Abstract
Linear furocoumarins, also known as psoralens, are clinically useful photo-activated pharmaceuticals employed to address hyperproliferative skin diseases. Seven diverse cytotoxic pharmacophores have been synthetically attached to 8-methoxypsoralen via a 5-amino functionality. The resulting unique set of compounds was evaluated for dark and light toxicity against PAM212 keratinocytes in culture.
Graphical Abstract

The natural product furocoumarin, known as methoxsalen or 8-MOP, has a long history as a nonionic reversible DNA intercalator(1,2) which demonstrates modest anticancer effects in the absence of light(3,4) but powerful cytotoxic effects upon photoactivation(5-7). A photocatalyzed mechanism through dual 2+2 cyclobutane formation between the proximal and distal ends of the psoralen and the pyrimidine bases, thereby cross-linking the DNA, has been invoked to explain the cytotoxicity.(1)
The synthetic incorporation of traditional anti-cancer pharmacophores onto the furocoumarin’s intercalator backbone might be expected to enhance the dark- and/or the light-activated cytotoxicity of the linear three-ring system. The 3,3-dialkyl-1-triazene group(8,9), the alpha-haloacetamide construct(10), the beta-chloroethylamine moiety(11-13) and the arylazide(14-15) functionality, all have a history of utility as anti-cancer pharmacophores through their incorporation in many other candidate chemotherapeutic platforms. The chemotherapeutic dacarbazine (or DTIC) is the most established drug containing the dialkyltriazene group. A photo-induced enhancement of its activity has recently been reported.(16) Many azide-containing molecules are cytotoxic with the antiviral azidothymidine (Zidovudine) being the most prominent(17) and, of course, the beta-chloroethylamino construct is found in many anticancer agents including Alkeran and Estramustine.(11, 18)
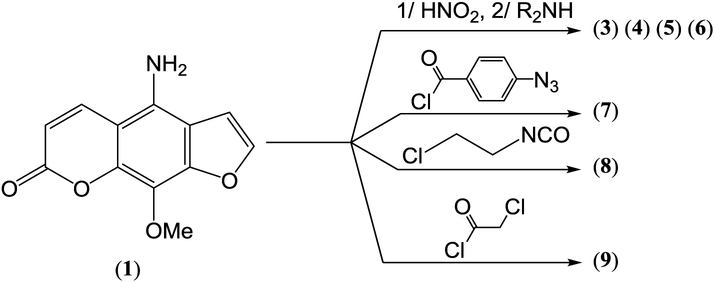
All of our methoxsalen chemotherapeutic candidates were synthesized in three or four steps from the parent psoralen. Careful nitration of methoxsalen, compound (2), followed by low-pressure hydrogenation of the pendant nitro group, provided a 38% yield of compound (1), the 5-amino-8-methoxypsoralen (a.k.a., 4-amino-9-methoxy-7H-furo[3,2-g][1]benzopyran-7-one, or 5-amino-8-MOP or 5-aminomethoxsalen).(19) The 5-amino functionality on this key intermediate constitutes a convenient synthetic handle to which can be attached the indicated cytotoxic pharmacophores: the triazeno group [– N=N–NR2], the alpha-haloacetamido group [–NH–CO–CH2Cl], the beta-chloroethylamino functionality [–NH–CH2CH2–Cl] and the aryl azido group [–NH–CO–Ph–N3-p]. Synthetic procedures for these attachments are described in the experimental section included in the supporting information and illustrated on Scheme 1.
Scheme 1.
