Abstract
The prevalence of type 2 diabetes mellitus (T2D) has risen in the United States and worldwide, with an increase in global prevalence from 4.7% to 8.5% between 1980 and 2014. A variety of antidiabetic drugs are available with different mechanisms of action, and multiple drugs are often used concomitantly to improve glycemic control. One of the newest classes of oral antihyperglycemic agents is the sodium glucose cotransporter-2 (SGLT2) inhibitors or “flozins”. Recent clinical guidelines have suggested the use of SGLT2 inhibitors as add-on therapy in patients for whom metformin alone does not achieve glycemic targets, or as initial dual therapy with metformin in patients who present with higher glycated hemoglobin (HbA1c) levels. The FDA has approved fixed-dose combination (FDC) tablets with each of the three available SGLT2 inhibitors (canagliflozin, dapagliflozin, and empagliflozin) and metformin. Both drug classes are associated with the rare but serious life-threatening complications that result from metabolic acidosis, including lactic acidosis (with metformin) and euglycemic diabetic ketoacidosis (with SGLT2 inhibitors). This review summarizes the current literature on the pharmacokinetics and the molecular targets of metformin and SGLT2 inhibitors. It also addresses the common adverse effects and highlights the molecular mechanisms by which this dual antihyperglycemic therapy contributes to high anion gap metabolic acidosis. In conclusion, while the combination of metformin and SGLT2 inhibitors would be a better option in improving glycemic control with a low risk of hypoglycemia, an increase in the risk of metabolic acidosis during combination therapy may be borne in mind.
Keywords: Metformin, Sodium glucose cotransporter-2 Inhibitors, Hepatic gluconeogenesis, Renal tubular glucose reabsorption, High anion gap metabolic acidosis
1. Introduction
Type 2 diabetes mellitus (T2D) is a chronic metabolic disorder associated with high blood glucose levels, eventually leading to microvascular and macrovascular complications. It is characterized by progressive β-cell dysfunction and loss of insulin secretion, as well as peripheral insulin resistance (American Diabetes Association, 2017). The CDC reports that in 2016, over 29 million Americans were living with diabetes and 90–95% of these cases were type 2 (CDC, 2016). Global prevalence of this disease is also rising, with an increase from 4.7% to 8.5% between 1980 and 2014 (WHO, 2016). Diabetes is estimated to be the 7th leading cause of death in the United States (CDC, 2016). The majority of subjects who develop T2D are overweight or obese, or carry extra body fat in the abdominal region. Additional risk factors include lack of physical activity, dyslipidemia, and hypertension. Individuals with a family history of T2D or those of certain high-risk ethnicities are also more likely to develop this disease (American Diabetes Association, 2017).
The currently available antidiabetic drugs improve glycemic control by targeting different organs, including liver, skeletal muscle, adipose tissue, pancreas, kidney, and intestine. The classical example of an antidiabetic drug that lowers blood glucose level by inhibiting hepatic glucose output is metformin. The drugs that exhibit insulin-sensitizing effects include thiazolidinediones and metformin, which improve insulin action in peripheral tissues such as skeletal muscle and adipose tissue. In addition, antidiabetic drugs function as insulin secretagogues [e.g., sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1 (GLP-1) receptor agonists] to stimulate insulin secretion by the pancreatic β-cells. While insulin promotes glucose uptake in peripheral tissues (e.g., skeletal muscle and adipose tissue) through glucose transporter-4 (GLUT-4), the recently approved sodium glucose cotransporter-2 (SGLT2) inhibitors that target renal SGLT2 exhibit their antihyperglycemic effects in an insulin-independent manner. Recent clinical guidelines have suggested the use of SGLT2 inhibitors as add-on therapy in patients for whom metformin alone does not achieve glycemic targets, or as initial dual therapy with metformin in patients who present with higher glycated hemoglobin (HbA1c) levels (American Diabetes Association, 2017; Garber et al., 2017). The FDA has approved fixed-dose combination (FDC) tablets with each of the three available SGLT2 inhibitors (canagliflozin, dapagliflozin, and empagliflozin) and metformin.
Metformin is considered the first-line drug therapy for patients with T2D due to its high efficacy in glycemic control and low risk of hypoglycemia (American Diabetes Association, 2017). Although metformin has been in clinical use for over five decades (Buse et al., 2016), its mechanism of action appears to involve multiple molecular targets to lower blood glucose level, as outlined below. The most common adverse effects of metformin are nausea and diarrhea, and a dose titration is recommended to limit these effects. A more serious risk, although rare (< 10 cases per 100,000 patient-years), is metformin-associated lactic acidosis (MALA) (DeFronzo et al., 2016). The black box warning for metformin indicates that the risk of lactic acidosis is higher for those with renal or hepatic impairment, congestive heart failure, and increased age. It is currently available in immediate release and extended release formulations.
The SGLT2 inhibitor, canagliflozin, was approved by the FDA in March 2013. Subsequently, two more SGLT2 inhibitors, dapagliflozin and empagliflozin, were approved in the following year. SGLT2 inhibitors improve glycemic control by reducing renal tubular reabsorption of glucose into the systemic circulation, as outlined below. In May 2015, the FDA issued a warning that SGLT2 inhibitors could lead to diabetic ketoacidosis (DKA) based on 20 cases, which were reported from the time of canagliflozin approval until June 2014 (Rosenstock & Ferrannini, 2015). In a subsequent report, the incidence of DKA was found to be 0.522 and 0.763 per 1,000 patient-years in subjects on canagliflozin 100 mg and 300 mg, respectively (Erondu et al., 2015).
