Abstract
Pneumococcal polysaccharide vaccine has been licensed for use in the United States for >30 years, and two-thirds of the elderly population in the United States have received this vaccine. Observational studies have demonstrated that pneumococcal polysaccharide vaccine reduces the risk of invasive pneumococcal disease in immunocompetent elderly individuals, but neither observational studies nor clinical trials have demonstrated consistent evidence for a reduction in the incidence of pneumonia in vaccinated older adults. The introduction of pneumococcal protein conjugate vaccine among children has led to a herd immunity effect that has resulted in a 38% decrease in the rate of invasive pneumococcal disease among elderly adults. The high efficacy of pneumococcal protein conjugate vaccine in children has renewed interest in evaluating pneumococcal protein conjugate vaccines in adults for prevention of invasive pneumococcal disease and pneumonia. Moreover, the recognition of the presence and function of noncapsular pneumococcal protein antigens and the increasing availability of adjuvants highlight the promise of new vaccination strategies to decrease the burden of pneumococcal infection in this high-risk population.
More than 30 years after licensure, pneumococcal polysaccharide vaccine (PPV) is now given to two-thirds of elderly people in the United States (figure 1) [1]. Fairly consistent results from observational studies have demonstrated that PPV reduces the risk of invasive pneumococcal disease (IPD) in immunocompetent older adults (table 1) [16]. However, questions remain regarding the degree to which the vaccine’s effectiveness against IPD varies by age of recipient, time since vaccination, and the presence of underlying disease and, in particular, whether PPV prevents nonbacteremic pneumococcal pneumonia. The value of revaccination is also uncertain. Moreover, the changing epidemiology of IPD following the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7) in children has altered the vaccine-preventable disease burden in older adults and, correspondingly, the potential magnitude of the benefit of PPV. Finally, newer vaccines, such as PCVs [17, 18] or those employing serotype-independent antigens, offer the potential to provide clinical protection against pneumococcal infection in the growing population of older adults in the 21st century.
Figure 1.
Pneumococcal polysaccharide vaccine coverage among elderly adults in the United States, 1989–2007. Data adapted from the Behavioral Risk Factor Surveillance System survey [1].
Table 1.
Observational studies of pneumococcal polysaccharide vaccine effectiveness (VE) against invasive pneumococcal disease (IPD) in older adults.
| Reference, study design | Study population | Subgroup | No. of cases of IPD | VEa (95% CI) | |
|---|---|---|---|---|---|
| Shapiro and Clemens [2] | |||||
| Case control | Adults with an indication for pneumococcal vaccination admitted to Yale-New Haven hospital in Connecticut | All | 90 | 67 (13–87) | |
| Immunocompromised | 20 | 0 (−1228 to 93 | |||
| Forrester et al. [3] | |||||
| Case control | Persons ≥55 years of age admitted to the Denver Veterans Administration hospital | All | 89 | −34 (−176 to 35) | |
| Indirect cohortb | Persons ≥55 years of age admitted to the Denver Veterans Administration hospital | All | 89 | −21 (−221 to 55) | |
| Sims et al. [4] | |||||
| Case control | Immunocompetent persons ≥55 years of age admitted to 1 of 5 hospitals in Pennsylvania | All | 122 | 70 (36–86) | |
| Shapiro et al. [5] | |||||
| Case control | Adults with an indication for pneumococcal vaccination admitted to 1 of 11 hospitals in Connecticut | All | 983 | 56 (42–67) | |
| Immunocompetent | 808 | 61 (47–72) | |||
| Immunocompromised | 175 | 21 (−55 to 60) | |||
| Indirect cohortb | Adults with an indication for pneumococcal vaccination admitted to 1 of 11 hospitals in Connecticut | All | 932 | 48 (3–72) | |
| Butler et al. [6] | |||||
| Indirect cohortb | Persons ≥5 years of age in the United States | All | 2837 | 57 (45–66) | |
| Immunocompetent, ≥65 years of age | 525 | 75 (57–85) | |||
| Anatomic asplenia, excluding persons with sickle cell disease | 166 | 77 (14–95) | |||
| Farr et al. [7] | |||||
| Case control | Persons ≥2 years of age with an indication for pneumococcal vaccination admitted to the University of Virginia Health Sciences Center in Virginia | All | 85 | 81 (34–94) | |
| Benin et al. [8] | |||||
| Case control | Navajo adults ≥18 years of age with an indication for vaccination | All | 108 | 26 (−29 to 58) | |
| Indirect cohortb | Navajo adults ≥18 years of age | All | 278 | 35 (−33 to 69) | |
| Jackson et al. [9] | |||||
| Cohort | Community-dwelling members of a Washington State health plan ≥65 years of age | All | 61 | 44 (7–67) | |
| Immunocompetent | 39 | 54 (13–76) | |||
| Immunocompromised | 22 | 22 (−87 to 68) | |||
| Hedlund et al. [10] | |||||
| Cohort | Residents of Stockholm County, Sweden, ≥65 years of age who were invited to receive influenza and pneumococcal vaccination in the fall of 1998 | All | 40 | 48 (3–72) | |
| Andrews et al. [11] | |||||
| Indirect cohortb | Persons ≥65 years of age in Victoria, Australia | All | 98 | 79 (−14 to 96) | |
| Dominguez et al. [12] | |||||
| Case control | Persons ≥65 years of age admitted to 12 hospitals in Catalonia, Spain | All | 149 | 70 (48–82) | |
| Immunocompetent | 103 | 76 (51–88) | |||
| Immunocompromised | 31 | 50 (−44 to 82) | |||
| Vila-Córcoles et al. [13] | |||||
| Cohort | Community-dwelling adults ≥65 years of age in Catalonia, Spain | All | 22 | 40 (−165 to 78) | |
| Singleton et al. [14] | |||||
| Indirect cohortb | Native Alaskan adults ≥20 years of age | All | 300 | 75 (27–91) | |
| Mooney et al. [15] | |||||
| Screening method | Persons ≥65 years of age in Scotland | Immunocompetent | 135 | 62 (45–73) | |
| Immunocompromised | 35 | 37 (−80 to 70) | |||
VE is a percentage calculated as 1 – relative risk of disease in vaccinated versus nonvaccinated individuals. A negative VE estimate indicates a higher risk among vaccinated individuals, compared with nonvaccinated persons; a 0% VE estimate indicates no difference in risk between vaccinated and nonvaccinated individuals; and a positive VE indicates a lower risk among vaccinated individuals, compared with nonvaccinated persons. If the 95% CI for the VE estimate extends from negative to positive or includes zero, the VE estimate is not considered to be statistically significant.
