nih.gov/pubmed/20116305 [DOI] [PubMed] [Google Scholar]
- 9.Mwenge G.B., Rombaux P., Dury M., Lengelé B., Rodenstein D. Targeted hypoglossal neurostimulation for obstructive sleep apnoea: a 1-year pilot study. Eur Respir J. 2013;41:360–367. doi: 10.1183/09031936.00042412. http://www.ncbi.nlm.nih.gov/pubmed/22599356 [DOI] [PubMed] [Google Scholar]
- 10.Dedhia R.C., Strollo P.J., Soose R.J. Upper airway stimulation for obstructive sleep apnea: past, present, and future. Sleep. 2015;38:899–906. doi: 10.5665/sleep.4736. http://www.ncbi.nlm.nih.gov/pubmed/25409109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietzsch J.B., Liu S., Garner A.M., Kezirian E.J., Strollo P.J. Long-term cost-effectiveness of upper airway stimulation for the treatment of obstructive sleep apnea: a model-based projection based on the STAR Trial. Sleep. 2015;38:735–744. doi: 10.5665/sleep.4666. http://www.ncbi.nlm.nih.gov/pubmed/25348126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eastwood P.R., Barnes M., Walsh J.H. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34:1479–1486. doi: 10.5665/sleep.1380. http://www.ncbi.nlm.nih.gov/pubmed/22043118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiser C., Maurer J.T., Hofauer B., Sommer J.U., Seitz A., Steffen A. Outcomes of upper airway stimulation for obstructive sleep apnea in a multicenter German Postmarket Study. Otolaryngol Head Neck Surg. 2017;156:378–384. doi: 10.1177/0194599816683378. http://www.ncbi.nlm.nih.gov/pubmed/28025918 [DOI] [PubMed] [Google Scholar]
- 14.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. http://www.ncbi.nlm.nih.gov/pubmed/26061376 [DOI] [PubMed] [Google Scholar]
- 15.Sterne J.A., Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. http://www.ncbi.nlm.nih.gov/pubmed/11576817 [DOI] [PubMed] [Google Scholar]
- 16.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. http://www.ncbi.nlm.nih.gov/pubmed/9310563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Freeman M.F., Tukey J.W. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–611. [Google Scholar]
- 19.Friedman M., Jacobowitz O., Hwang M.S. Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: six-month results. Laryngoscope. 2016;126:2618–2623. doi: 10.1002/lary.25909. http://www.ncbi.nlm.nih.gov/pubmed/27010361 [DOI] [PubMed] [Google Scholar]
- 20.Heiser C., Knopf A., Bas M., Gahleitner C., Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274:1727–1734. doi: 10.1007/s00405-016-4297-6. http://www.ncbi.nlm.nih.gov/pubmed/27619823 [DOI] [PubMed] [Google Scholar]
- 21.Kezirian E.J., Goding G.S., Malhotra A. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. J Sleep Res. 2014;23:77–83. doi: 10.1111/jsr.12079. http://www.ncbi.nlm.nih.gov/pubmed/24033656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodenstein D., Rombaux P., Lengele B., Dury M., Mwenge G.B. Residual effect of THN hypoglossal stimulation in obstructive sleep apnea: a disease-modifying therapy. Am J Respir Crit Care Med. 2013;187:1276–1278. doi: 10.1164/rccm.201211-2129LE. http://www.ncbi.nlm.nih.gov/pubmed/23725628 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz A.R., Bennett M.L., Smith P.L. Therapeutic electrical stimulation of the hypoglossal nerve in obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2001;127:1216–1223. doi: 10.1001/archotol.127.10.1216. http://www.ncbi.nlm.nih.gov/pubmed/11587602 [DOI] [PubMed] [Google Scholar]
- 24.Soose R.J., Gillespie M.B. Upper airway stimulation therapy: a novel approach to managing obstructive sleep apnea. Laryngoscope. 2016;126(Suppl 7):S5–S8. doi: 10.1002/lary.26258. http://www.ncbi.nlm.nih.gov/pubmed/27572120 [DOI] [PubMed] [Google Scholar]
- 25.Soose R.J., Woodson B.T., Gillespie M.B. Upper airway stimulation for obstructive sleep apnea: self-reported outcomes at 24 months. J Clin Sleep Med. 2016;12:43–48. doi: 10.5664/jcsm.5390. http://www.ncbi.nlm.nih.gov/pubmed/26235158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffen A., Sommer J.U., Hofauer B., Maurer J.T., Hasselbacher K., Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. Laryngoscope. 2018;128:509–515. doi: 10.1002/lary.26688. http://www.ncbi.nlm.nih.gov/pubmed/28561345 [DOI] [PubMed] [Google Scholar]
- 27.Strollo P.J., Gillespie M.B., Soose R.J. Upper airway stimulation for obstructivesleep apnea: durability of the treatment effect at 18 months. Sleep. 2015;38:1593–1598. doi: 10.5665/sleep.5054. http://www.ncbi.nlm.nih.gov/pubmed/26158895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Heyning P.H., Badr M.S., Baskin J.Z. Implanted upper airway stimulation device for obstructive sleep apnea. Laryngoscope. 2012;122:1626–1633. doi: 10.1002/lary.23301. http://www.ncbi.nlm.nih.gov/pubmed/22549513 [DOI] [PubMed] [Google Scholar]
- 29.Woodson B.T., Soose R.J., Gillespie M.B. Three-year outcomes of cranial nerve stimulation for obstructive sleep apnea: the STAR trial. Otolaryngol Head Neck Surg. 2016;154:181–188. doi: 10.1177/0194599815616618. http://www.ncbi.nlm.nih.gov/pubmed/26577774 [DOI] [PubMed] [Google Scholar]
- 30.Gottlieb D.J., Yenokyan G., Newman A.B. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. http://www.ncbi.nlm.nih.gov/pubmed/20625114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T., Palta M., Dempsey J. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–249. [PMC free article] [PubMed] [Google Scholar]
- 32.Heiser C., Hofauer B. Predictive success factors in selective upper airway stimulation. Orl. 2017;79:121–128. doi: 10.1159/000455728. [DOI] [PubMed] [Google Scholar]
- 33.Woodson B.T., Gillespie M.B., Soose R.J. Randomized controlled withdrawal study of upper airway stimulation on OSA: short- and long-term effect. Otolaryngol Head Neck Surg. 2014;151:880–887. doi: 10.1177/0194599814544445. http://www.ncbi.nlm.nih.gov/pubmed/25205641.http://www.ncbi.nlm.nih.gov/pubmed/26235158 [DOI] [PubMed] [Google Scholar]
- 34.Gillespie M.B., Soose R.J., Woodson B.T. Upper airway stimulation for obstructive sleep apnea: patient-reported outcomes after 48 months of follow-up. Otolaryngol Head Neck Surg. 2017;156:765–771. doi: 10.1177/0194599817691491. http://www.ncbi.nlm.nih.gov/pubmed/28194999 [DOI] [PubMed] [Google Scholar]
- 35.Certal V.F., Zaghi S., Riaz M. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015;125:1254–1264. doi: 10.1002/lary.25032. http://www.ncbi.nlm.nih.gov/pubmed/25389029 [DOI] [PubMed] [Google Scholar]
- 36.Strohl M., Strohl K., Palomo J.M., Ponsky D. Hypoglossal nerve stimulation rescue surgery after multiple multilevel procedures for obstructive sleep apnea. Am J Otolaryngol. 2016;37:51–53. doi: 10.1016/j.amjoto.2015.08.008. http://www.ncbi.nlm.nih.gov/pubmed/26700261 [DOI] [PubMed] [Google Scholar]