Syntheses of Compounds (3) to (9):
For biological evaluation, since the linear furocoumarins (psoralens) are most noted for their clinical activity against pathological skin conditions, the PAM212 keratinocyte cell line was chosen as a screening model for both a dark and a light-catalyzed cytotoxicity study with compounds 2 to 9. The PAM212 line is a murine cutaneous squamous cell carcinoma with a long history of use as a surrogate for human skin hyperproliferative disease.(20) Photo- and non photo-biological activity was assayed with this cell line grown in monolayer culture as previously described.(21, 22)
It is immediately evident, see Table 1, column [B], that only one of the compounds (9) demonstrates any cytotoxicity following a short dark exposure. The highly electrophilic alpha-chloroacetamido pharmacophore, incorporated in compound (9), is a well-known cytotoxic, genotoxic, and mutagenic construct frequently ranked near the top of similar active-site directed (alkylator) inhibitors. This functionality is one of the most reactive participants in SN2 reactions and has been incorporated in a large number of active-site directed protein kinase inhibitors.(23) It requires no light activation and it manifests toxicity when incorporated in a wide-variety of structures.(10, 24, 25)
Table 1.
Effect of 8-methoxypsoralen derivatives on keratinocyte growth*:
| Cpd | R-group | Purity# & Physical Aspect mp (°C) |
[A] IC50 with UVA light (30 min contact) |
[B] IC50 w/o UVA light (30 min contact) |
[C] IC50 w/o UVA (5 day contact) |
|---|---|---|---|---|---|
| (2) | H (8-MOP) | Pale yellow crystals 148-149 | 0.1 μM | >100 μM | >100 μM |
| (3) | -N=N-NMe2 | Yellow crystals 187-189 | >100 μM | >100 μM | 20 μM |
| (4) | -N=N-NEt2 | Orange crystals 123-124 | >100 μM | >100 μM | 6 μM |
| (5) | -N=N-N(Me)(Et) | Yellow crystals 145-147 | >70 μM | >100 μM | 10 μM |
| (6) | -N=N-N(Me)(nBu) | Dark yellow crystals 66-67 | 4 μM | >100 μM | 7 μM |
| (7) | -NH-CO-(4-N3-Ph) | Light tan solid 185-187 | 33 μM | >100 μM | >300 μM |
| (8) | -NH-CO-NH(CH2)2Cl | White crystals 242-244 | >100 μM | >100 μM | >300 μM |
| (9) | -NHCOCH2Cl | Beige crystals 270-272 | 40 μM | 50 μM | 4 μM |
IC50 = concentration of psoralen inhibiting growth by 50%
All compounds had combustion analyses (C,H,N) within ± 0.3% of theoretical.
With UVA exposure (column [A]) compounds 2, 5, 6, 7, and 9 displayed phototoxicity. Of course, methoxsalen 2 as an established photo-activated DNA cross-linker, displayed its expected effect.(1,2, 5-7) Compound (9), the chloroacetamido which possesses dark-reaction cytotoxicity, was slightly more cytotoxic with brief UVA exposure than it had been in the brief dark exposure (column [B]) this confirming the photosensitization effects also seen in the other methoxsalen analogs 5, 6, and 7. This photosensitized behavior is not necessarily a reflection of the parent tri-cyclic aromatic (methoxsalen) molecule’s light-induced post-intercalation crosslinking. An alternative explanation is possible for the IC50 of 33 μM compound (7). Independent of any presumed sensitization deriving from the methoxsalen core, organic azides can independently photolyze to nitrenes, a highly electrophilic DNA-alkylator species.(26) In addition, as noted above, the dialkyltriazene has its own unique photolability contributing to its cytotoxicity.(16)
Table 1 reports the keratinocyte inhibitory effects with UVA activation, column [A]; with brief dark exposure of the agents to the keratinocyte cultures, column [B]; and with five-day dark incubation of all agents and keratinocytes, column [C].