The indirect cohort design includes only cases of IPD; VE is estimated by comparing the distribution of pneumococcal serotypes in vaccinated and nonvaccinated persons with IPD. This serotype distribution method is based on the assumption that, if the vaccine is effective, vaccine serotypes will be less common among vaccinated persons, than among nonvaccinated persons.
EVIDENCE FOR PPV EFFECTIVENESS IN OLDER ADULTS
The licensure of a 14-valent PPV in the United States in 1977 (replaced by the current 23-valent formulation in 1983) was supported primarily by the results of clinical trials conducted among a unique population of young adults with very high rates of pneumococcal infection, gold miners in South Africa [19, 20]. These results are not necessarily generalizable to older adults and persons with chronic illness. For this reason, evidence regarding vaccine effectiveness in groups targeted for vaccination in the United States and other developed countries is derived from studies conducted or reported after PPV was licensed. Those studies have included observational studies, such as case-control and cohort studies, most of which have assessed vaccine effectiveness against the outcome of IPD (defined by isolation of Streptococcus pneumoniae from a normally sterile site, such as the bloodstream or CSF), as well as clinical trials, which have typically evaluated the more common but less specific outcome of all-cause pneumonia.
Effectiveness against IPD.
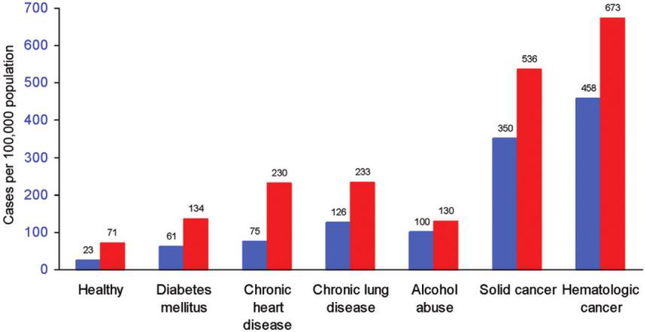
The ~24,000 cases of IPD yearly among adults aged ≥50 years in the United States result in 4500 deaths [21]. Observational studies provide fairly consistent support for the effectiveness of PPV in reducing the risk of IPD in immunocompetent older adults (table 1). These data provide the basis for the recommendations to administer PPV to older adults and adults with chronic illness. The largest case-control study of PPV effectiveness was reported by Shapiro et al. [5] and evaluated a total of 983 cases of IPD; the study identified a 56% reduction in the risk of IPD due to the vaccine serotypes of S. pneumoniae among immunocompetent older adults, which is consistent with the results of most other case-control and cohort studies performed among older adults [7, 9, 12], as well as those of a study that employed a novel indirect-cohort methodology [6]. In contrast, observational studies involving immunocompromised older adults, who are at a greatly increased risk of IPD (figure 2) [22], do not support a protective effect of PPV against IPD.
Figure 2.
Risk of invasive pneumococcal disease in elderly adults, by age group and chronic illness category. Blue bars, aged 65–79 years; red bars, aged ≥80 years. Figure adapted from Kyaw et al. [22].
Among immunocompetent older adults, vaccine effectiveness may decrease both with age and with time since vaccination, as demonstrated in the study by Shapiro et al. (figure 3) [5]. However, in a large, indirect-cohort study that included primarily adults >50 years of age, vaccine effectiveness was stable over time [6]. Although the results provided by these 2 studies are inconsistent, because pneumococcal capsular antibody levels decrease substantially with time from vaccination and because decreases in clinical efficacy over time have been demonstrated with other unconjugated polysaccharide vaccines, it can be postulated that the clinical effectiveness of PPV in older adults also diminishes over time.
