Abstract
Background and objectives
The prevalence of patients with ESKD who receive extracorporeal kidney replacement therapy is rising worldwide. We compared government reimbursement for hemodialysis and peritoneal dialysis worldwide, assessed the effect on the government health care budget, and discussed strategies to reduce the cost of kidney replacement therapy.
Design, setting, participants, & measurements
Cross-sectional global survey of nephrologists in 90 countries to assess reimbursement for dialysis, number of patients receiving hemodialysis and peritoneal dialysis, and measures to prevent development or progression of CKD, conducted online July to December of 2016.
Results
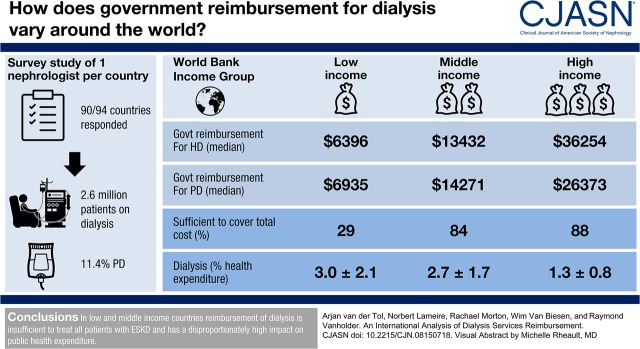
Of the 90 survey respondents, governments from 81 countries (90%) provided reimbursement for maintenance dialysis. The prevalence of patients per million population being treated with long-term dialysis in low- and middle-income countries increased linearly with Gross Domestic Product per capita (GDP per capita), but was substantially lower in these countries compared with high-income countries where we did not observe an higher prevalence with higher GDP per capita. The absolute expenditure for dialysis by national governments showed a positive association with GDP per capita, but the percent of total health care budget spent on dialysis showed a negative association. The percentage of patients on peritoneal dialysis was low, even in countries where peritoneal dialysis is better reimbursed than hemodialysis. The so-called peritoneal dialysis–first policy without financial incentive seems to be effective in increasing the utilization of peritoneal dialysis. Few countries actively provide CKD prevention.
Conclusions
In low- and middle-income countries, reimbursement of dialysis is insufficient to treat all patients with ESKD and has a disproportionately high effect on public health expenditure. Current reimbursement policies favor conventional in-center hemodialysis.
Keywords: government reimbursement for dialysis services, hemodialysis, peritoneal dialysis, worldwide survey, dialysis health economic, chronic dialysis, prevention CKD, prevalence of dialysis
Visual Abstract
Introduction
When CKD progresses to ESKD, survival and quality of life are usually maintained by kidney replacement therapy (KRT), which includes hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation. In suitable patients, transplantation offers improved quality of life, longer survival, and lowest costs as compared with dialysis (1). However, most patients in need of KRT are treated with dialysis due to deficiencies in infrastructure, a scarcity of donor organs, and contraindications to transplantation (2,3). Even in those countries most active in transplantation, about 30%–50% of patients in need of KRT depend on dialysis (4,5). In most countries the proportion of patients treated with dialysis is much higher, and in some—especially low- and middle-income countries—transplantation is available so rarely that patients depend entirely on dialysis (5–7). Moreover, in countries where government reimbursement for dialysis is unavailable or limited, patients contribute a substantial amount of their own resources for dialysis care (8,9), or have to refrain from treatment for financial reasons. Thus, many dialysis candidates worldwide remain untreated and presumably die prematurely (7).
Many high-income countries have a static or even decreasing incidence of KRT (10). By contrast, in lower-income countries, the incidence of KRT is rising dramatically, both because of the increasing prevalence of noncommunicable diseases that lead to CKD (7,11), and increased capacity to pay for KRT as these countries’ economies grow. Allocation of health care resources in these countries is often faced with equally urgent competing priorities, so an accurate understanding of the strategies different governments use to finance dialysis services could help in formulating appropriate health care policies.
Some international socio-economic assessments of average dialysis costs have been published (12,13), but no comprehensive worldwide comparison of national government reimbursement strategies for dialysis care has been done using data collected at a particular moment in time to provide a snapshot view. Furthermore, most previously published analyses have included data from a period before extracorporeal KRT became more accessible in some low- and middle-income countries (14), and before the last large worldwide economic crisis (2007–2008) imposed health care rationing even in high-income countries (15). The Global Kidney Health Atlas was developed to assess worldwide approaches to care for CKD, but as far as we know has not reported on absolute amounts of dialysis reimbursement (2).
This study surveyed government reimbursement fees for HD and PD, criteria that are used to reimburse dialysis, incentives for autodialysis, measures to prevent development or progression of CKD, and the prevalence of dialysis per country. We aimed to provide possible solutions for policy makers to help make dialysis reimbursement and organization more equitable and more sustainable by cost-saving strategies, such as PD-first policies (financial or policy stimuli designed to increase the numbers of patients who start KRT with PD rather than HD). Costs for PD are generally lower than those of HD (3,13,16); PD requires less infrastructure and personnel than in-center HD and can be provided to patients who live long distances from dialysis centers (13).
Materials and Methods
We undertook an online survey of the heads of nephrology divisions of university hospitals from 94 countries worldwide, to assess health system payment policies and government reimbursement for dialysis. One of the authors (R.V.) personally invited contacts made through the European Renal Association and European Dialysis and Transplant Association, European Renal Best Practice network, and continued medical education activities, whom we considered opinion leaders, to reply to a questionnaire with open-ended questions about financial regulation and organization of dialysis in their respective countries (Table 1). We selected one person per country, on the basis of their position in the nephrologic society of their country or one of the large international nephrologic associations. The questionnaire was developed from a previous survey that we used to compare dialysis reimbursement in seven countries, with additional questions developed from our work in the European Kidney Health Alliance (17,18). All financial data were expressed in US$ or converted from Euro or local currencies into US$ by conversion factors that were applicable on October 1, 2016 (i.e., the middle of the observation period), by use of a web-based converter (xe.com). All data were checked independently by two of the authors (A.v.d.T. and R.V.) to see whether the amounts spent were within the same range as those in nearby countries and countries with a similar economic status. When responses were unclear, we contacted the survey respondent by Email for clarification and corrected the information in the database.