The pharmacological relationship of DNA intercalators to the inhibition of cell growth is, of course, well established. Across any similarly structured family of intercalators, the most DNA-affinic members often seem to possess the highest cellular toxicity.(27, 28)
The structural relationships, however, which make molecules good intercalators are more complex, many and varied. In addition to being flat, fused three (or more) ring molecules, successful DNA intercalators frequently exhibit a large area of π electron delocalization. Few of them contain loci of high charge concentration within the zone of intercalation although charged moieties attached peripherally onto the intercalator core can enhance avidity (if positively charged) or reduce avidity (if negatively charged). The best intercalators are often good electron acceptors since the AT and GC base pairs with which they associate are good electron donors. This donor-acceptor feature, if present, results in very favorable aromatic stacking interactions between these two systems. Favorable intercalation characteristics have been reviewed.(27, 29)
In whole cell toxicity studies, any given structural class of intercalators often shows a correlation of cytotoxicity to lipophilicity. Whether the enhanced lipophilicity is linked to facilitated membrane permeation or an enhanced DNA-binding has never been determined but many examples of this lipophilic correlation are known.(30-32) In our study of the five-day, dark exposure of the candidate chemotherapeutics (see column [C] of Table 1), compounds (3) to (6) constitute a narrow structural class with only minor variations in the dialkyltriazeno sidechain. These do show such a correlation with lipophilcity. The computed logPs (cLogPs) obtained from the ChemDraw Professional, version 17.0.0.206 (121), are 4.47 for the most lipophilic compound, (6), whose IC50 is 7 μM and 2.88 for the least lipophilic analog, (3), whose IC50 was 20 μM. Compound (4) with cLogP of 3.94 had an IC50 of 6 μM and the methyl/ethyl triazeno analog, (5), with cLogP of 3.41 had IC50 of 10 μM. Here again compounds (7) and (8) were inactive and the highly electrophilic active site inhibitor (9) was best-inclass.
None of the compounds is as toxic with light (Table 1, Column [A]) as is the parent methoxsalen for which the dual 2+2 cyclobutane formation with pyrimidine bases requires pre-intercalation and photoactivation. (1, 2) One would not expect a significant electronic perturbation of the photo-adduct proclivities arising as a result of substitution at C5 (compared to methoxsalen as the reference compound). All these side chain pharmacophores have insignificant electronic interaction with the furanocoumarin platform, as measured by their Hammett constants. For example the σp values for –N=N–NMe2 (−0.03), – NHCOCH2Cl (−0.03), and –NH–CO–Ph (−0.06) are practically indistinguishable from –H (0.00) as in the parent methoxsalen.(33) Also, in 1H-NMR the chemical shifts of the respective protons on C3 – a position vinylogously linked to the C5 substituent – are isochronous at 6.38 ± 0.11 ppm thus implying very similar electron donations.
However, photo-toxicity – as manifest in bis-adduct formation arising from the psoralen double bonds – requires much more than just suitable electronic features. It requires a good intercalative fit and in our case attachments on compounds (3) to (9) are likely inhibitors of such binding. Ramaiah showed that in a series of six photoactive acridine intercalators, with varying-sized bulky groups at carbon-9, that DNA-binding decreased with the steric bulk of the substituents.(34) In a pyranocoumarin system we, too, showed that a single strategically-placed methyl at the edge of an intercalatable molecule thwarted DNA binding and substantially reduced phototoxicity.(35) The conclusion is that the phototoxicity enhanced effects seen in compounds (5), (6) and (7), compared to their dark-reaction toxicities arise from the functional group itself at C5 and not from the intercalation of its carrier psoralen.
For compound (8), the beta-chloroethylurea – a pharmacophore found in drugs such as cyclophosphamide and estramustine – is known to require a metabolic cleavage at the carbonyl, usually effected in the liver, before the chloroethylamine alkylating species is released.(36, 37) In our cell culture model such hydrolysis is apparently not possible and no cytotoxic activity is seen. There are no reports in the literature of photoactivation of beta-chloroethyl amides/ureas and no such activity was seen in our study.
The highly toxic alpha-chloroacetamido moiety (Compound 9) needs no UVA activation (compare columns [A] and [B]) and given sufficient time (column [C]) can manifest an impressive inhibition of keratinocyte growth. That functionality damages proteins by multiple pathways, at room temperature, and in the dark. Mass spectral studies have demonstrated that chloroacetamides alkylate at cysteine and the nitrogen termini of aspartate, glutamate, histidine, and lysine as well as perform oxidations at methionine, tyrosine, and tryptophan.(38)
Supplementary Material
Figure 1.