Figure 3.
Pneumococcal vaccine effectiveness against invasive pneumococcal disease by age of recipient and time since vaccination. The point estimate of vaccine effectiveness and 95% CI are indicated. Within each age group, 3 data points represent the vaccine effectiveness at <3 years, 3–5 years, and >5 years since vaccination, from left to right. Data adapted from Shapiro et al. [5].
Effectiveness against all-cause pneumonia.
Nonbacteremic pneumonia comprises the majority of morbidity and mortality associated with pneumococcal disease. In older adults, pneumococcal bacteremia is usually associated with pneumonia, but only 10%–15% of all cases of pneumococcal pneumonia are associated with documented bacteremia. Overall, S. pneumoniae is believed to be the leading cause of community-acquired pneumonia in older adults. Because community-acquired pneumonia is common, with ~915,000 cases per year in the elderly population in the United States [23], the burden of nonbacteremic pneumococcal pneumonia is substantial. Determining the efficacy of PPV against nonbacteremic pneumococcal pneumonia is of considerable clinical importance, but these efforts have been complicated by the limitations of available diagnostic tests for accurately defining the etiology of nonbacteremic pneumonia. Thus, for most of the clinical trials performed, the primary outcome has been all-cause pneumonia.
Among 6 clinical trials that evaluated vaccine effectiveness against all-cause pneumonia in older adults (table 2) [24–30], only 1 [25] demonstrated a reduction in risk in the group that received PPV. That study, which evaluated residents of long-term care institutions in France, did not include a placebo group, and neither subjects nor investigators were blinded to vaccine assignment. Those limitations of the study design have raised questions regarding the validity of the findings [16, 33]. None of the other 5 trials, including an NIH-sponsored trial involving 13,600 older adults [24] and a trial in Finland involving 26,925 elderly adults [29], have demonstrated a significant vaccine-associated reduction in risk of all-cause pneumonia. Many of the trials also evaluated an end point of pneumococcal pneumonia, which was variably defined depending on the trial. However, none found a significant difference in the risk of pneumococcal pneumonia in prespecified analyses of all subjects. The clinical trial results suggest that PPV is not highly effective against nonbacteremic pneumococcal pneumonia but leaves open the possibility that the vaccine does lead to a reduction in risk of pneumococcal pneumonia that is not reflected by a detectable difference in risk of the broader outcome of all-cause pneumonia evaluated in the trials.
Table 2.
Vaccine effectiveness (VE) against all-cause pneumonia reported by clinical trials in older adults.
| Reference | Vaccine valency | Study population | VEa (95% CI) | No. of cases of pneumonia/no. of vaccinated persons | No. of cases of pneumonia/no. of nonvaccinated person |
|---|---|---|---|---|---|
| Austrian [24]b | |||||
| Study 1 | 12 | Inpatients at the Dorothea Dix psychiatric hospital in Raleigh, North Carolina | −22 (−49 to 0) | 154/607 | 144/693 |
| Study 2 | 12 | Members of the Kaiser Permanente Health Plan in San Francisco ≥45 years of age | 2 (−16 to 7) | 268/6782 | 274/6818 |
| Gaillat et al. [25]c | 14 | Residents of 48 long-term care institutions in France | 79 (53 to 91) | 7/937 | 27/749 |
| Simberkoff et al. [26] | 14 | US veterans, immunocompetent, and either aged ≥55 years or with renal, hepatic, cardiac, or pulmonary disease; alcoholism; or diabetes mellitus | −39 (−110 to 8) | 56/1145 | 41/1150 |
| Koivula et al. [27] | 14 | Residents of age of a small town in Finland ≥60 years | −17 (−66 to 17) | 69/1364 | 64/1473 |
| Örtqvist et al. [28] | 23 | Immunocompetent persons 50–85 years of age who had been previously discharged after a hospitalization for community-acquired pneumonia in Sweden | −20 (−72 to 11) | 63/339 | 57/352 |
| Honkanen et al. [29] | 23 | Persons ≥65 years of age in Northern Finland | −20 (−50 to 10) | 145/13980 | 116/12945 |
| Alfageme et al. [30] | 23 | Immunocompetent patients with COPD 61–73 years of age in Seville, Spain | 3 (−52 to 38) | 33/298 | 34/298 |
NOTE. COPD, chronic obstructive pulmonary disease.
VE is a percentage calculated as 1 – relative risk of disease in vaccinated versus nonvaccinated individuals. A negative VE estimate indicates a higher risk among vaccinated individuals, compared with nonvaccinated persons; a 0% VE estimate indicates no difference in risk between vaccinated and nonvaccinated individuals; and a positive VE indicates a lower risk among vaccinated individuals, compared with nonvaccinated persons. If the 95% CI for the VE estimate extends from negative to positive or includes zero, the VE estimate is not considered to be statistically significant.
Study results obtained from Broome [31].
Study results obtained from Fedson and Liss [32].
