Abstract
Host manipulation is a parasite-induced alteration of a host's phenotype that increases parasite fitness. However, if genetically encoded in the parasite, it should be under selection in the parasite. Such host manipulation has often been assumed to be energetically costly, which should restrict its evolution. Evidence of such costs, however, remains elusive. The trophically transmitted cestode Schistocephalus solidus manipulates the activity of its first intermediate copepod host to reduce its predation susceptibility before the parasite is ready for transmission. Thereafter, S. solidus increases host activity to facilitate transmission to its subsequent fish host. I selected S. solidus for or against host manipulation over three generations to investigate the evolvability of manipulation and identify potential trade-offs. Host manipulation responded to selection, confirming that this trait is heritable in the parasite and hence can present an extended phenotype. Changes in host manipulation were not restrained by any obvious costs.
Keywords: host manipulation, Schistocephalus solidus, energetic costs, extended phenotype, response to selection, experimental selection
1. Background
The phenotypes, including the behaviour, of an infected and an uninfected individual often differ. Such alterations that are caused by an infection can benefit the host, the parasite, both or neither. Trophically transmitted parasites, for example, can reduce or even reverse their intermediate host's innate fear of (certain) predators to facilitate transmission [1–4]. Ensuing benefits to the parasite can be accidental due to host responses or side effects, but the parasite might engage in true host manipulation, which alters its host phenotype, such as its behaviour, to increase its own fitness. Central to this definition is that the trait in question is controlled by the parasite to some degree; it is an extended phenotype (i.e. a trait that is genetically encoded in one organism, the parasite, but whose phenotype is expressed elsewhere, the host) [5,6]. In practice, determining whether any trait expressed by an infected host which seems to benefit the parasite is indeed controlled by the parasite or a side-effect with accidental benefits to the parasite can be challenging [3,7–12]. Host manipulation that is encoded by the parasite's genes should be under selection acting on the parasite rather than the host. This is usually assumed to bear a cost for the parasite, which could be either physiological (i.e. a cost due to the very process of manipulation which might be energetically costly for the parasite [3,10,13–15]) or ecological (e.g. through increasing mortality through dead-end predation [16–19]). Clear evidence for energetic costs has been elusive and is restricted to correlational evidence of potential trade-offs with other traits [20–23], which might not always be related to costs [21,24].
To better understand how readily host manipulation might evolve, I conducted an experimental selection experiment using the cestode Schistocephalus solidus and its copepod host Macrocyclops albidus. Schistocephalus solidus is known to manipulate its first intermediate copepod host to modify its predation susceptibility according to its need. Before the parasite becomes infective to its subsequent fish host, it reduces host activity and predation susceptibility [21,25–29]. Once the parasite is infective, host manipulation switches to increase host activity and hence enhance predation to facilitate transmission to the next host [25,30,31], albeit this seems to differ between different parasite populations [27,29]. I selected the parasite either for high or low levels of host manipulation, and measured various other fitness-related parasite traits to assess potential trade-offs. Selection indeed resulted in altered host manipulation throughout the parasite's development.
2. Material and methods
(a). Hosts
Copepods (Macrocyclops albidus) stemmed from a laboratory culture that originated from the Neustädter Binnen See, northern Germany (54°06′49.6″ N 10°48′28.0″ E). On the day prior to infection, copepods were distributed to individual wells of 24-well cell culture plates with about 1 ml of water each. I used new copepods from the same laboratory culture for each generation. Only adult male copepods were used to reduce variability with regard to the host. I checked for dead copepods, cleaned wells if necessary and fed copepods every second to third day with 5 Artemia sp. naupili each.
(b). Parasites and infection
Schistocephalus solidus originated from the same population as the copepods (Neustädter Binnen See, northern Germany). Parasites for the initial generation (F0) came from nine different parasite families, parts of them half sibs that had been bred in an in vitro system in the laboratory [32]. Their parents originated from a total of seven different parasite families bred from parasites dissected from fish caught in 2013 and 2014 (see electronic supplementary material, figure S1 for a pedigree of parasite families). In subsequent generations, I used eggs from selected parasites to infect the next generation (see below). After breeding, parasite eggs were stored in the fridge (4°C) [33]. Prior to use, they were incubated for three weeks and exposed to light overnight to induce hatching. One hatched coracidium was given to each copepod for infection.
(c). Behavioural recordings and trait measurements
On days 6, 7 and 8 (when parasites are not yet infective and suppress predation) and on days 13, 14 and 15 (when parasites are infective and enhance predation) post infection, I recorded copepod behaviour by placing a plate with copepods on an apparatus that dropped it by about 3 mm to simulate a predator attack [21,25–27,34]. This drop took place once the plate had been on the apparatus for 1 min. Video recordings started just before the drop, lasted 15 min and were made with an HD-camera (MHD-13MG6SH-D, Mintron, Taiwan). From these recordings, I extracted one image every 2 s for 90 s in ImageJ [35], starting 10 s after the simulated predator attack to exclude the initial reaction. I only used this first 90 s of the recording after the drop because this should comprise the time when copepods should expect a predator to be present and during which host manipulation would be most crucial. These images were analysed with a custom-made Python program (available at: https://github.com/ferhah/copepodtracking) to automatically record copepod position [29]. To exclude random noise, I only considered a copepod moving if it moved by at least 5 pixels (about one copepod length) from one frame to the next. To obtain copepod activity, I calculated the proportion of time each copepod spent moving (i.e. the number of times a copepod moved divided by the number of images) during the 90 s following the simulated predator attack for each trial. Then, I computed the average activity of each copepod over the trials before (6–8 days post infection) and after (13–15 days post infection) parasites reached infectivity.
