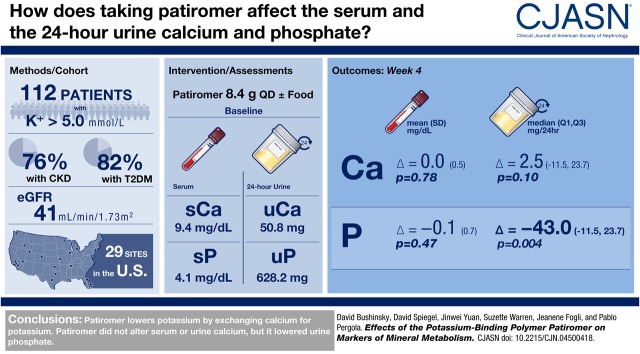
Abstract
Background and objectives
Patiromer is a sodium-free, nonabsorbed, potassium-binding polymer that uses calcium as the counter-exchange ion and is approved for treatment of hyperkalemia. The 4-week TOURMALINE study in patients with hyperkalemia previously demonstrated that patiromer administered once daily reduces serum potassium similarly when given with or without food. We report a prespecified exploratory efficacy analysis as well as a post hoc efficacy and safety analysis of the TOURMALINE study on circulating markers of mineral metabolism.
Design, setting, participants, & measurements
Adults with hyperkalemia (potassium >5.0 mEq/L) were randomized to once-daily patiromer 8.4 g without/with food for 4 weeks, with doses adjusted to achieve and maintain serum potassium 3.8–5.0 mEq/L. Baseline and week 4 serum and 24-hour urine markers of mineral metabolism are reported for all patients combined (evaluable for efficacy, n=112; evaluable for safety, n=113). P values were calculated using a paired t test for change from baseline, unless otherwise specified.
Results
Mean (SD) baseline eGFR was 41±26 ml/min per 1.73 m2. Mean (SD) changes from baseline to week 4 were 0.0±0.5 mg/dl (P=0.78; n=100) for albumin-corrected serum calcium, −0.2±0.2 mg/dl (P<0.001; n=100) for serum magnesium, and −0.1±0.7 mg/dl (P=0.47; n=100) for serum phosphate. Median (quartile 1, quartile 3) changes in 24-hour creatinine-normalized urine calcium and phosphate from baseline to week 4 were 2.5 (−11.5, 23.7) mg/24 h (P=0.10; n=69) and −43.0 (−162.6, 35.7) mg/24 h (P=0.004; n=95), respectively. Median (quartile 1, quartile 3) changes in intact parathyroid hormone and 1,25-dihydroxyvitamin D from baseline to week 4 were −13 (−31, 4) pg/ml (P<0.001; n=97) and −2 (−9, 3) pg/ml (P=0.05; n=96), respectively. There were no changes in fibroblast growth factor-23 or 25-hydroxyvitamin D. In patients (n=16) with baseline serum phosphate >4.8 mg/dL, the mean (SD) changes in serum and 24-hour creatinine-normalized urine phosphate from baseline to Week 4 were −0.6±0.8 mg/dl (n=13) and −149.1±162.6 mg/24hr (n=9), respectively.
Conclusions
Patiromer lowered urine phosphate in all patients, and lowered both serum and urine phosphate in a small subset of patients with hyperphosphatemia. Intact parathyroid hormone and 1,25-dihydroxyvitamin D decreased, with no change in serum calcium.
Keywords: Hyperkalemia, patiromer, potassium, mineral metabolism, Sodium, fibroblast growth factor 23, creatinine, Magnesium, Fibroblast Growth Factors, Polymers, Calcifediol, 25-hydroxyvitamin D, Vitamin D, Phosphates, parathyroid hormone, Albumins, Minerals
Visual Abstract
Introduction
Patiromer is a sodium-free, nonabsorbed, potassium-binding polymer that uses calcium as the counter-exchange ion. It is approved for the treatment of hyperkalemia, including in the United States, Switzerland, the European Union, and Australia (1,2). Studies have shown that patiromer effectively and safely lowers serum potassium in patients with hyperkalemia, including those with CKD, heart failure, diabetes mellitus, and hypertension, and those on renin-angiotensin-aldosterone system (RAAS) inhibitors (1,3–5). The use of calcium as the counter-exchange ion in patiromer—rather than sodium, the exchange cation in sodium polystyrene sulfonate (6)—avoids the potential for increases in sodium absorption and retention in patients at risk for volume overload, such as those with hypertension, CKD, and/or heart failure (6–10).