In contrast with the clinical trials, observational studies that evaluated vaccine effectiveness against all-cause pneumonia have yielded somewhat conflicting results. Neither a cohort study involving 47,365 older adults in a health maintenance organization in Washington State [9] nor a case-cohort study of elderly patients in Australia [34] identified a reduction in the risk of pneumonia associated with vaccination. In contrast, a cohort study involving 11,241 older adults in Spain reported that PPV was associated with reductions in the risk of all community-acquired pneumonia, hospitalization with community-acquired pneumonia, and pneumonia mortality [13]. Although a study involving 1898 elderly individuals with chronic lung disease in Minnesota reported a 43% reduction in the risk of pneumonia hospitalization and a 29% reduction in the risk of all-cause mortality [35], an assessment of the subgroup of 1298 older patients with lung disease from the cohort evaluated in Spain did not find a significant reduction of the risk of pneumonia with vaccination [36]. Thus, observational study assessments of the association of PPV with risk of all-cause pneumonia do not provide consistent support for vaccine effectiveness.
CHANGING EPIDEMIOLOGY OF IPD
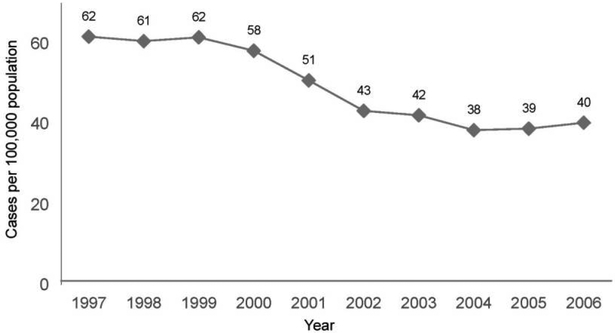
The introduction of PCV7 among children in 2000 has led to important changes in the epidemiology of IPD in older adults in the United States. Of the ~92 different S. pneumoniae serotypes, 7 of the most common serotypes causing IPD in children are included in PCV7 (4, 6B, 9V, 14, 18C, 19F, and 23F). The 23-valent PPV includes those 7 serotypes, as well as an additional 16 (1, 2, 3, 5, 7F, 8, 9N, 10A, 11A, 12F, 15B, 17F, 19A, 20, 22F, and 33F), which were selected primarily because of their contribution to IPD in adults. Following the introduction of PCV7 for children, overall rates of IPD have decreased markedly in the elderly population (figure 4), and those decreases are attributable to reductions in the rates of disease caused by the PCV7 serotypes. Rates of disease due to those 7 serotypes among older adults decreased from 34.5 cases per 100,000 population per year during 1998–1999 to 8.2 cases per 100,000 population in 2004 (table 3) [38]. During this interval, rates of IPD due to some non-PCV7 serotypes have increased modestly, including rates of disease due to serotypes included in PPV (especially 19A [39]) and serotypes not present in either vaccine (such as 23A). However, the overall effect of these changes is that the rate of IPD due to the 23 serotypes of S. pneumoniae represented in PPV has decreased in older adults, from 51.7 cases per 100,000 population during 1998–1999 to 26.9 cases per 100,000 population in 2004. Assuming that the reductions in disease risk in the elderly population following PCV7 introduction in children are attributable to decreases in the transmission of PCV7 strains in the population, the magnitude of the potential benefit derived from PPV in older adults has also decreased, because older adults are now at lower risk of acquiring disease due to the PCV7 strains. This also implies that previous estimates of the cost-effectiveness of PPV administration in elderly adults [40] may need to be updated to reflect the current epidemiology of vaccine-preventable disease.
Figure 4.
Rates of invasive pneumococcal disease in adults ≥65 years of age in the United States, 1997–2006. The 7-valent pneumococcal conjugate vaccine was introduced for children in 2000. Data adapted from the Centers for Disease Control and Prevention Active Bacterial Core Surveillance System [37].
Table 3.
Epidemiology of invasive pneumococcal disease (IPD) in adults ≥65 years of age before (1998–1999) and after (2004) the introduction of 7-valent pneumococcal conjugate vaccine (PCV7) in young children in 2000.
| 1998–1999 | 2004 | |||
|---|---|---|---|---|
| Pneumococcal serotypes | No. of IPD cases per 100,000 population | Percentage of all IPD cases | No. of IPD cases per 100,000 population | Percentage of all IPD cases |
| All | 61.5 | 100 | 38.0 | 100 |
| 7 Serotypes in PCV7 (which are also in PPV) | 34.5 | 56 | 8.2 | 22 |
| 16 Serotypes in PPV but not in PCV7 | 17.2 | 28 | 18.7 | 49 |
| All 23 serotypes in PPV | 51.7 | 84 | 26.9 | 71 |
| Serotypes not in PPV | 9.8 | 16 | 11.1 | 29 |
NOTE. PPV, pneumococcal polysaccharide vaccine. Data adapted from Hicks et al. [38].
