Abstract
Previous research showed that increasing the proportion of docosahexaenoic acid (DHA) in marine lipid supplements significantly reduces associated health benefits compared with balanced eicosapentaenoic acid (EPA):DHA supplementation Dasilva et al., 2015 [1]. It was therefore hypothesized that the EPA and DHA molecules might have differential resistance to oxidation during gastric digestion and that the oxidation level achieved could be inversely correlated with intestinal absorption and, hence, with the resultant health benefits. Accordingly, we tested this proposed mechanism of action by investigating the degree of oxidation in the stomach, and the levels of bioaccessible lipids, of varying molar proportions of DHA and EPA (2:1, 1:1 and 1:2) using the dynamic gastrointestinal tract model TIM-1. In addition, small intestine enterocyte absorption and metabolism were simulated by Caco-2 cell monolayers that were incubated with these same varying proportions of DHA and EPA, and comparing oxidized and nonoxidized polyunsaturated fatty acids (PUFAs). The results show an inverse correlation between lipid oxidation products in the stomach and the levels of bioaccessible lipids. The balanced 1:1 EPA:DHA diet resulted in lower oxidation of PUFAs during stomach digestion relative to the other ratios tested. Finally, cell-based studies showed significantly lower assimilation of oxidized EPA and DHA substrates compared to nonoxidized PUFAs, as well as significant differences between the net uptake of EPA and DHA. Overall, the present work suggests that the correct design of diets and/or supplements containing marine lipids can strongly influence the stability and bioaccessibility of PUFAs during gastrointestinal digestion and subsequent absorption. This could modulate their health benefits related with inflammation, oxidative stress and metabolic disorders.
Keywords: Caco-2 cells, Lipid peroxidation, Digestion, Bioaccessibility, Bioavailability, TIM, ω-3 PUFA
1. Introduction
Although fish oil consumption is generally considered beneficial, there is also some evidence that highly unsaturated substrates like polyunsaturated fatty acids (PUFAs) could lead to increased consumption of endogenous antioxidants, consequently leading to oxidative stress [1,2]. However, the effects of fish oil on antioxidant status are based mainly on in vitro observations, which may not accurately represent the in vivo situation. A comparison between highly unsaturated ω−3 fish oil diets and less unsaturated ω−6 vegetal diets showed a decrease in cardiovascular disease (CVD) risk factors after fish oil supplementation without increasing oxidative stress markers [3]. Fish oil diets also showed a protective effect against oxidative-stress-induced DNA damage [4]. It has been reported that regular consumption of ω−3 marine lipids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), modulates lipid biomarkers of inflammation and oxidative stress, processes involved in the development and progression of metabolic disorders including CVD, diabetes, obesity and even cancer and Alzheimer’s disease [5,6]. Despite the growing evidence of the health benefits of EPA and DHA consumption, no agreement about a recommended proportion of EPA vs. DHA in the diet has yet been achieved [7], although the European Food Safety Authority and the Food and Agriculture Organization have recommended a minimumconsumption of EPA and DHA of 0.25 g/day. In previous research using a rodent model, we observed noteworthy differences in the health effects exerted by diets containing different proportions of EPA and DHA. In particular, we found that higher proportions of DHA in the diet appeared to diminish the benefits of fish oil related to eicosanoid and docosanoids levels, plasma and membrane inflammatory indexes [8]. Moreover, other rodent studies by our group and others have suggested that oxidative stress parameters [9,10], CVD risk factors [11] and metabolic disorders [12,13] may also be modulated by the relative amounts of dietary EPA or DHA. The underlying mechanisms that give rise to these effects are not clear.
The question of the fate of marine oils in the gastrointestinal tract has been addressed due to their oxidability. Studies that have mimicked gastric conditions in vitro suggest that the stomach may act as a bioreactor [14], acelerating the peroxidation of lipids and other dietary constituents due to the temperature, presence of catalysts and acid pH [15,16]. Indeed, the use of antioxidants such as polyphenols has been shown to protect lipids from oxidation during gastric digestion. In fact, Maestre et al. [17] showed that the inclusion of a polyphenol-rich grape seed extract during digestion decreased the amount of oxidation products from fish lipids in the stomach dialysates, and Lapidot et al. [18] also showed the antioxidant effect of added ascorbic acid in lipid oxidation under gastric conditions. The production of primary oxidation metabolites such as lipid hydroperoxides generates free radicals that may catalyze the oxidation of new substrates [19]. Although Penumetcha et al. reported no differences in the cellular uptake of oxidized and nonoxidized linoleic acid [20], Maestre et al. [17] demonstrated a significant decrease in Caco-2 cell assimilation of DHA which had been previously oxidized with lipooxydase enzymes. Therefore, we hypothesized that pro-oxidant conditions of the stomach may increase PUFA degradation during digestion, leading to poorer assimilation efficiency by small intestine enterocytes. The reduction of PUFA bioavailability could thus limit the health benefits associated with dietary marine lipids.
The limitations of simple in vitro digestion models include their being monocompartmental, static and nonrepresentative of the continuously changing variables of digestion. By contrast, gastric digestion mimicked by the TNO dynamic gastrointestinal tract model (TIM-1 system) [21] more closely recapitulates the physiological processes occurring within the lumen of the stomach and small intestine. Contrary to static models, TIM-1 reproduces in several compartments the main changes and parameters that control digestion, and it has been validated by microbial, pharmaceutical and nutritional studies [22,23]. Nevertheless, an important limitation of the TIM-1 model is the absence of a cellular system, which therefore does not reproduce the passive and active absorption of nutrients occurring in the small intestine. Therefore, to simulate the uptake of bioactive compounds across the apical plasma membrane of the enterocyte, Caco-2 human intestinal cells have been used. When grown in culture, Caco-2 cells spontaneously develop many functions characteristic of mature villus cells of the small intestine epithelium; including formation of a polarized monolayer of columnar epithelium with intercellular tight junctions, an apical membrane with a brush border of organized microvilli and the basolateral secretion of lipoprotein particles [24]. Thus, a combination of the TIM-1 model with Caco-2 cells can provide physiologically relevant information about the digestion, absorption and intestinal metabolism of nutrients and drugs [22,25,26].
To further understand the mechanism underlying our previous observations, in which specific proportions of EPA and DHA in the diet appear to promote different health benefits [7], in the present studies, we analyzed gastrointestinal oxidative stability and intestinal enterocyte assimilation of EPA, DHA and different levels of both PUFAs. It was hypothesized that changing EPA/DHA would modify their oxidation and, thereby, absorption of the mixture. Specifically, we hypothesized that the greater unsaturation of DHA may lead to higher concentrations of oxidation products and lower intestinal assimilation, relative to EPA. Thus, the TIM-1 model firstly allows simulation of the digestion of marine oil meals in order to analyze the oxidation of PUFAs under gastric conditions as a function of the EPA:DHA ratio and, secondly, enables the quantification of the amount of “bioaccessible” lipids in the small intestine compartments using molecular size cutoff filters. In order to mimic the diets used in our previous studies in rats [8], we used the identical chow as the source of macronutrients; the main ingredients were wheat, corn, gluten and soy oil. This was mixed with the indicated oil supplements, which also mimicked the previous studies. In addition to the TIM-1 studies, Caco-2 cells incubated with radiolabeled oxidized and nonoxidized EPA, DHA and EPA:DHA mixtures were used to determine the influence of PUFA type, ratio and oxidation status on the cellular uptake and metabolism of the lipids.