In the phase 2 and 3 clinical trials of patiromer, patients were instructed to take patiromer with meals. The TOURMALINE study was a phase 4, prospective, open-label, 4-week, randomized trial designed to compare the efficacy and safety of patiromer when administered without food versus with food. The primary results demonstrated that food has no effect on either the efficacy or safety profile of patiromer (11). The study was performed in a general population with hyperkalemia, which afforded the opportunity to obtain information on the effects of patiromer on markers of mineral metabolism. Here we report the prespecified exploratory efficacy analysis of the changes observed for serum phosphate, 25-hydroxyvitamin D (25-D), 1,25-dihydroxyvitamin D (1,25-D), fibroblast growth factor-23 (FGF-23), and 24-hour creatinine-normalized urine phosphate as well as safety analysis for serum calcium, magnesium, intact parathyroid hormone (iPTH), and 24-hour creatinine-normalized urine calcium.
Materials and Methods
The methodology of the open-label TOURMALINE study (Clinicaltrials.gov identifier NCT02694744) has been published (11). Briefly, eligible patients were ≥18 years old with hyperkalemia (defined as two local whole blood potassium values >5.0 mEq/L; each obtained from separate venipunctures). Patients receiving RAAS inhibitors, β-blockers, or diuretics were required to be on stable doses for at least 14 days before screening. Patients could have clinically stable CKD, heart failure, diabetes mellitus (type 1 or type 2), and/or hypertension, but none of these disorders were a requirement for study entry. The use of calcium or potassium dietary supplements was an exclusion criterion; use of magnesium dietary supplements was allowed. Eligible patients were randomized in a 1:1 ratio to receive patiromer once daily, either without food or with food (11). Patients were allowed to continue their usual diets without study-prescribed dietary counseling.
After the screening visit, patients fasted overnight and returned the next day for the baseline visit (randomization and baseline blood sampling). On day 2, patients began their randomly assigned patiromer dosing of 8.4 g once daily without food (n=57) or with food (n=57), and completed visits on day 3 and at 1, 2, 3, and 4 weeks after starting patiromer treatment. For safety, patients were followed for 2 weeks after the last dose of patiromer (two visits). During the study, patiromer daily doses could be titrated on day 3 and weekly afterward by 8.4 g once daily to a maximum of 25.2 g once daily, to achieve and maintain serum potassium levels within the target range of 3.8–5.0 mEq/L.
The study was conducted in accordance with US Food and Drug Administration regulations, the International Conference on Harmonization Guideline for Good Clinical Practice, the Declaration of Helsinki, and institutional review board or independent ethics committee requirements.
Serum Electrolytes
Serum chemistry panels were analyzed by a central laboratory at baseline, day 3, weeks 1, 2, 3, and 4, and during follow-up visits. This included inorganic phosphate, magnesium, potassium, and serum calcium. Serum calcium was corrected for albumin as follows: if serum albumin was ≥4.0 g/dl, then corrected calcium equaled serum calcium; if serum albumin was <4.0 g/dl, then corrected calcium=serum calcium+(0.8×[4−serum albumin]) (12). For these calculations, calcium is in milligrams per deciliter and albumin is in grams per deciliter. Mean serum potassium (the primary study end point) and phosphate (the exploratory efficacy end point, including its subgroup analysis in patients with baseline eGFR<45 ml/min per 1.73 m2) values over time were prespecified. Mean change in serum phosphate from baseline to week 4 in the subgroup of patients with hyperphosphatemia (phosphate >4.8 mg/dl) was performed as a post hoc analysis. In addition, in this subgroup, post hoc safety analyses of mean change from baseline to week 4 for serum calcium and magnesium were conducted. Additional information on methods for assessment of serum electrolytes is provided in Supplemental Material.
Urine Electrolytes
We collected 24-hour urine samples starting from the day before the baseline visit and the day before the week 4 visit. For the baseline visit, patients were requested to collect 24-hour urine samples after the screening visit (day −1) until arrival at the baseline visit (day 1); for the week 4 visit, patients collected 24-hour urine samples through the interval after the final dose of patiromer. For additional information on methods for assessment of urine electrolytes, see Supplemental Material.
Two preplanned corrections were made to urine data. First, a time correction was made to adjust the actual duration of the urine collections to 24 hours as protocol directions to minimize errors in the start and stop times lead to the baseline collection, which started at the end of the screening visit, being slightly less than 24 hours. The calcium and phosphate values were divided by the sample collection time (in hours), then multiplied by 24 hours to obtain 24-hour values (24-hour–adjusted value=[(test value)/(sample collection time in hours)]×24).
Second, to adjust for possible missed urine collection during the collection period, and to allow for a quantitative comparison within individual participants, the time-corrected values for calcium and phosphate were normalized to the mean urine creatinine concentration for the two samples according to the following: 24-hour creatinine-normalized electrolyte=(24-hour–adjusted electrolyte)/[(24-hour–adjusted creatinine)/M24Cr]. Here, M24Cr is the mean of the 24-hour–adjusted urine creatinine values at baseline and week 4. This was on the basis of the assumption that over a 4-week period, the 24-hour urine creatinine excretion should not change. For a sensitivity analysis, 24-hour urine calcium-to-creatinine and phosphate-to-creatinine ratios were also calculated according to the following: 24-hour urine electrolyte-to-creatinine ratio (mg/g)=(24-hour electrolyte)/(24-hour creatinine).
