Abstract
Background and objectives
The sodium glucose cotransporter 2 (SGLT-2) inhibitor dapagliflozin is a novel drug for the treatment of diabetes mellitus. Recent studies suggest that SGLT-2 inhibitors affect phosphate homeostasis, but their effects on phosphate-regulating hormones in patients with diabetic kidney disease are still unclear.
Design, setting, participants, & measurements
We performed a post-hoc analysis of a double-blind, randomized, crossover trial in patients with type 2 diabetes with early-stage diabetic kidney disease on stable renin–angiotensin–aldosterone system blockade, with an albumin-to-creatinine ratio between 100 and 3500 mg/g, eGFR≥45 ml/min per 1.73 m2, and glycosylated hemoglobin≥7.2% and <11.4%. Patients were randomized to dapagliflozin 10 mg/d or placebo during consecutive 6-week study periods, separated by a 6-week wash-out. We investigated effects on circulating phosphate, calcium, parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23), 25-hydroxyvitamin D (25[OH]D), and 1,25-dihydroxyvitamin D (1,25[OH]2D) levels.
Results
Thirty-one patients (age 62 years; 23% female) were analyzed. Compared with placebo, dapagliflozin increased serum phosphate by 9% (95% confidence interval, 4% to 15%; P=0.002), PTH increased by 16% (3% to 30%; P=0.01), FGF23 increased by 19% (0.3% to 42%; P=0.05), and serum 1,25(OH)2D decreased by −12% (−25% to 4%; P=0.12). Calcium and 25(OH)D were unaffected. We found no correlation between changes in markers of phosphate homeostasis and changes in eGFR or 24-hour albumin excretion during dapagliflozin treatment.
Conclusions
Dapagliflozin increases serum phosphate, plasma PTH, and FGF23. This effect was independent of concomitant changes in eGFR or 24-hour albumin excretion.
Keywords: Phosphate homeostasis; Diabetic kidney disease; SGLT-2 inhibitors; FGF23; fibroblast growth factor 23; Diabetic Nephropathies; creatinine; Glycated Hemoglobin A; Diabetes Mellitus, Type 2; Renin-Angiotensin System; Cross-Over Studies; Double-Blind Method; Fibroblast Growth Factors; Calcifediol; parathyroid hormone; 25-hydroxyvitamin D; Vitamin D; Phosphates; Homeostasis; Albumins; Glucose; Sodium
Visual Abstract
Introduction
Dapagliflozin, a sodium glucose cotransporter 2 (SGLT-2) inhibitor, is a novel drug for the treatment of diabetes mellitus. Selective inhibition of SGLT-2 lowers plasma glucose and glycosylated hemoglobin (HbA1c) by inhibiting the reabsorption of glucose and sodium in the proximal tubule (1–3). Furthermore, beneficial effects on other cardiovascular risk markers have been reported including lowering of systolic BP, body weight, and albuminuria (4–7).
Previous studies indicated that SGLT-2 inhibitors also increase serum phosphate levels (8,9), fueling the hypothesis that these drugs also influence regulators of bone and mineral homeostasis (10). This hypothesis was recently confirmed in a randomized, crossover study in healthy individuals, which showed that canagliflozin influences the fibroblast growth factor 23 (FGF23)/1,25-dihydroxyvitamin D (1,25[OH]2D)/parathyroid hormone (PTH) axis (11). These effects may be of relevance for patients with diabetes and kidney disease, who are susceptible to develop hyperphosphatemia (12). However, it is unknown whether these effects are drug- or class-specific, or whether similar effects occur in patients with diabetic kidney disease.
Under physiologic conditions, phosphate homeostasis is closely regulated by the hormones FGF23, PTH, and vitamin D. PTH and FGF23 rise in response to phosphate loading (13,14), and subsequently induce phosphaturia by downregulating Npt2a transporters in tubular epithelial cells (15). 1,25[OH]2D is involved in phosphate regulation through the suppression of PTH and induction of FGF23, and promotes intestinal phosphate uptake. It is unclear how SGLT-2 inhibitors affect these phosphate-regulating hormones in patients with diabetic kidney disease. Importantly, SGLT-2 inhibitors induce greater glucosuria and have a greater effect on serum phosphate in patients with diabetes than in healthy individuals. This may imply that SGLT-2 inhibitors may also induce greater changes in bone and mineral homeostasis in these patients (11,16). Moreover, it is not known whether the effects of SGLT-2 inhibitors on serum phosphate are associated with their effects on kidney function or albuminuria (17).
