Abstract
Emerging evidence indicates that ectopic skeletal muscle adiposity may be a risk factor for type 2 diabetes (T2D), especially in persons of African ancestry. In vitro studies suggest that a Wnt pathway inhibitor, Dickkopf-related protein 1 (DKK1), plays a role in adiposity regulation and could be a biomarker for adiposity in humans. The objective of this study was to test whether serum DKK1 levels relate to adiposity measures in a cohort from an African ancestry population at high risk for T2D. Fasting serum DKK1 was measured in a sample of 159 men of African ancestry aged ≥40 years (mean age ± SD, 63.5 ± 8.2 years; mean body mass index, 27.8 ± 4.5 kg/m2). Anthropometrics included total-body and trunk adiposity measured by dual-energy x-ray absorptiometry and lower-leg skeletal muscle density measured by CT [which reflects the intramuscular adiposity content (mg/cm3)]. Serum DKK1 was positively correlated with BMI (r = 0.20; P = 0.01), waist circumference (r = 0.15; P = 0.046), DXA total-body adiposity (r = 0.24; P = 0.003), and DXA trunk adiposity (r = 0.21; P = 0.009), independent of age and height. In addition, serum DKK1 was inversely correlated with skeletal muscle density (r = −0.25; P = 0.002), independent of age, BMI, and calf muscle area. No significant correlation was found between serum DKK1 and fasting serum glucose or insulin levels or insulin resistance estimated by homeostasis model assessment. These findings suggest that higher levels of serum DKK1 may be associated with greater overall, central, and ectopic skeletal muscle adiposity. Further studies are needed to unravel the potential role of DKK1 in the regulation of adiposity in humans.
Keywords: WNT, DKK1, adiposity, skeletal muscle, muscle density, African ancestry
General and central adiposity are well-established risk factors for type 2 diabetes (T2D) [1]. Evidence has emerged that accumulation of excessive adipose tissue within and around organs, known as “ectopic adiposity,” may also contribute to the risk for T2D and related metabolic complications [2]. Skeletal muscle adiposity infiltration, also known as myosteatosis, is an ectopic adiposity depot detectable much earlier than the onset of T2D [3]. Despite the emerging role of skeletal muscle adiposity in development of T2D [3], the mechanisms behind its pathogenesis remain largely unknown. Certain race/ethnic populations, such as those of African ancestry, are disproportionately affected by T2D compared with those of European ancestry [4–8], and these racial disparities are not fully explained by traditional risk factors for T2D. Interestingly, compared with European ancestry, African ancestry individuals have less visceral adiposity [9] and more skeletal muscle adiposity [10, 11]. This suggests that skeletal muscle adiposity may be an ectopic adiposity depot of particular relevance in the African-ancestry populations, which further necessitates an investigation into the factors involved in its pathogenesis.
The Wnt pathway is an evolutionarily conserved pathway with a possible critical role in determination of body composition in humans through its effects on bone formation, myogenesis, and adipogenesis [12]. Although largely studied in the context of bone metabolism, recent evidence suggests that the relative balance between various Wnt modulators may play a key role in the distribution of adiposity [13]. This area needs further investigation as Wnt modulators may represent novel therapeutic targets for preventing and treating T2D and other metabolic complications associated with obesity. Dickkopf-related proteins are a family of Wnt pathway inhibitors that exert their action by binding to the coreceptor LRP5/6, thereby blocking the binding of Wnt proteins [14]. One such inhibitor, Dickkopf-related protein 1 (DKK1), has been shown in vitro to promote adipogenesis [15]. However, its relationship with adiposity in humans has not yet been studied.
Therefore, in the current study, we sought to further understand the potential relationship between the Wnt pathway inhibitor DKK1 and adiposity in a sample from an African-ancestry cohort at high risk for T2D. We also investigated the relationship between serum DKK1 and circulating measures of glucose metabolism.