This review summarizes the current literature on the pharmacokinetics and the molecular targets of metformin and SGLT2 inhibitors. It also addresses the common adverse effects of metformin and SGLT2 inhibitors, and highlights the molecular mechanisms by which this dual antihyperglycemic therapy contributes to high anion gap metabolic acidosis in diabetic patients. Furthermore, it describes the fixed dose combination (FDC) of these two drugs and the place in therapy toward achieving the recommended HbA1c goal in patients with T2D.
2. Pharmacokinetics of metformin versus SGLT2 inhibitors
As shown in Table 1, metformin and SGLT2 inhibitors have very little overlap in pharmacokinetic parameters, indicating that significant drug-drug interactions are unlikely when given together as a fixed-dose combination (FDC). Metformin does not bind to plasma proteins, while the SGLT2 inhibitors’ plasma protein binding ranges from 86.2 to 99%; therefore, there should be no competition for these binding sites (Glucophage, 2015; Jardiance, 2016; Farxiga, 2016; Invokana, 2017). In addition, metformin is not metabolized in the liver, while SGLT2 inhibitors undergo significant hepatic glucuronidation. Furthermore, metformin is eliminated through active tubular secretion via organic cation transporter-2 (OCT2), for which the SGLT2 inhibitors are neither known to be substrates nor to inhibit at clinically relevant concentrations (Kimura et al., 2005).
Table 1.
Pharmacokinetics of metformin versus SGLT2 inhibitors.
| SGLT2 Inhibitors | ||||
|---|---|---|---|---|
| Metformin | Empagliflozin | Dapagliflozin | Canagliflozin | |
| [Glucophage] | [Jardiance] | [Farxiga] | [Invokana] | |
| Absorption | F = 50–60% | F not published | F = 78% | F = 65% |
| Distribution | Vd=654±358 L NoPPB Partitions into erythrocytes |
Vd = 73.8 L 86.2% PPB |
Vd not published 91% PPB |
Vd=83.5 L 99% PPB |
| Metabolism | No hepatic metabolism |
Hepatic glucuronidation via UGT 2B7, 1A3, 1A8, and 1A9 |
Hepatic glucuronidation via UGT 1A9 |
Hepatic glucuronidation via UGT 1A9 and 2B4 |
| Elimination | Eliminated unchanged in the urine via active tubular secretion T1/2 (plasma)= 6.2 hr T1/2 (blood) = 17.6 hr |
41.2% eliminated in feces (majority unchanged) 54.4% eliminated in urine (half unchanged) T1/2 = 12.4 hr |
21% eliminated in feces (15% unchanged) 75% eliminated in urine (less than 2% unchanged) T1/2 = 12.9 hr |
41.5% eliminated in feces (majority unchanged) 33% eliminated in urine (less than 1% unchanged) T1/2 (100 mg dose) = 10.6 hr T1/2 (300 mg dose) = 13.1 hr |
F = absolute oral bioavailability; Vd = volume of distribution; PPB = plasma protein binding; UGT = UDP-glucuronosyltransferase; T1/2 = elimination half-life in hours.
3. Mechanism of action of metformin and/or SGLT2 inhibitors: mono versus dual therapy
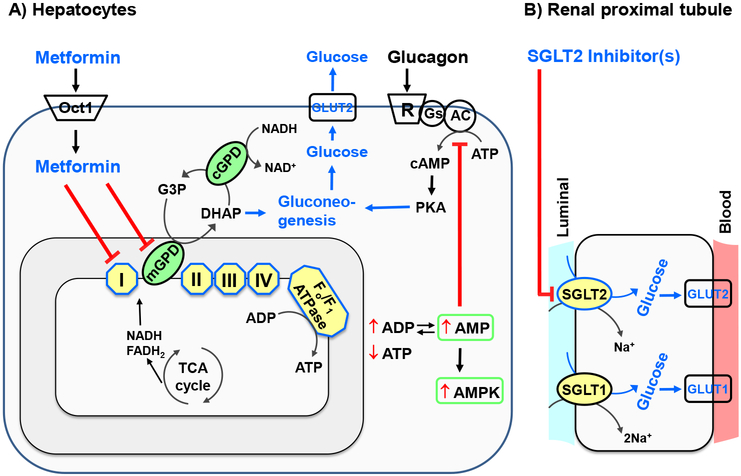
In T2D, the increase in fasting plasma glucose concentration results from enhanced hepatic glucose output, which in turn is primarily due to increased gluconeogenesis in insulin-resistant state (Bailey & Turner, 1996). In addition, T2D is associated with an upregulation of renal SGLT2 expression with ~2-fold increase in renal tubular glucose reabsorption, which is implicated in the maintenance of hyperglycemia (Vallon, 2015). From a mechanistic standpoint, antidiabetic drugs that target exaggerated hepatic glucose output and renal tubular glucose reabsorption may exhibit additive effects to improve glycemic control (Fig. 1A–B).
Fig. 1.
Molecular targets of metformin and SGLT2 inhibitors. A) After its uptake through Oct1 in hepatocytes, metformin inhibits gluconeogenesis (and thereby glucose output) through inhibition of complex I or mGPD, a decrease in ATP/ADP ratio, inhibition of AC by AMP, and activation of AMPK. B) SGLT2 inhibitors inhibit SGLT2-mediated reabsorption of glucose in the S1 segment of renal proximal tubule. I, II, III, IV, complexes I through IV; ADP, adenosine diphosphate; AMP, adenosine monophosphate; AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; cAMP, cyclic AMP; cGPD, cytosolic glycerophosphate dehydrogenase; DHAP, dihydroxyacetone phosphate; GLUT1, glucose transporter-1; GLUT2, glucose transporter-2; G3P, glycerol-3-phosphate; mGPD, mitochondrial glycerophosphate dehydrogenase; Oct1, organic cation transporter-1; PKA, protein kinase A; SGLT1, sodium glucose co-transporter-1; SGLT2, sodium glucose co-transporter-2; ⊥, inhibition; ↑, increase; ↓ decrease.