Core furocoumarin structure and the most common numbering system:
Acknowledgments:
This work was funded in part by the National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH Grant number U54AR055073). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.IARC (2012), International Agency for Research on Cancer, World Health Organization, Pharmaceuticals Monograph Vol. 100A: 363–373. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono100A.pdf [Google Scholar]
- 2.Wittung P, Kim SK, Buchardt O, Nielsen P, Norden B, (1994), Interactions of DNA binding ligands with PNA-DNA hybrids. Nucleic Acid Res, 22(24): 5371–5377. 10.1093/nar/22.24.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong J, Yang H, Luo W, et al. , (2016), The anti-metastatic effect of 8-MOP on hepatocellular carcinoma is potentiated by the down-regulation of bHLH transcription factor DEC1. Pharmacol. Res, 105: 121–133. 10.1016/j.phrs.2016.01.025 [DOI] [PubMed] [Google Scholar]
- 4.Peng Y, Liu W, Xiong J, et al. , (2012), Down regulation of differentiated embryonic chondrocytes 1 (DEC1) is involved in 8-methoxypsoralen-induced apoptosis in HepG2 cells. Toxicology, 301(1-3): 58–64. 10.1016/j.tox.2012.06.022 [DOI] [PubMed] [Google Scholar]
- 5.Johnson R, Staiano-Coico L, Austin L, et al. , (1996), PUVA treatment selectively induces a cell cycle block and subsequent apoptosis in human T-lymphocytes. Photochem. Photobiol, 63(5): 566–571. 10.1111/j.1751-1097.1996.tb05657.x [DOI] [PubMed] [Google Scholar]
- 6.Wrześniok D, Beberok A, Rok J, et al. , (2017), UVA radiation augments cytotoxicity of psoralens in melanoma cells. Int. J. Radiat. Biol, 93(7): 734–735. 10.1080/09553002.2017.1297903 [DOI] [PubMed] [Google Scholar]
- 7.Dalla Via L, Gonzalez-Gomez JC, Perez-Mastolo LG, et al. , (2009), A new psoralen derivative with enlarged anti-proliferative properties. Bioorg. Med. Chem. Lett, 19(10): 2874–2876. 10.1016/j.bmcl.2009.03.073 [DOI] [PubMed] [Google Scholar]
- 8.Atkinson V, (2015), Medical management of malignant melanoma. Aust. Prescr, 38(3): 74–78. DOI: 10.18773/austprescr.2015.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang C, Cao H, Liu N, et al. , (2016), Oncolytic adenovirus expressing interleukin-18 improves antitumor activity of dacarbazine for malignant melanoma. Drug Des. Devel. Ther, 10: 3755–3761. 10.2147/DDDT.S115121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DD, Steiner MS, Veverko KA, Barrett C, Hong S, (2005), Anticancer compounds and methods of use thereof. U. S. Patent Application, Pub No US 2005/0209320 A1, September 22, 2005.