CONSIDERATIONS FOR REVACCINATION
Revaccination is attractive, because unconjugated polysaccha-ride vaccines likely do not confer long-lasting protection, and the incidence of IPD among adults increases dramatically with age; there is a 3-fold increase in adults aged ≥85 years, compared with adults aged 65–74 years [21]. The Advisory Committee on Immunization Practices recommendations for revaccination indicate that all elderly adults should receive a dose of PPV on or after their 65th birthday (and at least 5 years after an earlier dose for those vaccinated prior to 65 years of age), but they do not recommend that >1 PPV dose be given after the 65th birthday [41]. The policy of a single PPV for older adults has important implications, as reported in a modeling study by Fry et al. [42]. They assumed that 1-time vaccination of immunocompetent adults 65 years of age is associated with a vaccine effectiveness against IPD of 75% in the 5 years after vaccination, 37% in the next 5 years, 18% in the 5 years after that, and 0% thereafter. In addition, 85% of IPD in older adults was assumed to be due to PPV serotypes, and vaccine effectiveness was assumed to be 0% for immunocompromised persons. In this model, an ongoing strategy that achieved vaccination of nearly all (90%) of the adults 65 years of age in the United States would prevent only 21% of cases of IPD in persons ≥65 years of age, most of which would be prevented in the first 5 years after vaccination. If effective, revaccination would increase the proportion of IPD cases prevented by vaccination.
Current recommendations for revaccination reflect the uncertainty in estimating the benefit of revaccination. This uncertainty stems, in part, from concern that vaccination with unconjugated pneumococcal polysaccharide antigens may induce hyporesponsiveness on rechallenge. Several studies have documented that elderly patients who are given a second dose of PPV ≥4 years after an initial vaccination demonstrate a significant increase in serotype-specific anticapsular IgG antibody levels after the second vaccination [17, 43]. As summarized in an excellent recent review [44], there is concern that, although revaccination leads to an immunological response, administration of a first dose of unconjugated polysaccharide vaccine may blunt the immune response to subsequent doses. If this occurs, the lower antibody levels generated by a second vaccination, compared with those levels generated by the first vaccination, may potentially be associated with a corresponding decrease in the magnitude of clinical protection provided by revaccination.
Hyporesponsiveness may occur after administration of PPV in adults. In a longitudinal study involving 61 elderly individuals who received a second PPV dose an average of 5 years after a first dose, the serotype-specific antibody concentrations were consistently lower after the second vaccination, compared with after the first vaccination (figure 5) [43]. Moreover, in a recent clinical trial of PCV7 in elderly individuals, those who received PCV7 1 year after receiving PPV had lower postvaccination ELISA geometric mean concentrations and functional opsonophagocytic activity geometric mean titers than did PPV-naive older adults who were given PCV7 as their first pneumococcal vaccination [18]. The results from that trial suggest that administration of PPV blunted the immune response to PCV7 administered 1 year later. Whether the hyporesponsiveness induced by PPV may decrease with time after vaccination is not well defined.
Figure 5.
Geometric mean concentrations (GMCs) of serotype-specific antibodies measured by use of ELISA in adults ≥50 years of age who were observed through their first and second pneumococcal polysaccharide vaccinations. Note that the y-axis scale for serotype 14 differs from that for the other serotypes. Column indicators are defined as follows: A, before first vaccination; B, 4 weeks after first vaccination; C, one year after first vaccination; D, immediately prior to revaccination 4–7 years after first vaccination; E, 4 weeks after revaccination. Data adapted from Torling et al. [43].
The clinical significance, if any, of lower antibody levels after a second PPV dose, compared with those after a first PPV dose, is difficult to judge, because there is no immune correlate of protection for pneumococcal infection in adults. It would, therefore, be helpful to know whether the clinical effectiveness of a second PPV dose is lower than that of a first PPV dose; however, such studies are difficult to conduct, because IPD is a relatively uncommon outcome, and most adults receive only a single PPV dose. Two studies conducted in special populations with relatively high rates of revaccination have assessed the effectiveness of revaccination, but the limited data do not provide conclusive results. The first study, an indirect-cohort study of native Alaskan adults, reported similar point estimates of vaccine effectiveness (VE) for first vaccination (VE, 75%; 95% CI, 19%–92%) and revaccination (VE, 74%; 95% CI, <0% to 94%), although the estimates for revaccination were not statistically significant [14]. The second study, a case-control study of the risk of IPD in Navajo adults, reported that revaccination was not associated with a reduction in the risk of IPD (VE, 40%; 95% CI, −27% to 72%), but that study also did not document an overall effectiveness of PPV, and the estimate of effectiveness following revaccination was not significantly different from that following any vaccination [8].
Finally, despite earlier reports of a greater-than-expected frequency and severity of local injection site reactions in healthy children and adults after revaccination, more-recent studies involving older adults indicate that a second vaccination given ≥5 years after a first vaccination is well tolerated [43, 45–47]. Although local reactions are more common after a second PPV dose in older adults, they are generally mild and self-limited [48]. The available data from 2 retrospective studies also indicate that a third PPV dose is not associated with an increased risk of medically attended adverse events in adults [49, 50].