2. Materials and methods
2.1. Chemicals
Sodium bicarbonate, chlorhydric acid, BSA (essentially FFA free), Triton X-100, pepsin A from porcine stomach mucosa (2500–3500 U/mg, P7012), trypsin from bovine pancreas (7500 N-α-benzoyl-L-arginine ethyl ester U/mg, T9201) and α-amylase Type II-A from Bacillus (1333 U/mg, A6380) were obtained from Sigma-Aldrich (Stockholm, Sweden). Fresh pig bile was obtained from TNO (Zeist, Netherlands). Pancreatin and Rhizopus lipase (150,000 U/mg, F-AP-15) was from Amano Enzyme (Nagoya, Japan). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), vitamins, nonessential amino acids (NEAA), penicillin (100 U/ml), streptomycin (100 mg/ml) and trypsin–EDTA were from Life Technologies. Unlabeled EPA and DHA were from Nu CheckPrep (Elysian, MN, USA). Radiolabeled 14C EPA and DHA were supplied by American Radiolabeled Chemicals (St. Louis, MO, USA). The Scint-AXF scintillation fluid was from Packard Instrument (Meriden, CT, USA). Sodium taurocholate was purchased from Calbiochem (La Jolla, CA, USA), and hexane, chlorophorm and methanol were from Fisher Scientific.
2.2. TIM-1 experiments
2.2.1. Preparation of EPA:DHA “meals”
Prior to TIM-1 in vitro digestion, meals were prepared by mixing a standard chow for rodents Teklad Global 2016S (caloric composition: 22% from protein, 66% from carbohydrate, and 12% from fat; Harlan Lab., South Easton, MA, USA) with soybean oil as a control diet (source of ω−6 LA) or with different proportions of the two main ω−3 FA from fish oils (1:1, 2:1 or 1:2 EPA:DHA); the lipid content of all TIM meals was in the form of triglyceride from the oils. Table 1 details the composition of the diets used. Oil supplements containing 1:1, 2:1 and 1:2 EPA:DHA (mol:mol) were prepared by blending appropriate proportions of the commercially available fish oils from AFAMPES 121 EPA (AFAMSA, Vigo-Spain), EnerZona Omega 3 RX (ENERZONA, Milano-Italy) and Oligen liquid DHA 80% (IFIGEN-EQUIP 98, Barcelona-Spain). Soybean oil, obtained from cold pressing unrefined organic soybean oil, was from Biolasi S.L. (Ordizia, Guipuzcoa-Spain). Meals were prepared by mixing 10g of chow with 1.7 g of corresponding oil and were kept at −20°C under N2 until used. All high-fat diets were high in PUFAs and delivered approximately 42% of calories from fat (34% from PUFAs of the supplements and 8% from the chow), 43% from carbohydrates and 14% from proteins (Supplementary Material S1). Analysis of the FA composition of the oil supplements is presented in Table 1. The results show that the molar percentages of SFA, MUFA and PUFA were constant, while the diets of 1:1, 2:1 and 1:2 ratios of EPA:DHA contained these desired proportions; the sum of ω−3 PUFA was fixed at approximately 50% of total fatty acid for all the test diets. Soybean oil provided 47% of total fatty acid as ω−6 LA (Table 1). Initial tocopherol level of fish oils was 2.84 mg tocopherol/g oil. Because PUFAs are easily oxidized, the initial peroxide value of the oils was checked by the ferric thiocyanate method [27] and was found to be below 5 mEq O2/kg of oil.
Table 1.
Fatty acid composition of soybean oil and fish oil mixtures (EPA:DHA 1:1, 2:1 and 1:2) used for TIM-1 in vitro digestion experiments
| Ratio 1:1(EPA:DHA) | Ratio 2:1(EPA:DHA) | Ratio 1:2(EPA:DHA) | Soybean oil | |||||
|---|---|---|---|---|---|---|---|---|
| Fatty acid | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. |
| 14:0 | 4.37 | 0.05 | 3.97 | 0.01 | 4.73 | 0.02 | 1.00 | 0.02 |
| 16:0 (palmitic) | 10.15 | 0.20 | 9.09 | 0.03 | 10.98 | 0.10 | 18.23 | 0.10 |
| 18:0 (stearic) | 2.94 | 0.03 | 2.95 | 0.00 | 2.97 | 0.01 | 2.12 | 0.01 |
| 20:0 | 0.32 | 0.01 | 0.39 | 0.01 | 0.20 | 0.00 | 0.00 | 0.00 |
| Total SFA | 18.52 | 0.20 | 17.05 | 0.02 | 19.68 | 0.10 | 21.35 | 0.10 |
| 16:1 ω7 (PA) | 4.99 | 0.04 | 4.57 | 0.02 | 5.39 | 0.03 | 0.92 | 0.03 |
| 18:1 ω9 (OA) | 6.41 | 0.06 | 6.18 | 0.00 | 6.61 | 0.04 | 19.22 | 0.03 |
| 18:1 ω7 (vaccenic) | 1.91 | 0.03 | 1.93 | 0.02 | 1.95 | 0.02 | 1.52 | 0.02 |
| 20:1 ω9 | 0.98 | 0.03 | 1.39 | 0.01 | 0.63 | 0.02 | 1.46 | 0.09 |
| 22:1 ω11 | 1.14 | 0.01 | 1.58 | 0.02 | 0.45 | 0.01 | 1.10 | 0.00 |
| 22:1 ω9 | 0.28 | 0.03 | 0.37 | 0.03 | 0.19 | 0.02 | 0.25 | 0.02 |
| 24:1 ω9 | 0.38 | 0.00 | 0.55 | 0.02 | 0.25 | 0.01 | 0.28 | 0.05 |
| Total MUFA | 17.22 | 0.10 | 17.14 | 0.07 | 17.43 | 0.10 | 24.78 | 0.10 |
| 18:2 ω6 (LA) | 0.65 | 0.01 | 0.61 | 0.00 | 0.65 | 0.02 | 48.75 | 0.01 |
| 20:2 ω6 | 0.21 | 0.00 | 0.28 | 0.01 | 0.17 | 0.01 | 0.20 | 0.05 |
| 20:3 ω6 (DGLA) | 0.22 | 0.01 | 0.27 | 0.01 | 0.15 | 0.00 | 0.00 | 0.00 |
| 20:4 ω6 (ARA) | 1.68 | 0.04 | 1.98 | 0.03 | 1.16 | 0.02 | 0.41 | 0.02 |
| Total ω6 PUFA | 2.76 | 0.01 | 3.14 | 0.01 | 2.14 | 0.01 | 49.36 | 0.02 |
| 18:3 ω3 (ALA) | 0.36 | 0.01 | 0.32 | 0.00 | 0.33 | 0.02 | 4.10 | 0.04 |
| 18:4 ω3 | 1.51 | 0.02 | 1.56 | 0.02 | 1.64 | 0.00 | ||
| 20:4 ω3 | 1.02 | 0.02 | 1.31 | 0.02 | 0.75 | 0.01 | ||
| 20:5 ω3 (EPA) | 25.09 | 0.10 | 32.43 | 0.06 | 17.33 | 0.03 | ||
| 22:5 ω3 (DPA) | 4.30 | 0.05 | 5.24 | 0.02 | 2.60 | 0.10 | ||
| 22:6 ω3 (DHA) | 25.70 | 0.20 | 17.98 | 0.03 | 34.85 | 0.10 | ||
| Total ω3 PUFA | 57.97 | 0.07 | 58.84 | 0.03 | 57.51 | 0.05 | 4.10 | 0.04 |
| ω6/ω3 | 0.05 | 0.05 | 0.04 | 12.00 | ||||
| Total PUFA | 64.26 | 0.30 | 65.81 | 0.08 | 62.90 | 0.20 | 53.46 | 0.03 |
Results are expressed as percentage of total fatty acids (mg/100 mg of total FA). Values are shown as means±S.D. 16:0 (palmitic acid), 16:1 ω7 (palmitoleic acid, PA), 18:0 (stearic acid), 18:1 ω9 (oleic acid, OA), 18:1 ω7 (vaccenic acid), 18:2 ω6 (LA), 18:3 ω3 (ALA), 20:3 ω6 (DGLA), 20:4 ω6 (ARA), 20:5 ω3 (EPA), 22:5 ω3 (DPA), 22:6 ω3 (DHA).