To identify potential trade-offs that could hint at an energetic cost to host manipulation, I recorded a number of fitness-relevant parasite traits. On day 8 post infection, I checked copepods for parasite infection and, if infected, the presence or absence of a cercomer (electronic supplementary material, figure S2), which is an indicator of parasite development [27,36,37]. On day 15 post infection, I took photos of copepods under a microscope to measure parasite size (electronic supplementary material, figure S2). To do so, I outlined each parasite's shape without the cercomer and measured the area within this shape [38] in ImageJ [35]. All measurements were conducted blindly with regard to selection line.
(d). Selection and parasite breeding
I selected only the parasites for host manipulation without inducing any selection on the copepod host. In the initial generation (F0), I exposed 597 copepods, 148 of which became infected. With these infected copepods, three parasite selection lines were created (numbers in brackets represent sample sizes of exposed/infected copepods in each generation and treatment): high line (selection for host manipulation, F1: 580/170, F2: 480/153, F3: 260/95), low line (selection against host manipulation, F1: 647/156, F2: 480/145, F3: 260/107) and control (no selection on host manipulation, F1: 213/61, F2: 160/54, F3: 223/70). Additionally, 27 (F0), 48 (F1), 32 (F2) and 49 (F3) copepods were not exposed and randomly distributed over all plates to measure the behaviour of uninfected copepods.
Not-yet-infective S. solidus reduce host activity to suppress predation while infective ones increase it to enhance predation. Hence, to obtain a single measure of host manipulation irrespective of parasite development, I calculated the magnitude of the change from predation suppression before the parasite became infective to predation enhancement after the parasite became infective. More precisely, I subtracted the mean host activity before the parasite became infective and suppressed predation (6–8 days post infection) from the mean host activity after the parasite reached infectivity and enhanced predation (13–15 days post infection). This allowed me to select for predation suppression and predation enhancement simultaneously, because ultimately both contribute to host manipulation. Additionally, this ensured that I did not simply select for parasites that increased or decreased host activity irrespective of their life stage.
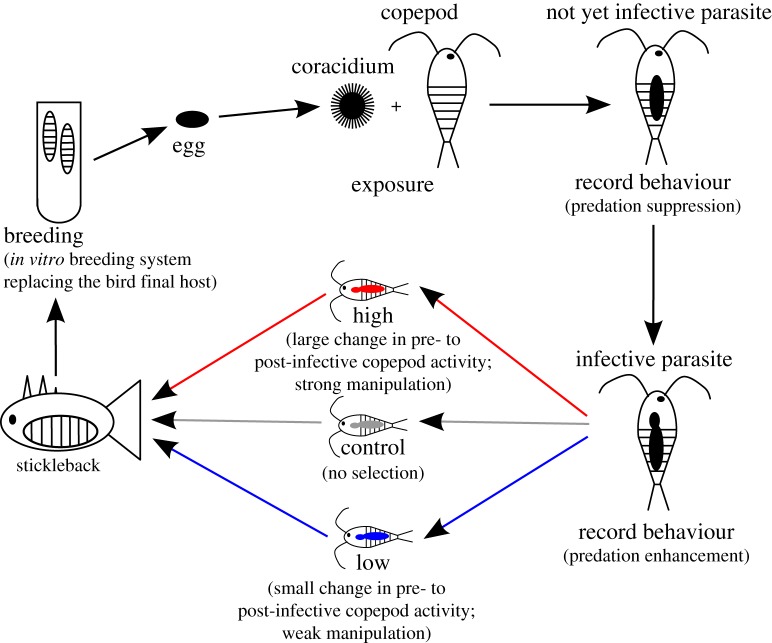
All parasites whose hosts survived until 15 days post infection were included in the selection pool. To create the F1 generation, initially, one-quarter of parasites were randomly selected to serve as controls (i.e. no selection on host manipulation). From the remaining parasites, the one-third that exhibited the strongest host manipulation was selected as the high selection line, while the one-third that showed the weakest host manipulation was the low selection line (figure 1). Any remaining parasites (and their copepod hosts) were discarded. Selected parasites from each selection line were randomly assigned to four artificial replicate populations with parasites from each selection line in each replicate. In each subsequent generation, selection and mating took place within these replicates within each line to obtain four replicates for each selection line. While this might have increased inbreeding, it allowed me to control for responses that could have occurred in single lines due to chance, which otherwise would have been impossible to distinguish from any actual response to selection. To set up F2 and F3, I used all parasites that successfully infected copepods in the control lines, the one-third of parasites who showed the highest host manipulation in the high selection lines, and the one-third of parasites which excerted the lowest host manipulation from the low selection lines. If too many copepods were available for any particular selection line and replicate after selection, I randomly selected a subset (controls) or took the ones with the highest (high) or lowest (low) host manipulation.
Figure 1.
Experimental set up to select parasites for or against host manipulation. Only parasites were selected in different selection lines (presented in different colours) and the copepods harbouring these selected parasites fed to sticklebacks for the parasites to continue their life cycle (bottom left in the drawing) and bred in pairs in an in vitro system to produce eggs (top left in the drawing). The offspring that hatched from these eggs of selected parasites was then used to infect naive, unselected copepods from the stock population in every generation (top middle of the drawing).