To further elucidate the effects of SGLT-2 inhibitors on phosphate homeostasis, we investigated the effects of the SGLT-2 inhibitor dapagliflozin on serum phosphate, PTH, FGF23, 25-hydroxyvitamin D (25[OH]D), and 1,25(OH)2D in a post-hoc analysis of a randomized, placebo-controlled, crossover trial in patients with type 2 diabetes and early-stage diabetic kidney disease. We subsequently evaluated whether the effects on these parameters of mineral metabolism were associated with effects of SGLT-2 inhibition on kidney function or albuminuria.
Materials and Methods
This is a post-hoc analysis of the IMPROVE trial (Netherlands Trial Register: NTR 4439), a prospective, randomized, double-blind, placebo-controlled, crossover, single-center clinical trial. The study protocol, sample size calculation, and primary outcomes have previously been published in detail (17). The study was approved by the Ethics Committee of the University Medical Center Groningen and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Before any study procedures, all participants provided written informed consent.
Study Population
Between September of 2014 and February of 2016, we enrolled 34 patients, of whom 33 completed the study (17). Two participants were excluded from this post-hoc study due to lack of samples for further measurements, leaving 31 subjects for the present analysis. Participants had type 2 diabetes and early-stage kidney disease, were between 18 and 75 year old, had a first morning void albumin-to-creatinine ratio (UACR) ≥100 and <3500 mg/g, had eGFR≥45 ml/min per 1.73 m2, had HbA1c levels between 7.2% and 11.3% (55 and 100 mmol/mol), and were treated with a stable dose of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for at least 4 weeks. Participants were recruited from the outpatient clinic of the Department of Internal Medicine of Ziekenhuis-Groep Twente, Almelo/Hengelo, The Netherlands. Key exclusion criteria were systolic/diastolic BP >180/110 mm Hg; a cardiovascular event during the past 6 months; and current use of pioglitazone, GLP-1 analogs, DDP-IV inhibitors, or SGLT-2 inhibitors. Patients were randomly allocated to one of two double-blinded treatment orders. For each sequence/treatment group, study medication was identical in packaging and appearance. No participants deviated from the original sequence allocation. All study personnel and patients remained blinded to the sequence allocation.
Study Design and Measurements
Eligible patients were randomized after a screening visit and a run-in period of 4 weeks if subjects were not on a stable dose of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or glucose-lowering medication. The study employed consecutive crossover treatment periods of 6 weeks each, in which patients were treated with dapagliflozin 10 mg/d or placebo, with wash-out periods of 6 weeks in between. No interim analyses were planned or conducted. Study medication was dispensed at the beginning of each treatment period. Patients were instructed to take the tablet in the morning. Adherence to study medication was excellent, as previously reported (17). All efforts were made to keep the use and dosage of all concomitant medication stable during follow-up.
Patients collected 24-hour urine samples at the beginning and end of each treatment period. Urine phosphate excretion was measured from these urine samples and fractional tubular reabsorption of phosphate was calculated using the equation (100%−[urine PO4×plasma creatinine×100]÷[plasma PO4×urine creatinine]). Blood samples were taken in the morning, in fasting condition, at the beginning and end of each treatment period. Total FGF23 (C-terminal+intact FGF23) was measured in plasma with commercially available ELISA kits (Immutopics, San Clemente, CA). The interassay coefficient of variation of this assay in our laboratory was <2.5%. Serum 1,25(OH)2D levels were measured with 2D-liquid chromatography tandem mass spectrometry as described by Dirks et al. (18), with minor modifications, at the Department of Laboratory Medicine, Radboud University Medical Center, Nijmegen, The Netherlands. The eGFR was calculated from the Modification of Diet in Renal Disease equation using the serum creatinine concentration measured at the beginning and end of each treatment period (19).
Statistical Analyses
We evaluated the effect of dapagliflozin treatment, compared with placebo treatment, on the changes in serum calcium and phosphate; plasma FGF23, PTH, and 25(OH)D; and serum 1,25(OH)2D. All analyses were conducted on a modified intention-to-treat population, comprising 31 individuals for whom samples were available for measurement of bone and mineral markers.
Mixed effects repeated measures analyses were used to assess percentage change during each treatment period, as well as the effect of dapagliflozin compared with placebo. The model included the sequence of interventions, period, treatment, and subject as factors, and baseline levels of serum phosphate, calcium, plasma PTH, FGF23, 25(OH)D, or 1,25(OH)2D as covariate. All variables with the exception of eGFR were log-transformed before being entered into the repeated measures model, and are presented as percentage change. The between-group geometric mean change was derived by 100×(exp [least square mean change]−1), and the same transformation was applied to the 95% confidence limits. We assessed correlation between changes in markers of phosphate regulation and changes in eGFR and 24-hour albumin excretion using Pearson’s correlation. All statistical analyses were performed with SAS software version 82 (SAS Institute, Inc., Cary, NC). All statistical tests were two-sided, with a statistical significance level of 5%.
