1. Introduction
The European rabbit (Oryctolagus cuniculus) has been widely used as a model for studies of human diseases. Recent reviews have described development of therapeutic and diagnostic applications of rabbit monoclonal antibodies [1], and examples of infectious diseases where rabbit is among the best animal models [2]. These include bacterial (syphilis and tuberculosis) and viral infections (HIV, papillomavirus, herpes simplex virus, poxvirus, and norovirus). Here we extend documentation of past and present utility of rabbits for development of diagnostics and therapeutics with examples from diagnosing and treating human disease with rabbit polyclonal and monoclonal antibodies (pAbs and mAbs, respectively), from studies of cardiovascular, autoimmune, ophthalmological, neoplastic diseases, and from new insights into the role of endogenous retroviruses.
2. Diagnosing and treating human disease with rabbit polyclonal and monoclonal antibodies
In addition to serving as models for a variety of human diseases, rabbits have been used to investigate immunological questions and to develop immunological techniques for more than 100 years. The rabbit antibody repertoire, first in form of pAbs and, more recently, as mAbs, remains a major source of diagnostic and therapeutic antibodies with broad utility. This is driven by their exceptional affinity and specificity and by our ability (i) to mine rabbit antibody repertoires by hybridoma, phage display, and single B-cell technologies, and (ii) to humanize rabbit mAbs [1, 3] (Figure 1).
Figure 1. Origin and composition of rabbit mAbs for diagnostic and therapeutic applications.
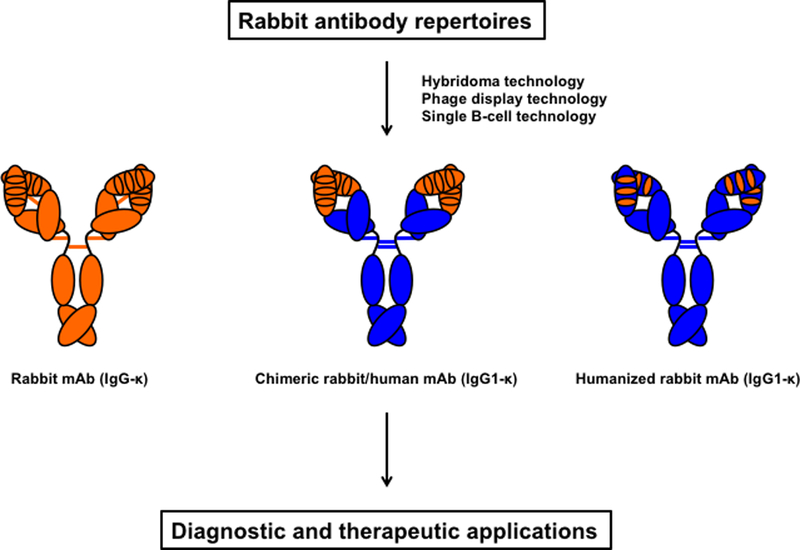
Rabbit antibody repertoires are mined by hybridoma, phage display, and single B-cell technologies to yield rabbit mAbs (here shown in the most common IgG-κ format with 5 interdomain disulfide bridges) or chimeric rabbit/human mAbs (here shown in the most common IgG1-κ format with 4 interdomain disulfide bridges). Rabbit variable (depicted with its 3 CDRs) and constant domains are shown in orange, human domains in blue. While rabbit antibody repertoires from immunized rabbits are the most common source of rabbit mAbs, a large naïve rabbit antibody repertoire that circumvents immunization has recently been generated for mining rabbit mAbs by phage display technology (Peng et al 2017). Chimeric rabbit/human mAbs combine rabbit variable domains of light and heavy chain with human constant domains. Humanized rabbit mAbs, which are preferred for therapeutic applications, are generated by grafting the 6 CDRs into human framework regions. For detailed information on rabbit heavy and light chain isotypes as well as rabbit mAb generation and humanization, please see Weber et al 2017.
An attractive feature of rabbit antibody repertoires is their complementary paratope collection, i.e. their collective ability to either engage different epitopes of human antigens or to engage these epitopes differently in comparison with murine and human antibodies. In addition, whereas murine mAbs to human antigens may not cross-react with other species, rabbit mAbs specific for the same antigen often cross-react with murine antigens. This can expedite preclinical toxicity assessments in murine models of human disease [1]. The early development of the primary rabbit B-cell and antibody repertoire occurs in gut-associated lymphoid tissues (GALT) and is stimulated by gut flora. Diversification by somatic gene conversion and somatic hypermutation already occurs by mechanisms similar to those used for affinity maturation in response to specific immunogens. The finding that the development of the rabbit B-cell repertoires significantly differs from that of other mammals provides the foundation for this unique paratope repertoire (reviewed in [4. and 5]. The neonatal rabbit B-cell and antibody repertoire in the bone marrow is shaped by VH-D-JH and VL-JL recombination similar to other mammals. However, lack of diversity due to a highly biased usage of VH1, the most D-proximal VH gene, is partially compensated for by more extensive nontemplated nucleotide addition to VH-D, D-JH, and VL-JL junctions, resulting in longer HCDR3 and LCDR3 loops compared to murine antibodies (reviewed in [6]). Collectively, these peculiarities of rabbit antibodies have made them attractive alternative reagents for both diagnostic and therapeutic application as discussed below.