Mean change in 24-hour creatinine-normalized urine phosphate from baseline to week 4 was a prespecified exploratory efficacy end point. Mean change in 24-hour creatinine-normalized urine calcium from baseline to week 4 was analyzed post hoc. Mean change in 24-hour creatinine-normalized urine electrolytes from baseline to week 4 in the subgroup of patients with hyperphosphatemia (phosphate >4.8 mg/dl) was analyzed post hoc.
Markers of Mineral Metabolism
Markers of mineral metabolism including 25-D, 1,25-D, and FGF-23 were collected as prespecified exploratory efficacy end points at baseline and week 4 (or at early termination). iPTH was a safety end point and was analyzed post hoc. For additional information see Supplemental Material.
Safety Assessments
Laboratory safety assessments conducted for this analysis included percent of patients in the normal range, and percent below the lower limit of normal (LLN) or above the upper limit of normal (ULN) for serum magnesium (LLN 1.8 mg/dl, ULN 2.4 mg/dl) and phosphate (LLN 2.5 mg/dl, ULN 4.8 mg/dl) at baseline and week 4. For serum calcium, in this analysis the percent of patients with albumin-adjusted central laboratory calcium values >10.2 and <8.5 mg/dl were assessed rather than >10.5 mg/dl ULN and <8.5 mg/dl LLN.
Statistical Analyses
Analyses of serum calcium, magnesium, iPTH, and 24-hour creatinine-normalized urine calcium were conducted in the safety population (n=113), which included all randomized patients who had taken at least one dose of patiromer. Analyses of serum phosphate, 24-hour creatinine-normalized urine phosphate, FGF-23, and vitamin D were conducted in the efficacy population (the safety population, excluding one patient who had a protocol deviation and no postbaseline serum potassium). The primary analysis of the TOURMALINE study demonstrated that the primary end point (serum potassium in target range 3.8–5.0 mEq/L) was achieved by similar proportions of patients who received patiromer without food and with food (11). Outcomes examined here combined the without food and with food study arms and are referred to as the overall study population. Additional analyses examined serum and urine calcium and phosphate data according to the randomized treatment groups (without food and with food study groups). All analyses are on the basis of observed cases. Unless otherwise specified, descriptive statistics were summarized as mean and SD for serum electrolytes, as mean (SD) for urine calcium and phosphate values at baseline and week 4 and median (quartile 1, quartile 3 [Q1, Q3]) for change from baseline, as median (Q1, Q3) for markers of mineral metabolism, and as proportions for categorical variables. The mean change from baseline to week 4 was examined by paired t test. For parameters that were not normally distributed (i.e., iPTH and FGF-23 change from baseline in the overall population), a Wilcoxon signed-rank sum test was used. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), with statistical significance set at P<0.05.
Results
Baseline Demographics and Clinical Characteristics
As reported previously (11), 114 patients were randomized, with 113 included in the safety population, and 112 in the efficacy population; 103 completed the study. Among the 11 patients who did not complete the study, reasons for early termination were adverse events (AEs; n=3), investigator’s decision (n=3), withdrawal by patient (n=3), lost to follow-up (n=1), and failure to disclose prohibited medications (n=1). Not all data were reported for all patients by the central laboratory because of missing laboratory data; therefore, sample sizes for each parameter are indicated.
Most patients were white (82%), men (65%), and had diabetes (82%; Table 1). The majority had hypertension (94%), and 59% were taking RAAS inhibitors. One patient in each treatment group was on the phosphate binder sevelamer; no other binders were used. Calcium-based binders were excluded medications, and no one took lanthanum carbonate. Mean (SD) age was 67 (12) years. A total of 76% of patients had CKD, with a mean (SD) eGFR of 41 (26) ml/min per 1.73 m2. At baseline, the mean (SD) serum potassium was 5.39 (0.40) mEq/L and 41% of patients had serum potassium ≥5.5 mEq/L.
Table 1.