Table 1.
Questionnaire
| (1) Is in your country only dialysis for acute kidney failure reimbursed, or is there also reimbursement for chronic (end-stage) kidney failure? |
| (2) Is reimbursement foreseen for all valid candidates, or only for selected groups, and in that case, which? |
| (3) Does the reimbursement system stimulate certain strategies like e.g., PD? |
| (4) Is specific reimbursement foreseen for less labor-intensive dialysis strategies like home HD or self-care (low-care, autodialysis)? |
| (5) Can you give a rough indication of the reimbursement fee for one hospital HD session and for 1 d of standard PD (continuous ambulatory PD)? Please provide the currency. |
| (6) Is this sufficient to cover costs? |
| (7) Is there any form of payment if certain quality parameters are reached (e.g., a given threshold Kt/V or hemoglobin, the use of AV-fistula), or a restriction of reimbursement for the opposite? Please specify which parameters. |
| (8) Is your government taking any initiative to stimulate prevention of CKD, and in that case which ones? |
| (9) How many patients in your country are on dialysis (HD+PD) and how many inhabitants are there? |
Because standard HD in many countries is done in three sessions per week, we calculated the annual cost for reimbursement of HD sessions per patient by multiplying the amount refunded for one public-sector hospital HD session by 156 (i.e., three times 52). We did not include reimbursement for home HD, or additional reimbursement for specific strategies such as online hemodiafiltration, in our analyses. We calculated annual reimbursement for PD per patient by multiplying the amount of reimbursement for 1 day of continuous ambulatory PD by 365. We did not include reimbursement for specific PD modalities such as automated PD. In some low- and lower-middle–income countries, where government reimbursement for dialysis is on the basis of a more limited package per year (e.g., the Philippines, where public provision of HD is limited to 90 sessions per patient per year), we calculated costs accordingly. We calculated each country’s prevalence of dialysis provision (PD and HD) by dividing the total number of patients receiving dialysis treatment by the country’s per million population on the basis of the United Nations population list per country (19). We calculated government reimbursement for dialysis for each responding country as the sum of ([number of patients receiving PD×annual reimbursement for PD]+[number of patients receiving HD×reimbursement for HD]). We used World Bank data for 2016 for public health expenditure as a percentage of Gross Domestic Product (GDP) (20). We calculated the percentage of public health expenditure on dialysis provision (PD and HD) per country by (number of patients receiving PD times annual reimbursement for PD+number of patients receiving HD times annual reimbursement for HD)/(public health expenditure in %GDP×GDP).
Countries were classified according to income groups defined by the World Bank according to Gross National Income per capita (2016) as low-income countries (<US$1025), lower-middle–income countries (US$1026–US$4035), upper-middle–income countries (US$4036–US$12,475), and high-income countries (>US$12,476) (21). We grouped low- and lower-middle–income countries together for our analysis because Burundi and Senegal were the only low-income countries that provided data.
We calculated the HD/PD reimbursement ratio per country, in order to compare the costs to government of both HD and PD independently of exchange rates. We analyzed both nonfinancial and financial incentives that governments offered to KRT providers to attempt to increase use of PD. Because in most countries reimbursement for PD is lower than reimbursement for HD, we defined financial incentives aimed to increase use of PD as reimbursement for PD that was >110% of HD reimbursement per patient per year.
We defined screening for CKD as initiatives that were designed to detect patients with reduced eGFR or presence of proteinuria/albuminuria, and divided countries’ strategies for CKD prevention into the following categories: absence of screening or prevention strategies; unspecified screening or prevention strategies; screening for CKD in high-risk populations in primary care; pay-for-performance by governments to general practitioners for achievement of targets in CKD prevention; and awareness campaigns highlighting the association between lifestyle behaviors and CKD aimed at the general population, the population at risk for CKD, or health professionals.
Statistical Analyses
Descriptive statistics for all numeric variables, including means, medians, SD, and minimum and maximum values, were calculated in Microsoft Excel. SPSS 24 software was used to perform the t test to compare independent group means. When the continuous variables were not normally distributed, the Mann–Whitney U test was used. When more than two groups were compared, a one-way ANOVA and post hoc Scheffé test were applied. SPSS 24 software was used to calculate Spearman rho correlations between two parameters. Statistical significance was accepted at P<0.05. Chart builder (SPSS 24) and Excel software were used to plot graphs.
Results
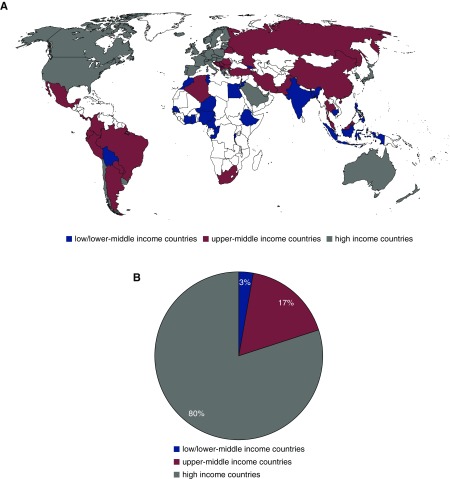
Between July 1, 2016, and Dec 31, 2016, 90 respondents answered 94 invitations, representing approximately 6.0 billion inhabitants (81% of the global population): eight in North America, eight in South America, 38 in Europe, 19 in Asia, 15 in Africa, and two in Oceania (Figure 1, Supplemental Table 1).
Figure 1.