Immunohistochemistry (IHC) with rabbit antibodies has been a particularly useful companion diagnostic for mAb therapy. FDA-approved rabbit pAbs and mAbs that aid treatment decisions are compiled in Table 1. For example, a rabbit anti-human c-Kit pAb and a rabbit anti-human PD-L1 mAb are used to detect c-Kit and PD-L1 expression in cancer, respectively, to support targeted intervention with tyrosine kinase inhibitors and mAbs. An additional 7 rabbit mAbs have received FDA approval for diagnosing human disease. Well established and refined IHC and other immunological techniques that can detect rabbit antibodies with high signal-to-noise ratios have fueled their popularity for diagnostic and basic research applications.
Table 1.
FDA-approved rabbit pAbs and mAbs for diagnostic applications.1
| Antibody | Manufacturer | Assay2 | Antigen | Diagnostic application | FDA approval |
|---|---|---|---|---|---|
| rabbit pAb | PCL-RIA | RIA | human thyroid stimulating hormone | measurement of thyroid stimulating hormone concentration in blood | 1978 |
| rabbit pAb | PCL-RIA | RIA | digoxin | measurement of digoxin concentration in blood | 1978 |
| rabbit pAb | PCL-RIA | RIA | Human triiodothyronine | measurement of triiodothyronine concentration in blood | 1978 |
| rabbit pAb | Dako | ELISA and IHC | human Ig light chain | measurement of free Ig light chain in blood and tissues | 1995 |
| rabbit mAb | Ventana | IHC | human HER2 | companion diagnostic for anti-HER2 mAb (trastuzumab) therapy of breast cancer | 2000 |
| rabbit pAb | Dako | IHC | human CD117 | companion diagnostic for imatinib mesylate therapy of gastrointestinal stromal tumor | 2005 |
| rabbit mAb | Lab Vision | IHC | human progesterone receptor | companion diagnostic for hormone therapy of breast cancer | 2006 |
| rabbit mAb | Lab Vision | IHC | human estrogen receptor | companion diagnostic for hormone therapy of breast cancer | 2006 |
| rabbit mAb | Dako | IHC | human estrogen receptor | companion diagnostic for hormone therapy of breast cancer | 2009 |
| rabbit mAb | Ventana | IHC | Helicobacter pylori | detection of Helicobacter pylori infection | 2011 |
| rabbit mAb | Ventana | IHC | human progesterone receptor | companion diagnostic for hormone therapy of breast cancer | 2011 |
| rabbit mAb | Ventana | IHC | human estrogen receptor | companion diagnostic for hormone therapy of breast cancer | 2012 |
| rabbit mAb | Dako | IHC | human PD-L1 | companion diagnostic for anti-PD1 mAb (nivolumab) therapy of lung cancer and melanoma | 2015, 2016 |
| rabbit mAb | Ventana | IHC | human PD-L1 | companion diagnostic for anti-PD-L1 mAb (atezolizumab and durvalumab) therapy of bladder and lung cancer | 2016, 2017 |
Based on a search (“rabbit antibody”) of Devices@FDA via www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm on June 13, 2018.
Abbreviations: RIA, radioimmunoassay; ELISA, enzyme-linked immunosorbant assay; IHC, immunohistochemistry
Over the past two decades, therapeutic mAbs have become clinically and commercially highly successful drugs. Five of the Top 10 and 7 of the Top 20 bestselling drugs in 2018 are mAbs (www.igeahub.com). More than 60 antibody-based therapies have been approved by the FDA and are collectively approaching global revenues of $100 billion [7]. Although currently all FDA-approved therapeutic mAbs have either murine, humanized murine, or human variable domain amino acid sequences, rabbit-based therapeutic mAbs have entered various clinical trials (Table 2). While immunogenicity in human patients will be difficult to compare between therapeutic mAbs derived from rabbit and murine antibody repertoires, there is currently no evidence that rabbit or humanized rabbit mAbs are more immunogenic than their murine counterparts. Thus, there is a strong rationale for mining the unique paratope collection of rabbit antibodies for therapeutic mAbs.
Table 2.