In each generation, copepods that had been selected were fed to the subsequent host, three-spined sticklebacks (Gasterosteus aculeatus), to allow them to grow to maturity. This took place 17 days post infection when all parasites should be able to infect sticklebacks. I used laboratory-bred sticklebacks whose parents had been caught in the Große Plöner See, northern Germany (54°08′09.1″ N 10°24′58.9″ E). I randomly used fish from 4 (F0) or 7 (F1 and F2) different fish families. Two days prior to exposure, fish were spine clipped (first or second spine) for later identification (see below) and distributed to small individual 1 l tanks. Each fish was then individually exposed to one infected copepod. Two days later, fish were returned to 16 l tanks with 9–15 fish per tank. Fish that received copepods from the same selection line and replicate shared tanks. In the F2 generation, I additionally infected fish from the same families with copepods from the high and low selection lines that had not been selected. These fish were used only to measure infection rates in fish, but not to obtain the next generation of parasites.
Two to three months after exposure, fish were killed and dissected to obtain mature parasites. I measured fish weight and length. Unless they were small, I also weighed the gonads to obtain fish weight without the potentially large weight of the female gonads. Parasites were weighed and bred in pairs with a size-matched individual from their own selection line and replicate using an in vitro breeding system [32,39]. Fertility was measured by counting eggs using a Z2 Coulter particle counter and size analyser (Beckman Coulter Inc., USA). Since parasites were bred in pairs, I was unable to obtain individual measurements of egg output, but rather obtained one value for each parasite pair.
To identify fish and thereby determine the identity of the copepod for each parasite, I took a tail clip during dissection for subsequent DNA extraction. DNA from tail clips and the spine clips obtained prior to exposure was extracted with the Qiagen DNeasy 96 Blood and Tissue Extraction Kit, following the manufacturer's protocol, and used to type fish for nine microsatellites to identify fish [40].
(e). Statistical analysis
All statistical analyses were conducted in R [41].
I analysed various response variables separately using different subsets of the data. To analyse copepod behaviour with respect to host manipulation (i.e. the difference in host activity between copepods with not-yet-infective and copepods with infective parasites) and host activity (i.e. the raw host activity either in copepods with not-yet-infective or with infective parasites), I excluded all copepods that had died or been lost during the experiment or in which exposure had not resulted in an infection. To analyse infection success in copepods, I used all copepods that had been exposed to a parasite and for which I knew their infection status (i.e. that had survived long enough to be checked for infection). For parasite development and size in copepods, I used all infected copepods for which I was able to obtain the relevant information. To analyse infection success and size in fish, I excluded fish that died within a few weeks after exposure because their parasites might have been too small to determine their infection status or which survived until dissection, respectively. To analyse parasite fecundity, I used all parasite families bred during the experiment. Since parasites did not belong to any treatment in the initial generation prior to selection, they were randomly assigned to a selection line which did not necessarily correspond to the selection line for which they were selected in order to facilitate statistical analysis which required that all treatments were present in each generation.
To each parasite trait, I applied general (host manipulation, host activity, parasite size in copepods and fish, and parasite fertility in fish) or generalized linear mixed models with a binomial error family (infection success in copepods and fish and development) using the lme4 package [42]. Mixed models were used in order to account for replicate which was included as a random factor. Generation and selection line and their interaction were included as fixed factors. To analyse host manipulation and host activity, I additionally included the infection status of the copepod and all its interactions with fixed factors that did not involve any interaction between infection and selection line for selection on parasites. The model for host activity included two additional factors: a fixed factor parasite stage (i.e. predation suppression by not-yet-infective parasites versus predation enhancement by infective parasites) and all its interactions with the other fixed factors, and a random factor copepod identity to account for the fact that each copepod was measured twice (i.e. before and after reaching infectivity). I chose to analyse host manipulation and activity in two separate models despite substantial overlap because these models serve to answer two different questions. The former served to test whether there were any changes in the actual trait under selection, host manipulation, the latter to test to what extend these changes affected host activity during predation suppression and predation enhancement and whether, as expected, they changed into different directions. To model parasite size in fish and parasite fecundity, I additionally accounted for time spent in fish. If, in the case of parasite fecundity, the two parasites in a pair had spent different amounts of time in their fish host, I used the mean time they had spent in the fish.
To obtain p-values for the combined effect of each fixed factor in each analysis, I compared each model to a less complicated model using likelihood ratio tests from the ANOVA function from the R base package [41]. Additionally, I report the estimates for the best model in each case, meaning the least complicated model that explained the data significantly better than any less complicated model (table 2; electronic supplementary material, tables S2 and S5). To identify between which selection lines significant differences occurred if either selection line and/or its interaction with any other factor was significant, I fitted separate general or generalized linear mixed models to each response variable within each generation and, for host activity, parasite stage (not-yet-infective parasites, i.e. predation suppression, versus infective parasites, i.e. predation enhancement), using selection line and infection (host manipulation and host activity only) as fixed factor on which I could subsequently apply post hoc tests using Tukey corrections for multiple testing (glht, multcomp [43]) to compare between individual selection lines. To keep the results easier to read, only the most important details on the statistical outputs of the models are presented in tables 1 and 2, and electronic supplementary material, tables S1–S6.