PCVS
The remarkable success of pneumococcal polysaccharide protein conjugate vaccines among young children [51, 52] has led to renewed interest in the possible use of these vaccines in older adults. Although evaluations of earlier PCV formulations did not consistently demonstrate higher antibody responses to PCV in older adults, compared with responses to PPV [53], more-recent evaluations of the licensed PCV7 formulation have shown such differences. A clinical trial comparing PPV with PCV7 in pneumococcal vaccine–naive adults aged ≥70 years found consistently higher ELISA geometric mean concentrations and opsonophagocytic activity geometric mean titers to the 7 serotypes of S. pneumoniae common to both vaccines after PCV7 administration [18]. In addition, although antibody levels decreased in the year after PCV7 administration, administration of a second PCV7 dose 1 year after the first dose led to an increase in antibody levels to values that were comparable with those achieved after the first immunization.
Another trial compared the response to varying dosages of PCV7 with the response to PPV in adults ≥70 years of age who had received PPV at least 5 years earlier [17]. There was little difference in immunological response to the standard pediatric dose of PCV7, compared with the response to PPV. However, there was a dose response to PCV7, and elderly individuals who were given twice the standard dose of PCV7 had a greater immune response to most of the PCV7 serotypes, compared with the individuals in the PPV group.
Possible advantages of PCVs in elderly adults may include higher levels of protection against the vaccine serotypes after vaccination and the ability to prolong the duration of protection by use of repeated vaccinations over time. The effectiveness of PCVs against pneumonia in children also raises the possibility that PCVs may protect against nonbacteremic pneumococcal pneumonia in adults. A limitation of PCVs, however, is that fewer serotypes are included in those vaccines than in PPV. A 13-valent PCV, which offers greater serotype coverage than PCV7, is currently being evaluated in clinical trials [54, 55]. However, if the routine use of this vaccine in children is associated with a herd immunity effect similar to that seen with PCV7, then the potential impact of direct vaccination of adults would be diminished. In that circumstance, the magnitude of the direct benefit from vaccination of adults would depend on the degree to which adults remained at risk for vaccine-preventable disease.
Serotype replacement, in which decreases in disease due to vaccine-type S. pneumoniae are counterbalanced by increases in disease due to non-vaccine serotypes, may also limit the impact of vaccines based on the capsular polysaccharides. This limitation would not be mitigated by enhancing vaccine potency by the use of more-effective adjuvants or by increasing the duration of protection by repeated vaccination. The impact of serotype replacement may potentially be overcome by use of a broader range of bacterial antigens and engagement of innate receptors and alternate mechanisms of defense, stimuli which may more fully recapitulate natural immunity against pneumococcal disease. Earlier vaccines that protected otherwise healthy but high-risk South African gold miners against pneumonia [19, 20] may have retained noncapsular antigens that are purified out of the current vaccine preparations. Indeed, antibodies that are elicited by colonization [56] to surface-intercalated proteins are associated with reduced carriage in mice and experimental colonization in humans [57]. Moreover, common pneumococcal proteins identified very recently by exploiting molecular microbiological and immunological techniques suggest intriguing new vaccine directions [58]. Engagement of innate immune receptors, such as toll-like receptors, by shared pneumococcal antigens, such as pneumolysin and cell wall polysaccharide that serve as both specific antigens and adjuvants, may enhance and broaden the protective response [59]. Pneumococcal pili and lysins provide other potential vaccine possibilities, as does induction of non–antibody-mediated CD4+ T cell–dependent mechanisms [60, 61]. Thus, integration of bacterial genomics, clinical and experimental animal and human immunology, and functional outcomes are providing new options for vaccine development, even while capsule-based vaccine development efforts are striving to improve serotype coverage, immunogenicity, and duration of response.
In summary, the decrease in rates of pneumonia [62, 63] and IPD among young children after the introduction of PCV7 indicates that eliciting protective immunity against invasive and noninvasive pneumococcal infections by vaccination is a realistic goal. The unconjugated polysaccharide vaccine has reduced the risk of IPD among older adults, and protein-conjugated vaccines have provided substantial direct benefits to children and indirect benefits to adults. Future opportunities to further reduce the risk of pneumococcal infections in adults will depend on advances in our understanding of the mechanisms of protective responses to S. pneumoniae in the systemic and, particularly, in the respiratory mucosal compartments. Elucidating and overcoming the limitations in immune responses imposed by advanced age, environmental injury (e.g., smoking), and underlying disease (e.g., malnutrition, organ dysfunction, cancer, and immunosuppressive therapy) will guide development of vaccines that can elicit more robust primary and memory responses in the bloodstream and lung in individuals with the greatest attendant morbidity and mortality from pneumococcal infection, our elderly adults.
Acknowledgments
Financial support. Veterans Affairs Research Service, Mucosal and Vaccine Research Program Colorado (to E.N.J.), and the National Institutes of Health (R21-AI077069 and R01-AI48796 to E.N.J.).
Footnotes
Potential conflicts of interest. L.A.J. has served as a consultant to Merck, the manufacturer of 23-valent pneumococcal polysaccharide vaccine. L.A.J. and E.N.J. have received research funding from and served as consultants to Wyeth, the manufacturer of 7-valent pneumococcal conjugate vaccine.