Clinically investigated rabbit antibodies for therapeutic applications.1
| Antibody | Format2 | Names | Manufacturers and developers | Antigen | Indication | Year of FDA approval or highest stage of clinical trials |
|---|---|---|---|---|---|---|
| rabbit pAb | Polyclonal IgG | anti-thymocyte globulin [rabbit] (ATG; Thymoglobulin®) | Sanofi, Genzyme | human thymocytes | transplantation (prophylaxis and treatment) | 1998 |
| humanized rabbit mAb | scFv | brolucizumab (ESBA1008) | Novartis, Alcon, ESBATech | human VEGF | eye diseases (treatment) | Phase III |
| humanized rabbit mAb | aglycosylated IgG1 | eptinezumab (ALD403) | Alder | human CGRP | migraine (prophylaxis) | Phase III |
| humanized rabbit mAb | scFv | LME636 (ESBA1622) | Novartis, Alcon, ESBATech | human TNFα | eye diseases (treatment) | Phase II |
| humanized rabbit mAb | IgG1 | sevacizumab (APX003; TK001) | Simcere, Jiangsu T-mab, Apexigen, Epitomics | human VEGF | cancer (treatment) eye diseases (treatment) | Phase II |
| humanized rabbit mAb | IgG1 | APX005M | Apexigen, Epitomics | human CD40 | cancer (treatment) | Phase II |
| humanized rabbit mAb | aglycosylated IgG1 | clazakizumab (ALD518; BMS - 945429) | Vitaeris, Bristol-Myers Squibb, Alder | human IL-6 | inflammation (treatment) transplantation (treatment) | Phase II |
| humanized rabbit mAb | IgG1 | ontuxizumab (MORAb-004) | Morphotek | human CD248 | cancer (treatment) | Phase II |
| humanized rabbit mAb | IgG1 | SSS07 (APX001) | 3SBio, Apexigen, Epitomics | human TNFα | inflammation (treatment) | Phase I |
| humanized rabbit mAb | IgG1 | YYB101 | YooYoung | human HGF | cancer (treatment) | Phase I |
| rabbit mAb | scFv CAR-T | R12 | Fred Hutchinson Cancer Research Center | human ROR1 | cancer (treatment) | Phase I |
Only rabbit antibodies in clinical trials registered at ClinicalTrials.gov were included.
Abbreviations: IgG, immunoglobulin G; scFv, single-chain variable fragment; CAR-T, chimeric antigen receptor T cell.
3. Cardiovascular diseases
The developm ent of current diagnostics and therapeutics for cardiovascular diseases greatly benefitted from studies using rabbit models including cholesterol-fed rabbits and the Watanabe hyperlipidemic rabbit strain (reviewed in Fan et al) [8], [9], Nimi et al [10]. A valuable overview of the history of discoveries related to the development of statins by Akira Endo [11], leads back to a 1913 report by Anitschkow [12] that cholesterol-fed rabbits developed hypercholesterolemia and atherosclerosis. This paper has been cited more than 400 times over the years. The 1985 Nobel Prize in Physiology or Medicine was awarded to Michael S. Brown and Joseph L. Goldstein “for their discoveries concerning the regulation of cholesterol metabolism”. According to their Nobel lecture [13], they “began … work in 1972 in an attempt to understand a human genetic disease, familial hypercholesterolemia”. In these patients the concentration of cholesterol in blood is elevated many times above normal and heart attacks occur early in life. The 1973 discovery of inhibitors of cholesterogenesis produced by Penicillium citrinium reported by Endo et al [14] in 1976, was followed by a series of discoveries that are reviewed in Endo’s detailed historical perspective [11] and by Shiomi et al. [15]. Lovastatin, the first of the family of statins, was approved by the FDA in 1986. Statins such as atorvastatin are now among the most highly prescribed therapeutics. Recently, rabbits with knock-outs of cholesteryl ester transfer protein (CETP) were produced to investigate its role in susceptibility to high cholesterol induced atherosclerosis [16]. Compared to wild type, the knock-out rabbits fed a high cholesterol diet for 18 weeks had reduced plasma cholesterol and reduced atherosclerosis in both coronary and aortic arteries. Tests of three different CETP inhibitors failed to identify a useful therapeutic. However, tests of treatment with a fourth (anacetrapib) added to intensive statin treatment in patients with atherosclerotic vascular disease yielded significantly lower incidence of major coronary events than the addition of placebo during a 4.1-year period [17]. Current Controlled Trials number, ISRCTN48678192 ; ClinicalTrials.gov number, NCT01252953; and EudraCT number, 2010–023467-18). A commentary by R.A Hegle [18] in the same issue of the New England Journal of Medicine notes that there may be a need for a replication study plus evaluation of possible long-term effects. It remains to be seen whether the data on hand now about anacetrapib will lead to an FDA-approved therapeutic to manage cardiovascular diseases.