Results
Baseline characteristics of the 31 participants included in the present post-hoc analysis are shown in Table 1. This subset of participants was highly similar to the original IMPROVE study cohort (n=33) in age (current subcohort 62±8 versus original study population 61±9 years), sex (number of females/males: 7/24 versus 8/25), HbA1c (7.3±2.9 versus 7.4±3.1%), body mass index (31.5 versus 31.6 kg/m2), eGFR (72±19 versus 72±21 ml/min per 1.73 m2), and urinary albumin excretion (UAE) (521 [234–997] versus 470 [191–997] mg/24 hours).
Table 1.
Baseline characteristics of 31 participants with early diabetic kidney disease in the IMPROVE trial
| Characteristics | n=31 |
|---|---|
| General characteristics | |
| Age, yr | 62 (8) |
| Sex (n, female/male) | 7/24 |
| HbA1c, % | 7.3 (2.9) |
| Diabetes duration, yr | 10 (7) |
| Systolic BP, mm Hg | 143 (15) |
| Diastolic BP, mm Hg | 77 (6) |
| Weight, kg | 94 (22) |
| Body mass index, kg/m2 | 31 (5.6) |
| eGFR, ml/min per 1.73 m2 | 72 (19) |
| 24-h urine albumin excretion, mg/24 ha | 521 (234–997) |
| Markers of bone and mineral metabolism | |
| Serum phosphate, mg/dl | 3.3 (0.6) |
| Serum calcium, mg/dl | 9.4 (0.4) |
| Serum PTH, pg/mla | 45 (35–64) |
| Plasma FGF23, RU/mla | 104 (84–166) |
| Plasma 25(OH)D, ng/ml | 18 (8) |
| Plasma 1,25(OH)2D, pg/ml | 31 (25–39) |
Data are given mean (SD), except where indicated. HbA1c, glycosylated hemoglobin; PTH, parathyroid hormone; FGF23, fibroblast growth factor 23; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Data given as median (interquartile range).
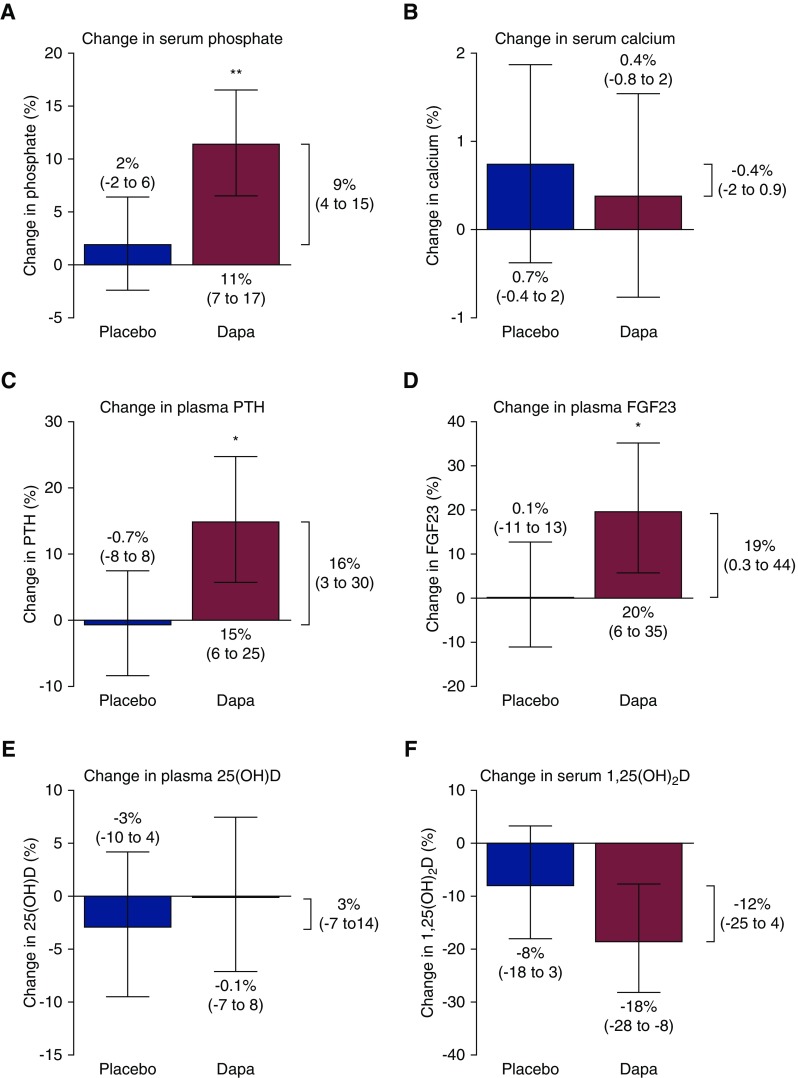
Effects of Dapagliflozin on Phosphate and Calcium
The levels of mineral metabolism and kidney function biomarkers at the beginning (week 0) and end (week 6) of each treatment period are shown in Table 2. Mean serum phosphate was 3.3±0.2 mg/dl at the start of both placebo and dapagliflozin treatment. Placebo treatment did not change serum phosphate (change, 2%; 95% confidence interval [95% CI], −2% to 6%; P=0.38). However, dapagliflozin increased serum phosphate by 11% (95% CI, 7% to 17%; P<0.001 versus start of dapagliflozin, P=0.002 versus placebo; Figure 1A). Fractional tubular reabsorption of phosphate was 84% (interquartile range [IQR], 78–87) at the start of placebo treatment and 84% (IQR, 79–88) at the start of dapagliflozin treatment, and was unchanged by either placebo (change, 0.5%; 95% CI, −2 to 3; P=0.68) or dapagliflozin treatment (change, −0.2%; 95% CI, −3% to 2%; P=0.86 versus start of dapagliflozin, P=0.53 versus placebo; Table 2).