Baseline characteristics of TOURMALINE study patients included in the efficacy population
| Parameter | Value, N=112 |
|---|---|
| Age | |
| Years, mean (SD) | 67 (12) |
| ≥65 yr, n (%) | 73 (65) |
| Men, n (%) | 73 (65) |
| Race, n (%) | |
| White | 92 (82) |
| Black | 14 (13) |
| Other | 4 (4) |
| Ethnicity, n (%) | |
| Hispanic/Latino | 63 (56) |
| Non-Hispanic/Latino | 49 (44) |
| Serum potassium, mEq/L, mean (SD) | 5.39 (0.40)a |
| <5.5, n (%) | 66 (59) |
| ≥5.5, n (%) | 46 (41) |
| eGFR, mean (SD), ml/min per 1.73 m2 | 41 (26) |
| CKD, n (%) | 85 (76) |
| eGFR≥45 ml/min per 1.73 m2 | 16 (14) |
| eGFR<45 ml/min per 1.73 m2 | 69 (62) |
| Diabetes mellitus, n (%) | 92 (82) |
| Heart failure, n (%) | 10 (9) |
| Hypertension, n (%) | 105 (94) |
| RAAS inhibitors, n (%) | 66 (59) |
| β-Blocking agents, n (%) | 58 (52) |
| Non-RAAS diuretic, n (%) | 40 (36)b |
RAAS, renin-angiotensin-aldosterone system.
Baseline serum potassium is defined as the mean of serum potassium from the central laboratory on two consecutive days (day −1 and day 1) immediately before the first dose of patiromer.
All non-RAAS diuretics were potassium wasting.
Serum Electrolyte Concentration and 24-Hour Urine Excretion
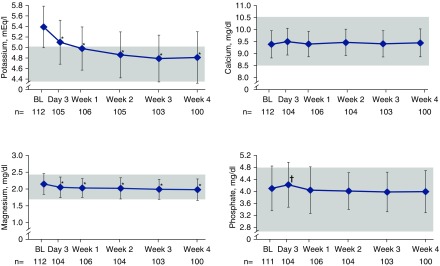
Mean serum potassium decreased during patiromer treatment (Figure 1), whereas mean (SD) albumin-corrected serum calcium and serum phosphate did not change significantly from baseline values of 9.4 (0.6) and 4.1 (0.7) mg/dl, respectively. Mean (SD) change in serum calcium at week 4 was 0.0 (0.5) mg/dl (P=0.78, paired t test; n=100), and mean (SD) change in serum phosphate was −0.1 (0.7) mg/dl (P=0.47, paired t test; n=100). Unadjusted serum calcium was similar to albumin-corrected serum calcium (Supplemental Table 1). Consistent results were observed for serum calcium and serum phosphate when the randomized treatment groups were examined separately (without food and with food treatment groups; Supplemental Table 2). Mean (SD) serum magnesium was 2.2 (0.3) mg/dl at baseline (n=112); the mean (SD) change from baseline to week 4 was −0.2 (0.2) mg/dl (P<0.001, paired t test; n=100).
Figure 1.
Mean serum levels of potassium, magnesium, serum albumin-corrected calcium, and phosphate over time. All values are mean±SD; shaded boxes represent target range for each electrolyte. *P<0.001; †P<0.05. BL, baseline.
The mean (SD) 24-hour urine collection times were 21.1 (2.9) hours (n=109) at baseline and 23.7 (2.8) hours (n=98) at week 4. Overall, mean (SD) 24-hour creatinine-normalized urine calcium was 50.8 (55.1) mg/24 h at baseline (n=73) and 58.5 (59.4) mg/24 h at week 4 (n=78; Figure 2). There was a small numerical increase from baseline to week 4; the median (Q1, Q3) change was 2.5 (−11.5, 23.7) mg/24 h (P=0.10, paired t test; n=69). The mean (SD) 24-hour urine calcium-to-creatinine ratio was 41.4 (43.8) mg/g at baseline (n=85), and demonstrated a small median (Q1, Q3) increase from baseline to week 4 of 2.1 (−10.7, 26.3) mg/g (P=0.04, paired t test; n=69). Mean urine calcium changes evaluated by both methods (24-hour creatinine-normalized urine calcium and 24-hour urine calcium-to-creatinine ratio) in the without food and with food treatment groups were nonsignificant (Supplemental Tables 3 and 4).
Figure 2.
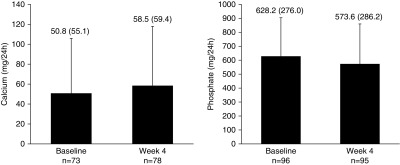
Mean 24-hour creatinine-normalized urine calcium and urine phosphate at baseline and week 4. Bars represent one-sided SD. Values above bars are mean (SD). Observed cases, no imputation.
Overall, the mean (SD) 24-hour creatinine-normalized urine phosphate was 628.2 (276.0) mg/24 h at baseline (n=96) and 573.6 (286.2) mg/24 h at week 4 (n=95; Figure 2). There was a significant decrease from baseline to week 4, with a median (Q1, Q3) change of −43.0 (−162.6, 35.7) mg/24 h (P=0.004, paired t test; n=95). The decrease from baseline in 24-hour creatinine-normalized urine phosphate was similar in the with and without food groups, but was only significant in patients randomized to the without food treatment group (Supplemental Table 3). Mean change in the 24-hour urine phosphate-to-creatinine ratio was also significant in the combined groups at week 4 (Supplemental Table 4).