1. Methods
A. Study Population
Men in this analysis were from the Tobago Bone Health Study, a population-based, prospective cohort study of bone health among 2589 community-dwelling men aged 40 years and older, residing on the Caribbean island of Tobago, who underwent baseline whole-body dual-energy x-ray absorptiometry (DXA) between 2000 and 2003 [16]. Men had to be ambulatory, noninstitutionalized, and not terminally ill to be able to participate in the study. As part of follow- up to the Tobago Bone Health Study, 1726 Tobago men underwent another whole-body DXA examination and baseline peripheral quantitative CT (pQCT) exam between 2010 and 2013. Of these men, 304 were randomly selected for CT of the chest to assess coronary artery calcification as a part of a cardiovascular disease ancillary study. During this visit, demographic data, health history, and anthropometric characteristics were assessed by trained staff. Morning blood samples were obtained after an overnight fast and were immediately divided into aliquots and frozen at −80°C. Serum DKK1 was measured in a sample of 159 men from the cardiovascular disease ancillary study and form the basis of our current analysis.
The Institutional Review Boards of the University of Pittsburgh and the Tobago Ministry of Health and Social Services approved this study and written informed consent was obtained from each participant by using forms and procedures approved by the University of Pittsburgh Institutional Review Board, the US Surgeon General’s Human Use Review Board, and the Tobago Division of Health and Social Services Institutional Review Board.
B. Adiposity Assessment
Adiposity was measured by using clinical anthropometric examination [body weight, body mass index (BMI), and waist circumference], whole-body DXA (total percentage fat and percentage fat in the trunk), and pQCT of the calf (calf skeletal muscle density). Body weight was measured to the nearest 0.1 kg on a balance beam scale, and standing height was measured to the nearest 0.1 cm using a wall-mounted stadiometer, both without participants wearing shoes. BMI was calculated as body weight in kilograms divided by standing height in meters squared. Waist circumference was measured at the level of the umbilicus or greatest circumference by using a flexible tape measure.
Total body fat percentage and percentage fat in the trunk were measured via DXA using a Hologic QDR 4500 W densitometer (Hologic, Bedford, MA). Calf muscle density was measured via pQCT performed with a Stratec XCT-2000 scanner (Orthometrix, Inc., White Plains, NY). This method has been well validated in previous studies using magnetic resonance spectroscopy and muscle biopsy [17, 18]. Details of the pQCT procedure have been published previously [19]. Briefly, a site at 66% of the calf length, proximal to the terminal end of the tibia was scanned because it has the largest circumference and the lowest variability in composition between individuals [20]. All images were analyzed with STRATEC analysis software, version 5.5D (Orthometrix, Inc.) and performed by a trained investigator who was unaware of the participant’s health status. Skeletal muscle density is indicative of intramuscular fat, such that lower skeletal muscle density indicates higher intramuscular fat content [18].
C. Biochemical Analysis
Blood samples were collected from participants in the morning after an overnight fast. Serum was separated and the samples were divided into aliquots and immediately frozen at −80°C. Serum DKK1 levels were measured in duplicate in previously unthawed specimens according to the manufacturer's protocol by using a validated sandwich ELISA (R&D Systems, Minneapolis, MN) [21]. Intra- and interassay coefficients of variation within our study were 6.9% and 8.5%, respectively. Fasting serum glucose was measured by using an enzymatic procedure, and fasting serum insulin was measured by using a radioimmunoassay procedure developed by Linco Research. The degree of insulin resistance was estimated by homeostasis model assessment (HOMA-IR) according to the method described by Matthews et al. [22].
D. Other Measurements
Trained interviewers and nurses administered questionnaires to participants. Race/ethnicity was self-reported, and participants provided detailed information on the racial/ethnic origin of their parents and grandparents. The population of Tobago has very low non-African admixture compared with a more heterogeneous African-American population (6% vs 17% to 23%, respectively) [23]. Men were asked to report their history and current use of diabetes-related medications.