Proportion of total amount (57 billion US$) of dialysis reimbursement (number of patient times annual funding) in low- and lower-middle, upper-middle and high-income countries. (A) Worldwide distribution of responding countries; blue: low- and lower-middle–income countries, red: upper-middle–income countries, gray: high-income countries (representing 81% of global population). (B) Proportion of reimbursement spent for HD and PD (number of patients times annual funding) in low- and lower-middle– (blue), upper-middle– (red), and high-income countries (gray).
Respondents from nine countries (Cambodia, Democratic Republic Congo, Ethiopia, Ghana, Ivory Coast, Niger, Nigeria, Pakistan, and Rwanda) reported that patients pay out of pocket (around the equivalent of US$25–US$250) for one HD session, or US$24–US$50 for 1 day of PD without government reimbursement, so we did not include their data. In some countries—e.g., China, Cameroon, and Bangladesh—all patients have to pay out of pocket a variable fraction of the total cost of their dialysis care. In countries such as India, Burundi, Mexico, and Malaysia, some patients’ dialysis is reimbursed by the government (e.g., for civil servants), is provided by nongovernmental organizations, or all costs for their dialysis are paid out of pocket. For reimbursement analysis we used data from 81 countries (Supplemental Table 2). For analysis of CKD prevention strategies, we used data from all 90 respondent countries. Our results suggest that approximately 2,600,000 patients are on dialysis worldwide (11.3% of these on PD), for which total government expenditure amounts to US$57.1 billion per year (US$4.8 billion for PD) (Table 2).
Table 2.
Results of survey of government reimbursement of dialysis by World Bank income group
| Low-income Countries (n=14) | Middle-income Countries (n=26) | High-income Countries (n=41) | All (n=81) | |
|---|---|---|---|---|
| 2615±1275a | 7532±2863a | 35,168±17,152 | 20,671±19,256 | |
| GDP per Capita (US$) | ||||
| Hemodialysis, per patient | ||||
| Mean±SD | 8198±5374a | 17,377±17,406a | 42,113±22,045 | 28,311±23,445 |
| Median | 6396 | 13,432 | 36,254 | 19,812 |
| Interquartile | 9111 | 9083 | 37,290 | 30,315 |
| Min | 1560 | 5070 | 12,363 | 1560 |
| Max | 18,720 | 93,600 | 89,958 | 93,600 |
| Peritoneal Dialysis, per patient | ||||
| Mean±SD | 9281±6510a | 14,942±8370a | 33,340±21,013 | 24,073±19,077 |
| Median | 6935 | 14,271 | 26,373 | 19,284 |
| Interquartile | 6172 | 12,514 | 24,214 | 17,990 |
| Min | 2508 | 2346 | 10,220 | 2346 |
| Max | 24,617 | 41,029 | 99,280 | 99,280 |
| Sufficient to cover total cost as reported by Director of Nephrology (%) | 4 of 14 (29)b | 21 of 25 (84) | 36 of 41 (88) | 61 of 80 (76) |
| Prevalence of dialysis, per million population | ||||
| Mean±SD | 312±283c | 556±276 | 762±500 | 612±436 |
| Median | 280 | 561 | 633 | 544 |
| Interquartile | 398 | 378 | 487 | 417 |
| Min | 2 | 110 | 198 | 2 |
| Max | 823 | 1155 | 2553 | 2553 |
| Hemodialysis patients, N | 286,708 | 890,446 | 1,173,115 | 2,350,269 |
| Peritoneal Dialysis patients, N | 14,975 | 180,308 | 107,526 | 302,809 |
| Peritoneal Dialysis in overall dialysis population, %c | 3.3±3.7d | 12.7±14.5 | 13.2±12.3 | 11.3±12.5 |
| Total Hemodialysise | 1505 | 8706 | 42,069 | 52,280 |
| Total Peritoneal Dialysise | 30 | 1281 | 3487 | 4798 |
| N countries funding home and self-care hemodialysis (%) | 0 of 14a | 3 of 26 (12)a | 20 of 41 (49) | 23 of 81 (28) |
| Health expenditure (%GDP) | 2.4±1.5b | 4.3±1.3a | 6.5±2.1 | 5.0±2.3 |
| Dialysis (%health expenditure on dialysis funding) | 3.0±2.1d | 2.7±1.7d | 1.3±0.8 | 2.1±1.6 |
All GDP and cost data are provided in US dollars. Min, lowest amount; Max, highest amount.
P<0.001 compared with High.
P<0.001 compared with Middle and High.
Percentage of PD patients among total dialysis population.
P<0.05 compared with High.
Total amount of reimbursement spent for HD and PD (no. of patients × annual funding) in million US$.