4. Diseases of the eye
Rabbits have eyes that are larger than those of rodents. They have been used for studies of human ocular diseases including dry eye syndrome, glaucoma, age-related macular degeneration, light-induced retinopathies, cataracts and uveitis (reviewed in Zernii et al) [19]. In addition, the use of HLA transgenic rabbits for studies of potentially blinding recurrent herpes stromal keratitis was described in a previous review [2]. Therapeutic applications of humanized rabbit monoclonal antibodies that are listed in Table 2, include three for eye diseases (brolucizumab, LME636, and sevacizumab). An April 2018 press release from Novartis about the single chain Fv fragment brolucizumab as a therapeutic for neovascular age-related macular degeneration (nAMD) stated that they “look forward to continuing to advance brolucizumab through regulatory approvals as a welcome new option for treatment of nAMD, which is a leading cause of blindness”. New reports of the use of rabbit models of diseases of the eye continue to appear. Okumura et al, [20]. reported a cell-based approach in a rabbit model for treating Fuchs endothelial corneal dystrophy. In this condition, patients develop vision loss (bullus keratopathy) that is currently treated by a surgical endothelial allograft to replace the single layer of corneal endothelium with donor endothelium. used rabbits to develop methods for inducing proliferation of corneal endothelial cells and. The same group followed up their studies in rabbits and reported a small “proof-of-concept” human trial that used cultured corneal endothelial cells supplemented with a rho-associated protein kinase (ROCK) inhibitor Kinoshita et al, [21]. The study was accompanied by an editorial entitled “A New Frontier in Curing Corneal Blindness” [22]. This editorial also raises a series of questions that remain to be answered including acceptable age range of cell donors, density of cells seeded, and to what extent the ROCK inhibitor contributed to the observed clinical improvement.
5. Endogenous retroviruses in rabbits and other lagomorphs
The role of endogenous retroviruses (ERVs) in human diseases is an emerging area of great interest as it applies to several diseases. Retroviral DNAs and retrovirus-like transposable elements are present in the genomes of all eukaryotes and constitute as much as 8% of the total genetic content of many species [23]. ERVs provide a ‘fossil record’ of past retroviral infections dating back many millions of years, suggesting a never-ending stream of retroviral challenges for vertebrates [24]. ERVs can have positive effects such as provision of functions necessary for placenta formation and resistance to novel retrovirus infections as well as negative effects on hosts including tumor induction and genome instability [25]., [26]. Induction of ERV expression, may have a central role in efficacy of cellular responses to infectious agents rather than direct effects on specific genes [27]. Evolutionary studies indicate that an arms race between viruses and hosts has taken place [24]. ERVs are inherited cellular genes that could encode retroviral gene products or replication-competent retroviruses. Over time, ERVs have been subjected to repeated amplification and transposition events giving rise to multicopies that are distributed within the DNA of all cells.
RELIK, the first endogenous lentivirus described in mammals, was discovered in the European rabbit [28]. It is simpler than other lentiviruses, with only two accessory proteins, tat and rev, in addition to the main coding domains gag, pol and env. Sequencing of this virus in several other leporids showed that the endogenization event occurred in the ancestral of these species more than 12 million years ago [29], [30]. All the main coding domains were shown to be defective with several stop codons and frameshifts. Another rabbit endogenous retrovirus, (RERV-H) was first identified in human tissues and only later recognized as an Oryctolagus endogenous retrovirus [31]. It is present in multiple copies spread throughout the Oryctolagus genome(s). Screening for the presence of RERV-H revealed that it was also present in the genomes of Bunolagus and Pentalagus. This suggests that the virus was endogenized in their common ancestor, roughly 9 million years ago [32]. There are several examples that the insertion of ERVs can shape the host innate immune system. Although the mechanism of infection used by RELIK is still unknown, it was suggested that its presence in rabbits and hares conditioned the evolution of TRIM5α of these species [33]. The residues identified under positive selection in the PRYSPRY domain of lagomorphs’ TRIM5α, and the ability of hare TRIM5α to inhibit the replication of several retroviruses, suggest that as in primates, the selective pressure in these species was exerted by aTRIM5-sensitive virus, such as RELIK [33]., [34]. Indeed, the RELIK capsid-containing virions are susceptible to TRIM5α inhibition, and the TRIM5α from Sylvilagus and Oryctolagus inhibit RELIK more efficiently than the TRIM5α from Ochotona (which lack endogenous RELIK sequences [35]. It has been shown that ERVs have shaped the evolution of a transcriptional interferon response and that lineage-specific ERVs have dispersed numerous IFN-inducible enhancers independently in diverse mammalian genomes [36]. The likely involvement of endogenous retroviruses in several diseases is addressed below.
6. Multiple Sclerosis
MS is one of the most frequently referred to diseases with etiological links to human endogenous retroviruses (HERVs). A recent critical meta-analysis by Morandi et al, [37] concluded that their “systematic review and meta-analysis shows an association between expression of HERVS and MS”. The most significant findings were based on 25 reports concerning members of the HERV-W family of MS-associated retroviruses, with findings of envelope mRNA in peripheral blood mononuclear cells, MSRV/HERV-W polymerase mRNA in CSF (four studies each), and from 6 studies of MSRV/HERV-W polymerase mRNA in serum/plasma. The remaining implicated HERV were 13 to HERV-H, 9 to HERV-K, 5 to HRES-1 and 1 to the HERV-15 family. A therapeutic mAb, GNbAC1 anti-HERV-W-Env is currently being evaluated in a a long-term safety study in patients with MS (ClinicalTrials.gov Identifier: NCT03239860).