3.1. Metformin
The antihyperglycemic action of metformin is primarily attributed to its inhibitory effect on hepatic glucose output (Bailey & Turner, 1996; Hundal et al., 2000). Studies over the past two decades have identified multiple targets in the liver/hepatocytes from different species to explain how metformin inhibits endogenous glucose production (Fig. 1A). After its uptake through organic cation transporter-1 (Oct1) in hepatocytes (Wang et al., 2002), metformin has been shown to inhibit gluconeogenesis by the following mechanisms: i) inhibition of mitochondrial respiratory chain complex 1 (El-Mir et al., 2000; Owen et al., 2000); ii) inhibition of mitochondrial glycerophosphate dehydrogenase (mGPD) (Madiraju et al., 2014); iii) AMP-mediated inhibition of adenylyl cyclase (Miller et al., 2013); iv) activation of AMP-activated protein kinase (AMPK) (Zhou et al., 2001); and v) AMPK-independent inhibition of glucose production (Foretz et al., 2010). In particular, metformin inhibits mitochondrial respiratory chain complex 1, which would decrease ATP/ADP ratio to diminish gluconeogenesis (e.g., by inhibiting pyruvate carboxylase) (El-Mir et al., 2000; Owen et al., 2000). Metformin has also been shown to diminish mGPD activity, which would inhibit the conversion of glycereol-3-phosphate (G3P) to dihydroxyacetone phosphate (DHAP), thereby preventing the contribution of glycerol (a gluconeogenic precursor) to gluconeogenesis (Madiraju et al., 2014). In addition, metformin inhibition of mGPD would result in an increase in cytosolic redox state (↑ cytosolic NADH/NAD+ ratio), which would facilitate conversion of pyruvate to lactate to enhance lactate/pyruvate ratio thereby inhibiting gluconeogenesis from lactate (another gluconeogenic precursor) (Madiraju et al., 2014). Thus, metformin has been shown to inhibit complex 1 and mGPD (El-Mir et al., 2000; Owen et al., 2000; Madiraju et al., 2014), integral components of the mitochondrial respiratory chain (Mracek et al., 2013). In a different study, metformin has been shown to inhibit glucagon-induced cAMP production and PKA activation (and thereby inhibition of gluconeogenesis) by facilitating the accumulation of AMP, which inhibits adenylyl cyclase activity to decrease the conversion of ATP to cAMP (Miller et al., 2013). Furthermore, metformin has been shown to enhance the activity of AMPK (Zhou et al., 2001), which would suppress ATP-consuming anabolic pathways (Hardie et al., 2012), including gluconeogenesis. In AMPK-null mouse hepatocytes, metformin inhibits gluconeogenesis through a decrease in energy state (↓ ATP), suggesting an AMPK-independent mechanism (Foretz et al., 2010). Together, metformin has been shown to inhibit mitochondrial respiratory chain components (e.g., complex 1 and mGPD), increase cytosolic NADH/NAD+ and lactate/pyruvate ratios, decrease ATP/ADP ratio, increase AMPK activity, increase AMP level, and decrease adenylyl cyclase activity. Thus, it inhibits glucagon action in the liver and prevents endogenous glucose production from key gluconeogenic precursors, lactate and pyruvate.
In contrast to the currently available metformin [immediate-release or extended-release formulation] that inhibits hepatic gluconeogenesis following its absorption through duodenum and jejunum (Graham et al., 2011), a recent study has shown that delayed-release metformin exhibits its antihyperglycemic effect predominantly through its exposure in the lower gut (e.g., ileum) (Buse et al., 2016). The use of delayed-release formulation of metformin allows its delivery/exposure in the ileum and beyond, where metformin absorption is relatively less thereby contributing to low systemic exposure. Although delayed-release metformin can enhance GLP-1 secretion/release from the intestinal L cells [cited in (McCreight et al., 2016; Henry et al., 2018)], the molecular mechanism by which it lowers blood glucose level remains unclear. A recent Phase 2b study has compared fasting plasma glucose and HbA1c lowering with delayed-release metformin versus immediate-release metformin and placebo (Henry et al., 2018). While immediate-release metformin produced greater HbA1c improvement, it also produced approximately 3-fold higher plasma metformin exposure. After normalizing to overall systemic exposure, delayed-release metformin improved fasting plasma glucose and HbA1c levels to a greater degree than immediate-release metformin in patients with T2D. This suggests that delayed-release metformin may provide clinical benefit for patients who are not otherwise candidates for metformin due to concerns of systemic over-exposure.
3.2. SGLT2 inhibitor(s)
The antihyperglycemic action of SGLT2 inhibitors is attributed to its inhibitory effect on renal tubular reabsorption of glucose (Fig. 1B). These drugs inhibit SGLT2 expressed in the S1 segment of proximal tubule (Ferrannini & Solini, 2012). Inhibition of SGLT2 results in the inhibition of reabsorption of filtered glucose, thereby accounting for its blood glucose-lowering effect with an accompanying glycosuria. In addition, SGLT2 inhibitor has been shown to inhibit SGLT2 protein expressed in the pancreatic α-cells, thereby enhancing glucagon release and hepatic gluconeogenesis (Bonner et al., 2015).
3.3. Dual therapy
As illustrated in Fig. 1A–B, combination therapy with metformin and SGLT2 inhibitor(s) would improve glycemic control by inhibiting hepatic gluconeogenesis and renal tubular glucose reabsorption, respectively. Thus, the dual action in suppressing hepatic glucose output and promoting renal glycosuria may account for the observed improvement in HbA1c reduction (by ~0.3 to 0.5%) with combination therapy in recent trials, as discussed above (Henry et al., 2012; Hadjadj et al., 2016; Rosenstock et al., 2016). Under these conditions, metformin would also suppress enhanced hepatic glucagon signaling toward gluconeogenesis, which may occur upon SGLT2 inhibition in pancreatic α-cells (Bonner et al., 2015). The antagonizing effect of metformin on SGLT2 inhibitor-mediated hepatic gluconeogenesis may result in further HbA1c reduction, compared with SGLT2 inhibitor alone.