Table 2.
Biomarker levels at the start and end of each study period, and the difference in change between the two study periods
| Biomarker | Placebo | Dapagliflozin | Difference in Change, %a | P Valueb | ||
|---|---|---|---|---|---|---|
| Week 0 | Week 6 | Week 0 | Week 6 | |||
| Serum phosphate, mg/dl | 3.3±0.2 | 3.4±0.2 | 3.3±0.2 | 3.7±0.2 | 9 (4 to15) | 0.002 |
| Serum calcium, mg/dl | 9.4±0.4 | 9.5±0.4 | 9.4±0.4 | 9.5±0.4 | 0.4 (−2 to 0.9) | 0.56 |
| Plasma PTH, pg/ml | 42 (35–62) | 44 (32–62) | 46 (38–63) | 53 (39–75) | 16 (3 to 30) | 0.002 |
| Plasma FGF23, RU/ml | 111 (84–157) | 106 (91–181) | 113 (90–207) | 129 (103–166) | 19 (0.3 to 42) | 0.05 |
| Plasma 25(OH)D, ng/ml | 17 (13–24) | 18 (13–22) | 18 (12–24) | 16 (12–23) | 39 (−7 to 14) | 0.55 |
| Plasma 1,25(OH)2D, pg/ml | 29 (25–36) | 31 (21–37) | 34 (24–41) | 26 (19–34) | −12 (−25 to 4) | 0.12 |
| Urine phosphate, mEq/24 h | 41.4 (24.5–68.5) | 54.6 (39.8–73.5) | 45.8 (23.2–72.4) | 53.0 (35.9–79.4) | −12 (−24 to 2) | 0.10 |
| Fractional tubular reabsorption of phosphate | 0.84 (0.78–0.87) | 0.83 (0.80–0.86) | 0.84 (0.79–0.88) | 0.83 (0.80–0.87) | −0.7 (−3 to 2) | 0.53 |
| eGFR, ml/min per 1.73 m2 | 74±21 | 73±19 | 71±18 | 66±20 | −5 (−8 to −3) | 0.004 |
| Urinary albumin excretion, mg/24 h | 477 (208–1063) | 559 (271–1320) | 537 (201–973) | 283 (118–579) | −43 (−54 to −31) | <0.001 |
Data are shown as mean±SD or median (interquartile range). PTH, parathyroid hormone; FGF23, fibroblast growth factor 23; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Difference (95% confidence interval) between dapagliflozin and placebo treatment assignments in the relative change in biomarkers from beginning to end of the study periods.
Figure 1.
Dapagliflozin increases serum phosphate, plasma PTH and plasma FGF23, compared with placebo. The bars show the difference between week 0 and week 6 of each treatment period for serum phosphate (A), calcium (B), PTH (C), FGF23 (D), 25(OH)D (E), and 1,25(OH)2D (F). The difference in change between the dapagliflozin (Dapa) and placebo intervention, assessed by mixed effects repeated measures analysis, is shown to the right of each graph. Data shown as mean (95% confidence interval). *P versus placebo <0.05; **P versus placebo <0.01. 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; Dapa, dapagliflozin; FGF23, fibroblast growth factor 23; PTH, parathyroid hormone.
Mean serum calcium was 9.4±0.4 mg/dl before both treatments and was unaffected by placebo (change, 0.7%; 95% CI, −0.4% to 2%; P=0.19) or dapagliflozin (change, 0.4%; 95% CI, −0.8% to 2%; P=0.51 versus start of treatment, P=0.56 versus placebo; Figure 1B).