2.2.2. Fatty acid analysis of the oil supplements
To determine the fatty acid (FA) composition of the oil supplements, 0.6 mg of lipid was methylated following the methodology of Lepage and Roy [28]. Briefly, methylatedFAs (FAMEs) were extracted into toluene. The FAMEs were analyzed by gas chromatography (GC/FID, Clarus 500, Perkin-Elmer) using an SP-2330 fused silica capillary column (30 m × 0.25 mm i.d., 0.20 μm; Supelco Inc., Bellefonte, USA). The fatty acid nonadecanoic acid was used as an internal standard. Results are shown in Table 1.
2.2.3. TIM-1 in vitro digestion
The in vitro digestion was performed using the dynamic TIM-1 system (TNO, Zeist, Netherlands) which consists of four successive compartments simulating human stomach, duodenum, jejunum and ileum. The main parameters of human digestion, including pH; body temperature; peristaltic mixing and transport; gastric, biliary and pancreatic secretions; and passive absorption of small molecules and water are reproduced as accurately as possible [21]. The TIM was programmed to reproduce the digestion of a solid meal in a healthy human adult. In detail, the compartments are temperature regulated at 37°C. The peristalsis is mimicked by changing the water pressure controlled by computer. The secretions to the stomach (HCl 1 M, NaCl 3.1 g/L, KCl 1.1 g/L, CaCl2·0.15 g/L, pepsin 132 mg/L, lipase 124 mg/L) and small intestine (NaHCO3 1 M, bile, pancreatin 17.5 g/L, NaCl 1.25 g/L, KCl 0.15 g/L, CaCl2·0.075 g/L) are also controlled by pumps, and rates were set at 0.5 ml/min for the stomach and l ml/min for the intestine. PH is controlled by three electrodes according to pH profiles of human digestion. In the stomach, initial pH was 4.8 and final pH was 1.7. The duodenal pH was set at 6.5. Samples from the stomach compartment (1.5 g) were collected in duplicate at 0, 30, 60, 90, 120 and 150 min following the addition of diet to the first (stomach) compartment. Hollow fiber filtration devices connected to jejunal and ileal compartments were composed of semipermeable membranes (0.05 μm pore size, Spectrum Milikros) that simulate absorption of released/digested water and fat soluble compounds less than 50 nm in size [23]. During digestion (5 h), samples were collected hourly in duplicate (5–10 g) from dialysates absorbed through jejunal and ileal filtration devices; these dialysates represent the bioaccessible digested products [23]. For the experiments, 11.7 g of experimental diets described above were pulverized (6×10 s) using a food blender. Then, the powder was stirred for 1 min on ice with 5 g artificial saliva (11.5 g amylase, 30 ml citrate buffer pH 5.5), 95 g electrolyte solution (5 g/L NaCl, 0.6 g/L KCl, and 0.3 g/L CaCl2) and 200 g MiliQ water up to a final volume of 300 ml using a Turrax homogenizer. The mixture was introduced into the stomach compartment, and digestion was initiated. The digestion experiments were done four separate times for each diet (n=4).
2.2.4. Determination FA oxidation in the stomach and intestinal bioaccessible lipids
To follow the development of lipid oxidation products in the stomach, lipids were extracted from 1.5 g of gastric fluid using the Bligh and Dyer procedure [29]. After that, the conversion of FA into oxidized lipids was spectrophotometrically monitored by conjugated dienes (234 nm) and trienes (268 nm) [30]. Results are expressed as mmol linoleic acid hydroperoxides/kg lipid. To determine the amount of bioaccessible digested products in the small intestine, 5 g of jejunal and 10 g of ileal fluids were subjected to Bligh and Dyer extraction [29]. The concentration of lipids in the organic layer was gravimetrically determined [31]. Then, the total volume of the jejunal and ileal dialysates was used to calculate the mg of digested lipids that went through the intestinal filters.
2.3. Caco-2 cells experiments
2.3.1. Cell culture
Caco-2 human colon cancer cells were obtained from American Type Culture Collection (Rockville, MD, USA). Stock cultures at passage 24 were grown in DMEM with 25 mM glucose, 4 mM glutamine, 1% penicillin, 1% streptomycin, 1% NEAA, 1% vitamins and 20% FBS at 37°C in a humidified atmosphere of air/CO2 (95:5, v/v) as described previously [32]. For experiments, cells were plated at a density of 4×104 cells per well in 6-well plates and used at 14–15 days postconfluence as described by Ho et al. [25]. Experiments were done three separate times for each treatment.
2.3.2. Uptake of oxidized and nonoxidized PUFAs by Caco-2 cells
Radiolabeled FA uptake media (15 ml) were prepared with a final FA concentration of 30 μM in 10 mM taurocholate/PBS following the protocol previously described [33]. Labeled FAs were 5% of total FA, and the specific activities were 55 mCi/mmol. Sixteen uptake media were prepared immediately prior to use, including oxidized and nonoxidized FA, for the uptake assays of individual EPA and DHA, and different proportions of EPA:DHA are summarized in Supplementary Material S2. It is important to note that only one of the PUFA in each mixture, EPA or DHA, was radiolabeled in these mixtures. To prepare the oxidized uptake media, the protocol was modified as follows: 1 ml of the media was incubated under simulated stomach conditions (1 N HCl and pH 1.5 for 2.5 h at 37°C) [14,34] in order to oxidize the FA. The conversion of FA into oxidized lipids was spectrophotometrically monitored by following the production of conjugated dienes at 234 nm of absorbance [30]. Sodium bicarbonate and taurocholate/PBS solution were then added to a final volume of 15 ml, concentration of 30 μM FA and pH=7 [33].