- 11.Potish R, Adcock L, Brooker D, et al. , (1980), Sequential surgery, radiation therapy, and Alkeran in the management of epithelial carcinoma of the ovary. Cancer, 45: 2754–2758. [DOI] [PubMed] [Google Scholar]
- 12.San Miguel JF, Schlag R, Khuageva NK, et al. , (2008), Bortezomelo plus melphalan and prednisone for initial treatment of multiple myeloma. New England J. Medicine, 359(9): 906–917. DOI: 10.1056/NEJMoa0801479 [DOI] [PubMed] [Google Scholar]
- 13.Sullivan RD, Jones R Jr., Schnabel TG, Mc Shorey JC, (1954), The treatment of human cancer with intra- arterial nitrogen mustard (methylbis(2- chloroethyl)amine hydrochloride). Utilizing a simplified catheter technique. Cancer, 6: 121–134. [DOI] [PubMed] [Google Scholar]
- 14.Francuz J, Kovacevic I, Popsavin M, et al. , (2017), Design, synthesis and in vitro antitumour activity of new goniofufurone and 7-epi-goniofufurone mimics with halogen or azido groups at the C-7 position. Eur. J. Med. Chem, 128: 13–24. 10.1016/j.ejmech.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 15.Kinski E, Marzenell P, Hofer W, et al. , (2016), 4-Azidobenzylferrocenylcarbamate as an anticancer prodrug. J. Inorg. Biochem, 160: 218–224. 10.1016/j.jinorgbio.2016.02.023 [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto T, Hiraku Y, Okuda M, Kawanishi S, (2008), Mechanism of UVA-dependent DNA damage induced by an antitumor drug dacarbazine in relation to its photogenotoxicity. Pharm. Res 25(3): 598–604. DOI: 10.1007/s11095-007-9413-2 [DOI] [PubMed] [Google Scholar]
- 17.Pereira J, Levy D, Ruiz JL, Brocardo GA, Ferreira KA, Costa RO, Queiroz RG, Maria DA, Neto AE, Chamone DA, Bydlowski SP, (2013), Azidothymidine is effective against human multiple myeloma: a new use of an old drug? Anticancer Agents Med. Chem, 13(1): 186–192. DOI: 10.2174/1871520611307010186 [DOI] [PubMed] [Google Scholar]
- 18.Qin Z, Li X, Zhang J, Tang J, et al. , (2016), Chemotherapy with or without estramustine for treatment of castration-resistant prostate cancer: A systematic review and meta-analysis. Medicine (Baltimore), 95(39): e4801 DOI: 10.1097/MD.0000000000004801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heindel ND, Foster M, Varkey T, (1986), Nitrations of 4',5'-dihydropsoralens: a route to radiopharmaceutical precursors. J. of Heterocyclic Chem, 23(5): 1579–1582. 10.1002/jhet.5570230564 [DOI] [Google Scholar]
- 20.Ridd K, Dhiur S, Smith AG, Gant TW, (2010), Defective TPA signalling compromises HaCat cells as a human in vitro skin carcinogenesis model. Toxicol. In Vitro, 24: 910–915. 10.1016/j.tiv.2009.11.017 [DOI] [PubMed] [Google Scholar]
- 21.Mariano TM, Vetrano AM, Gentile SL, Heck DE, Whittemore MS, Guillon CD, Jabin I, Rapp RD, Heindel ND, Laskin JD, (2002), Cell-impermeant pyridinium derivatives of psoralens as inhibitors of keratinocyte growth. Biochem. Pharmacol, 63(1): 31–39. 10.1016/S0006-2952(01)00855-3 [DOI] [PubMed] [Google Scholar]
- 22.Black AT, Gray JP, Shakarjian MP, Laskin DL, Heck DE, Laskin JD, (2008), Increased oxidative stress and antioxidant expression in mouse keratinocytes following exposure to paraquat. Toxicol. Appl. Pharmacol, 231: 384–392. 10.1016/j.taap.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, Gray NS, (2013), Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol, 20(2): 146–159. 10.1016/j.chembiol.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pals JA, Wagner ED, Plewa MJ, Xia M, Attene-Ramos MS, (2017), Monohalogenated acetamide-induced cellular stress and genotoxicity are related to electrophilic softness and thiol/thiolate reactivity. J. Environ. Sci., (China) 58: 224–230. 10.1016/j.jes.2017.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vennerstrom JL, Holmes TJ Jr., (1987), Preparation and Evaluation of Electrophilic Derivatives of Phenylbutazone as Inhibitors of Prostaglandin-H Synthase. J. Med. Chem, 30: 563–567. DOI: 10.1021/jm00386a020 [DOI] [PubMed] [Google Scholar]