National Reimbursement of KRT as Related to GDP per Capita
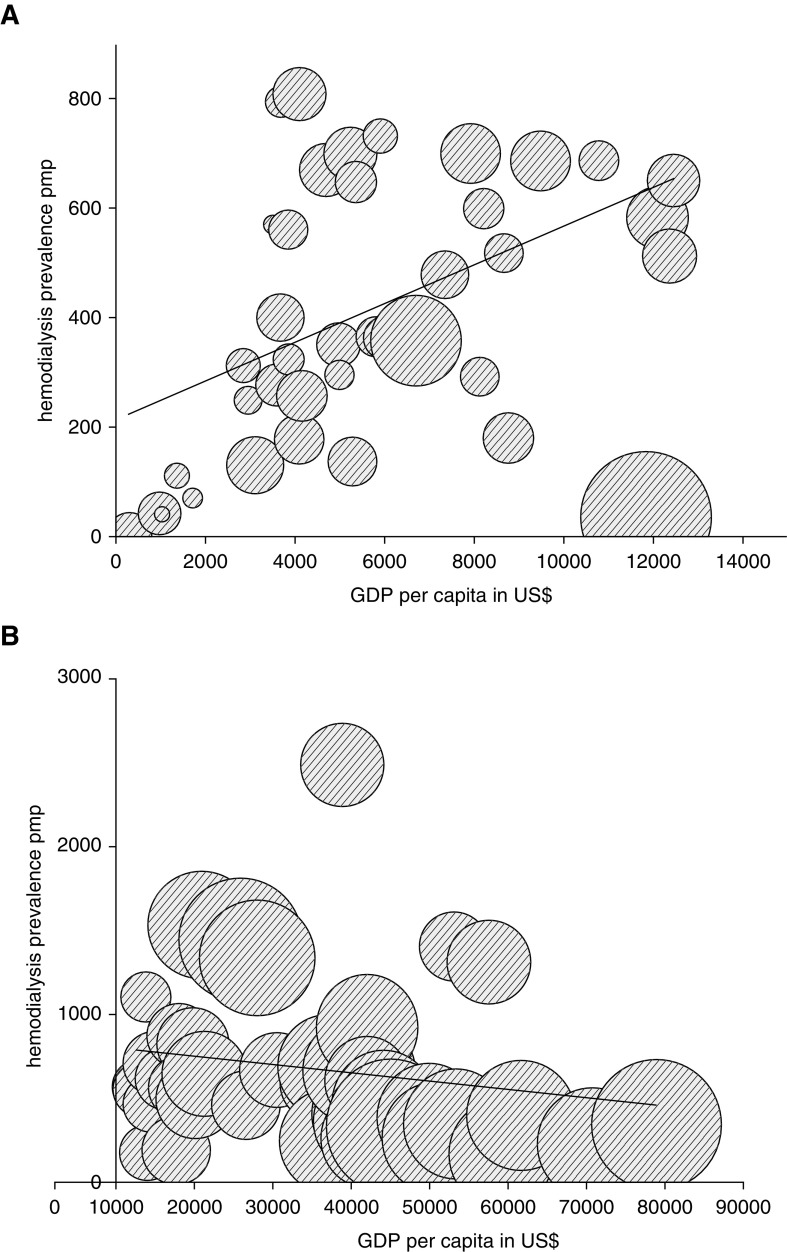
Analysis of government reimbursement for dialysis according to countries’ World Bank income groups (Figure 2A) showed a correlation between prevalence of HD and national GDP per capita in low-income countries and middle-income countries (Spearman rho, 0.49; n=40; P=0.001), but this association was not seen in high-income countries (Spearman rho, −0.29; n=41; P=0.06) (Figure 2B). An association was found between prevalence of PD use and national GDP per capita in low-income countries and middle-income countries (Spearman rho, 0.57; n=36; P<0.001), but this association was not seen in high-income countries (Spearman rho, 0.17; n=40; P=0.28).
Figure 2.
Prevalence of patients receiving HD increases with GDP per capita in low- and middle-income countries. (A) Association between GDP per capita (x axis) in US$ and prevalence of patients receiving HD per million population (y axis) in low-income countries, lower-middle–income countries, and upper-middle–income countries (Spearman rho, 0.49; n=40; P<0.001). The size of the bubble represents degree of government funding for HD in that country. Reimbursement for HD increases with GDP per capita (Spearman rho, 0.49; n=40; P<0.001). The difference in prevalence of HD for a comparable GDP per capita is not explained by differences in reimbursement. (B) Prevalence of patients receiving HD is independent of GDP per capita in high-income countries. Association between GDP per capita (x axis) in US$ and prevalence of patients receiving HD per million population (y axis) in high-income countries (Spearman rho, 0.29; n=41; P=0.06). The size of the bubble represents degree of government funding for HD in that country. Reimbursement for HD increases with GDP per capita (Spearman rho, 0.65; n=41; P<0.001). pmp, prevalence rate per million population.
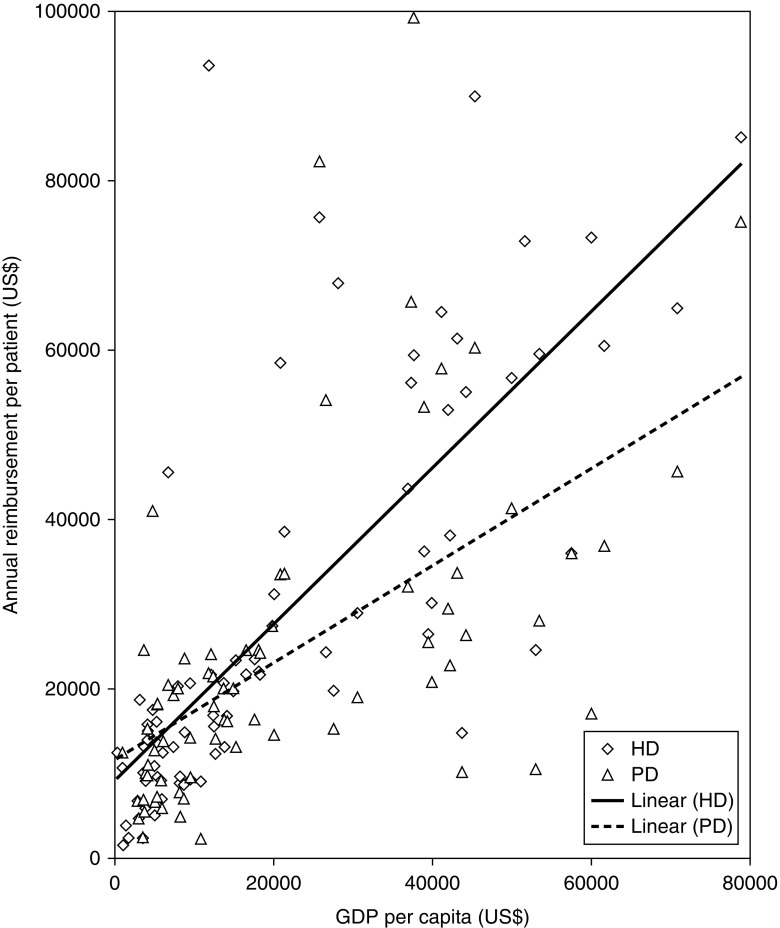
Reimbursement per annum by government positively associated with GDP per capita both for HD (Spearman rho, 0.83; n=8176; P<0.001) and for PD (Spearman rho, 0.67; n=73; P<0.001) (Figure 3). When asked whether government reimbursement was sufficient to cover the costs of all dialysis sessions for each patient treated in their unit, respondents in 76% of countries (61 of 80) found government reimbursement sufficient, but this proportion was substantially lower in lower-middle– and low-income countries (Table 2). Many countries do not reimburse for home HD or self-care HD (Table 2). In nine of the surveyed countries, government reimbursement depends in part on surrogate measures for clinical or dialysis quality, such as hemoglobin or Kt/Vurea (Supplemental Table 3). Although the high-income countries spent a higher proportion of their national GDP on public health expenditure (6.5%) than upper-middle–income countries (4.3%), and lower-middle–income and low-income countries together (2.4%), high-income countries spent a smaller proportion of their public health expenditure on reimbursement for dialysis care than upper-middle–income countries, or lower-middle–income and low-income countries combined (1.3% versus 2.7% and 3.0%, respectively) (Figure 4, Table 2).