Effects of Dapagliflozin on PTH and FGF23
As indicated in Table 2, median PTH before placebo treatment was 42 pg/ml (IQR, 35–62); PTH was unchanged by placebo treatment (change, −0.7%; 95% CI, −8% to 8%; P=0.85). Median PTH at the start of dapagliflozin was 46 pg/ml (IQR, 38–63). After dapagliflozin treatment, PTH was increased by 15% (95% CI, 6% to 25%; P=0.002 versus start of dapagliflozin, P=0.002 versus placebo; Figure 1C).
Median FGF23 before placebo treatment was 111 Relative Units/ml (RU/ml) (IQR, 84–157) and FGF23 was unchanged by placebo treatment (change, 0.1%; 95% CI, −11% to 13%; P=0.94). Median FGF23 before dapagliflozin treatment was 113 RU/ml (IQR, 90–207). After dapagliflozin treatment, FGF23 levels had increased by 20% (95% CI, 6% to 35%; P<0.01 versus start of dapagliflozin, P=0.05 versus placebo; Figure 1D). Neither change in PTH nor change in FGF23 correlated with change in serum phosphate (r −0.08, P=0.67 and r −0.09, P=0.62, respectively).
Effects of Dapagliflozin on Vitamin D
Median plasma 25(OH)D was 17 ng/ml (IQR, 13–24) before placebo treatment and 18 ng/ml (IQR, 12–24) before dapagliflozin treatment. Neither placebo (change, −3%; 95% CI, −10% to 4%; P=0.40) nor dapagliflozin (change, −0.1%; −7% to 8%; P=0.89 versus start of dapagliflozin, P=0.55 versus placebo) altered 25(OH)D levels (Figure 1E).
Median serum 1,25(OH)2D was 29 pg/ml (IQR, 25–36) at the start of placebo treatment. Placebo did not affect 1,25(OH)2D levels (change, −8.0%; −18.0% to 3%; P=0.15). Median serum 1,25(OH)2D levels were 34 pg/ml (IQR, 24–41) before dapagliflozin treatment. Dapagliflozin induced a decrease in 1,25(OH)2D levels (change, −19%; 95% CI, −28 to −8%; P=0.002), but this effect was NS compared with placebo (P=0.12; Figure 1F). The change in 1,25(OH)2D was inversely correlated with the change in serum phosphate (r −0.47, P=0.02), tended to correlate with the change in FGF23 (r −0.36, P=0.07), and was positively correlated with change in PTH (r 0.45, P=0.02).
Change in Markers of Mineral Metabolism and Change in Kidney Function Parameters
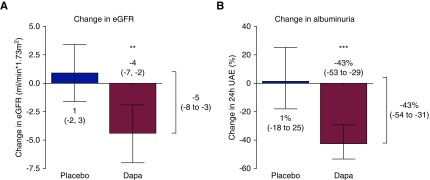
Mean eGFR before placebo treatment was 74 (±21) ml/min per 1.73 m2 and eGFR was unchanged by placebo (change, 1 ml/min per 1.73 m2; 95% CI, −2 to 3). Mean eGFR before dapagliflozin treatment was 71 (±18) ml/min per 1.73 m2. Dapagliflozin treatment reduced eGFR by −4 (95% CI, −7 to −2) ml/min per 1.73 m2 (P<0.001, P=0.004 versus placebo; Figure 2A).
Figure 2.
Dapagliflozin reduces eGFR and albuminuria, compared with placebo. The bars show the difference between week 0 and week 6 of each treatment period for eGFR and albuminuria. The difference in change between the dapagliflozin (Dapa) and placebo intervention, assessed by mixed effects repeated measures analysis, is shown to the right of each graph. Data shown as mean (95% confidence interval). **P versus placebo <0.01; ***P versus placebo <0.001. Dapa, dapagliflozin; UAE, urinary albumin excretion.
Albuminuria was 559 mg/24 hours (IQR, 271–1320) before placebo and was unaffected by placebo (change, 1%; 95% CI, −18% to 25%; P=0.89). Albuminuria was 537 (IQR, 201–973) before dapagliflozin treatment, and dapagliflozin decreased albuminuria by −43% (95% CI, −53 to −29%; P<0.001 versus start of dapagliflozin and versus placebo; Figure 2B).
No correlation was found between the change in eGFR and the change in serum phosphate (r 0.08, P=0.66), change in PTH (r 0.10, P=0.60), change in FGF23 (r 0.02, P=0.91), or change in 1,25(OH)2D (r 1.13, P=0.53). Similarly, no significant correlation was found for changes in albuminuria and change in serum phosphate (r −0.20, P=0.28), change in PTH (r 0.11, P=0.57), change in FGF23 (r 0.27, P=0.15), or change in 1,25(OH)2D (r 0.36, P=0.07).