Caco-2 cells were incubated for 2 and 6 h with the indicated media in triplicate for each treatment, and net uptake of oxidized or nonoxidized FA was measured as previously described [33]. Each experiment was repeated three separate times. Briefly, monolayers were cultured overnight in serum-free media before the experiment. Cells were washed twice with PBS and then incubated with the specific media. After the designated incubation time, cells were washed with cold BSA (×3) and cold PBS (×3), and then each well was scraped into 400 μl 0.05% Triton X-100 with 0.5% of protease inhibitor cocktail (Sigma P8340). Finally, cells were sonicated for 15 s using a Branson sonifier equipped with a microtip (Danbury, CT, USA). The amount of FA taken up was quantified by comparing the radioactivity of the cells and incubation media. Samples were mixed with Scint-AXF and counted in a Beckman LS5000TD liquid scintillation spectrometer. Protein content of sonicated cells was determined by the method of Lowry et al. with BSA as the standard [35], and FA uptake was expressed as μmol FA/mg protein.
2.3.3. Metabolism of oxidized and nonoxidized PUFAs in Caco-2 cells
Total lipids were extracted from approximately 400 μl of sonicated cells (representing approximately 0.2 mg of cell protein) using the method of Bligh and Dyer [29]. Organic extracts were dried under N2, redissolved in 1:1 chlorophorm:methanol (v/v) and kept at −20°C until used. The incorporation of radiolabeled EPA and DHA from the uptake solution into lipid metabolites produced by the cells was analyzed using TLC to separate the lipid classes. Briefly, the same amount of radioactivity species from each sample was spotted under N2 onto 20×20-cm TLC plates (K5 silica gel 150A, 250 μm; Whatman, Hillsboro, OR, USA). TLC plates were activated prior to sample application and developed as described previously [32]. The radioactivity in each lipid class was measured using a Storm 840 phosphorimager (Amersham Biosciences). The partitioning of radiolabeled lipid into individual lipid species was expressed as a percentage of total label incorporated.
2.4. Statistical analysis
Data are expressed as mean±S.D. Statistical analyses were performed by one-way analysis of variance with R free software (version 386–3.1.0). Nonparametric Kruskal– Wallis analyses were required when data distribution did not fit a Gaussian model or heterogeneity was found in variances. The means were further compared by post hoc Fisher least square difference, and significant differences were set at Pb.05 or below.
3. Results
3.1. TIM-1 experiments
3.1.1. FA oxidation during TIM-1 stomach digestion as a function of the dietary EPA:DHA ratio
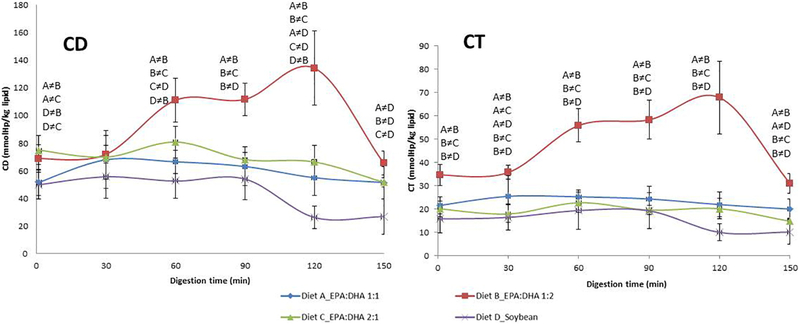
Fig. 1 shows the evolution of conjugated diene and triene levels during the 150 min of TIM-1 stomach digestion for diets containing 1:1, 2:1 or 1:2 EPA:DHA molar ratios or soybean oil. It was found that the 1:2 diet displayed almost twofold greater production of oxidized products after 60 min, which remained elevated throughout the digestive period; the highest levels of CD on the 1:2 diet were found at 120 min, where levels were approximately fourfold higher than those from the soybean oil diet. All other diets showed similar levels of conjugated dienes and trienes, with no notable oxidation during the entire gastric digestion period, with the exception of the 2:1 EPA:DHA diet at 60 min. The soybean oil control diet was the most resistant to oxidation under these stomach conditions, and it generated the lowest levels of CD at all sampling points, although not statistically different compared with 1:1 and 2:1 diets. Similar results were obtained for the 1:1 meal, and intermediate levels were produced by the 2:1 meal, although these were not statistically different from the 1:1 diet. After 150 min of digestion, the CD levels decreased in the 1:2 diet, reaching similar levels as the other meals. Conjugated diene and triene levels in jejunal and ileal dialysates (small intestine) did not show significant differences at any time point (data not shown). The proportion of PUFAs oxidized in the stomach ranged from 1.7% to 4.3% mol/mol of total dietary PUFAs, evaluated by the maximum level of conjugate dienes formed.
Fig. 1.
Concentrations of conjugate dienes (CD) and conjugate trienes (CT) evolution during the in vitro stomach digestion in TIM-1 for supplements 1:1, 2:1, 1:2 EPA:DHA and soybean oil. Results are expressed as mmol of produced linoleic acid hydroperoxides/kg lipid. Values are shown as means±S.D. (n=4).). ≠=significant differences between diets at P<.01 (CD) and P<.001 (CT) are shown on the figure with letters for individual sampling times.
3.1.2. Bioaccessibility of FA digestive products in simulated small intestine depends on the dietary EPA:DHA ratio
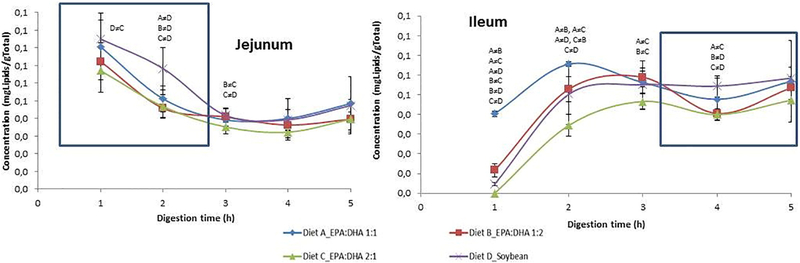
After digestion in the TIM-1 stomach compartment, lipids were filtered by semipermeable membranes that represent the jejunal and ileal sections of the small intestine, with the lipids found in the filtrates representing the “bioaccessible” compounds [23]. Fig. 2 depicts the concentration of bioaccessible lipids in the jejunal and ileal compartments during the 5 h of digestion for each diet. All diets showed the same trend during the digestion period: the concentration of bioaccessible compounds was higher at the beginning (1–2 h) in the more proximal part of the intestine (jejunal) and decreased to near zero after 2–3 h. On the other hand, the concentration of bioaccessible compounds in the distal part of the small intestine (ileal) was close to zero at the beginning (1 h), with the exception of the 1:1 ratio which was somewhat higher; all were increased in the ileal compartment by 2 to 3 h of digestion.