Figure 3.
Association between GDP per capita (x axis) in US$ and annual reimbursement for HD (diamonds) and PD (triangles) per patient year (y axis) in US$. The association of reimbursement for HD and GDP per capita was stronger (Spearman rho, 0.83; n=81; P<0.001, straight line) than for PD and GDP per capita (Spearman rho, 0.67; n=73; P<0.001, dotted line).
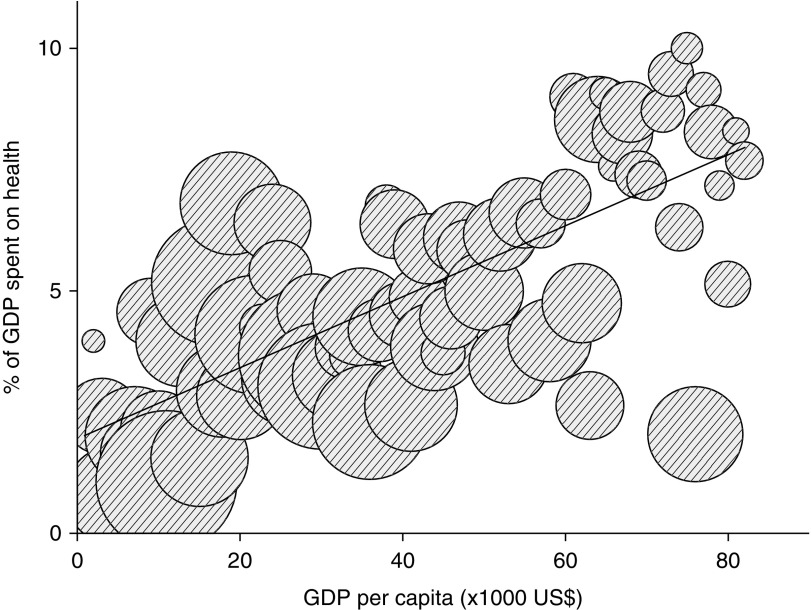
Figure 4.
Association between GDP per capita (x axis) in US$ and percentage of GDP spent on public health expenditure (y axis) (Spearman rho, 0.70; n=76; P<0.001). Bubble size is percentage of public health expenditure spent on dialysis (Spearman rho, −0.50; n=76; P<0.001). Countries with a higher income spend a higher percentage of their GDP to publicly funded health care while spending a lower percentage of their public health expenditure to fund dialysis than countries with lower income.
PD-First Policy
Countries that have a nonfinancial PD-first policy (Denmark, Hong Kong, Latvia, Malaysia, Mexico, Philippines, South-Africa, and Thailand) reported a higher prevalence and percentage of patients on PD than countries that do not have such a policy, with lower total dialysis costs for governments (Table 3).
Table 3.
Financial and policy incentives to increase PD use
| Incentive | PD-First Policy (n=8) | Financial to Hospitals (n=23) | No Incentive (n=50) |
|---|---|---|---|
| Prevalence of PD (pmp) | 202±215a | 38±23 | 71±65 |
| Prevalence of HD (pmp) | 435±327 | 601±472 | 524±389 |
| PD (%) | 28.1±22.2a | 6.7±3.6 | 10.8±11.1 |
| HD/PD reimbursement ratio | 1.45±0.47 | 0.74±0.16b | 1.59±0.81 |
| Annual PD reimbursement (in US$), mean±SD | 10,884±7898c | 27,880±21,593 | 24,500±18,380 |
HD, hemodialysis; PD, peritoneal dialysis; pmp, prevalence rate per million population.
P<0.001 compared with financial and no incentive.
P<0.05 compared with PD-first policy and no incentive.
P<0.05 compared with financial and no incentive.
Prevention of CKD
For the analysis of prevention of CKD, we included data from all 90 responding countries. We found that 73% of governments (66 of 90) take either no or only nonspecific measures to prevent development or progression of CKD (Supplemental Table 4).
Discussion
Our cross-sectional online survey of nephrologists from 90 countries reveals that, in low- and middle-income countries, economic strength is the dominant factor that determines the number of KRT patients who have dialysis treatment (Figure 2A). By contrast, in high-income countries, GDP per capita has no effect on dialysis prevalence (Figure 2B). Moreover, in low- and middle-income countries, government reimbursement for dialysis services uses a substantially higher proportion of public health expenditure than it does in high-income countries. In all countries surveyed, specific strategies to decrease the financial burden of ESKD—such as programs to stimulate prevention of progression of CKD, or promote the most cost-saving dialysis modalities (often PD)—are underutilized.