References
- 1.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire US Department of Health and Human Services. Available at: http://www.cdc.gov/brfss/questionnaires/english.htm. Accessed 25 September 2008. [Google Scholar]
- 2.Shapiro ED, Clemens JD. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Ann Intern Med 1984; 101:325–30. [DOI] [PubMed] [Google Scholar]
- 3.Forrester HL, Jahnigen DW, LaForce FM. Inefficacy of pneumococcal vaccine in a high-risk population. Am J Med 1987; 83:425–30. [DOI] [PubMed] [Google Scholar]
- 4.Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 1988; 108:653–7. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325:1453–60. [DOI] [PubMed] [Google Scholar]
- 6.Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy: an evaluation of current recommendations. JAMA 1993; 270:1826–31. [PubMed] [Google Scholar]
- 7.Farr BM, Johnston BL, Cobb DK, et al. Preventing pneumococcal bacteremia in patients at risk: results of a matched case-control study. Arch Intern Med 1995; 155:2336–40. [PubMed] [Google Scholar]
- 8.Benin AL, O’Brien KL, Watt JP, et al. Effectiveness of the 23-valent polysaccharide vaccine against invasive pneumococcal disease in Navajo adults. J Infect Dis 2003; 188:81–9. [DOI] [PubMed] [Google Scholar]
- 9.Jackson LA, Neuzil KM, Yu O, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348: 1747–55. [DOI] [PubMed] [Google Scholar]
- 10.Hedlund J, Christenson B, Lundbergh P, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up. Vaccine 2003; 21:3906–11. [DOI] [PubMed] [Google Scholar]
- 11.Andrews RM, Counahan ML, Hogg GG, McIntyre PB. Effectiveness of a publicly funded pneumococcal vaccination program against invasive pneumococcal disease among the elderly in Victoria, Australia. Vaccine 2004; 23:132–8. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez A, Salleras L, Fedson DS, et al. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: a case-control study. Clin Infect Dis 2005; 40:1250–7. [DOI] [PubMed] [Google Scholar]
- 13.Vila-Corcoles A, Ochoa-Gondar O, Hospital I, et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43:860–8. [DOI] [PubMed] [Google Scholar]
- 14.Singleton RJ, Butler JC, Bulkow LR, et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine 2007; 25:2288–95. [DOI] [PubMed] [Google Scholar]
- 15.Mooney JD, Weir A, McMenamin J, et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 2008; 8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson LA, Neuzil KM. Pneumococcal polysaccharide vaccine In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th edition Philadelphia: Saunders, 2008:569–604. [Google Scholar]
- 17.Jackson LA, Neuzil KM, Nahm MH, et al. Immunogenicity of varying dosages of 7-valent pneumococcal polysaccharide-protein conjugate vaccine in seniors previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine 2007; 25:4029–37. [DOI] [PubMed] [Google Scholar]
- 18.de Roux A, Schmoele-Thoma B, Siber GR, et al. Comparison of pneumococcal conjugate polysaccharide and free polysaccharide vaccines in elderly adults: conjugate vaccine elicits improved antibacterial immune responses and immunological memory. Clin Infect Dis 2008; 46: 1015–23. [DOI] [PubMed] [Google Scholar]
- 19.Smit P, Oberholzer D, Hayden-Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA 1977; 238:2613–6. [PubMed] [Google Scholar]
- 20.Austrian R, Douglas RM, Schiffman G, et al. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians 1976; 89:184–94. [PubMed] [Google Scholar]
- 21.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043–51. [DOI] [PubMed] [Google Scholar]
- 22.Kyaw MH, Rose CE Jr, Fry AM, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377–86. [DOI] [PubMed] [Google Scholar]
- 23.Jackson ML, Neuzil KM, Thompson WW, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis 2004; 39:1642–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austrian R Surveillance of pneumococcal infection for field trials of polyvalent pneumococcal vaccines. Report DAB-VDP-12–84. Bethesda, MD: National Institutes of Health, 1980:1–84. [Google Scholar]
- 25.Gaillat J, Zmirou D, Mallaret MR, et al. Clinical trial of an antipneumococcal vaccine in elderly subjects living in institutions [in French]. Rev Epidemiol Sante Publique 1985; 33:437–44. [PubMed] [Google Scholar]
- 26.Simberkoff MS, Cross AP, Al-Ibrahim M, et al. Efficacy of pneumococcal vaccine in high-risk patients: results of a Veterans Administration Cooperative Study. N Engl J Med 1986; 315:1318–27. [DOI] [PubMed] [Google Scholar]
- 27.Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single-blind population-based trial. Am J Med 1997; 103:281–90. [DOI] [PubMed] [Google Scholar]
- 28.Örtqvist A, Hedlund J, Burman LA, et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people: Swedish Pneumococcal Vaccination Study Group. Lancet 1998; 351:399–403. [DOI] [PubMed] [Google Scholar]
- 29.Honkanen PO, Keistinen T, Miettinen L, et al. Incremental effectiveness of pneumococcal vaccine on simultaneously administered influenza vaccine in preventing pneumonia and pneumococcal pneumonia among persons aged 65 years or older. Vaccine 1999; 17:2493–500. [DOI] [PubMed] [Google Scholar]
- 30.Alfageme I, Vazquez R, Reyes N, et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 2006; 61:189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broome CV. Efficacy of pneumococcal polysaccharide vaccines. Rev Infect Dis 1981; 3(Suppl):S82–96. [DOI] [PubMed] [Google Scholar]
- 32.Fedson DS, Liss C. Precise answers to the wrong question: prospective clinical trials and the meta-analyses of pneumococcal vaccine in elderly and high-risk adults. Vaccine 2004; 22:927–46. [DOI] [PubMed] [Google Scholar]
- 33.Hirschmann JV, Lipsky BA. The pneumococcal vaccine after 15 years of use. Arch Intern Med 1994; 154:373–7. [PubMed] [Google Scholar]
- 34.Skull SA, Andrews RM, Byrnes GB, et al. Prevention of community-acquired pneumonia among a cohort of hospitalized elderly: benefit due to influenza and pneumococcal vaccination not demonstrated. Vaccine 2007; 25:4631–40. [DOI] [PubMed] [Google Scholar]
- 35.Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med 1999; 159:2437–42. [DOI] [PubMed] [Google Scholar]
- 36.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine 2008; 26:1955–62. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Active Bacterial Core Surveillance System. Available at: http://www.cdc.gov/ncidod/dbmd/abcs. Accessed 25 September 2008.