Discussion
SGLT-2 inhibitors are a new class of drugs that have shown promising effects in slowing progression of kidney function decline in patients with type 2 diabetes. However, emerging data also suggest effects on phosphate homeostasis. This effect may be particularly relevant in patients with diabetes with CKD, who are susceptible to develop hyperphosphatemia.
In this study, we confirm earlier observations that dapagliflozin treatment increases serum phosphate. We extend on these observations by demonstrating that dapagliflozin also increases PTH and FGF23 levels. We also observed a nonsignificant trend in reduction of 1,25(OH)2D. These changes were independent of the concomitant effects of dapagliflozin on eGFR and albuminuria. Serum calcium and plasma 25(OH)D remained unchanged in this study.
Our results are consistent with findings from a recent intervention study in healthy volunteers by Blau et al. (11). In this study, canagliflozin 300 mg/d increased FGF23 and PTH levels within days of the start of treatment, and suppressed 1,25(OH)2D. Taken together, our study indicates that the effects of SGLT-2 inhibitors on bone and mineral homeostasis are likely a class effect. The magnitude of the changes was similar in both studies, despite the use of different drugs in different populations. Although SGLT-2 inhibitors induce more prominent glucosuria and may have greater effects on serum phosphate in patients with diabetes, in our study they reassuringly did not have stronger effects on phosphate homeostasis (11,16). Our study further adds to the findings by Blau et al. by showing that changes in phosphate homeostasis may persist over 6 weeks of treatment. A pertinent question is whether alterations of FGF23, PTH, and 1,25(OH)2D are sustained over the full duration of SGLT-2 treatment, especially because the effect of dapagliflozin on serum phosphate is consistent over multiple years (20). Unlike Blau et al. (11), we did not observe a change in fractional tubular reabsorption of phosphate. However, our study may have been less well suited to detect such a change. Dietary phosphate intake was not controlled for in our study, and urine samples were collected primarily to measure urine albumin levels and were not acidified and may have been affected by precipitation.
In our study, changes in phosphate, PTH, FGF23, and 1,25(OH)2D did not correlate with change in eGFR during dapagliflozin treatment. Additionally, the change in serum phosphate induced by dapagliflozin is disproportionately large for the concomitantly observed change in eGFR (21). Consequently, the effect of dapagliflozin on eGFR is unlikely to drive the effect on phosphate levels. Instead, our findings fit the hypothesis that SGLT-2 inhibition promotes tubular sodium/phosphate cotransport through increased availability of sodium in the proximal tubule (10,11). The observed increases in PTH and FGF23 are most likely part of a subsequent compensatory response to maintain phosphate homeostasis (14,22). Higher FGF23 levels, in turn, suppress 1,25(OH)2D through inhibition of CYP27B1 and stimulation of CYP24A1 (23,24). This effect on CYP27B1 may be partially counteracted by PTH (24), which translates into a positive correlation between change in PTH and change in 1,25(OH)2D in our study.
Our findings could also represent an effect of dapagliflozin on the Nampt/NAD+ pathway, which regulates the circadian rhythm of phosphate (25). The nadir of this circadian rhythm occurs around 8:00–11:00 am (26–28), coinciding with sample collection in this study. Our results may therefore reflect attenuation of the circadian rhythm rather than a consistent effect over time. Hyperglycemia affects kidney Nampt expression in mice (29), but the effect of SGLT-2 inhibition on the Nampt/NAD+ system remains unstudied.
Serum calcium and 25(OH)D were not affected by dapagliflozin, in concordance with previous studies of dapagliflozin (6–8). On the basis of the changes in PTH and 1,25(OH)2D in our study, as well as the recent findings in healthy volunteers (11), a change in urine calcium excretion might be expected. However, we could not investigate this because urine calcium measurements were not available in our study. The absence of effects on serum calcium and 25(OH)D fits with the hypothesis that altered tubular phosphate handling is the driving factor behind effects of SGLT-2 inhibition on phosphate homeostasis. Contrasting our results, canagliflozin has been reported to induce modest increases in serum calcium and 25(OH)D (9,30). Whether these effects are agent-specific or due to differences in populations or trial design cannot be ascertained in the absence of published head-to-head trials.