Societal willingness to pay for a therapy can be defined as the cost that society considers acceptable, assuming that as many potential candidates as possible will receive this treatment if needed (22). In the past, dialysis has frequently been used as a maximum allowable threshold for cost-effectiveness of medical technologies (23). However, dialysis is not affordable for governments in low-income countries and might come at the expense of other, more cost-effective interventions, which could have a much greater effect on the health of the population—such as malaria prevention (24). Some countries apply multitiered programs—such as dialysis at low reimbursement rates provided in the public sector for poor people, with a costlier private system for those insured—creating disparities in treatment availability among population groups (8,9).
Government reimbursement for dialysis services is influenced by different but concurrent macroeconomic factors. The total cost of in-center HD is much more dependent on labor costs—which themselves are linked to the overall strength of an economy—than PD, where expenditure is more closely related to the costs of consumables (i.e., bags of sterile fluid and tubings) (12,13). Therefore, in low- and middle-income countries where most consumables are imported, paid for in foreign currency, and transported over long distances, HD is less expensive than PD (12). Consequently, the prevalence of PD in these countries is low. To increase use of PD, costs of PD need to be reduced by local production and supply of PD fluids, or reduced taxes on imported fluids, which might be made even more efficient by the introduction of an active PD-first policy (25).
The prevalence of patients on PD is much lower than that of patients on HD worldwide. Financial incentives that aim to change health care practices can be directed at patients and health care providers (26,27). Our results support previous studies that show that financial incentives aimed solely at hospitals or care providers are not sufficient to increase use of PD (28,29). In countries where the percentage of patients on PD is high, governmental policy often promotes PD as the first modality, even if reimbursement is lower than for HD (Table 3). In view of the labor costs involved in provision of in-center HD, increasing HD at home could further reduce costs of KRT while also benefiting patients’ quality of life and possibly survival and complication rates (30).
More than half of the countries we surveyed do not invest in prevention of CKD, despite evidence that such measures might help to avoid ESKD (31). Such preventive measures might be most useful in the poorest countries where access to dialysis is unaffordable, while CKD risk is exacerbated by a rising prevalence of diabetes and insufficiently treated hypertension (32). Absence of central regulation leaves prevention of CKD and noncommunicable diseases to personal and local initiatives, which might reduce the potential of any intervention and cause unequal distribution of the possible effects. In nine countries reimbursement for dialysis depends on achievement of a set of targets. Use of thresholds to define reimbursement is problematic because the evidence base that supports these parameters might change over time (33). With the exception of predialysis patient education, none of the proxies for value-based care assess real quality of care, by contrast with outcomes such as mortality, rate of hospital admission, need for vascular access intervention, patient satisfaction, or quality of life (34).
A strength of our approach is that we collected data on the actual amounts reimbursed by governments for dialysis care from local nephrologists, in contrast to most previous reports that were on the basis of literature searches and included studies with heterogeneous methodology (12,13). Our approach allowed us to compare results from all countries within the same time period, by contrast with previous studies which collected results over several decades. Our data represent 81% of the world population, including many different economic and health care systems. Finally, we made an approximate calculation of total government expenditure for dialysis worldwide, which provides a benchmark for analysis of future trends.
Our study has limitations. First, the analysis is on the basis of the personal evaluation of one respondent per country, which might have created bias. Unfortunately, we could not find enough candidates with comparable expertise for a double check. Second, assessment of dialysis costs for individual countries would vary depending on the respondent’s inclusion or exclusion of items such as nephrologists’ fees, medication, treatment frequency, or laboratory tests. Third, in some countries—such as Spain, the Russian Federation, Canada, and Australia—reimbursement differs between regions (i.e., states or provinces), and because cost estimations were provided by one correspondent these regional variations might not have been accurately reflected.
In conclusion, our study shows that dialysis reimbursement policies in most countries are focused on conventional in-center HD, although home HD and PD might contribute to improved quality of life and cost savings. Nephrology professionals, scientific associations, policy makers, and industry must collaborate to implement cheaper ways to provide dialysis care to patients in need of KRT. Initiatives to improve CKD care should also focus on improving access to transplantation, increasing provision of prevention strategies to reduce progression from CKD to ESKD, and improving the quality of supportive care for ESKD that does not involve dialysis.
Disclosures
R.L.M.: Funding by the Australian National Health and Medical Research Council Fellowship #1150989. Speaker’s honoraria from Baxter Healthcare and Amgen Australia in the last 3 years (but not related to this study). W.V.B.: Speaker fees and travel grants from Fresenius Medical Care and Gambro/Baxter (but not related to this study). R.V.: Travel costs: Nikisho; travel costs and speaker’s honorarium: Braun; advisor: Astra-Zeneca (but not related to this study).