Table 2.
Summary of the model containing all fixed effects and significant interactions. Infection and its interaction with parasite stage (host activity only) were removed from this model because this information is also contained in selection line. Comparisons are with not-yet-infective (host activity only) parasites from the control line.
| host manipulation | host activity | |||
|---|---|---|---|---|
| random effects | ||||
| factor | variance ± s.d. | variance ± s.d. | ||
| identity (intercept) | 0.0060 ± 0.0775 | |||
| replicate (intercept) | 0.0002 ± 0.0127 | 0.0011 ± 0.0329 | ||
| residual | 0.0431 ± 0.2076 | 0.0216 ± 0.1470 |
| factor | estimate ± s.e. | t | estimate ± s.e. | t |
|---|---|---|---|---|
| fixed effects | ||||
| intercept | 0.2354 ± 0.0185 | 12.697 | 0.2897 ± 0.0216 | 13.418 |
| selection line (high) | 0.0147 ± 0.0172 | 0.859 | −0.0046 ± 0.0270 | −0.170 |
| selection line (low) | −0.0737 ± 0.0172 | −4.275 | 0.0271 ± 0.0271 | 0.999 |
| selection line (uninfected) | −0.2261 ± 0.0351 | −6.448 | 0.2301 ± 0.0366 | 6.287 |
| generation | 0.0277 ± 0.0155 | 1.791 | 0.0011 ± 0.0124 | 0.086 |
| parasite stage | 0.2360 ± 0.0174 | 13.540 | ||
| parasite stage: generation | 0.0286 ± 0.0155 | 1.849 | ||
| parasite stage: selection line (high) | 0.0138 ± 0.0172 | 0.806 | ||
| parasite stage: selection line (low) | −0.0742 ± 0.0173 | −4.298 | ||
| parasite stage: selection line (uninfected) | −0.2285 ± 0.0351 | −6.516 | ||
| infection: generation | −0.0646 ± 0.0168 | −3.846 | 0.0209 ± 0.0135 | 1.545 |
| parasite stage: generation: infection | −0.0655 ± 0.0168 | −3.905 |
Table 1.
General linear mixed models to analyse copepod behaviour in response to infection by parasites selected for or against host manipulation. Initial model for host manipulation: response approximately 1 + (1/population). Initial model for host activity: response approximately 1 + (1/population) + (1/copepod identity). Test statistics and p-values were obtained using likelihood ratio tests and are always for the comparison with the preceding (i.e. less complicated) model. n: host manipulation: 1231 copepods in 4 populations; host activity: 2462 observations on 1231 copepods in 4 populations. Significant p-values are highlighted in bold.
| factor | df | χ2 | p |
|---|---|---|---|
| host manipulation (df: 3) | |||
| + generation | 4.1 | 20.28 | <0.0001 |
| + infection | 5.1 | 27.44 | <0.0001 |
| + selection line | 7.2 | 41.96 | <0.0001 |
| + infection: generation | 8.1 | 14.88 | 0.0001 |
| + selection line: generation | 10.2 | 3.59 | 0.1658 |
| host activity (df: 4) | |||
| + parasite stage (predation suppression versus predation enhancement) | 5.1 | 428.66 | <0.0001 |
| + generation | 6.1 | 1.77 | 0.1436 |
| + generation: parasite stage | 7.1 | 20.39 | <0.0001 |
| + infection | 8.1 | 132.17 | <0.0001 |
| + selection line | 10.2 | 1.87 | 0.8362 |
| + infection: generation | 11.1 | 1.44 | 0.2460 |
| + selection line: generation | 13.2 | 0.91 | 0.5638 |
| + infection: parasite stage | 14.1 | 27.50 | <0.0001 |
| + selection line: parasite stage | 16.2 | 41.77 | <0.0001 |
| + generation: infection: parasite stage | 17.1 | 15.23 | 0.0001 |
| + generation: selection line: parasite stage | 19.2 | 3.62 | 0.1635 |
I calculated selection differential for each selection line and generation as the difference between the mean host manipulation within each replicate and the mean host manipulation of selected copepods. To estimate the heritability of host manipulation by not-yet-infective parasites (suppressing predation) by infective parasites (enhancing predation) and overall, I combined data from all three generations and calculated mid-parent and mean offspring values for each trait. Subsequently, I applied a linear regression in R to obtain heritability.