Markers of Mineral Metabolism
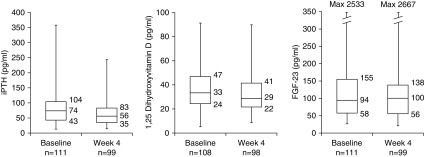
At baseline the median (Q1, Q3) iPTH level was 74 (43, 104) pg/ml (n=111; Figure 3). The median (Q1, Q3) change from baseline to week 4 was −13 (−31, 4) pg/ml (P<0.001, Wilcoxon signed-rank sum test; n=97). The iPTH reduction was numerically greater in patients receiving the 16.8 and 25.2 g once-daily doses at week 4 relative to the 8.4 g once-daily dose at week 4 (Supplemental Figure 1). Median (Q1, Q3) serum 1,25-D at baseline was 33 (24, 47) pg/ml (n=108). The median (Q1, Q3) change from baseline to week 4 was −2 (−9, 3) pg/ml (P=0.05, paired t test; n=96). Median (Q1, Q3) serum 25-D was 29 (17, 41) ng/ml at baseline (n=109). Median (Q1, Q3) change in 25-D at week 4 was −1 (−3, 2) ng/ml (P=0.49, paired t test; n=98). Median (Q1, Q3) serum levels of FGF-23 were 94 (58, 155) pg/ml at baseline (n=111) and 100 (56, 138) pg/ml at week 4 (n=99); the median (Q1, Q3) change from baseline was 3 (−27, 21) pg/ml (P=0.62, Wilcoxon signed-rank sum test; n=98). Similarly, FGF-23 did not change significantly in patients with CKD or hyperphosphatemia (Supplemental Table 5).
Figure 3.
Median values for serum markers of mineral metabolism at baseline and week 4. Boxes represent median (center line), Q1 (lower bound), and Q3 (upper bound). Whiskers represent minimum and maximum values. Observed cases, no imputation. Max, maximum.
Patients with Hyperphosphatemia or Low eGFR
There was a significant (P<0.001) effect modification of the change in serum phosphate at week 4 by baseline serum phosphate. In the subgroup of patients with baseline hyperphosphatemia (n=16), there were clinically relevant reductions in serum and 24-hour creatinine-normalized urine phosphate (Table 2). In patients with hyperphosphatemia, mean (SD) serum phosphate was 5.3 (0.4) mg/dl at baseline (n=16) and decreased into the normal range to 4.7 (0.9) mg/dl at week 4 (n=13); the mean (SD) decrease from baseline to week 4 was 0.6 (0.8) mg/dl (n=13). In patients with baseline serum phosphate ≤4.8 mg/dl (Table 2), mean (SD) serum phosphate was 3.9 (0.6) mg/dl at baseline (n=95) and 3.9 (0.6) mg/dl at week 4 (n=87). In the subgroup with hyperphosphatemia, the mean (SD) baseline urine phosphate-to-creatinine ratio was 574.1 (229.0) mg/g at baseline (n=14). The mean (SD) change in urine phosphate-to-creatinine ratio from baseline at week 4 was −140.2 (186.4) mg/g (n=9). Mean (SD) serum magnesium decreased by 0.1 (0.1) mg/dl in this subgroup (Table 2).
Table 2.
Electrolyte serum concentrations and 24-hour creatinine-normalized urine excretion at baseline and week 4 in patients with baseline serum phosphate >4.8 mg/dl (hyperphosphatemia) and baseline serum phosphate ≤4.8 mg/dl
| Parameter | Baseline | Week 4 | Change from Baseline to Week 4 | |||
|---|---|---|---|---|---|---|
| Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | |
| Patients with baseline serum phosphate >4.8 mg/dl | ||||||
| Serum electrolytes, mg/dl | ||||||
| Calciuma | 9.1 (0.5) | 16 | 9.3 (0.3) | 13 | 0.1 (0.4) | 13 |
| Magnesium | 2.3 (0.4) | 16 | 2.1 (0.4) | 13 | −0.1 (0.1) | 13 |
| Phosphate | 5.3 (0.4) | 16 | 4.7 (0.9) | 13 | −0.6 (0.8) | 13 |
| 24-h creatinine-normalized urine electrolytes, mg/24 h | ||||||
| Calcium | 30.5 (19.9) | 6 | 32.3 (20.0) | 6 | 1.8 (12.3) | 6 |
| Phosphate | 722.8 (331.9) | 9 | 573.7 (304.7) | 9 | −149.1 (162.6) | 9 |
| Patients with baseline serum phosphate ≤4.8 mg/dl | ||||||
| Serum electrolytes, mg/dl | ||||||
| Calciuma | 9.4 (0.6) | 95 | 9.5 (0.6) | 87 | 0.0 (0.5) | 87 |
| Magnesium | 2.1 (0.3) | 95 | 2.0 (0.3) | 87 | −0.2 (0.2) | 87 |
| Phosphate | 3.9 (0.6) | 95 | 3.9 (0.6) | 87 | 0.0 (0.6) | 87 |
| 24-h creatinine-normalized urine electrolytes, mg/24 h | ||||||
| Calcium | 52.7 (56.9) | 67 | 60.7 (61.2) | 72 | 9.3 (44.6) | 63 |
| Phosphate | 604.8 (277.5) | 94 | 573.6 (286.1) | 86 | −49.9 (194.4) | 86 |
Serum albumin-corrected calcium performed according to the following: if serum albumin ≥4.0 g/dl, corrected calcium=serum calcium; if serum albumin <4.0 g/dl, corrected calcium=serum calcium+(0.8×[4−serum albumin]). For calculations, calcium units were milligrams per deciliter and albumin units were grams per deciliter.