Type 1 Diabetes
A recent report [38] suggests that HERV-W-Env retroviral protein may be a new therapeutic target for type 1 diabetes (T1D). HERV-W-Env protein was detected in 70% of patients’ sera, and HERV-W-Env RNA was detected in 57% of peripheral blood mononuclear cells. In addition, they observed inhibition of insulin secretion by HERV-W-Env in human Langerhans islets. They also found a significant correlation between HERV-W-Env expression and macrophage infiltrates in the exocrine part the human pancreas and expression by pancreatic acinar cells in 75% of human T1D patients. The same therapeutic mAb GNbAC1 anti-HERV-W-Env that is being evaluated for treatment of MS is also being tested as a new therapeutic approach for T1D. [39]). It is hypothesized that it “will inhibit the potential inflammatory and cytotoxic actions of HERV‐W‐Env, which may play a role in T1D pathophysiology”, and lead to a “truly disease‐modifying treatment of T1D without impact on the immune system”. A phase 2 clinical trial is in progress (ClinicalTrials.gov Identifier: NCT03179423)
7. Systemic Lupus Erythematosus
During the 1990s, it was considered likely that Epstein-Barr virus (EBV) played some role in development of Systemic Lupus Erythematsus (SLE) [40]. Early pioneering work by James et al [41] described immunization of rabbits with peptides PPPGMRPP and PPPGIRGP from the Smith Sm B/B’ subunit of a spliceosomal autoantigen. Such antibodies are frequently found in the sera of SLE patients. They observed spliceosome autoimmunity after peptide immunization of rabbits, autoimmune disease-induced epitope spreading, SLE-like autoantibody production, and clinically observed seizures. Continued studies by this group [42] supported the hypothesis that infection with EBV contributes to the etiology of SLE. They reported that autoantibodies similar to those developed in SLE patients were elicited in both mice and rabbits by immunization with multi-antigen poly-lysine constructs (MAP) bound to fragments of the EBV nuclear antigen EBNA-1 with sequence PPPGRRP that is similar to and cross-reactive with the Smith antigen Smith Sm B/B’. A 2012 review by James and Robertson [43} updates data concerning the role of EBV.
In addition to using a MAP-8-PPPGMRPP immunogen used in the previous studies, Rai and coworkers [44] chose a new immunogen termed GR-MAP-8 based on a 2001 report by DeGiorgio and coworkers [45] that some anti-DNA antibodies cross-react with the NMDA glutamate receptor. Rabbits could be selectively bred within the colony of pedigreed, immunoglobulin allotype-defined but non-inbred rabbits in the Laboratory of Immunology at NIAID. We suggested that the genetic heterogeneity of the pedigreed animals may correspond to that found among patients of a given ethnicity. Immunization with the GR-MAP-8 led to lupus-like autoantibody production including anti-Sm, -RNP, -SS-A, -SS-B and -dsDNA. Some neurological symptoms in form of seizures and nystagmus were observed. Selected responders were used to breed several generations that were similarly immunized [46]. Details about gene expression, genetics, and cellular studies are reviewed in a chapter in an open access volume on SLE [47].
After development of the rabbit model initiated at NIAID, Dr. Rai’s laboratory initiated translational studies toward “identification, optimization and evaluation of disease processes aimed at amelioration of the disease state in human beings”. Chauhan et al. [48] addressed the role of Toll like Receptors and found that different expression profiles of TLR7 and TLR9 correlated with SLE patients’ autoantibody expression profiles. In addition, their “characterization of novel markers in SLE using genomic and proteomic approaches” including miRNA and global transcriptome sequencing studies have helped provide a strong basis for distinguishing 3 distinct subgroups of SLE with a potential of influencing decisions about therapy [49].
8. Cancer
Among the rabbit antibodies for therapeutic applications registered at ClinicalTrials.gov that are listed in Table 2, five are for cancer treatment. Three in phase II are humanized monoclonal antibodies (eptinezumab, anti-human VEGF, APX005M anti-human CD40, and ontuxizumab anti-human CD248 (TEM1/endosialin/). Two in phase I are a humanized monoclonal YYB101 anti-human HGF and the last one R1,2, is specific for human ROR1 in the form of a single chain Fv-CAR-T construct.
Human oncogenic viruses” is the title of a Theme issue of Philosophical Transactions of the Royal Society B that includes consideration of ERVs in cancer. Kassiotis and Stoye [50] suggest that “HERVs, despite their oncogenic past, now represent potential targets for therapeutics via “immune-mediated anti-tumor mechanisms”. They suggest that “the depth at which cancer genomes are currently analysed suggests that HERVs have entirely lost their ability to cause cancer by mechanisms involving novel integration leading to insertional mutagenesis”. Although HERVs may still contribute to the pathological processes leading to cancer by other mechanisms, they suggest an alternative potential role for HERVs in preventing tumor development. These could for example, enhance tumor antigenicity and immunogenicity through elevated expression of HERV nucleic acid and protein products in cancer cells. They refer to such a sole role for HERVs as akin to an in-built ‘warning system’, alerting the cell, as well as the immune system, to any genetic damage or epigenetic dysregulation.
In addition, in a new 2018 Frontiers in Microbiology Research Topic “Endogenous Viral Elements. Links Between Autoimmunity and Cancer?”, Balestrieri et al [51] describe HERV-K and the role of HERV-K in the crosstalk between cancer cells and the tumor microenvironment. They suggest this crosstalk has implications for combination therapy targeting HERVs in addition to standard approaches.