3. Results and discussion
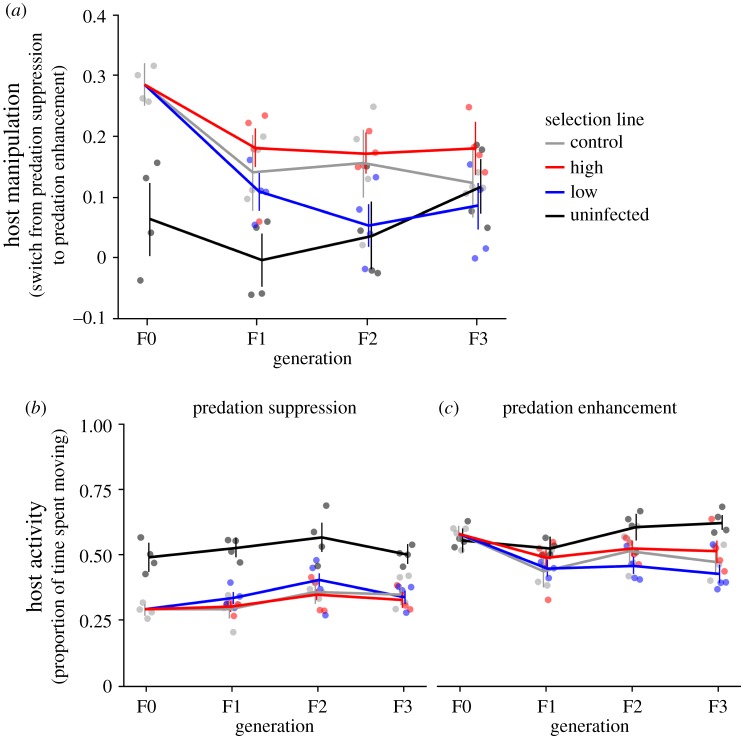
The parasite Schistocephalus solidus responded to selection on host manipulation. Selection was based on host manipulation, which, in order to obtain a single measurement, is here considered as the change in host manipulation as parasites became infective and switched from predation suppression by not-yet-infective parasites to predation enhancement by infective parasites (see Material and methods). The resulting selection lines had a significant effect on host manipulation (p < 0.001; figure 2a, tables 1 and 2); selection for high host manipulation (high selection lines) increased it by 0.015 ± 0.017 (estimate ± s.e.), while selection against host manipulation (low selection lines) decreased host manipulation by 0.074 ± 0.017 (estimate ± s.e.) compared with unselected controls. This confirms that selection on the parasite only was successful in changing the host's phenotype. Within infected copepods, differences between those infected by parasites selected for more or less host manipulation appeared within a single generation (t = −3.01, p = 0.013; figure 2a) and became more pronounced in subsequent generations (p < 0.005; figure 2a; electronic supplementary material, table S3). The behaviour of copepods infected with parasites from control lines was intermediate and never differed from high selection lines (p > 0.2; figure 2a; electronic supplementary material, table S3) and differed significantly from low selection lines during the second generation only (t = −2.92, p = 0.017; figure 2a). This could be some indication that host manipulation more easily decreases than increases. However, given that power to detect differences involving control lines was much lower than power to detect differences between the two selected lines due to a smaller sample size for control lines, variation in the data and the relatively small effect size makes any interpretation of the lack of significant differences between control lines and selected lines difficult to interpret. Somewhat surprisingly, host manipulation and the effect of infection on host manipulation decreased between generations (p < 0.0001). Some variation in copepod behaviour within and between experiments does not seem uncommon in this system [21,25,26,29,34]. Host manipulation might additionally have been affected by the breeding regime resulting in some inbreeding. It is unknown whether inbreeding can affect host manipulation. However, these changes occurred equally in all selection regimes (p = 0.1658; tables 1 and 2), so they are unlikely to change any of the findings with regard to selection on host manipulation.
Figure 2.
Host manipulation over generations. (a) Host manipulation. (b) Predation suppression (i.e. days 6–8 post infection, not-yet-infective parasites), (c) Predation enhancement (i.e. days 13–15 post infection, infective parasites). Error bars represent 95% confidence intervals. n: F0: uninfected: 27, infected: 132, F1: uninfected: 47, control: 60, high: 158, low: 149, F2: uninfected: 28, control: 49, high: 144, low: 135, F3: uninfected: 46, control: 65, high: 90, low: 101. Points indicate replicate populations.
Differences between selection lines could be caused by differences in host manipulation either before or after the parasite became infective or both. In order to distinguish between these scenarios, I also analysed host activity depending on parasite stage (not-yet-infective versus infective). There was no main effect of selection line on host activity (p = 0.393; figure 2b,c, tables 1 and 2), but there was a significant interaction between parasite stage (not-yet-infective and suppressing predation versus infective and enhancing predation) and selection line (p < 0.001; figure 2b,c, tables 1 and 2). Selection line had opposite effects on host activity depending on parasite maturity. More precisely, not-yet-infective parasites from high selection lines induced lower activity (i.e. higher levels of manipulation) than not-yet-infective parasites from low selection lines (figure 2b), albeit these differences were only significant during the second generation (t = 3.36, p = 0.004; electronic supplementary material, table S3) and seemed to disappear thereafter (t = 0.63, p = 0.920; electronic supplementary material, table S3). Once infective, the pattern was reversed in that infective parasites from high selection lines induced higher activity (i.e. higher levels of manipulation) than infective parasites from low selection lines (figure 2c). These differences became significant during the second generation (t = −3.03, p = 0.013; electronic supplementary material, table S3) and increased during the third (t = −3.28, p = 0.005; electronic supplementary material, table S3). Hence, both the ability of not-yet-infective parasites to reduce host activity to suppress predation and the ability of infective parasites to increase host activity to enhance predation changed in the expected direction. Controls were mostly intermediate and never significantly differed from either selection line (p > 0.1; electronic supplementary material, table S3).