Since the recently reported delayed-release metformin does not appear to have a direct inhibitory effect on hepatic gluconeogenesis (Buse et al., 2016), future studies should determine whether the combination therapy with delayed-release metformin and SGLT inhibitor(s) offers an advantage over monotherapy with regard to significant HbA1c reduction.
4. Adverse effects of metformin versus SGLT2 inhibitors
4.1. Common adverse effects:
As shown in Table 2, the most common adverse effects of metformin are gastrointestinal in nature and include nausea, diarrhea, and abdominal discomfort (DeFronzo, 1999). These events can be mitigated by using a slow upward dose titration when initiating metformin therapy. Long-term metformin use has also been associated with vitamin B12 deficiency (Aroda et al., 2016). As discussed below, the most serious adverse event associated with metformin use, while rare, is lactic acidosis (DeFronzo et al., 2016).
Table 2.
Adverse effects of metformin versus SGLT2 inhibitors.
| Metformin | SGLT2 Inhibitors | |
|---|---|---|
| Dehydration | -- | ↑ |
| Intravascular Volume | -- | ↓ |
| Serum Creatinine | -- | ↑ |
| eGFR | -- | ↓ |
| Gastrointestinal Events | ↑ | -- |
| (diarrhea, nausea, vomiting) | ||
| Urinary Tract Infection, | -- | ↑ |
| Pyelonephritis, and Urosepsis | ||
| Genital Mycotic Infection | -- | ↑ |
| Orthostatic Hypotension | -- | ↑ |
| Hypoglycemia | -- | -- |
| Vitamin B-12 Level | ↓ | -- |
| LDL-C Level | ↓ | ↑ |
| Hematocrit Level | ↓ | ↑ |
| Bone Fracture | -- | ↑ |
| Lactic Acidosis | ↑ | -- |
| Ketoacidosis | -- | ↑ |
, increase;
decrease.
The most common adverse effects of the SGLT2 inhibitors include renal insufficiency (increases in serum creatinine and decreases in GFR may be noted after the start of therapy), genital mycotic infections, and urinary tract infections (including some cases of pyelonephritis) (Henry et al., 2012; Hadjadj et al., 2016; Rosenstock et al., 2016). The SGLT2 inhibitors may decrease intravascular volume due to diuresis, and certain patients may be at risk for orthostatic hypotension and dehydration. An increased risk of bone fracture has also been associated with the use of canagliflozin (Watts et al., 2016). Unlike metformin that decreases LDL-cholesterol and hematocrit (Wulffele et al., 2004; Diabetes Prevention Program Research, 2012), SGLT2 inhibitors show modest increases in LDL-C and hematocrit levels that may be secondary to hemoconcentration (Lavalle-Gonzalez et al., 2013). Finally, as discussed below, the SGLT2 inhibitors have been implicated in rare cases of euglycemic DKA (euDKA) (Peters et al., 2015).
4.2. Metabolic acidosis
Metabolic acidosis is characterized by a decrease in blood pH following a primary decrease in plasma bicarbonate concentration (HCO3−) and a secondary decrease in the partial pressure of carbon dioxide (pCO2) (Kraut & Madias, 2010). Cases of metabolic acidosis are typically divided into two categories: high anion gap and hyperchloremic with normal anion gap. The mnemonic MUDPILES is frequently used to remember common causes of high anion gap metabolic acidosis, although an updated mnemonic, GOLDMARK, has been more recently proposed (Mehta et al., 2008). Acute forms of metabolic acidosis are often the result of accumulation of organic acids (e.g., lactate and ketone bodies). Lactic acidosis and diabetic ketoacidosis are among the most common causes of acute high anion gap metabolic acidosis and are represented in both mnemonics (Table 3). Along with obtaining a thorough patient history, calculation of the plasma anion gap is valuable in discerning the cause of acidosis. The pH, pCO2, pO2, and electrolytes can be measured in blood samples using the blood gas analyzer. HCO3− values can be calculated using Henderson-Hasselbalch equation as follows: pH = pK + log HCO3− / 0.03(pCO2). Anion gap (AG) can be calculated using the following equation: AG = Na+ - (Cl− + HCO3−). Since K+ is excluded in the calculation, the normal value for AG ranges between 8 and 16 mEq/L.
Table 3.
Common causes of high anion gap metabolic acidosis.
| MUDPILES | GOLDMARK |
|---|---|
| Methanol |
Glycols (ethylene and propylene) |
| Uremia | Oxoproline |
| Diabetes | L-lactate |
| Paraldehyde | D-lactate |
| Iron (and Isoniazid) | Methanol |
| Lactate | Aspirin |
| Ethylene glycol | Renal failure |
| Salicylate | Ketoacidosis |
4.2.1. Lactic acidosis
Lactic acidosis is characterized by an increase in arterial lactate level (> 5 mmol/L), a decrease in blood pH (< 7.35), and an increase in anion gap. However, it is important to note that while these factors are indicative of lactic acidosis, their absence cannot be used to rule out the diagnosis. Patients may present with a normal anion gap while having elevated blood lactate, or the blood pH may appear less acidotic due to a coexisting acid-base disorder. An elevated blood lactate is necessary to confirm the diagnosis, but the upper limit of normal may vary between laboratories. The causes of lactic acidosis are numerous and are often divided into two categories.