Although the laboratory rabbits bred, immunized, and maintained for years at NIAID rarely developed cancer, a valuable rabbit model of cancer is ulcerative gastric cancer with peritoneal carcinomatosis resembling human peritoneal carcinoma (PC) was described by Mei and coworkers [52]. A rabbit VX2 carcinoma was used to establish gastric cancer with PC. Having established this rabbit model, the same group went on to evaluate therapeutic cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC) in their model [53]. Nine days after inoculation with tumor cells, they initiated therapies to compare maximal removal of tumor nodules in a CRS alone group, and a maximal CRS plus hyperthermic intraperitoneal chemoperfusion with docetaxel (10 mg/rabbit) and carboplatin (40 mg/rabbit) at 42.0 ± 0.5°C for 30 min in the CRS + HIPEC group. They concluded that in this rabbit model of gastric cancer with PC, CRS alone could not bring benefit while “CRS + HIPEC with docetaxel and carboplatin could significantly prolong survival with “acceptable safety”.
9. Concluding remarks
Their larger size and greater similarity to human than rodents at the genomic level have made the rabbit an important animal model for studies of human diseases. In this review, we have chosen a few examples of human disease-related discoveries that have been made using the rabbit as a model. We summarize the important roles that rabbit monoclonal antibodies (mAbs) play now for diagnostics, and the promise of humanized rabbit mAbs as therapeutics. In addition, the discovery of endogenous retroviruses (ERVs) in rabbits, and studies about ERVS in rabbits and other members of the Order Lagomorpha have now contributed to the current realization that ERVS may contribute to the etiology of and/or provide therapeutic targets for several human diseases.
Acknowledgements
This research was supported in part by the Intramural Research program of the NIH, NIAID (R. Mage), by the project AGRIGEN – NORTE-01–0145-FEDER-000007, supported by Norte Portugal Regional Operational Programme (NORTE2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF)” (PJ Esteves), by the Fundação para a Ciência e Tecnologia (FCT) that supported the FCT Investigator grants of P.J. Esteves (IF/00376/2015) and grants: NIH R01 CA174844, NIH R01 CA181258, and NIH R01 CA204484 (C. Rader). We thank Dr. Michael Mage for helpful suggestions about the manuscript.
References
- 1.Weber J, Peng H, Rader C, 2017. From rabbit antibody repertoires to rabbit monoclonal antibodies. Experimental & Molecular Medicine 49, e305; doi:10.1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteves PJ, Abrantes J, Baldauf HM, BenMohamed L, Chen Y, Christensen N, González-Gallego J, Giacani L, Hu J, Kaplan G, Keppler OT, Knight KL, Kong XP, Lanning DK, Le Pendu J, de Matos AL, Liu J, Liu S, Lopes AM, Lu S, Lukehart S, Manabe YC, Neves F, McFadden G, Pan R, Peng X, de Sousa-Pereira P, Pinheiro A, Rahman M, Ruvoën-Clouet N, Subbian S, Tuñón MJ, van der Loo W, Vaine M, Via LE, Wang S, Mage R 2018. The wide utility of rabbits as models of human diseases. Exp Mol Med 22 50–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Liu H, Guan Q, Wang L, Yuan H 2017. Advances in the Isolation of Specific Monoclonal Rabbit Antibodies. Front Immunol 8, 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mage RG, Lanning D, Knight KL 2006. .B cell and antibody repertoirs development in rabbits:The requirement of gut-asssosciated lymphoid tissurd. Developmenta; and comprative Immunology 30,137–153. [DOI] [PubMed] [Google Scholar]
- 5.Lanning DK, Knight KL 2015. Diversification of the Primary Antibody Repertoire by AID-Mediated Gene Conversion. In: Hsu E, Du Pasquier L (eds) Pathogen-Host Interactions: Antigenic Variation v. Somatic Adaptations. Results and Problems in Cell Differentiation, vol 57 Springer, Cham; [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro A, Lanning D, Alves PC, Mage RG, Knight KL, van der Loo W, Esteves PJ 2011. Molecular bases of genetic diversity and evolution of the immunoglobulin heavy chain variable region (IGHV) gene locus in leporids. Immunogenetics 63, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter PJ ., Lazar GA 2018. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat Rev Drug Discov 17 197–223. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Kitajima S, Watanabe T et al. 2015. Rabbit models for the study of human atherosclerosis: From paththophysiological mechanisms to translational medicine. Pharmacology &Therapeutics 146 104–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan J, Chen Y, Yan H et al. 2018. Principles and Applications of Rabbit Models for Atherosclerosis Research. J Atheroscler Thromb 25 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niimi M, Yang D, Kitajima S, et al. 2016. ApoE knockout rabbits: a novel model for the study of human hyperlipidemia. Atherosclerosis 245, 187–193. [DOI] [PubMed] [Google Scholar]
- 11.Endo A 2010. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci 86 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anitschkow N 1913. Uber die Veranderungen der Kannichenaorta bei experimentaeller Cholesterinsteatose Beitrz Path Anatalllg Pathol 379–404.