There were no clinically relevant changes in mean serum calcium and 24-hour creatinine-normalized urine calcium from baseline to week 4 (Table 2). The mean (SD) urine calcium-to-creatinine ratio was 25.4 (26.6) mg/g at baseline (n=10), with a change at week 4 of 2.4 (14.8) mg/g (n=6). Mean iPTH levels were numerically higher in patients with hyperphosphatemia compared with patients who had baseline serum phosphate ≤4.8 mg/dl; the mean (SD) change from baseline to week 4 was −21 (50) pg/ml in this subgroup of patients (Supplemental Table 5).
There was a significant (P=0.02) effect modification of the change in serum phosphate at week 4 by baseline eGFR <45 ml/min per 1.73 m2. In patients with baseline eGFR <45 ml/min per 1.73 m2 (n=72), mean (SD) serum phosphate was 4.3 (0.7) mg/dl at baseline and 4.0 (0.8) mg/dl at week 4; the mean (SD) change at week 4 was −0.3 (0.6) (P=0.002, paired t test; n=62).
Safety
Albumin-adjusted central laboratory serum calcium values >10.2 mg/dl were observed in 9.8% of patients before patiromer treatment and in 9.4%, 5.8%, 5.8%, and 9.0% of patients at week 1 to week 4, respectively. Of the ten patients with calcium values >10.2 mg/dl at baseline, seven had serum calcium ≤10.2 mg/dl by week 4 (Supplemental Table 6). As reported previously, no patients had AEs of hypocalcemia or hypercalcemia (11).
No patients had hypophosphatemia (<2.5 mg/dl) during the treatment period in the study. An elevation of serum phosphate (defined as serum phosphate above the ULN [4.8 mg/dl]) was observed in 14.2% of patients before patiromer treatment and in 11.5%, 8.8%, 6.2%, and 10.6% of patients at week 1 to week 4, respectively. Of the 15 patients with serum phosphate above the ULN at baseline (who also had at least one postbaseline serum phosphate assessment), nine had serum phosphate in the normal range by week 4 (Supplemental Table 6).
Eleven patients with serum magnesium in the normal range at baseline had values below the LLN (1.8 mg/dl) at week 4 (Supplemental Table 6). In addition, 11 patients with low serum magnesium at baseline remained low at week 4. As previously reported, serum magnesium <1.4 mg/dl occurred in five patients, four of whom had serum magnesium <1.8 mg/dl at baseline; two were reported as moderate hypomagnesemia AEs and patiromer was continued without a dose change, and the AEs were resolved with over-the-counter magnesium replacement (11). In addition, four patients received magnesium supplements since the beginning of the study and none had abnormally low serum magnesium.
Discussion
In this analysis we show that in the overall population, 24-hour creatinine-normalized urine phosphate excretion decreased significantly, consistent with phosphate binding in the gastrointestinal tract by some of the calcium released when patiromer binds to potassium. The magnitude of the decrease, about 8% in this population of patients, most of whom had mild-to-moderate CKD (mostly with normal serum phosphate at baseline), was about a third of the 22% reported by Block et al. (13) in a similar population where phosphate binders were given three times per day with meals and uptitrated in an attempt to achieve a significant reduction in serum phosphate. The decrease in serum and urine phosphate in the small number of patients with hyperphosphatemia in this study is consistent with previous patiromer studies in both healthy volunteers evaluating net balance showing a reduction in urine phosphate (14), and in patients with CKD requiring both potassium and phosphate binders (15). In healthy volunteers, treatment with patiromer leads to an increase in fecal phosphate elimination, which likely occurs via binding of patiromer-released calcium to intestinal phosphate (14).