Fig. 2.
Concentrations of bioaccessible digested lipids in the jejunal and ileal sections of small intestine during the in vitro digestion in TIM-1 for supplements 1:1, 2:1, 1:2 EPA:DHA and soybean oil. Results are expressed as mg of digested lipids/g of dialysate. Values are shown as means±S.D. (n=4). ≠=significant differences between diets at P<.001 (P<.05 at time 3 h) are shown on the figure with letters for individual sampling times.
A tendency toward higher amounts of bioaccessible lipids was found at all time points in jejunal dialysates for the soybean and 1:1 EPA:DHA diets compared with the 1:2 and 2:1 diets. Differences were significant at 1–2 h, when jejunal absorption principally occurs. Interestingly, ileal dialysates analyzed after 4 h of digestion showed the same tendency: higher concentrations of bioaccessible lipids in the soybean and 1:1 diets than in the others.
3.2. Caco-2 cell experiments
3.2.1. Influence of PUFA oxidation by gastric conditions on the uptakeand metabolism of EPA and DHA by Caco-2 cells
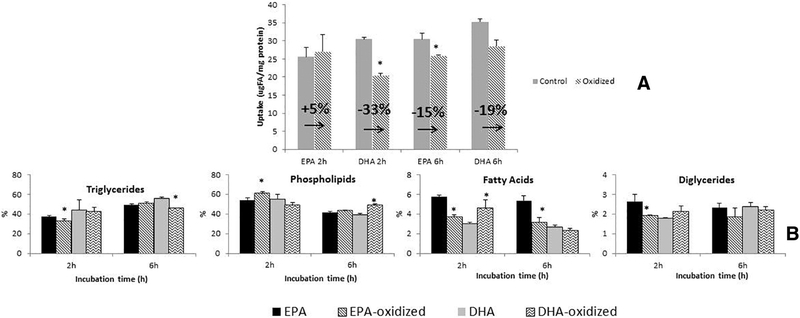
In these experiments, our purpose was to test whether the extent of oxidation of ω−3 EPA and DHA occurring under gastric conditions correlates with effects on intestinal absorption efficiency and further metabolism of these individual PUFAs. Monolayers of Caco-2 cells and fatty acids solubilized in taurocholate micelles were used to mimic the physiological presentation of FAs in the intestinal lumen [32]. Cells were incubated for 2 and 6 h with control nonoxidized EPA and DHA, and oxidized EPA and DHA. Oxidation of the PUFAs was achieved by prior incubation of the individual PUFAs under gastric conditions of pH and temperature, as described in Methods. CD production after this gastric incubation reached 33%–34% of the added PUFA for both EPA and DHA (data not shown). This level is likely higher than an in vivo gastric oxidation.
The results in Fig. 3A show that, as expected, the net uptake for all FA was generally increased at the longer 6-h incubation time compared with 2 h. At the 6-h time point, the uptake of both oxidized PUFAs was reduced by 15%–19% compared with uptake of the nonoxidized PUFAs; the percent reduction was quite similar for both EPA and DHA. Interestingly, at the 2-h time point, it was observed that while oxidized DHA uptake was significantly less (33%) compared with control DHA, oxidized EPA uptake was essentially unchanged (5%) relative to control nonoxidized EPA. Thus, EPA uptake was less affected than DHA uptake by the gastric oxidation process.
Fig. 3.
Net uptake (A) and lipid metabolism (B) of Caco-2 cells incubated during 2 and 6 h with control (nonoxidized) and oxidized radiolabeled EPA and DHA. Oxidation achieved incubating the taurocholate/PUFAs medium under gastric conditions (37°C, 2.5 h and pH 1.5). The uptake is expressed as μg FA/mg of protein, and variation of uptake between control/ oxidized PUFAs is expresed as percentage. Results for lipid metabolism are expressed as percentage of each lipid class by total lipids. Standard deviations (n=3) are shown on the figure, and significant differences between oxidized and nonoxidized PUFAs at P<.05 (A) or P<.001 (B) are expressed as *.
An examination of the lipid classes produced by Caco-2 cell metabolism of control and oxidized EPA and DHA (Fig. 3B) shows that the major lipids into which the PUFA were incorporated were triglycerides (TG) and phospholipids (PL), followed by diglycerides (DG) and free fatty acids (FFA) in smaller proportions. Some small differences in incorporation of oxidized vs. nonoxidized PUFA into cellular lipid metabolites were found, with somewhat different patterns observed for EPA compared to DHA. For example, after 2 h of incubation, the incorporation of oxidized EPA into TG, DG and FFA was lower and the incorporation into PL was higher relative to nonoxidized EPA. For DHA, no differences in metabolite incorporation after 2 h of incubation were found except for an increase of label in FFA. By 6 h of incubation, no differences in metabolite incorporation were found between oxidized and nonoxidized EPA; however, a decreased incorporation into TG and an increased incorporation into PL were found for oxidized relative to nonoxidized DHA.
3.2.2. Influence of PUFA oxidation by gastric conditions and EPA:DHA ratios on the uptake and metabolism of EPA and DHA by Caco-2 cells
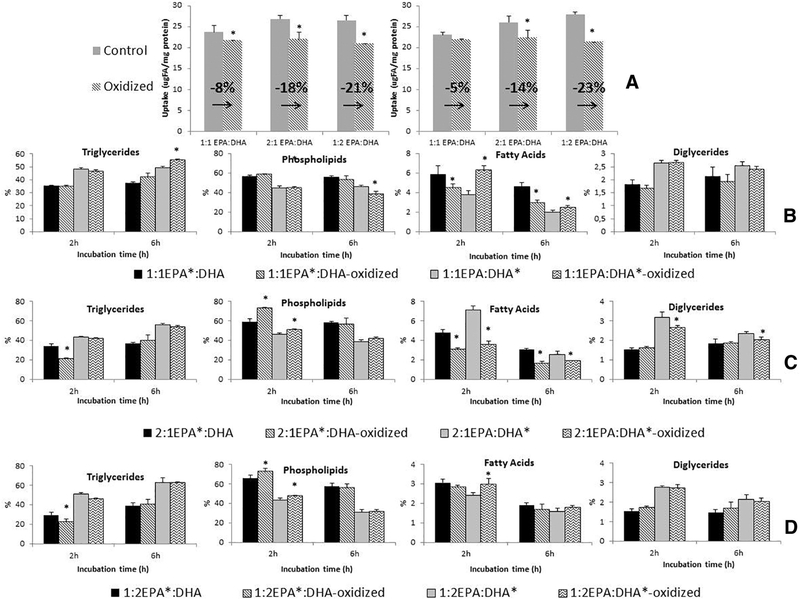
Results showed that the longer incubation times resulted in greater uptake from both oxidized and control uptake solutions, similar to that observed for individual EPA and DHA incubations. Generally, the uptake of oxidized PUFAs from media containing the EPA:DHA combinations was significantly lower than uptake from nonoxidized PUFAs for both 2- and 6-h incubation times. Nevertheless, interesting differences were found in the total uptake depending on the ratio of EPA to DHA. It was observed that the largest decrease in uptake was found after oxidation of 1:2 EPA:DHA (21%–23% at 2–6 h, respectively), followed by 2:1 EPA:DHA (18%–14%), and finally 1:1 EPA:DHA absorption was the least reduced (8%–5%) (Fig. 4A).