- 13.Brown MS and Goldstein JL 1986. A receptor-mediated pathway for cholesterol homeostasis. (Nobel Lecture) Angewandte Chemie International 25 583–660. [DOI] [PubMed] [Google Scholar]
- 14.Endo A, Kuroda M, Tsujita Y 1976. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. Journal of Antibiotics (Tokyo) 29, 1346–1348. [DOI] [PubMed] [Google Scholar]
- 15.Shiomi M, Koike T, Ito T, 2013. Contribution of the WHHL rabbit, an animal model of familial hypercholesterolemia, to elucidation of the anti-atherosclerotic effects of statins. Atherosclerosis 231, 39–47. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Niimi M, Yang D, Liang J, Xu J, Kimura T, Mathew AV, Guo Y, Fan Y, Zhu T, Song J, Ackermann R, Koike Y, Schwendeman A, La i L., Pennathur S, Garcia-Barrio M, Fan J, Chen YE 2017. Deficiency of Cholesteryl Ester Transfer Protein Protects against Atherosclerosis in Rabbits. Arteriosclerosis, Thrombosis, and Vascular Biology 37,1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HPS3/TIMI55–REVEAL Collaborative Group, Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ, Collaborators (2325). 2017. N Engl J Med 377 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegele RA 2017. CETP Inhibitors — A New Inning? N Engl J Med 377 1284–1285. [DOI] [PubMed] [Google Scholar]
- 19.Zernii E, Bajsheeva V, Iomdina E, et al. 2016, Rabbit Models of Ocular Diseases: New Relevance for Classical Approaches. CNS & Neurological Disorders - Drug Targets, 267–291. [DOI] [PubMed]
- 20.Okumura N, Matsumoto D, Fukui Y et al. 2018. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PLoS ONE 13 (1): e0191306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kinoshita S, Koizumi N, Ueno M et al. 2018. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy N Engl J Med 378 995–1003. [DOI] [PubMed] [Google Scholar]
- 22.Dana R 2018. A New Frontier in Curing Corneal Blindness. N Engl J Med 378 1057–1058. [DOI] [PubMed] [Google Scholar]
- 23.Hayward A, Grabherr M, Jern P, 2013. Broad-scale phylogenomics provides insights into retrovirus–host evolution. PNAS 110 20146–20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeke JD, Stoye JP, 1997. Retrotransposons, endogenous retroviruses, and the evolution of retroelements, in: Coffin JM, Hughes SH, Varmus HE (eds), Retroviruses Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- 25.Stoye JP, 2012. Studies of endogenous retroviruses reveal a continuing evolutionary saga. Nat. Rev. Microbiol 10, 395–406. [DOI] [PubMed] [Google Scholar]
- 26.Chuong EB, Elde NC, Feschotte C, 2017. Regulatory activities of transposable elements: from conflicts to benefits. Nat. Rev. Genet 18 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mager DL, Lorincz MC, 2017. Epigenetic modifier drugs trigger widespread transcription of endogenous retroviruses. Nat. Genet 49, 974–975. [DOI] [PubMed] [Google Scholar]
- 28.Katzourakis A, Tristem M, Pybus OG, Gifford RJ, 2007. Discovery and analysis of the first endogenous lentivirus. Proc. Natl. Acad. Sci. U S A 104, 6261–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keckesova Z, Ylinen LM, Towers GJ, Gifford RJ, Katzourakis A 2009. Identification of a RELIK orthologue in the European hare (Lepus europaeus) reveals a minimum age of 12 million years for the lagomorph lentiviruses. Virology 384, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Loo W, Abrantes J, Esteves PJ, 2009. Sharing of endogenous lentiviral gene fragments among leporid lineages separated for more than 12 million years (genera Lepus, Sylvilagus, Bunolagus and Oryctolagus). J. Virol 83, 2386–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voisset C, Myers RE, Carne A, Kellam P, Griffiths DJ, 2003. Rabbit endogenous retrovirus-H encodes a functional protease. J Gen Virol 84(Pt 1), 215–25. [DOI] [PubMed] [Google Scholar]
- 32.de Sousa-Pereira P, Abrantes J, Baldauf. HM, Esteves PJ (2017) Evolutionary studies on the betaretrovirus RERV-H in the Leporidae family reveal an endogenization in the ancestor of Oryctolagus, Bunolagus and Pentalagus at 9 million years ago. Virus Res pii: S0168–1702(17)30174–0. doi: 10.1016/j.virusres.2017.12.