- 26.Wild D, Dirr A, Fasshauer I, Henschler D, (1989), Photolysis of arylazides and generation of highly electrophilic DNA-binding and mutagenic intermediates. Carcinogenesis, 10(2): 335–341. 10.1093/carcin/10.2.335 [DOI] [PubMed] [Google Scholar]
- 27.Sponer J, Leszezynski J, Hobza P, (1997), Thioguanine and thiouracil: hydrogen bonding and stacking properties, J. Phys. Chem., A, 101: 9489–9495. DOI: 10.1021/jp9720404 [DOI] [Google Scholar]
- 28.Wilder PT, Weber DJ, Winstead A, et al. , (2018), Unprecedented anticancer activities of organorhenium sulfonato and carboxylato complexes against hormone-dependent MCF-7 and hormone-independent triple-negative MDA-MB-231 breast cancer cells. Mol. & Cell. Biochem, 441: 151–163. 10.1007/s11010-017-3181-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rescifina A, Zagni C, Varrica MG, et al. , (2014), Recent advances in small organic molecules as DNA intercalating agents: Synthesis, activity, and modeling. Eur. J. Med. Chem, 74: 95–115. 10.1016/j.ejmech.2013.11.029 [DOI] [PubMed] [Google Scholar]
- 30.Gabano E, Gama S, Mendes F, et al. , (2013), Study of the synthesis, antiproliferative properties, and interaction with DNA and polynucleotides of cisplatin-like Pt(II) complexes containing carcinogenic polyaromatic amines. J. Biol. Inorg. Chem, 18(7): 791–801. 10.1007/s00775-013-1022-4 [DOI] [PubMed] [Google Scholar]
- 31.Mazuryk O, Magiera K, Rys B, et al. , (2014), Multifaceted interplay between lipophilicity, protein interaction and luminescence parameters of non-intercalative ruthenium(II) polypyridyl complexes controlling cellular imaging and cytotoxic properties. J. Biol. Inorg. Chem, 19(8): 1305–1316. 10.1007/s00775-014-1187-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajendra-Prasad VV, Peters GJ, Lemos C, et al. , (2011), Cytotoxicity studies of some novel fluoro acridone derivatives against sensitive and resistant cancer cell lines and their mechanistic studies. Eur. J. Pharm. Sci, 43(4): 217–224. 10.1016/j.ejps.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 33.Hansch C, Leo A, Taft RW, (1991), A survey of Hammett substituent constants, resonance, and field parameters. Chem. Rev, 91(2): 165–195. https://pubs.acs.org/doi/10.1021/cr00002a004 [Google Scholar]
- 34.Joseph J, Kuruvilla E, Ahhuthan AT, Ramaiah D, Schuster GB, (2004), Tuning of intercalation and electron-transfer processes between DNA and acridinium derivatives through steric effects. Bioconj. Chem, 15(6): 1230–1235. DOI: 10.1021/bc0498222 [DOI] [PubMed] [Google Scholar]
- 35.Laskin JD, Jan Y-H, Jetter MM, Guillon CD, Mariano TM, Heck DE, Heindel ND, (2018), Identification of a pyranocoumarin photosensitizer that is a potent inhibitor of keratinocyte growth, Photochem. Photobiol, 94: 577–582. 10.1111/php.12882 [DOI] [PubMed] [Google Scholar]
- 36.Andersson SB, Gunnarsson PO, Nilsson T, Forshell GP, (1981), Metabolism of estramustine phosphate (Estracyt) in patients with prostatic carcinoma. Eur. J. Drug Metab. Pharmacokinet., 6(2): 149–154. DOI: 10.1007/BF03189482 [DOI] [PubMed] [Google Scholar]
- 37.Alberts DS, Einspahr JG, Struck R, Bignami G, Young L, Surwit EA, Salmon SE, (1984), Comparative in vitro cytotoxicity of cyclophosphamide, its major active metabolites and the new oxazaphosphorine ASTA Z 7557 (INN mafosfamide). Invest. New Drugs, 2(2): 141–148. 10.1007/BF00232343 [DOI] [PubMed] [Google Scholar]
- 38.Hains PG, Robinson PJ, (2017), The Impact of Commonly Used Alkylating Agents on Artifactual Peptide Modification. J. Proteome Res, 16(9): 3443–3447. https://pubs.acs.org/doi/10.1021/acs.jproteome.7b00022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.