Supplementary Material
Acknowledgments
The following correspondents contributed to this analysis with data about their country: Albania: Merita Rroji; Algeria: Messaoud Saidani; Argentina, Claudio Mascheroni; Aruba: Romeo Kock; Australia: Peter Kerr; Austria: Friedrich Prischl; Bangladesh: Harun Ur-Rashid; Belarus: Aleh Kalachyk; Belgium: Raymond Vanholder; Bolivia: Rolando Claure-Del Granado; Bosnia-Herzegowina: Halima Resic; Brazil: Roberto Pecoits-Filho; Bulgaria: Evgueniy Vazelov; Burundi: Joseph Nyandwi; Cambodia: Toru Hyodo; Cameroon: Gloria Ashuntantang; Canada: Peter Magner; Chile: Ronald Wainstein; China (People’s Republic): Zhihong Liu; Colombia: Rafael Alberto Gomez Acevedo; Congo (Democratic Republic): Ernest Sumaili; Costa Rica: Fabio Hernandez; Croatia: Petar Kes; Curaçao: Willem Develter; Czech Republic: Ivan Rychlik; Denmark: Bo Feldt-Rasmussen; Dubai: Mona Al Rukhaimi; Egypt: Gamal Saadi; Estonia: Mai Rosenberg; Ethiopia: Tadesse Yewondwossen; Finland: Eero Honkanen; Former Yugoslavian Republic of Macedonia: Goce Spasovski; France: Thierry Hannedouche; Georgia: Irma Tchokhonelidze; Germany: Andreas Kribben; Ghana: Dwomoa Adu; Greece: Christos Iatrou; Hong Kong: Philip Li; Hungary: Judit Nagy; Iceland: Runólfur Pálsson; India: Vishwanath Billa; Indonesia: Aida Lydia Sutranto; Iran: Behrooz Broumand; Ireland: Liam Plant; Israel: Michal Dranitzki-Elhalel; Italy: Carlo Basile; Ivory Coast: Hubert Yao; Japan: Hideki Kawanishi; Jordan: Mohammed Ghnaimat; Latvia: Aivars Petersons; Lebanon: Robert Najem; Lithuania: Inga Bumblyte; Kosovo: Ymer Elezi; Malaysia: Lai-Seong Hooi; Mexico: Guillermo Garcia-Garcia; Montenegro: Marina Ratkovic; Morocco: Mohammed Benghanem Gharbi; the Netherlands: Frank van der Sande; New Zealand: Robert Walker; Niger: Moussa Diongolé Hassane; Nigeria: Babatunde Salako; Norway: Egil Hagen; Pakistan: Rubina Naqvi; Panama: Regulo Valdes; Paraguay: Francisco Santa Cruz; Peru: Abdias Hurtado; Phillipines: Susan Anonuevo; Poland: Andrzej Wiecek; Portugal: Anibal Ferreira; Romania: Ionut Nistor; Russian Federation: Elena Zakharova; Rwanda: Jerome Muhizi; Saudi-Arabia: Faisal Shaheen, Besher Attar; Senegal: Niang Abdou; Serbia: Nada Dimkovic; Singapore: Lina Choong Hui Lin; Sint Maarten: Maite Gil Gonzalez; Slovenia: Rafael Ponikvar; Slowakia: Miroslav Mydlik; South Africa: Charles Swaenepoel; South Korea: Eunah Hwang; Spain: Alejandro Martin-Malo; Sweden: Stefan Jacobson; Switzerland: Michel Burnier; Thailand: Paweena Susantitaphong; Tunisia: Faiçal Jarraya; Turkey: Mehemt Sukru Sever, Fatih Kircelli; United Kingdom: Andrew Davenport; United States of America: Raj Mehrotra; Uruguay: Raul Lombardi. Medical writer Philippa Berman was paid by the Nephrology Section, Department of Internal Medicine, Ghent University Hospital.
No funding.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Influence of Reimbursement Policies on Dialysis Modality Distribution around the World,” on pages 10–12.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08150718/-/DCSupplemental.
Supplemental Material
Supplemental Table 1. Responding countries (n=90).
Supplemental Table 2. Funding and country characteristics. HD and PD funding, annual funding per patient for hemodialysis and peritoneal dialysis in US$; GDPc, gross domestic product per capita in US$; Public HE (%GDP), % of GDP spent on public health expenditure; Dialysis funding (%HE), % public health expenditure spent on public funding of dialysis; HD pmp, prevalence of hemodialysis per million population; PD pmp, prevalence of peritoneal dialysis per million population; POP, Population in million inhabitants.
Supplemental Table 3. Countries with a pay-for-quality system for dialysis. Government funding is made dependent on parathyroid hormone (PTH) level, urea clearance Kt/V, dialysis access type, calcium and/or phosphate level (Ca and/or P), pre-dialysis education delivered, haemoglobin (Hb), dialysis length (time), water quality, and/or infection control.
Supplemental Table 4. Prevention strategies according to different World Bank income groups. LIC, low income countries; LMIC, lower-middle income countries; UMIC, upper-middle income countries; HIC, high income countries; CKD, chronic kidney disease.
References
- 1.Wong G, Howard K, Chapman JR, Chadban S, Cross N, Tong A, Webster AC, Craig JC: Comparative survival and economic benefits of deceased donor kidney transplantation and dialysis in people with varying ages and co-morbidities. PLoS One 7: e29591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, Fox CS, Gansevoort RT, Heerspink HJL, Jardine M, Kasiske B, Kottgen A, Kretzler M, Levey AS, Luyckx VA, Mehta R, Moe O, Obrador G, Pannu N, Parikh CR, Perkovic V, Pollock C, Stenvinkel P, Tuttle KR, Wheeler DC, Eckardt KU; ISN Global Kidney Health Summit participants : Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 390: 1888–1917, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Vanholder R, Lameire N, Annemans L, Van Biesen W: Cost of renal replacement: How to help as many as possible while keeping expenses reasonable? Nephrol Dial Transplant 31: 1251–1261, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Cusumano AM, Rosa-Diez GJ, Gonzalez-Bedat MC: Latin American Dialysis and Transplant Registry: Experience and contributions to end-stage renal disease epidemiology. World J Nephrol 5: 389–397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pippias M, Kramer A, Noordzij M, Afentakis N, Alonso de la Torre R, Ambühl PM, Aparicio Madre MI, Arribas Monzón F, Åsberg A, Bonthuis M, Bouzas Caamaño E, Bubic I, Caskey FJ, Castro de la Nuez P, Cernevskis H, de Los Ángeles Garcia Bazaga M, des Grottes JM, Fernández González R, Ferrer-Alamar M, Finne P, Garneata L, Golan E, Heaf JG, Hemmelder MH, Idrizi A, Ioannou K, Jarraya F, Kantaria N, Kolesnyk M, Kramar R, Lassalle M, Lezaic VV, Lopot F, Macario F, Magaz Á, Martín de Francisco AL, Martín Escobar E, Martínez Castelao A, Metcalfe W, Moreno Alia I, Nordio M, Ots-Rosenberg M, Palsson R, Ratkovic M, Resic H, Rutkowski B, Santiuste de Pablos C, Seyahi N, Fernanda Slon Roblero M, Spustova V, Stas KJF, Stendahl ME, Stojceva-Taneva O, Vazelov E, Ziginskiene E, Massy Z, Jager KJ, Stel VS: The European renal association - European dialysis and transplant association