The selection differential confirmed that selection was similarly strong in either selection line, albeit, in both lines, it decreased over generations (selection differential for each generation (F0/F1/F2): high: 0.256/0.214/0.100; low: 0.261/0.201/0.062) and there was little selection on control lines (0.016/0.004/0.006). This decrease could be due to inbreeding and/or because the limit in genetic variation in host manipulation was reached which might well be connected. The fact that S. solidus responded to selection indicates that host manipulation must be heritable. Indeed, the heritability estimate (estimate ± s.e.) for overall manipulation was significantly positive (h2 = 0.166 ± 0.042, t1,78 = 3.91, p < 0.001). Heritability was similar for manipulation by not-yet-infective parasites (h2 = 0.147 ± 0.070, t1,78 = 2.10, p = 0.039) and by infective parasites (h2 = 0.143 ± 0.056, t1,78 = 2.56, p = 0.012). Heritability for manipulation by not-yet-infective parasites corresponds well to previous estimates of heritability in this system calculated on comparisons between full sibships [27], indicating that host manipulation is mostly an additive trait. In the same study, however, Benesh [27] found no evidence that host manipulation by infective parasites to enhance predation was heritable. This discrepancy could be due to the fact that Benesh [27] used a different parasite population, whose host manipulation once parasites are infective differs from the parasite population used in this study [27,29].
To identify potential costs of host manipulation, I measured potential trade-offs with a number of other traits. Increased ability to manipulate the host did not result in any clear trade-offs between host manipulation and other fitness-related traits in parasites (see electronic supplementary information for details). If anything, parasites from the high selection line seemed to perform better during the first and second generation, albeit these differences were not significant and disappeared in the third generation. Selection over further generations would be necessary to judge whether costs would eventually emerge, but they do not seem to be obvious or present a strong hindrance to at least some changes in the level of host manipulation.
The response to selection suggests standing genetic variation for host manipulation in the parasite population. How can such variation be maintained when there seem to be no energetic costs restraining increases in host manipulation? Rather than energetic costs, ecological factors could shape host manipulation. Trade-offs between enhanced transmission to a correct subsequent host and increased risk of dead-end predation (i.e. by predators other than the appropriate subsequent host) [18,44,45] could, for example, limit the level of host manipulation by infective parasites to enhance predation (and thereby transmission). Host manipulation by not-yet-infective parasites that suppresses predation must have a natural limit as host activity can only be reduced so far, especially because it is also in the parasite's interest for the host to continue performing some normal functions such as feeding [44]. However, this might not be due to any limit imposed by the host because a not-yet-infective nematode with the same first intermediate copepod host and similar host manipulation reduces host activity much more than S. solidus [34]. The benefits and ecological costs of host manipulation should vary with environmental factors, such as the prevalence of dead-end predators, correct subsequent hosts (and possibly their size [46]) and the availability of alternative food items for these predators. These factors might vary in time and space. The final host of S. solidus, a bird, provides parasite dispersion resulting in unpredictability with regard to the environment. Additionally, parasites could have to trade off their ability to manipulate a certain host genotype or species (S. solidus can infect various cyclopoid copepods [33,47]) versus their ability to manipulate another host genotype or species. Such genotype-by-genotype interactions are frequent when it comes to host–parasite interactions [48–50].
4. Conclusion
Host manipulation responded to selection on the parasite and does not seem to be constrained by any obvious physiological costs. This confirms that host manipulation is an extended parasite phenotype that can evolve in response to selection on the parasite rather than on the host. Given ecological selection pressure, host manipulation can and will respond to selection to better accommodate a parasite's need for certain host behaviour, even at the expense of the host.
Supplementary Material
Acknowledgements
I would like to thank R. Derner, G. Schmiedeskamp and M. Schwarz for technical assistance, D. Martens and R. Schmuck for fish keeping, M. Ritter and D. Schmid for providing fish, F. Hahmann for help with data analysis, M. Milinski and D. P. Benesh for insightful comments on a previous version of this manuscript, and M. Gopko and an anonymous reviewer for suggestions that helped improve this manuscript.
Ethics
Animal experiments were conducted with the permission of the ‘Ministry of Energy, Agriculture, the Environment and Rural Areas' of the state of Schleswig-Holstein, Germany (reference number: V 313-72241.123-34).
Data accessibility
All data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.v273bt1 [51].
Competing interests
I have no competing interests.
Funding
Financial support was provided by the Max Planck Society, and I was partially supported by a DFG fellowship (HA 8471/1-1).