- 38.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis 2007; 196:1346–54. [DOI] [PubMed] [Google Scholar]
- 39.Moore MR, Gertz RE Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis 2008; 197:1016–27. [DOI] [PubMed] [Google Scholar]
- 40.Sisk JE, Moskowitz AJ, Whang W, et al. Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA 1997; 278:1333–9. [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 1997; 46(RR-8):1–24. [PubMed] [Google Scholar]
- 42.Fry AM, Zell ER, Schuchat A, Butler JC, Whitney CG. Comparing potential benefits of new pneumococcal vaccines with the current polysaccharide vaccine in the elderly. Vaccine 2002; 21:303–11. [DOI] [PubMed] [Google Scholar]
- 43.Torling J, Hedlund J, Konradsen HB, Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine 2003; 22: 96–103. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis 2007; 7:597–606. [DOI] [PubMed] [Google Scholar]
- 45.Davidson M, Bulkow LR, Grabman J, et al. Immunogenicity of pneumococcal revaccination in patients with chronic disease. Arch Intern Med 1994; 154:2209–14. [PubMed] [Google Scholar]
- 46.Rodriguez R, Dyer PD. Safety of pneumococcal revaccination. J Gen Intern Med 1995; 10:511–2. [DOI] [PubMed] [Google Scholar]
- 47.Lackner TE, Hamilton RG, Hill JJ, Davey C, Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatr Soc 2003; 51:240–5. [DOI] [PubMed] [Google Scholar]
- 48.Jackson LA, Benson P, Sneller VP, et al. Safety of revaccination with pneumococcal polysaccharide vaccine. JAMA 1999; 281:243–8. [DOI] [PubMed] [Google Scholar]
- 49.Jackson LA, Nelson JC, Whitney CG, et al. Assessment of the safety of a third dose of pneumococcal polysaccharide vaccine in the Vaccine Safety Datalink population. Vaccine 2006; 24:151–6. [DOI] [PubMed] [Google Scholar]
- 50.Walker FJ, Singleton RJ, Bulkow LR, Strikas RA, Butler JC. Reactions after three or more doses of pneumococcal polysaccharide vaccine in adults in Alaska. Clin Infect Dis 2005; 40:1730–5. [DOI] [PubMed] [Google Scholar]
- 51.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737–46. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease Control and Prevention. Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction—eight states, 1998–2005. MMWR Morb Mortal Wkly Rep 2008; 57:144–8. [PubMed] [Google Scholar]
- 53.Jackson LA, Siber G. Immunogenicity and safety in adults In: Käyhty H, Siber G, eds. Pneumococcal conjugate vaccines. Washington, DC: ASM Press, 2008:245–60. [Google Scholar]
- 54.Scott D, Ruckle J, Dar M, Baker S, Kondoh H, Lockhart S. Phase 1 trial of 13-valent pneumococcal conjugate vaccine in Japanese adults. Pediatr Int 2008; 50:295–9. [DOI] [PubMed] [Google Scholar]
- 55.Scott DA, Komjathy SF, Hu BT, et al. Phase 1 trial of a 13-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2007; 25: 6164–6. [DOI] [PubMed] [Google Scholar]
- 56.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004; 4: 144–54. [DOI] [PubMed] [Google Scholar]
- 57.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med 2002; 195:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giefing C, Meinke AL, Hanner M, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med 2008; 205:117–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malley R, Henneke P, Morse SC, et al. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc Natl Acad Sci USA 2003; 100:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci USA 2005; 102:4848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lipsitch M, Whitney CG, Zell E, Kaijalainen T, Dagan R, Malley R. Are anticapsular antibodies the primary mechanism of protection against invasive pneumococcal disease? PLoS Med 2005; 2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med 2007; 161:1162–8. [DOI] [PubMed] [Google Scholar]
- 63.Grijalva CG, Nuorti JP, Arbogast PG, Martin SW, Edwards KM, Griffin MR. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 2007; 369:1179–86. [DOI] [PubMed] [Google Scholar]