Fig. 4.
Net uptake (A) and lipid metabolism (B-C-D) of Caco-2 cells incubated during 2 and 6 h with control (nonoxidized) and oxidized EPA:DHA ratios. Superscript * indicates the PUFA that is radiolabeled in each mixture EPA:DHA. (A) Net uptake of EPA:DHA ratios calculated as the sum of individual radiolabeled mixtures (EPA:DHA + EPA:DHA*). (B) Lipid metabolism of 1:1 EPA*:DHA and 1:1 EPA:DHA*. (C) Lipid metabolism of 2:1 EPA*:DHA and 2:1 EPA:DHA*. (D) Lipid metabolism of 1:2 EPA*:DHA and 1:2 EPA:DHA*. Oxidation achieved incubating the taurocholate/PUFAs medium under gastric conditions (37°C, 2.5 h and pH 1.5). The uptake is expressed as μg FA/mg of protein, and variation of uptake between control/ oxidized PUFAs is expresed as percentage. Results for lipid metabolism are expressed as percentage of each lipid class by total lipids. Standard deviations (n=3) are shown on the figure, and significant differences between oxidized and nonoxidized PUFAs at P<.05 (A) or P<.001 (B) are expressed as * above the bars.
Analysis of cell metabolites for the mixtures of EPA and DHA showed incorporation into the same lipid classes as cells incubated singly with EPA and DHA (Fig. 4B–D). When control EPA:DHA mixtures were compared with oxidized mixtures at 2 h of incubation, results showed that the oxidation process affected the metabolite profiles of cells incubated with EPA:DHA ratios of 2:1 and 1:2; the changes followed similar trends as observed for single EPA and DHA: oxidation decreased the incorporation into TG, DG and FFA of EPA and increased incorporation of EPA and DHA into PL. In contrast to the 1:2 and 2:1 mixtures, the nonoxidized and oxidized 1:1 ratio incubations showed similar metabolite profiles with the exception of unesterified FFA percentages: oxidation increased the percentage of DHA present at 2 h but reduced levels of EPA. After 6 h of incubation, TG, DG and PL percentages were generally not modified by the oxidation process, although the 1:1 and 2:1 ratios showed a decreased level of EPA and an increased level of DHA secondary to oxidation.
4. Discussion
It is generally thought that ω−3 EPA and DHA consumption may positively modulate inflammation, oxidative stress, metabolic disorders or even cancer, although reservations concerning their use have also been raised [5,6,36,37]. Currently, there is a lack of agreement about the relative amounts of EPA vs. DHA in the diet which potentiate their benefits [7]. Results obtained in previous research suggested noteworthy health outcomes using balanced EPA:DHA supplements instead of greater amounts of DHA or EPA [8]. In addition, several studies have demonstrated that the stomach is a bioreactor that promotes the peroxidation of unsaturated lipids, and we and others have shown a possible decrease in the uptake of oxidized relative to unoxidized lipids. Therefore, it was hypothesized that PUFA oxidation during stomach digestion and subsequent changes in their bioavailability, assimilation and perhaps metabolism by intestinal enterocytes may influence the health benefits of marine lipid diets. To examine this hypothesis, in the present study, gastric digestion was mimicked by the dynamic gastrointestinal tract model (TIM-1 system), and small intestine enterocyte absorption and metabolism were simulated using monolayers of Caco-2 cell cultures. Experiments were designed to examine the relationship between gastric oxidation of PUFA and subsequent assimilation by the enterocyte. We further examined different combinations of EPA vs. DHA to assess the influence of the mixture in the oxidative stability and bioavailability of these marine oil PUFAs.
When the stability of EPA:DHA diets under simulated stomach conditions was examined, results showed a significant increase in CD generation when the dietary proportion of DHA is more elevated, with lower levels produced by the balanced 1:1 diet. It was also observed that a diet enriched with linoleic acid (LA) was the most resistant to gastric oxidation. Since diet composition only differed in PUFA profile, the different oxidation curves may be explained by the differential oxidizability of single PUFAs. The results indicate that the stability of fatty acids under the pro-oxidant conditions of the stomach was inversely proportional to the degree of unsaturation and the number of carbons of the molecule [38]. Accordingly, DHA (22:6 ω−3) is longer and more unsaturated than EPA (20:5 ω−3) and LA (18:2 ω−6), and thus, it appears to be more readily oxidized. In concurrence, the 1:2 EPA:DHA diet showed the highest levels of CD. Furthermore, the control diet enriched with the shortest and least unsaturated PUFA, 18:2, resulted in the lowest CD level. These results are in agreement with previous studies that showed elevated plasma levels of lipid oxidation derivates with increasing amounts of dietary DHA [8,13]. According to the differential oxidizability of single PUFAs, mixtures with higher proportions of EPA should produce lower CD levels than other EPA:DHA combinations; nevertheless, the 1:1 EPA:DHA ratio showed a tendency toward lower CD values than 2:1 EPA:DHA during stomach digestion (not statistically different), suggesting that the combination of both PUFAs also influences the general stability of the mixture; overall, a balanced ratio of EPA:DHA seems to be the most resistant to oxidation.
As expected, an analysis of jejunal and ileal TIM-1 dialysates showed that fatty acids were mainly taken up in the jejunal section at the beginning of digestion, and the highest absorption in the last hours occurred in the ileal section. Higher levels of bioaccessible PUFAs were found in soybean and 1:1 diets, while lowest levels were found in dialysates of the 1:2 and 2:1 diets. Thus, there is an inverse correlation between the extent of stomach oxidation and the level of bioaccesible PUFAs: the highest CD production in the stomach correlates with the lowest concentration of bioaccesible lipids. The TIM-1 small intestine model uses semipermeable membranes that discriminate the absorption of lipids by molecular size [23]. Accordingly, it is possible that the polarity of the newly formed oxidized PUFA may increase interactions between hydroperoxides and other food substrates, resulting in the generation of bigger complexes that do not cross the filters to become “bioaccessible.”
To better explore the assimilation of nonoxidized and oxidized PUFAs, and relative combinations of EPA and DHA specifically, the Caco-2 cell line was used. Caco-2 cells are considered a reasonable model for studying lipid assimilation by intestinal enterocytes [25]. We found a significant reduction in uptake when PUFAs were partially oxidized under conditions similar to those found in the stomach compared to nonoxidized PUFAs. These results are in agreement with Maestre et al. who found a dramatic reduction of DHA assimilation in Caco-2 cells after extensive in vitro oxidation using lipoxygenase [17]. Moreover, assimilation results in the cell model, and bioaccessibility data obtained in the TIM-1 experiment are closely correlated.