References
- 1.Moore J. 2002. Parasites and the behavior of animals. New York, NY: Oxford University Press. [Google Scholar]
- 2.Poulin R, Maure F. 2015. Host manipulation by parasites: a look back before moving forward. Trends Parasitol. 31, 563–570. ( 10.1016/j.pt.2015.07.002) [DOI] [PubMed] [Google Scholar]
- 3.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Study Behav. 41, 151–186. ( 10.1016/S0065-3454(10)41005-0) [DOI] [Google Scholar]
- 4.Heil M. 2016. Host manipulation by parasites: cases, patterns, and remaining doubts. Front. Ecol. Evol. 4, 80 ( 10.3389/fevo.2016.00080) [DOI] [Google Scholar]
- 5.Dawkins R. 1982. The extended phenotype. Oxford, UK: Oxford University Press. [Google Scholar]
- 6.Dawkins R. 1990. Parasites, desiderata lists and the paradox of the organism. Parasitology 100, S63–S73. ( 10.1017/S0031182000073029) [DOI] [PubMed] [Google Scholar]
- 7.Milinski M. 1990. Parasites and host decision-making. In Parasitism and host behaviour (eds Barnard CJ, Behnke JM), pp. 95–116. London, UK: Taylor & Francis Group. [Google Scholar]
- 8.Poulin R. 1995. ‘Adaptive’ parasitized changes in the behaviour of animals: a critical review. Int. J. Parasitol. 25, 1371–1383. ( 10.1016/0020-7519(95)00100-X) [DOI] [PubMed] [Google Scholar]
- 9.Poulin R, Thomas F. 1999. Phenotypic variability induced by parasites: extent and evolutionary implications. Parasitol. Today 15, 28–32. ( 10.1016/S0169-4758(98)01357-X) [DOI] [PubMed] [Google Scholar]
- 10.Thomas F, Adamo SA, Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Processes 68, 185–199. ( 10.1016/j.beproc.2004.06.010) [DOI] [PubMed] [Google Scholar]
- 11.Cézilly F, Thomas F, Médoc V, Perrot-Minnot M-J. 2010. Host-manipulation by parasites with complex life cycles: adaptive or not? Trends Parasitol. 26, 311–317. ( 10.1016/j.pt.2010.03.009) [DOI] [PubMed] [Google Scholar]
- 12.Moore J. 2013. An overview of parasite-induced behavioral alterations: and some lessons from bats. J. Exp. Biol. 216, 11–17. ( 10.1242/jeb.074088) [DOI] [PubMed] [Google Scholar]
- 13.Poulin R. 1994. The evolution of parasite manipulation of host behaviour: a theoretical analysis. Parasitology 109, S109–S118. ( 10.1017/S0031182000085127) [DOI] [PubMed] [Google Scholar]
- 14.Thomas F, Brodeur J, Maure F, Franceschi N, Blanchet S, Rigaud T. 2011. Intraspecific variability in host manipulation by parasites. Infect. Genet. Evol. 11, 262–269. ( 10.1016/j.meegid.2010.12.013) [DOI] [PubMed] [Google Scholar]
- 15.Vickery WL, Poulin R. 2009. The evolution of host manipulation by parasites: a game theory analysis. Evol. Ecol. 24, 773–788. ( 10.1007/s10682-009-9334-0) [DOI] [Google Scholar]
- 16.Milinski M. 1985. Risk of predation of parasitized sticklebacks (Gasterosteus aculeatus L.) under competition for food. Behaviour 93, 203–216. ( 10.1163/156853986X00883) [DOI] [Google Scholar]
- 17.Mouritsen KN, Poulin R. 2003. Parasite-induced trophic facilitation exploited by a non-host predator: a manipulator's nightmare. Int. J. Parasitol. 33, 1043–1050. ( 10.1016/S0020-7519(03)00178-4) [DOI] [PubMed] [Google Scholar]
- 18.Seppälä O, Valtonen ET, Benesh DP. 2008. Host manipulation by parasites in the world of dead-end predators: adaptation to enhance transmission? Proc. R. Soc. B 275, 1611–1615. ( 10.1098/rspb.2008.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dianne L, Perrot-Minnot M-J, Bauer A, Guvenatam A, Rigaud T. 2014. Parasite-induced alteration of plastic response to predation threat: increased refuge use but lower food intake in Gammarus pulex infected with the acanothocephalan Pomphorhynchus laevis. Int. J. Parasitol. 44, 211–216. ( 10.1016/j.ijpara.2013.11.001) [DOI] [PubMed] [Google Scholar]
- 20.Franceschi N, Bollache L, Cornet S, Bauer A, Motreuil S, Rigaud T. 2010. Co-variation between the intensity of behavioural manipulation and parasite development time in an acanthocephalan–amphipod system. J. Evol. Biol. 23, 2143–2150. ( 10.1111/j.1420-9101.2010.02076.x) [DOI] [PubMed] [Google Scholar]
- 21.Hafer N, Benesh DP. 2015. Does resource availability affect host manipulation? An experimental test with Schistocephalus solidus. Parasitol. Open 1, e3 ( 10.1017/pao.2015.3) [DOI] [Google Scholar]
- 22.Gopko M, Mikheev VN, Taskinen J. 2017. Positive density-dependent growth supports costs sharing hypothesis and population density sensing in a manipulative parasite. Parasitology 144, 1511–1518. ( 10.1017/S0031182017001020) [DOI] [PubMed] [Google Scholar]
- 23.Maure F, Brodeur J, Ponlet N, Doyon JJJ, Firlej A, Elguero E, Thomas FF. 2011. The cost of a bodyguard. Biol. Lett. 7, 843–846. ( 10.1098/rsbl.2011.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dheilly NM, et al. 2015. Who is the puppet master? Replication of a parasitic wasp-associated virus correlates with host behaviour manipulation. Proc. R. Soc. B 282, 20142773 ( 10.1098/rspb.2014.2773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammerschmidt K, Koch K, Milinski M, Chubb JC, Parker GA. 2009. When to go: optimization of host switching in parasites with complex life cycles. Evolution 63, 1976–1986. ( 10.1111/j.1558-5646.2009.00687.x) [DOI] [PubMed] [Google Scholar]