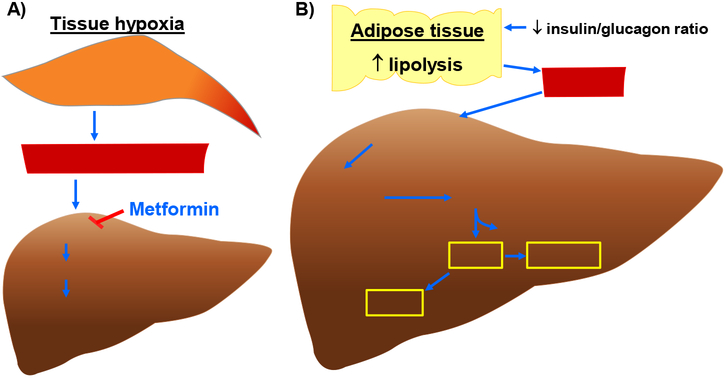
Type A lactic acidosis is associated with conditions of low oxygen delivery to tissues and the subsequent production of excess lactate by glycolysis in anaerobic conditions (Fig. 2A). It occurs most commonly in shock, sepsis, and advanced heart failure. In Type B lactic acidosis, lactate levels are elevated due to causes other than tissue hypoxia and, in addition, clearance of lactic acid is reduced. Type B lactic acidosis is often associated with alcoholic or diabetic ketoacidosis, liver disease, and metformin use (Fig. 2A) (Kraut & Madias, 2014; DeFronzo et al., 2016).
Fig. 2.
Molecular mechanisms that contribute to enhanced blood lactate and ketone body levels. A) Increase in blood lactate level (and thereby lactic acidosis) in hypoxic or metformin-treated conditions. A decrease in microcirculatory perfusion in target tissues (e.g., skeletal muscle) would result in tissue hypoxia. An increase in anaerobic glycolysis under hypoxic conditions would lead to enhanced lactate formation/release into the systemic circulation. In addition, lactate accumulation may result from its impaired hepatic clearance due to high concentration of plasma metformin, which would inhibit utilization of lactate toward hepatic gluconeogenesis. B) Synthesis of ketone bodies (e.g., βOHB, AcAc, and acetone) in DKA. A decrease in systemic insulin-to-glucagon ratio would promote lipolysis in adipose tissue to enhance FFA release and its uptake by the liver. In the hepatic mitochondria, HMGCS2 catalyzes the condensation reaction between AcAc-CoA and Acetyl-CoA (derived from β-oxidation of fatty acids) to form HMG-CoA. Subsequently, HMGCL cleaves HMG-CoA to generate AcAc. In the presence of increased NADH/NAD+ ratio, BDH1 catalyzes the conversion of AcAc to βOHB. In addition, AcAc undergoes nonenzymatic decarboxylation to form acetone. AcAc, acetoacetate; AcAc-CoA, acetoacetyl-CoA; BDH1, mitochondrial βOHB dehydrogenase; FFA, free fatty acids; HMG-CoA, 3-hydroxymethylglutaryl-CoA; HMGCL, HMG-CoA lyase; HMGCS2, mitochondrial HMG-CoA synthase.
As noted above, metformin (a biguanide) carries a black box warning for the risk of lactic acidosis (Fig. 2A). Metformin has been shown to increase plasma lactate levels by inhibition of hepatic mitochondrial respiration (DeFronzo et al., 2016). In addition, metformin can enhance plasma lactate levels by inhibiting the utilization of lactate toward gluconeogenesis in the liver. When metformin is implicated as the cause of lactic acidosis, plasma metformin levels > 5 μg/mL are often found, and higher plasma metformin levels have been correlated with higher lactate levels (Boucaud-Maitre et al., 2016). The risk factors for MALA include impaired renal function (causing poor clearance of metformin), impaired hepatic function (causing poor clearance of lactate), and conditions of increased lactate production such as sepsis. Both age-related changes in renal function, as well as acute changes in renal function (e.g. dehydration, surgery) have been implicated as risk factors for MALA (DeFronzo et al., 2016).
4.2.2. Diabetic ketoacidosis
Diabetic ketoacidosis (DKA) is another cause of high anion gap metabolic acidosis, and is a serious complication of diabetes mellitus. Approximately two-thirds of all cases of DKA occur in type 1 diabetes. DKA is often precipitated by infection, discontinuation of insulin, or other acute events such as myocardial infarction and stroke. Patients typically present with excessive thirst and urination, vomiting, dehydration, hypotension, and possible changes in mental status. Metabolic acidosis is present (pH < 7.35), and the calculated serum anion gap is typically > 10–12 mEq/L. Hyperglycemia (blood glucose > 250 mg/dL) is a key component of DKA, with blood glucose upon presentation commonly up to 800 mg/dL (Rosenstock & Ferrannini, 2015). In addition, DKA is associated with a rise in the circulating concentration of ketone bodies that result from enhanced ketogenesis in the liver (Laffel, 1999), as illustrated in Fig. 2B. The most important laboratory test in the diagnosis of DKA is the blood ketone measurement. The nitroprusside assay has been used in the past to detect acetone and acetoacetate (AcAc) levels, but it does not detect β-hydroxybutyrate (βOHB), a principal metabolite of enhanced hepatic ketogenesis in DKA. The measurement of serum βOHB levels is therefore recommended to aid diagnosis when available, although an exact serum level for diagnosis has not been established (Kitabchi et al., 2009). The use of capillary blood ketone measurement with point of care devices has also been advocated for this purpose of diagnosing DKA. The Joint British Diabetes Society guidelines suggest that a level > 3 mmol/L is indicative of ketoacidosis. However, studies have shown that the results of point of care testing may be less reliable at levels > 3 mmol/L (Kitabchi et al., 2009; Misra & Oliver, 2015).