It is unclear whether effects of SGLT-2 inhibition on serum phosphate, PTH, and FGF23 should be considered a safety signal. SGLT-2 inhibitor treatment has been shown to confer a survival benefit, and to reduce the risk of cardiovascular events (31). However, these benefits have been investigated in populations with cardiovascular disease, rather than kidney disease. In patients with CKD, higher serum phosphate levels, even when within the normal range, are associated with mortality (12,32). It has also been suggested that patients with CKD with diabetes are particularly susceptible to phosphate toxicity (12). In patients with diabetic kidney disease, a higher plasma FGF23 level has been associated with adverse kidney outcomes (33) and all-cause mortality (34,35). Importantly, although the average change in serum phosphate due to SGLT-2 inhibitor treatment is small, significant interindividual variability has been observed (10). Therefore, the benefits of SGLT-2 treatment might be attenuated by the effects on phosphate homeostasis in some patients. Lastly, it has been suggested that SGLT-2 inhibitor use is associated with an increased rate of bone fractures (9). However, this finding has not been replicated consistently (36). Data from clinical trials are eagerly awaited to establish whether SGLT-2 inhibition positively affects end points in patients with kidney disease.
Our study has a number of limitations. First, because we performed a post-hoc analysis, these results should be considered hypothesis-generating. Second, we were only able to evaluate changes in biomarkers of bone and mineral homeostasis, which are considered intermediate outcomes. The limited number of participants and the short duration of this study did not allow us to also study potential effects on clinical outcomes. Third, because only the clinically used dapagliflozin dose of 10 mg/d was administered in this study, a potential dose-response effect could not be assessed. Lastly, our study only included white patients with diabetes type 2 and residual proteinuria; caution is warranted when extrapolating our findings to different populations.
In conclusion, dapagliflozin induced a significant rise in serum phosphate, PTH, and FGF23 levels, independent of concomitant effects on eGFR. Serum calcium and 25(OH)D levels remained unchanged. Our findings support effects of dapagliflozin on phosphate homeostasis independent of its effect on kidney function. The long-term persistence of these effects and their potential clinical effects in patients with more advanced CKD should be further investigated.
Disclosures
G.L. received lecture fees from Sanofi, Astra Zeneca and Jansen, and has served as a consultant for Abbvie, Sanofi, Novo Nordisk, Astra Zeneca, Boehringer Ingelheim and MSD; he received a research grant from Astra Zeneca. D.d.Z. served on advisory boards and/or as a speaker for Bayer, Boehringer Ingelheim, Fresenius, and Mitsubishi-Tanabe; served on steering committees and/or as a speaker for AbbVie and Janssen; and served on data safety and monitoring committees for Bayer. H.J.L.H. has consultancy agreements with the following companies: Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen, Fresenius, Gilead, and Merck, and has a policy of honoraria going to his employer, has received grant support from Boehringer Ingelheim, AstraZeneca and Janssen (funding to his employer), and is a member of the steering committee for the DAPA-CKD study. M.H.d.B. has consultancy agreements with Amgen, Astra Zeneca, Bayer, Vifor Fresenius Medical Care Renal Pharma, and Sanofi Genzyme, and received grant support from Amgen and Sanofi Genzyme. The other authors reported no conflict of interest.
Acknowledgments
We thank all patients and research staff for their participation and assistance in this study. We thank Wendy Dam and Sandra Oude-Alink, technicians, for their technical assistance. We thank Astra Zeneca for providing study medication.
G.D.L. received lecture fees and/or research grants from Astra Zeneca. H.J.L.H. is supported by a VIDI (917.15.306) grant from The Netherlands Organization for Scientific Research.
The sponsor had no influence on the design, conduct, analysis, or manuscript of the study. H.J.L.H. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
All authors were involved in the design of the study. M.A.d.J. was responsible for data analysis and interpretation of the results and wrote the first draft of the manuscript. S.I.P. was responsible for data collection and critical revision of the manuscript. D.d.Z. and S.J.L.B. assisted in data interpretation and contributed in critical revision of the manuscript. A.E.v.H. and G.D.L. contributed to data collection and critical revision of the manuscript. H.J.L.H. and M.H.d.B. were responsible for data analysis, data interpretation, and final review of the manuscript. All authors read and approved the final manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Good Guys, Bad Guys, Guesses, and Near Misses in Nephrology,” on pages 7–9.