The small decrease in iPTH along with a decrease in serum 1,25-D levels suggests that some of the calcium released by patiromer is absorbed. The overall low urine calcium excretion is consistent with all studies in CKD (16). Prior studies of patiromer in healthy volunteers have shown that on the highest commercially available daily dose (25.2 g/d) about 73 mg of elemental calcium are absorbed daily (14). The recommended calcium intake in patients with CKD has not been well defined (17); however, recent data suggests that net calcium balance should be neutral throughout the stages of CKD (18). The limited data available also suggests that a dietary intake of 800–1200 mg of elemental calcium per day (the recommended amount in healthy adults) achieves neutral balance on average in patients with CKD (19–21). Excessive calcium intake has been shown to shift patients into positive calcium balance (19,20) and may promote vascular and soft tissue calcification (22–25), whereas inadequate calcium intake may worsen secondary hyperparathyroidism (18). In this study we did not measure dietary calcium intake and we are therefore unable to determine the effect of patiromer on net calcium balance. The long-term effect of the increase in calcium absorption, phosphate binding, and change in iPTH and 1,25-D on bone mineral metabolism and vascular calcification cannot be assessed in this short-term study. Furthermore, calcium balance needs to be evaluated on an individual patient basis with an understanding of overall calcium intake, vitamin D status, and mineral requirements.
As reported previously (11), some patients had low serum magnesium or AEs of hypomagnesemia, consistent with previous studies (3–5) and with the potential binding of cationic magnesium to patiromer. The magnitude of the serum magnesium decrease was similar to that seen in earlier studies (3,5,14,26). During the 2-week safety follow-up period after the last dose of patiromer, mean serum magnesium returned to baseline values. Other AEs in the TOURMALINE study were also similar to the known AE profile of patiromer.
A strength of this analysis is that the main outcomes reported here were prespecified in the statistical analyses plan, and thus the data were collected in a uniformly defined patient population and analyzed according to a predetermined protocol. Furthermore, an independent central laboratory analyzed all samples collected from TOURMALINE study centers, and thus the data are derived from a consistent application of sample procedures and detection assays.
There are several limitations of this study that affect our ability to fully understand the effects of patiromer on markers of bone and mineral metabolism. The TOURMALINE study was not powered to examine changes in markers of mineral metabolism, and the data in patients with hyperphosphatemia were from a small subset (n=16). In addition, the mean baseline urine collection time was 21.1 hours because of protocol procedures that may have introduced a systemic bias. However, the 24-hour creatinine-normalized values and the electrolyte-to-creatinine ratios give similar results. This study is not a balance study, and therefore we are unable to quantitate the net absorption of the calcium released when patiromer binds to potassium. Serum calcium is not a biomarker of calcium balance and cannot be utilized to measure net absorption or retention. Patients with CKD have a marked reduction of urine calcium excretion such that it does not reflect intake or absorption (16). Therefore, we are unable to determine if the changes in iPTH and 1,25-D were a reflection of changes in dietary intake of calcium or phosphorus or were due to the calcium released from patiromer in binding to potassium, and whether the changes in these markers reflected any overall change in calcium balance, either in a favorable or unfavorable direction. The TOURMALINE study did not have a placebo control arm. Thus, the observed differences in iPTH, 1,25-D, and phosphate may have been from treatment with patiromer or from other causes.
In conclusion, patiromer lowered urine phosphate in all patients in this study, and lowered both serum and urine phosphate in patients with hyperphosphatemia. There was no change in serum calcium levels or the 24-hour creatinine-normalized urine calcium. iPTH, 1,25-D, and serum magnesium decreased. When patiromer binds to potassium in the gastrointestinal tract, calcium is released (3,5,11,14). Our findings suggest that some of the released calcium binds to intestinal phosphate, lowering urine phosphate in all patients and lowering both serum and urine phosphate in patients with hyperphosphatemia. Although there were no AEs of hypercalcemia, some of the calcium may be absorbed, which could explain the decrease in iPTH and 1,25-D. Some of the calcium in patiromer appears to exchange for magnesium in the gastrointestinal tract, thereby lowering serum magnesium. Although this study is consistent with other studies evaluating the effects of patiromer on urine and serum calcium, magnesium, and phosphate, further studies will be necessary to determine if treatment of hyperkalemia with patiromer has any influence on the net balance of calcium, magnesium, and phosphate.