As noted above, SGLT2 inhibitor labeling has been updated to include the risk of DKA. It is important to note that initial reports indicated that patients on an SGLT2 inhibitor presenting with ketoacidosis had normal or only slightly elevated blood glucose, which differs from the typical DKA presentation (Rosenstock & Ferrannini, 2015). This phenomenon has been called euglycemic DKA (euDKA) and has led to a delay of diagnosis in some cases due to this unusual feature. EuDKA has been reported to occur in both type 1 and type 2 diabetes. Shortly after the FDA warning was released, the manufacturer of canagliflozin announced the total incidence of DKA in their clinical trials database (Erondu et al., 2015). Many of these patients were determined to have type 1 diabetes (in which the use of SGLT2 inhibitors is not FDA approved) or latent autoimmune diabetes of adulthood (LADA). Additional risk factors for euDKA were identified by Peters and colleagues and include reduction or discontinuation of insulin, major surgery, alcohol consumption, and low carbohydrate diet (Peters et al., 2015). The current AACE/ACE position statement on this issue, released in May 2016, concludes that while the risk of euDKA in type 2 diabetes does exist for patients on an SGLT2 inhibitor, the risk-benefit ratio is in favor of continuing use. The position statement does recommend discontinuing the SGLT2 inhibitor at least 24 hours prior to planned major surgeries, avoiding sudden decreases in insulin dose, and avoiding excessive alcohol intake or ketogenic diets (Handelsman et al., 2016).
4.2.3. Lactic acidosis in diabetic ketoacidosis
It is important to note that lactic acidosis is also commonly found in diabetic ketoacidosis. The mechanism by which this occurs is still debated, although it is thought to be due to a combination of hypoperfusion and altered glucose metabolism (Cox et al., 2012; Feenstra et al., 2014). A retrospective study found that as many as 68% of patients presenting to an emergency department with DKA also had lactic acidosis. However, lactic acidosis was defined as lactate level > 2.5 mmol/L. Still, 40% of patients presenting with DKA had a lactate level > 4 mmol/L. Interestingly, all patients who were on metformin at the time of presentation had a lactate level < 4 mmol/L, suggesting that metformin use was not responsible for the degree of lactic acidosis. The study also aimed to determine if lactate levels were predictive of prognosis, as higher lactate levels have been shown to predict severity of illness in sepsis, trauma, burns, and other critical illnesses. The authors concluded that there was no correlation between lactate level and ICU length of stay, suggesting that lactic acidosis may not be predictive of outcome in DKA (Cox et al., 2012).
Since metformin or SGLT2 inhibitor therapy alone has been shown to be associated with the rare but serious life-threatening complications that result from high anion gap metabolic acidosis, future studies are clearly warranted to determine whether the combination therapy would increase the risk of lactic acidosis and/or DKA in clinical practice.
5. Metformin and SGLT2 inhibitor(s): a fixed dose combination therapy
For the treatment of T2D, the first fixed-dose combination (FDC) tablet of an SGLT2 inhibitor (canagliflozin) with metformin was approved by the FDA in August 2014. This was followed by the FDC of dapagliflozin and metformin extended-release in October 2014 (Fala, 2015; Invokamet and Xigduo XR, 2015). Since that time, an extended-release combination of canagliflozin and metformin has been approved, as well as immediate- and extended-release combinations of empagliflozin with metformin. The FDCs are available in multiple strengths to allow for variable dosing, dose titration, and adjustments for renal impairment.
Using these FDCs, no phase III clinical trials have been published till date. However, SGLT2 inhibitors have been studied as add-on therapies in patients stabilized on metformin, as well as initial dual therapy with metformin. In a phase III trial published in 2016, Rosenstock et al. evaluated reductions in baseline HbA1c at 26 weeks using initial combination therapy of canagliflozin 100 mg or 300 mg with metformin extended-release versus canagliflozin alone and metformin extended-release alone. The combination arms showed an average reduction from baseline HbA1c of 1.77% and 1.78%, respectively, compared with 1.3% reduction in HbA1c on metformin extended-release alone (Rosenstock et al., 2016). In a different phase III trial published in 2016, Hadjadj et al. compared the initial combination therapy of empagliflozin with metformin (immediate-release) to empagliflozin alone and metformin alone. Groups initiated on combination therapy showed an average reduction in HbA1c of 1.9% to 2.1% at 24 weeks (Hadjadj et al., 2016). Similar reduction from baseline HbA1c (~2%) has also been shown in initial combination therapy of dapagliflozin and metformin extended-release (Henry et al., 2012).
6. Place in therapy
In their 2017 Standards of Care, the American Diabetes Association (ADA) recommends a goal HbA1c < 7.0% for many adults with T2D (American Diabetes Association, 2017). The American Association of Clinical Endocrinologists/American College of Endocrinology (AACE/ACE) have also published guidelines recommending an HbA1c ≤ 6.5% in patients who can achieve this goal safely, noting that more intensive glycemic control reduces the risk of microvascular complications but can also lead to an increased risk of adverse events in certain patient populations (Garber et al., 2017). HbA1c goals may be achieved with lifestyle interventions in addition to a variety of antihyperglycemic medications.
The ADA recommends monotherapy initially for patients with an HbA1c < 9.0% at the start of treatment, while the AACE/ACE recommends monotherapy only in those patients who present with an HbA1c < 7.5%. Metformin is the preferred agent for monotherapy by both organizations, although other agents such as the SGTL2 inhibitors may be considered for monotherapy in patients who are intolerant to metformin (Garber et al., 2017).
It is important to note that many patients will not meet their target HbA1c on metformin therapy alone. Some studies have suggested that up to half of patients initiated on metformin monotherapy are unable to achieve an HbA1c < 7.0% as recommended by the ADA (Cook et al., 2007). In addition, of patients who do achieve initial success with metformin monotherapy, more than 40% may experience treatment failure within the next 5 years as the disease progresses. Risk factors for treatment failure include younger age at initiation of therapy, longer duration of disease prior to initiation of therapy, and higher HbA1c at baseline (Brown et al., 2010).