Several studies have correlated an increase in oxidative stress with damage to cell structure and functionality. Higher concentrations of lipid hydroperoxides may induce a loss of membrane integrity, alterations in membrane fluidity and dysregulation of membrane transport mechanisms, including alterations in protein-mediated transport and changes in permeability [39,40]. Such membrane alterations may, in turn, reduce the efficiency of PUFA absorption.
The decrease in the assimilation of oxidized PUFA was more pronounced when cells were incubated with DHA than EPA. Thus, it is possible that the oxidized DHA molecule induced more oxidative damage to the cell membranes or to resident membrane transport proteins [39,40], thereby affecting uptake efficiency as Maestre et al. [17] observed. Interestingly, a similar trend was observed in the set of experiments that compared the absorption of oxidized and nonoxidized EPA:DHA ratios. The greatest reduction of uptake due to oxidation was achieved when the proportion of DHA was more elevated in the mixture. Nevertheless, the lowest reduction of the uptake due to the oxidation was not achieved with the higher proportion of EPA, but rather. it was produced by the 1:1 ratio of EPA:DHA. These data further indicate that not only the PUFA molecule itself but also the combination of both PUFAs strongly influences the stability and oxidation of the mixture, and thus, the equimolar ratio seems to be the most resistant to oxidation leading to higher PUFA assimilation.
An examination of the metabolic fate of EPA and DHA showed that DHA metabolism remained largely unaltered after the gastric oxidation as observed by Maestre et al. [17]. Nevertheless, oxidation of EPA produced some changes in PL, TG, DG and FFA levels. Interestingly, the oxidation of EPA:DHA 1:1 did not change the metabolic fate of PUFAs compared to nonoxidized one; however, oxidized 1:2 and 2:1 ratios produced some changes in the metabolism of EPA and DHA compared to nonoxidized mixtures. Balanced 1:1 diet showed the lower oxidation level and minor metabolite changes after oxidation. Further experiments will be needed to understand the underlying mechanism by which the oxidative stability of the PUFAs may be correlated with their metabolic fate.
In summary, an in vitro model of the gastrointestinal digestive tract and cultured small intestine enterocytes were used to delve into the potential mechanisms underlying the health effects of marine lipids. Taken together, the results of gastric stability, bioaccessibility and cellular PUFA assimilation support the hypothesis that an increase in the PUFA oxidation level during the gastric digestion phase leads to a decreased amount of bioaccessible products and a reduction of cell assimilation efficiency. Furthermore, the results highlight the different stability of EPA and DHA under simulated stomach conditions, and the importance of the EPA:DHA ratio in the intestinal assimilation of PUFAs. Interestingly, the balanced ratio of 1:1 EPA:DHA produces the lowest degree of PUFA oxidation and the highest level of absorption. Therefore, not only the specific characteristics of individual PUFAs but the proportion between EPA and DHA in the mixture can exert a strong influence in determining the final level of absorption. Interestingly, previous studies in rats comparing several EPA:DHA ratios showed that a balanced 1:1 diet was the most effective combination to mitigate inflammation, oxidative stress and metabolic disorders [8,13]. The present findings therefore shed light on the possible underlying mechanism behind these observations and highlight the importance of dietary design for improving PUFA stability during gastrointestinal digestion in order to potentiate their bioavailability and further health benefits.
Supplementary Material
Acknowledgments
This work was supported by the Spanish Ministerio de Economía y Competitividad (AGL2013-49079-C2-1-R) and the National Institutes of Health (R01-DK38389). The Consejo Superior de Investigaciones Científicas and the University of Santiago de Compostela are gratefully acknowledged for the doctoral fellowship to Gabriel Dasilva. Xunta de Galicia and Axencia Galega de Innovación are also thankfully recognized for the financial support to Gabriel Dasilva. The authors thank Ms. Sarala Kodukula and Ms. Yin Xiu Zhou for excellent technical assistance.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jnutbio.2017.11.007.
References
- [1].Guéraud F, Taché S, Steghens J-P, Milkovic L, Borovic-Sunjic S, Zarkovic N, et al. Dietary polyunsaturated fatty acids and heme iron induce oxidative stress biomarkers and a cancer promoting environment in the colon of rats. Free Radic Biol Med 2015;83:192–200. [DOI] [PubMed] [Google Scholar]
- [2].Rom O, Jeries H, Hayek T, Aviram M. Supplementation with linoleic acid-rich soybean oil stimulates macrophage foam cell formation via increased oxidative stress and diacylglycerol acyltransferase1-mediated triglyceride biosynthesis. Biofactors 2017;43:100–16. [DOI] [PubMed] [Google Scholar]
- [3].Tholstrup T, Hellgren LI, Petersen M, Basu S, Straarup EM, Schnohr P, et al. A solid dietary fat containing fish oil redistributes lipoprotein subclasses without increasing oxidative stress in men. J Nutr 2004;134:1051–7. [DOI] [PubMed] [Google Scholar]
- [4].Kikugawa K, Yasuhara Y, Ando K, Koyama K, Hiramoto K, Suzuki M. Protective effect of supplementation of fish oil with high n-3 polyunsaturated fatty acids against oxidative stress-induced DNA damage of rat liver in vivo. J Agric Food Chem 2003;51:6073–9. 10.1021/jf030141v. [DOI] [PubMed] [Google Scholar]
- [5].Brahmbhatt V, Oliveira M, Briand M, Perrisseau G, Schmid VB, Destaillats F, et al. Protective effects of dietary EPA and DHA on ischemia-reperfusion-induced intestinal stress. J Nutr Biochem 2013;24:104–11. 10.1016/j.jnutbio.2012.02.014. [DOI] [PubMed] [Google Scholar]
- [6].Neilson AP, Djuric Z, Ren J, Hong YH, Sen A, Lager C, et al. Effect of cyclooxygenase genotype and dietary fish oil on colonic eicosanoids in mice. J Nutr Biochem 2012; 23:966–76. 10.1016/j.jnutbio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, et al. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr 2009;139:804S–19S. 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dasilva G, Pazos M, García-Egido E, Gallardo JM, Rodríguez I, Cela R, et al. Healthy effect of different proportions of marine ω−3 PUFAs EPA and DHA supplementation in Wistar rats: lipidomic biomarkers of oxidative stress and inflammation. J Nutr Biochem 2015;26:1385–92. 10.1016/j.jnutbio.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [9].Méndez L, Pazos M, Gallardo JM, Torres JL, Pérez-Jiménez J, Nogués R, et al. Reduced protein oxidation in Wistar rats supplemented with marine ω3 PUFAs. Free Radic Biol Med 2013;55:8–20. 10.1016/j.freeradbiomed.2012.11.004. [DOI] [PubMed] [Google Scholar]