registry annual report 2014: A summary. Clin Kidney J 10: 154–169, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swanepoel CR, Wearne N, Okpechi IG: Nephrology in Africa--not yet uhuru. Nat Rev Nephrol 9: 610–622, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Kaur G, Prinja S, Ramachandran R, Malhotra P, Gupta KL, Jha V: Cost of hemodialysis in a public sector tertiary hospital of India. Clin Kidney J 11: 726–733, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luyckx VA, Naicker S, McKee M: Equity and economics of kidney disease in sub-Saharan Africa. Lancet 382: 103–104, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Pippias M, Jager KJ, Kramer A, Leivestad T, Sánchez MB, Caskey FJ, Collart F, Couchoud C, Dekker FW, Finne P, Fouque D, Heaf JG, Hemmelder MH, Kramar R, De Meester J, Noordzij M, Palsson R, Pascual J, Zurriaga O, Wanner C, Stel VS: The changing trends and outcomes in renal replacement therapy: Data from the ERA-EDTA Registry. Nephrol Dial Transplant 31: 831–841, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Thomas B, Wulf S, Bikbov B, Perico N, Cortinovis M, Courville de Vaccaro K, Flaxman A, Peterson H, Delossantos A, Haring D, Mehrotra R, Himmelfarb J, Remuzzi G, Murray C, Naghavi M: Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol 26: 2621–2633, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karopadi AN, Mason G, Rettore E, Ronco C: The role of economies of scale in the cost of dialysis across the world: A macroeconomic perspective. Nephrol Dial Transplant 29: 885–892, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Karopadi AN, Mason G, Rettore E, Ronco C: Cost of peritoneal dialysis and haemodialysis across the world. Nephrol Dial Transplant 28: 2553–2569, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Mushi L, Marschall P, Fleßa S: The cost of dialysis in low and middle-income countries: A systematic review. BMC Health Serv Res 15: 506, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen MJ, Friedman AN: The coming fiscal crisis: Nephrology in the line of fire. Clin J Am Soc Nephrol 8: 1252–1257, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Klarenbach S, Manns B: Economic evaluation of dialysis therapies. Semin Nephrol 29: 524–532, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Vanholder R, Davenport A, Hannedouche T, Kooman J, Kribben A, Lameire N, Lonnemann G, Magner P, Mendelssohn D, Saggi SJ, Shaffer RN, Moe SM, Van Biesen W, van der Sande F, Mehrotra R; Dialysis Advisory Group of American Society of Nephrology : Reimbursement of dialysis: A comparison of seven countries. J Am Soc Nephrol 23: 1291–1298, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Vanholder R, Annemans L, Brown E, Gansevoort R, Gout-Zwart JJ, Lameire N, Morton RL, Oberbauer R, Postma MJ, Tonelli M, Biesen WV, Zoccali C; European Kidney Health Alliance : Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat Rev Nephrol 13: 393–409, 2017 [DOI] [PubMed] [Google Scholar]
- 19.The World Bank: Population, total. Available at: https://data.worldbank.org/indicator/SP.POP.TOTL?locations=AF-DZ&view=chart. Accessed January 8, 2018
- 20.The World Bank: DataBank, World Development Indicators. Available at: http://databank.worldbank.org/data/indicator/NY.GDP.PCAP.CD/1ff4a498/Popular-Indicators. Accessed January 8, 2018
- 21.World Bank: World Bank Country and Lending Groups. Available at: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed January 8, 2018
- 22.Lee CP, Chertow GM, Zenios SA: An empiric estimate of the value of life: Updating the renal dialysis cost-effectiveness standard. Value Health 12: 80–87, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Winkelmayer WC, Weinstein MC, Mittleman MA, Glynn RJ, Pliskin JS: Health economic evaluations: The special case of end-stage renal disease treatment. Med Decis Making 22: 417–430, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S: Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bull World Health Organ 93: 118–124, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N: Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 13: 90–103, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Loewenstein G, Asch DA, Volpp KG: Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Aff (Millwood) 32: 1244–1250, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Lorincz IS, Lawson BCT, Long JA: Provider and patient directed financial incentives to improve care and outcomes for patients with diabetes. Curr Diab Rep 13: 188–195, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendelssohn DC, Langlois N, Blake PG: Peritoneal dialysis in Ontario: A natural experiment in physician reimbursement methodology. Perit Dial Int 24: 531–537, 2004 [PubMed] [Google Scholar]
- 29.Dor A, Pauly MV, Eichleay MA, Held PJ: End-stage renal disease and economic incentives: The International Study of Health Care Organization and Financing (ISHCOF). Int J Health Care Finance Econ 7: 73–111, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Walker RC, Howard K, Morton RL: Home hemodialysis: A comprehensive review of patient-centered and economic considerations. Clinicoecon Outcomes Res 9: 149–161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fink HA, Ishani A, Taylor BC, Greer NL, MacDonald R, Rossini D, Sadiq S, Lankireddy S, Kane RL, Wilt TJ: Screening for, monitoring, and treatment of chronic kidney disease stages 1 to 3: A systematic review for the U.S. Preventive Services Task Force and for an American College of Physicians Clinical Practice Guideline. Ann Intern Med 156: 570–581, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, Saran R, Wang AY, Yang CW: Chronic kidney disease: Global dimension and perspectives. Lancet 382: 260–272, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R; Hemodialysis (HEMO) Study Group : Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Breckenridge K, Bekker HL, Gibbons E, van der Veer SN, Abbott D, Briançon S, Cullen R, Garneata L, Jager KJ, Lønning K, Metcalfe W, Morton RL, Murtagh FEM, Prutz K, Robertson S, Rychlik I, Schon S, Sharp L, Speyer E, Tentori F, Caskey FJ: How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: An expert consensus meeting. Nephrol Dial Transplant 30: 1605–1614, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.