The ADA recommends that patients who do not achieve their HbA1c goal after 3 months of metformin monotherapy should proceed to dual therapy with an additional antihyperglycemic medication, chosen based on patient-specific factors. SGLT2 inhibitors are one of six drug classes recommended for consideration in dual therapy by the ADA. These six drug classes include thiazolidinediones, sulfonylureas, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, basal insulin, and SGLT2 inhibitors. Patients who present with an HbA1c ≥ 9.0% may be initiated on dual therapy without a trial of metformin monotherapy (American Diabetes Association, 2017). A notable difference from ADA guidelines is that the AACE/ACE recommends starting dual therapy with metformin in patients who present with an initial HbA1c ≥ 7.5%. Add-on therapies are graded by AACE/ACE in order by the strength of recommendation. The GLP-1 receptor agonists are given the strongest recommendation as an add-on to metformin, followed by the SGLT2 inhibitor(s). As the GLP-1 receptor agonists are injectable drugs, this means the SGLT2 inhibitor(s) have the strongest recommendation of any oral agent for dual therapy with metformin (Garber et al., 2017).
7. Conclusion
Metformin and the SGLT2 inhibitors do not carry a high risk for hypoglycemia when used alone or in combination (Henry et al., 2012; Hadjadj et al., 2016; Rosenstock et al., 2016). However, the risk of hypoglycemia increases substantially when used concomitantly with insulin or an insulin secretagogue (Wilding et al., 2013; Neal et al., 2015). It is noteworthy that insulin infusion is a rational treatment option for the management of ketoacidosis in diabetic subjects, as it is known to inhibit hepatic mitochondrial HMGCS2 to suppress ketone body formation and its release into the systemic circulation (Laffel, 1999). Further studies are needed to understand the hormonal and nutritional regulation of ketogenesis/ketoacidosis in the setting of diabetes with metformin-plus-SGLT2 inhibitor therapy, to facilitate the development of novel treatment strategies for the prompt resolution of ketoacidosis in vulnerable subjects. Since metformin or SGLT2 inhibitor therapy alone has been shown to be associated with the rare but serious life-threatening complications that result from high anion gap metabolic acidosis, future studies are clearly warranted to determine whether the combination therapy would increase the risk of lactic acidosis and/or DKA in clinical practice.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute/National Institutes of Health Grant (R01-HL-097090), University of Georgia Research Foundation Fund, and University of Georgia RC Wilson Pharmacy Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Diabetes Association: Standards of medical care in diabetes-2017. Diabetes Care 2017;40:S1–S135.27979885 [Google Scholar]
- Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, Bray GA, Schade DS, Temprosa MG, White NH, Crandall JP & Diabetes Prevention Program Research, G. (2016) Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J Clin Endocrinol Metab, 101, 1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CJ & Turner RC (1996) Metformin. N Engl J Med, 334, 574–579. [DOI] [PubMed] [Google Scholar]
- Bonner C, Kerr-Conte J, Gmyr V, Queniat G, Moerman E, Thevenet J, Beaucamps C, Delalleau N, Popescu I, Malaisse WJ, Sener A, Deprez B, Abderrahmani A, Staels B & Pattou F (2015) Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nature Med, 21, 512–517. [DOI] [PubMed] [Google Scholar]
- Boucaud-Maitre D, Ropers J, Porokhov B, Altman JJ, Bouhanick B, Doucet J, Girardin E, Kaloustian E, Lassmann Vague V & Emmerich J (2016) Lactic acidosis: relationship between metformin levels, lactate concentration and mortality. Diabet Med, 33, 1536–1543. [DOI] [PubMed] [Google Scholar]
- Brown JB, Conner C & Nichols GA (2010) Secondary failure of metformin monotherapy in clinical practice. Diabetes Care, 33, 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A & Fineman M (2016) The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care, 39, 198–205. [DOI] [PubMed] [Google Scholar]
- CDC: Diabetes, Working to Reverse the US Epidemic, At A Glance 2016. https://www.cdc.gov/chronicdisease/resources/publications/aag/diabetes.htm, National Center for Chronic Disease Prevention and Health Promotion, 2016.