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.de Matos AL, van der Loo W, Areal H, Lanning DK, Esteves PJ, 2011. Study of Sylvilagus rabbit TRIM5α species-specific domain: how ancient endoviruses could have shaped the antiviral repertoire in Lagomorpha. BMC Evol. Biol 11, 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fletcher AJ, Hué S, Schaller T, Pillay D, Towers GJ, 2010. Hare TRIM5α restricts divergent retroviruses and exhibits significant sequence variation from closely related lagomorpha TRIM5 genes. J. Virol 84, 12463–12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yap MW, Stoye JP 2013. Apparent effect of rabbit endogenous lentivirus type K acquisition on retrovirus restriction by lagomorph Trim5as. Phil Trans R Soc B 368: 20120498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuong EB, Elde NC, Feschotte C, 2016. Regulatory evolution of innate immunity through co-option of endogenous retroviruses. Science 351 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morandi E, Tanasescu R, Tarlinton RE, Constantinescu CS, Zhang W, et al. 2017. The association between human endogenous retroviruses and multiple sclerosis: A systematic review and meta-analysis. PLOS ONE 12(2): e0172415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levet S, Medina J, Joanou J, Demolder A Querue N, Réant K, Normand M, Seffals M, Dimier J, Germi R, Piofczyk T, Portoukalian J, Touraine JL, Perron H 2017 An ancestral retroviral protein identified as a therapeutic target in type-1 diabetes. JCI Insight 2017;2(17) e94387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtin F, Bernard C, Levet S, Perron H, Porchet H, Médina J, Malpass S, Lloyd D, Simpson R; RAINBOW-T1D investigators. 2018. A new therapeutic approach for type 1 diabetes: Rationale for GNbAC1, an anti-HERV-W-Env monoclonal antibody. DiabetesObes Metab 1–10. 10.1111/dom.13357 [DOI] [PubMed] [Google Scholar]
- 40.Marchini B, Dolcher MP., Sabbatini A, Klein G, Migliorini PJ 1994. Immune response to different sequences of the EBNA I molecule in Epstein-Barr virus-related disorders and in autoimmune diseases. Autoimmun 7, 179–191. [DOI] [PubMed] [Google Scholar]
- 41.James JA, Gross T, Scofield RH, Harley JB 1995. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B’-derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. Journal of Experimental Medicine, 181 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole BD, Templeton AK,. Guthridge JM, Brown EJ, Harley JB, et al. 2009. Aberrant Epstein-Barr viral infection in Systemic Lupus Erythematosus. Autoimmunity Rev 8: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James JA and Robertson JM, 2012. Lupus and Epstein-Barr. Current Opinion in Rheumatology, 24, 383–388. in [INFECTION AND AUTOIMMUNITY: Edited by Noel R. Rose] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rai G, Ray S, Shaw RE, DeGrange PF, Mage RG, Newman BA 2006. Models of systemic lupus erythematosus: Development of autoimmunity following peptide immunizations of noninbred pedigreed rabbits. J. Immunol 176, 660–667. [DOI] [PubMed] [Google Scholar]
- 45.DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B 2001. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature Med 7, 1189–1193. [DOI] [PubMed] [Google Scholar]
- 46.Rai G, Ray S, Milton J, Yang J, Ren P, Lempicki R, Mage RG 2010. Gene expression profiles in a rabbit model of systemic lupus erythematosus Autoantibody. J. Immunol 185, 4446–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mage RG, and Rai G 2012. A rabbit model of systemic lupus erythematosus, useful for studies of neuropsychiatric SLE. In: Systemic Lupus Erythematosus pp 201–216. InTech –Open Access Publisher, Rijeka, Croatia [Google Scholar]
- 48.Chauhan SK, Singh VV, Rai R Rai M, Rai G 2013. Distinct Autoantibody Profiles in Systemic Lupus Erythematosus Patients are Selectively Associated with TLR7 and TLR9 Upregulation. J Clin Immunol 33, 954–964. [DOI] [PubMed] [Google Scholar]
- 49.Chauhan SK, Singh VV, Rai R, Rai M, Rai G, 2014. Differential microRNA Profile and Post-Transcriptional Regulation Exist in Systemic Lupus Erythematosus Patients with Distinct Autoantibody Specificities. J Clin Immunol 34, 491–503. [DOI] [PubMed] [Google Scholar]
- 50.Kassiotis G, and Stoye JP, 2017. Making a virtue of necessity: the pleiotropic role of human endogenous retroviruses in cancer. Phil. Trans. R. Soc 372:0160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balestrieri E, Argaw-Denboba A, Gambacurta A, Cipriani C, Bei R, Serafino A, Sinibaldi-Vallebona P, Matteucci C 2018. Human Endogenous Retrovirus K in the Crosstalk Between Cancer Cells Microenvironment and Plasticity: A New Perspective for Combination Therapy. Frontiers in Microbiology 1448 doi: 10.3389/fmicb.2018.01448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mei LJ, Yang XJ, Tang L, Hassan AH, Yonemura Y, Li Y 2010. Establishment and identification of a rabbit model of peritoneal carcinomatosis from gastric cancer BMC Cancer 10,124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang L, Mei L-J, Yang X-J, Huang C-Q, Zhou YF, Yonemura Y, Li Y.2011. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of gastric cancer with peritoneal carcinomatosis: evidence from an experimental study. J Transl Med 9:53. doi: 10.1186/1479-5876-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]