Disclosures
D.A.B. reports personal fees from and past stock ownership of Relypsa, Inc., a Vifor Pharma Group Company; personal fees outside this work from Amgen, Sanofi/Genzyme, and Tricida; and stock outside of this work in Amgen and Tricida. He reports research support from the National Institutes of Health and from the Renal Research Institute outside of this work. D.M.S., J.Y., S.W., and J.F. are employees of Relypsa, Inc., a Vifor Pharma Group Company. P.E.P. reports receiving honoraria from Akebia, Astra-Zeneca, Keryx, Reata, and ExThera; and reports serving as a consultant or participating in advisory boards for Akebia, Vifor, and Keryx. As the principal investigator for many pharmaceutical companies, his institution has received research support.
Supplementary Material
Acknowledgments
Writing and editorial support services were provided by Impact Communication Partners, Inc., and funded by Relypsa, Inc., a Vifor Pharma Group Company.
This study was sponsored and funded by Relypsa, Inc., a Vifor Pharma Group Company.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04500418/-/DCSupplemental.
References
- 1.US Food and Drug Administration: Veltassa® (patiromer): Prescribing information, 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205739s016lbl.pdf. Accessed October 9, 2018
- 2.European Medicines Agency: Veltassa® (patiromer), 2017. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/004180/human_med_002141.jsp&mid=WC0b0. Accessed October 9, 2018
- 3.Bakris GL, Pitt B, Weir MR, Freeman MW, Mayo MR, Garza D, Stasiv Y, Zawadzki R, Berman L, Bushinsky DA; AMETHYST-DN Investigators : Effect of patiromer on serum potassium level in patients with hyperkalemia and diabetic kidney disease: The AMETHYST-DN randomized clinical trial. JAMA 314: 151–161, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Pitt B, Bakris GL, Bushinsky DA, Garza D, Mayo MR, Stasiv Y, Christ-Schmidt H, Berman L, Weir MR: Effect of patiromer on reducing serum potassium and preventing recurrent hyperkalaemia in patients with heart failure and chronic kidney disease on RAAS inhibitors. Eur J Heart Fail 17: 1057–1065, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir MR, Bakris GL, Bushinsky DA, Mayo MR, Garza D, Stasiv Y, Wittes J, Christ-Schmidt H, Berman L, Pitt B; OPAL-HK Investigators : Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 372: 211–221, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Sanofi-Aventis: Kayexalate (sodium polystyrene sulfonate) [Package Insert], 2014. Available at: http://products.sanofi.ca/en/kayexalate.pdf. Accessed October 9, 2018
- 7.Arcand J, Ivanov J, Sasson A, Floras V, Al-Hesayen A, Azevedo ER, Mak S, Allard JP, Newton GE: A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: A prospective follow-up study. Am J Clin Nutr 93: 332–337, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Appel LJ, Whelton PK: Sodium intake and all-cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol 68: 1609–1617, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK: Long term effects of dietary sodium reduction on cardiovascular disease outcomes: Observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334: 885–888, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon EJ, Bauer JD, Hawley CM, Isbel NM, Stowasser M, Johnson DW, Campbell KL: A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 24: 2096–2103, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pergola PE, Spiegel DM, Warren S, Yuan J, Weir MR: Patiromer lowers serum potassium when taken without food: Comparison to dosing with food from an open-label, randomized, parallel group hyperkalemia study. Am J Nephrol 46: 323–332, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 13.Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM: Effects of phosphate binders in moderate CKD. J Am Soc Nephrol 23: 1407–1415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bushinsky DA, Spiegel DM, Gross C, Benton WW, Fogli J, Hill Gallant KM, Du Mond C, Block GA, Weir MR, Pitt B: Effect of patiromer on urinary ion excretion in healthy adults. Clin J Am Soc Nephrol 11: 1769–1776, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushinsky DA, Rossignol P, Spiegel DM, Benton WW, Yuan J, Block GA, Wilcox CS, Agarwal R: Patiromer decreases serum potassium and phosphate levels in patients on hemodialysis. Am J Nephrol 44: 404–410, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E: Mineral metabolism parameters throughout chronic kidney disease stages 1-5–achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium : Dietary Reference Intakes for Calcium and Vitamin D, edited by Ross AC, Taylor CL, Yaktine AL, Del Valle HB, Washington, DC, National Academies Press, 2011 [PubMed] [Google Scholar]
- 18.Hill Gallant KM, Spiegel DM: Calcium balance in chronic kidney disease. Curr Osteoporos Rep 15: 214–221, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M: Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int 83: 959–966, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel DM, Brady K: Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int 81: 1116–1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bushinsky DA: Contribution of intestine, bone, kidney, and dialysis to extracellular fluid calcium content. Clin J Am Soc Nephrol 5[Suppl 1]: S12–S22, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Yang H, Curinga G, Giachelli CM: Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int 66: 2293–2299, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Giachelli CM: Vascular calcification mechanisms. J Am Soc Nephrol 15: 2959–2964, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM: Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ Res 109: 697–711, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ; PEARL-HF Investigators : Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J 32: 820–828, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.