- 26.Hafer N, Milinski M. 2015. When parasites disagree: evidence for parasite-induced sabotage of host manipulation. Evolution 69, 611–620. ( 10.1111/evo.12612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benesh DP. 2010. What are the evolutionary constraints on larval growth in a trophically transmitted parasite? Oecologia 162, 599–608. ( 10.1007/s00442-009-1507-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinreich F, Benesh DP, Milinski M. 2013. Suppression of predation on the intermediate host by two trophically-transmitted parasites when uninfective. Parasitology 140, 129–135. ( 10.1017/S0031182012001266) [DOI] [PubMed] [Google Scholar]
- 29.Hafer N. 2017. Differences between populations in host manipulation by the tapeworm Schistocephalus solidus: is there local adaptation? Parasitology 145, 762–769. ( 10.1017/S0031182017001792) [DOI] [PubMed] [Google Scholar]
- 30.Wedekind C, Milinski M. 1996. Do three-spined sticklebacks avoid consuming copepods, the first intermediate host of Schistocephalus solidus? An experimental analysis of behavioural resistance. Parasitology 112, 371–383. ( 10.1017/S0031182000066609) [DOI] [Google Scholar]
- 31.Urdal K, Tierney JF, Jakobsen PJ. 1995. The tapeworm Schistocephalus solidus alters the activity and response, but not the predation susceptibility of infected copepods. J. Parasitol. 81, 330–333. ( 10.2307/3283949) [DOI] [PubMed] [Google Scholar]
- 32.Smyth JD. 1946. Studies on tapeworm physiology I. The cultivation of Schistocephalus solidus in vitro. J. Exp. Biol. 23, 47–70. [DOI] [PubMed] [Google Scholar]
- 33.Dubinina MN. 1980. Tapeworms (Cestoda, Ligulidae) of the fauna of the USSR. New Delhi, India: Amerind Publishing Co. Pvt. Ltd. [Google Scholar]
- 34.Hafer N, Milinski M. 2016. Inter- and intraspecific conflicts between parasites over host manipulation. Proc. R. Soc. B 283, 20152870 ( 10.1098/rspb.2015.2870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benesh DP. 2010. Developmental inflexibility of larval tapeworms in response to resource variation. Int. J. Parasitol. 40, 487–497. ( 10.1016/j.ijpara.2009.10.001) [DOI] [PubMed] [Google Scholar]
- 37.Benesh DP, Hafer N. 2012. Growth and ontogeny of the tapeworm Schistocephalus solidus in its copepod first host affects performance in its stickleback second intermediate host. Parasit. Vectors 5, 90 ( 10.1186/1756-3305-5-90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wedekind C, Christen M, Schärer L, Treichel N. 2000. Relative helminth size in crustacean hosts: in vivo determination, and effects of host gender and within-host competition in a copepod infected by a cestode. Aquat. Ecol. 34, 279–285. ( 10.1023/A:1009976420423) [DOI] [Google Scholar]
- 39.Wedekind C. 1997. The infectivity, growth, and virulence of the cestode Schistocephalus solidus in its first intermediate host, the copepod Macrocyclops albidus. Parasitology 115, 317–324. ( 10.1017/S0031182097001406) [DOI] [PubMed] [Google Scholar]
- 40.Kalbe M, Eizaguirre C, Dankert I, Reusch TB, Sommerfeld RD, Wegner KM, Milinski M. 2009. Lifetime reproductive success is maximized with optimal major histocompatibility complex diversity. Proc. R. Soc. B 276, 925–934. ( 10.1098/rspb.2008.1466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Development Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 42.Bates DM, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.1177/009286150103500418) [DOI] [Google Scholar]
- 43.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biometric. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 44.Parker GA, Ball MA, Chubb JC, Hammerschmidt K, Milinski M. 2009. When should a trophically transmitted parasite manipulate its host? Evolution 63, 448–458. ( 10.1111/j.1558-5646.2008.00565.x) [DOI] [PubMed] [Google Scholar]
- 45.Seppälä O, Jokela J. 2008. Host manipulation as a parasite transmission strategy when manipulation is exploited by non-host predators. Biol. Lett. 4, 663–666. ( 10.1098/rsbl.2008.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Christen M, Milinski M. 2005. The optimal foraging strategy of its stickleback host constrains a parasite's complex life cycle. Behaviour 142, 979–996. ( 10.1163/1568539055010129) [DOI] [Google Scholar]
- 47.Clarke AS. 1954. Studies on the life cycle of the pseudophyllidean cestode Schistocephalus solidus. Proc. Zool. Soc. 124, 257–302. ( 10.1111/j.1469-7998.1954.tb07782.x) [DOI] [Google Scholar]
- 48.Lazzaro BP, Little TJ. 2009. Immunity in a variable world. Phil. Trans. R. Soc. B 364, 15–26. ( 10.1098/rstb.2008.0141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebert D. 2008. Host–parasite coevolution: insights from the Daphnia–parasite model system. Curr. Opin. Microbiol. 11, 290–301. ( 10.1016/j.mib.2008.05.012) [DOI] [PubMed] [Google Scholar]
- 50.Lambrechts L, Fellous S, Koella JC. 2006. Coevolutionary interactions between host and parasite genotypes. Trends Parasitol. 22, 12–16. ( 10.1016/j.pt.2005.11.008) [DOI] [PubMed] [Google Scholar]
- 51.Hafer-Hahmann N. 2019. Data from: Experimental evolution of parasitic host manipulation Dryad Digital Repository. ( 10.5061/dryad.v273bt1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hafer-Hahmann N. 2019. Data from: Experimental evolution of parasitic host manipulation Dryad Digital Repository. ( 10.5061/dryad.v273bt1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All data are available in the Dryad Digital Repository: https://doi.org/10.5061/dryad.v273bt1 [51].