- [10].Mendez L, Pazos M, Giralt M, Nogues MR, Perez-Jimenez J, Torres JL, et al. Targets of protein carbonylation in spontaneously hypertensive obese Koletsky rats and healthy Wistar counterparts: a potential role on metabolic disorders. J Proteomics 2014;106:246–59. 10.1016/j.jprot.2014.04.036. [DOI] [PubMed] [Google Scholar]
- [11].Lluís L, Taltavull N, Muñoz-Cortés M, Sánchez-Martos V, Romeu M, Giralt M, et al. Protective effect of the omega-3 polyunsaturated fatty acids: Eicosapentaenoic acid/docosahexaenoic acid 1:1 ratio on cardiovascular disease risk markers in rats. Lipids Health Dis 2013;12:1–8. 10.1186/1476-511X-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Molinar-Toribio E, Perez-Jimenez J, Ramos-Romero S, Lluis L, Sanchez-Martos V, Taltavull N, et al. Cardiovascular disease-related parameters and oxidative stress in SHROB rats, a model for metabolic syndrome. PLoS One 2014;9:e104637 10.1371/journal.pone.0104637ER. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dasilva G, Pazos M, García-Egido E, Pérez-Jiménez J, Torres JL, Giralt M, et al. Lipidomics to analyze the influence of diets with different EPA:DHA ratios in the progression of metabolic syndrome using SHROB rats as a model. Food Chem 2016;205:196–203. 10.1016/j.foodchem.2016.03.020. [DOI] [PubMed] [Google Scholar]
- [14].Gorelik S, Ligumsky M, Kohen R, Kanner J. The stomach as a “bioreactor”: when red meat meets red wine. J Agric Food Chem 2008;56:5002–7. 10.1021/jf703700d. [DOI] [PubMed] [Google Scholar]
- [15].Larsson K, Tullberg C, Alminger M, Havenaar R, Undeland I. Malondialdehyde and 4-hydroxy-2-hexenal are formed during dynamic gastrointestinal in vitro digestion of cod liver oils. Food Funct 2016;7:3458–67. 10.1039/C6FO00635C. [DOI] [PubMed] [Google Scholar]
- [16].Larsson K, Harrysson H, Havenaar R, Alminger M, Undeland I. Formation of malondialdehyde (MDA), 4-hydroxy-2-hexenal (HHE) and 4-hydroxy-2-nonenal (HNE) in fish and fish oil during dynamic gastrointestinal in vitro digestion. Food Funct 2016;7:1176–87. [DOI] [PubMed] [Google Scholar]
- [17].Maestre R, Douglass JD, Kodukula S, Medina I, Storch J. Alterations in the intestinal assimilation of oxidized PUFAs are ameliorated by a polyphenol-rich grape seed extract in an in vitro model and Caco-2 cells. J Nutr 2013;143:295–301. 10.3945/jn.112.160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lapidot T, Granit R, Kanner J. Lipid peroxidation by “free” iron ions and myoglobin as affected by dietary antioxidants in simulated gastric fluids. J Agric Food Chem 2005;53:3383–90. 10.1021/jf040402g. [DOI] [PubMed] [Google Scholar]
- [19].Frankel EN. Lipid oxidation. West Ferry, Dundee, Scotland: The Oily Press LTD; 1998. [Google Scholar]
- [20].Penumetcha M, Khan N, Sampath P. Dietary oxidized fatty acids: an atherogenic risk? J Lipid Res 2000;41:1473–80. [PubMed] [Google Scholar]
- [21].Minekus M, Marteau P, Havenaar R, Huisintveld JHJ. A multicompartmental dynamic computer-controlled model simulating the stomach and small-intestine. Altern Lab Anim 1995;23:197–209. [Google Scholar]
- [22].Deat E, Blanquet-Diot S, Jarrige J-F, Denis S, Beyssac E, Alric M. Combining the dynamic TNO-gastrointestinal tract system with a Caco-2 cell culture model: application to the assessment of lycopene and alpha-tocopherol bioavailability from a whole food. J Agric Food Chem 2009;57:11314–20. 10.1021/jf902392a. [DOI] [PubMed] [Google Scholar]
- [23].Ribnicky DM, Roopchand DE, Oren A, Grace M, Poulev A, Lila MA, et al. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem 2014;142:349–57. 10.1016/j.foodchem.2013.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pinto M, Robineleon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, et al. Enterocyte-like differentiation and polarization of the human-colon carcinoma cell-line Caco-2 in culture. Biol Cell 1983;47:323–30. [Google Scholar]
- [25].Ho SP, Storch J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am J Physiol Cell Physiol 2001;281:C1106–17. [DOI] [PubMed] [Google Scholar]
- [26].Delie F, Rubas W. A human colonic cell line sharing similarities with enterocytes as a model to examine oral absorption: advantages and limitations of the Caco-2 model. Crit Rev Ther Drug Carrier Syst 1997;14:221–86. [PubMed] [Google Scholar]
- [27].Chapman RA, Mackay K. The estimation of peroxides in fats and oils by the ferric thiocyanate method. J Am Oil Chem Soc 1949;26:360–3. 10.1007/BF02651444. [DOI] [Google Scholar]
- [28].Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 1986;27:114–20. [PubMed] [Google Scholar]
- [29].Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–7. [DOI] [PubMed] [Google Scholar]
- [30].Kim RS, Labella FS. Comparison of analytical methods for monitoring autoxidation profiles of authentic lipids. J Lipid Res 1987;28:1110–7. [PubMed] [Google Scholar]
- [31].Herbes SE, Allen CP. Lipid quantification of fresh-water invertebrates — method modification for microquantitation. Can J Fish Aquat Sci 1983;40:1315–7. [Google Scholar]
- [32].Trotter PJ, Storch J. Fatty-acid uptake and metabolism in a human intestinal-cell line (Caco-2) — comparison of apical and basolateral incubation. J Lipid Res 1991; 32:293–304. [PubMed] [Google Scholar]
- [33].Trotter PJ, Ho SY, Storch J. Fatty acid uptake by Caco-2 human intestinal cells. J Lipid Res 1996;37:336–46. [PubMed] [Google Scholar]
- [34].Gorelik S, Kohen R, Ligumsky M, Kanner J. Saliva plays a dual role in oxidation process in stomach medium. Arch Biochem Biophys 2007;458:236–43. 10.1016/j.abb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- [35].Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265–75. [PubMed] [Google Scholar]
- [36].Pérez-Martinez P, Pérez-Jimenez F, López-Miranda J. N-3 PUFA and lipotoxicity. Biochim Biophys Acta 2010;1801:362–6. 10.1016/j.bbalip.2009.09.010. [DOI] [PubMed] [Google Scholar]
- [37].McDaniel JC, Massey K, Nicolaou A. Fish oil supplementation alters levels of lipid mediators of inflammation in microenvironment of acute human wounds. Wound Repair Regen 2011;19:189–200. 10.1111/j.1524-475X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Di Nunzio M, Valli V, Bordoni A. Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot Essent Fatty Acids 2011;85:121–7. 10.1016/j.plefa.2011.07.005. [DOI] [PubMed] [Google Scholar]
- [39].Davies KJA, Lin SW, Pacifici RE. Protein damage and degradation by oxygen radicals. 4. Degradation of denatured protein. J Biol Chem 1987;262:9914–20. [PubMed] [Google Scholar]
- [40].Wijeratne SSK, Cuppett SL. Lipid hydroperoxide induced oxidative stress damage and antioxidant enzyme response in Caco-2 human colon cells. J Agric Food Chem 2006;54:4476–81. 10.1021/jf060475v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.