- Cook MN, Girman CJ, Stein PP & Alexander CM (2007) Initial monotherapy with either metformin or sulphonylureas often fails to achieve or maintain current glycaemic goals in patients with Type 2 diabetes in UK primary care. Diabet Med, 24, 350–358. [DOI] [PubMed] [Google Scholar]
- Cox K, Cocchi MN, Salciccioli JD, Carney E, Howell M & Donnino MW (2012) Prevalence and significance of lactic acidosis in diabetic ketoacidosis. Journal of Critical Care, 27, 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R, Fleming GA, Chen K & Bicsak TA (2016) Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism, 65, 20–29. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA (1999) Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med, 131, 281–303. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research, G. (2012) Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care, 35, 731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M & Leverve X (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem, 275, 223–228. [DOI] [PubMed] [Google Scholar]
- Erondu N, Desai M, Ways K & Meininger G (2015) Diabetic Ketoacidosis and Related Events in the Canagliflozin Type 2 Diabetes Clinical Program. Diabetes Care, 38, 1680–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fala L (2015) Invokamet (Canagliflozin plus Metformin HCl): First Fixed-Dose Combination with an SGLT2 Inhibitor Approved for the Treatment of Patients with Type 2 Diabetes. American Health & Drug Benefits, 8, 70–74. [PMC free article] [PubMed] [Google Scholar]
- Farxiga (R) [package insert]. AstraZeneca Pharmaceuticals LP, Wilmington, DE; 2016. [Google Scholar]
- Feenstra RA, Kiewiet MK, Boerma EC & ter Avest E (2014) Lactic acidosis in diabetic ketoacidosis. BMJ Case Reports, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrannini E & Solini A (2012) SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol, 8, 495–502. [DOI] [PubMed] [Google Scholar]
- Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, Sakamoto K, Andreelli F & Viollet B (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest, 120, 2355–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD & Umpierrez GE (2017) Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2017 Executive Summary. Endocr Pract, 23, 207–238. [DOI] [PubMed] [Google Scholar]
- Glucophage (R) [package insert]. Bristol Myers-Squibb Company, Princeton, NJ; 2015. [Google Scholar]
- Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P & Williams KM (2011) Clinical pharmacokinetics of metformin. Clin Pharmacokinet, 50, 81–98. [DOI] [PubMed] [Google Scholar]
- Hadjadj S, Rosenstock J, Meinicke T, Woerle HJ & Broedl UC (2016) Initial Combination of Empagliflozin and Metformin in Patients With Type 2 Diabetes. Diabetes Care, 39, 1718–1728. [DOI] [PubMed] [Google Scholar]
- Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, Ferrannini E, Fonseca VA, Garber AJ, Grunberger G, LeRoith D, Umpierrez GE & Weir MR (2016) AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY POSITION STATEMENT ON THE ASSOCIATION OF SGLT-2 INHIBITORS AND DIABETIC KETOACIDOSIS. Endocr Pract, 22, 753–762. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA & Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews. Molecular Cell Biology, 13, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RR, Frias JP, Walsh B, Skare S, Hemming J, Burns C, Bicsak TA, Baron A & Fineman M (2018) Improved glycemic control with minimal systemic metformin exposure: Effects of Metformin Delayed-Release (Metformin DR) targeting the lower bowel over 16 weeks in a randomized trial in subjects with type 2 diabetes. PLoS One, 13, e0203946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A & List JF (2012) Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract, 66, 446–456. [DOI] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR & Shulman GI (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes, 49, 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invokamet and Xigduo XR--two new combinations for type 2 diabetes. JAMA. 2015;313:620–1. [DOI] [PubMed] [Google Scholar]
- Invokana (R) [package insert]. Janssen Pharmaceuticals Inc., Titusville, NJ; 2017. [Google Scholar]
- Jardiance (R) [package insert]. Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT; 2016. [Google Scholar]
- Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T & Inui K (2005) Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metabolism and Pharmacokinetics, 20, 379–386. [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Umpierrez GE, Miles JM & Fisher JN (2009) Hyperglycemic crises in adult patients with diabetes. Diabetes Care, 32, 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut JA & Madias NE (2010) Metabolic acidosis: pathophysiology, diagnosis and management. Nat Rev Nephrol, 6, 274–285. [DOI] [PubMed] [Google Scholar]
- Kraut JA & Madias NE (2014) Lactic acidosis. N Engl J Med, 371, 2309–2319. [DOI] [PubMed] [Google Scholar]
- Laffel L (1999) Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev, 15, 412–426. [DOI] [PubMed] [Google Scholar]
- Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W & Meininger G (2013) Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia, 56, 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG & Shulman GI (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature, 510, 542–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreight LJ, Bailey CJ & Pearson ER (2016) Metformin and the gastrointestinal tract. Diabetologia, 59, 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AN, Emmett JB & Emmett M (2008) GOLD MARK: an anion gap mnemonic for the 21st century. Lancet, 372, 892. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chu Q, Xie J, Foretz M, Viollet B & Birnbaum MJ (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature, 494, 256–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S & Oliver NS (2015) Utility of ketone measurement in the prevention, diagnosis and management of diabetic ketoacidosis. Diabet Med, 32, 14–23. [DOI] [PubMed] [Google Scholar]
- Mracek T, Drahota Z & Houstek J (2013) The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta, 1827, 401–410. [DOI] [PubMed] [Google Scholar]
- Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, Desai M, Shaw W, Capuano G, Alba M, Jiang J, Vercruysse F, Meininger G, Matthews D & Group CTC (2015) Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care, 38, 403–411. [DOI] [PubMed] [Google Scholar]
- Owen MR, Doran E & Halestrap AP (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J, 348 Pt 3, 607–614. [PMC free article] [PubMed] [Google Scholar]
- Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC & Hirsch IB (2015) Euglycemic Diabetic Ketoacidosis: A Potential Complication of Treatment With Sodium–Glucose Cotransporter 2 Inhibition. Diabetes Care, 38, 1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstock J, Chuck L, Gonzalez-Ortiz M, Merton K, Craig J, Capuano G & Qiu R (2016) Initial Combination Therapy With Canagliflozin Plus Metformin Versus Each Component as Monotherapy for Drug-Naive Type 2 Diabetes. Diabetes Care, 39, 353–362. [DOI] [PubMed] [Google Scholar]
- Rosenstock J & Ferrannini E (2015) Euglycemic Diabetic Ketoacidosis: A Predictable, Detectable, and Preventable Safety Concern With SGLT2 Inhibitors. Diabetes Care, 38, 1638–1642. [DOI] [PubMed] [Google Scholar]
- Vallon V (2015) The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annual Review of Medicine, 66, 255–270. [DOI] [PubMed] [Google Scholar]
- Wang DS, Jonker JW, Kato Y, Kusuhara H, Schinkel AH & Sugiyama Y (2002) Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J Pharmacol Exp Ther, 302, 510–515. [DOI] [PubMed] [Google Scholar]
- Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G & Meininger G (2016) Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab, 101, 157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO: World Health Organization Global Report on Diabetes. http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1, 2016
- Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W & Meininger G (2013) Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract, 67, 1267–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulffele MG, Kooy A, de Zeeuw D, Stehouwer CD & Gansevoort RT (2004) The effect of metformin on blood pressure, plasma cholesterol and triglycerides in type 2 diabetes mellitus: a systematic review. J Intern Med, 256, 1–14. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ & Moller DE (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest, 108, 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]