References
- 1.DeFronzo RA, Hompesch M, Kasichayanula S, Liu X, Hong Y, Pfister M, Morrow LA, Leslie BR, Boulton DW, Ching A, LaCreta FP, Griffen SC: Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36: 3169–3176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF: Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: A randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J: Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 15: 853–862, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherney D, Lund SS, Perkins BA, Groop P-H, Cooper ME, Kaspers S, Pfarr E, Woerle HJ, von Eynatten M: The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia 59: 1860–1870, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC; EMPA-REG RENAL Trial Investigators : Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2(5): 369–384, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Yale J-F, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G: Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 15: 463–473, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heerspink HJL, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD: Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab 18: 590–597, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.List JF, Woo V, Morales E, Tang W, Fiedorek FT: Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 32: 650–657, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon H: Canagliflozin: Clinical Efficacy and Safety, United States Food and Drug Administration, Silver Spring, MD, 2013
- 10.Taylor SI, Blau JE, Rother KI: Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 3: 8–10, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blau JE, Bauman V, Conway EM, Piaggi P, Walter MF, Wright EC, Bernstein S, Courville AB, Collins MT, Rother KI, Taylor SI: Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 3: 1–14, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21(8): 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Vervloet MG, van Ittersum FJ, Büttler RM, Heijboer AC, Blankenstein MA, ter Wee PM: Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol 6(2): 383–389, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrukhova O, Zeitz U, Goetz R, Mohammadi M, Lanske B, Erben RG: FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51(3): 621–628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blau JE, Taylor SI: Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol 14: 473–474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL: The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab 19: 1363–1370, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Dirks NF, Martens F, Vanderschueren D, Billen J, Pauwels S, Ackermans MT, Endert E, Heijer MD, Blankenstein MA, Heijboer AC: Determination of human reference values for serum total 1,25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC-MS/MS method. J Steroid Biochem Mol Biol 164: 127–133, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration : Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53(4): 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Dekkers CCJ, Wheeler DC, Sjöström CD, Stefansson BV, Cain V, Heerspink HJL: Effects of the sodium–glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b–4 chronic kidney disease. Nephrol Dial Transplant 33: 2005–2011, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirillo M, Botta G, Chiricone D, De Santo NG: Glomerular filtration rate and serum phosphate: An inverse relationship diluted by age. Nephrol Dial Transpl 24: 2123–2131, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Perwad F, Azam N, Zhang MYH, Yamashita T, Tenenhouse HS, Portale AA: Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146: 5358–5364, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19(3): 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Bikle DD: Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol 21: 319–329, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyagawa A, Tatsumi S, Takahama W, Fujii O, Nagamoto K, Kinoshita E, Nomura K, Ikuta K, Fujii T, Hanazaki A, Kaneko I, Segawa H, Miyamoto KI: The sodium phosphate cotransporter family and nicotinamide phosphoribosyltransferase contribute to the daily oscillation of plasma inorganic phosphate concentration. Kidney Int 93: 1073–1085, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Kemp GJ, Blumsohn A, Morris BW: Circadian changes in plasma phosphate concentration, urinary phosphate excretion, and cellular phosphate shifts. Clin Chem 38: 400–402, 1992 [PubMed] [Google Scholar]
- 27.Markowitz M, Rotkin L, Rosen JF: Circadian rhythms of blood minerals in humans. Science 213(4508): 672–674, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Jubiz W, Canterbury JM, Reiss E, Tyler FH: Circadian rhythm in serum parathyroid hormone concentration in human subjects: Correlation with serum calcium, phosphate, albumin, and growth hormone levels. J Clin Invest 51: 2040–2046, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitada K, Nakano D, Ohsaki H, Hitomi H, Minamino T, Yatabe J, Felder RA, Mori H, Masaki T, Kobori H, Nishiyama A: Hyperglycemia causes cellular senescence via a SGLT2- and p21-dependent pathway in proximal tubules in the early stage of diabetic nephropathy. J Diabetes Complications 28: 604–611, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weir MR, Kline I, Xie J, Edwards R, Usiskin K: Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin 30: 1759–1768, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Eddington H, Hoefield R, Sinha S, Chrysochou C, Lane B, Foley RN, Hegarty J, New J, O’Donoghue DJ, Middleton RJ, Kalra PA: Serum phosphate and mortality in patients with chronic kidney disease. Clin J Am Soc Nephrol 5: 2251–2257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titan SM, Zatz R, Graciolli FG, dos Reis LM, Barros RT, Jorgetti V, Moysés RM: FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin J Am Soc Nephrol 6: 241–247, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz Mendoza J, Isakova T, Cai X, Bayes LY, Faul C, Scialla JJ, Lash JP, Chen J, He J, Navaneethan S, Negrea L, Rosas SE, Kretzler M, Nessel L, Xie D, Anderson AH, Raj DS, Wolf M; CRIC Study Investigators : Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int 91: 711–719, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan GC, Divers J, Russell GB, Langefeld CD, Wagenknecht LE, Hsu FC, Xu J, Smith SC, Palmer ND, Hicks PJ, Bowden DW, Register TC, Ma L, Carr JJ, Freedman BI: FGF23 concentration and APOL1 genotype are novel predictors of mortality in African Americans with type 2 diabetes. Diabetes Care 41: 178–186, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]