E. Statistical Analysis
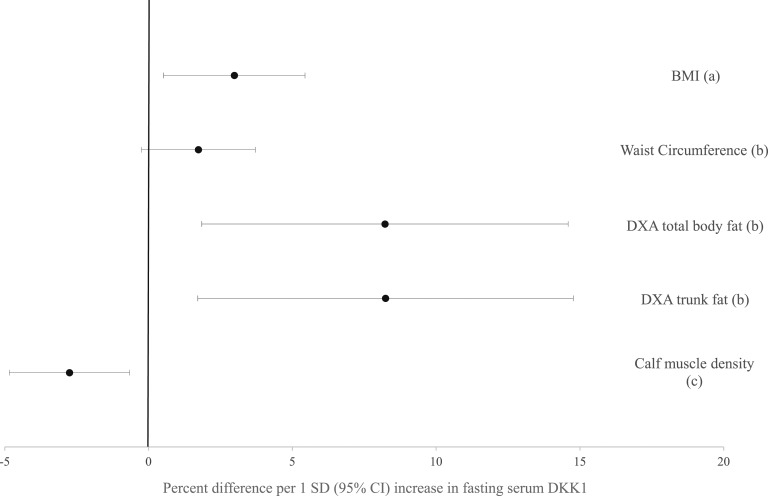
Data were analyzed by using Stata software, version 15 (Stata Corp., College Station, TX). All normally distributed continuous variables are reported as mean ± SD, and nonnormal variables are reported as median (interquartile range). Spearman correlations of serum DKK1 with measures of adiposity were tested, including BMI, DXA total fat, DXA trunk fat, and waist circumference, and were adjusted for age and height. Spearman correlations of serum DKK1 were also performed with muscle density and were adjusted for age, BMI, and calf muscle area. Correlations of serum DKK1 with measures of glucose metabolism, including fasting insulin levels, fasting blood glucose levels, and HOMA-IR, were adjusted for age and BMI. Finally, regression analysis was performed with serum DKK1 as the predictor variable to assess its relationship with measures of adiposity. To present our results in a more clinically meaningful way, we calculated the percentage difference in markers of body fat per SD difference in serum DKK1 (Fig. 1). A P value < 0.05 was considered to indicate a statistically significant difference.
Figure 1.
Percentage difference in adiposity measures per 1 SD (95% CI) increase in fasting serum DKK1.(a), adjusted for age; (b), adjusted for age and height; (c), adjusted for age, BMI, and calf muscle area.
2. Results
A. General Characteristics of Study Sample
Our study sample consisted of older (mean age, 63 years), predominantly overweight (mean BMI, 27.8 kg/m2) men of African ancestry (Table 1). The prevalence of diabetes was 25%, and 87% of the patients with diabetes reported receiving antidiabetic treatment. We found no significant difference between our study sample and the complete cohort of men (n = 1726) who participated in this clinic visit, including no meaningful differences in age, BMI, waist circumference, DXA total and trunk fat, diabetes status, fasting glucose, or insulin levels or HOMA.
Table 1.
General Characteristics of African-Ancestry Men
| Characteristic | Value (n = 149) |
|---|---|
| Age, y | 63.5 ± 8.2 |
| Anthropometrics | |
| Weight, kg | 85.6 ± 15.3 |
| Height, cm | 175.0 ± 7.1 |
| Waist circumference, cm | 98.7 ± 12.8 |
| BMI, kg/m2 | 27.8 ± 4.5 |
| DXA total body fat, kg | 23.8 ± 6.3 |
| DXA trunk fat, kg | 10.4 ± 4.5 |
| Comorbidities and metabolic features | |
| Patients with diabetes, % | 25 |
| Treated patients with diabetes, % | 87 |
| Fasting serum glucose levels, mg/dL | 91.0 (82.0, 108.0) |
| Fasting serum insulin levels, μU/mL | 12.9 (10.5, 17.4) |
| HOMA-IR | 3.1 (2.3, 4.5) |
| Calf skeletal muscle composition | |
| Muscle area, mm2 | 6974 ± 1311 |
| Muscle density, mg/cm3 | 72.3 (67.7, 75.6) |
Values are expressed as mean ± SD, median (range), or percentage.
B. Relationship of Adiposity and Diabetes-Related Measures With Fasting Serum DKK1
Table 2 shows Spearman correlation coefficients between serum DKK1 and adiposity measures (all men, n = 159) and biomarkers of glucose and insulin homeostasis (among nondiabetic men, n = 121). Serum DKK1 was positively associated with BMI independent of age (P = 0.011) and DXA-measured trunk and total body fat, independent of age and height (both P < 0.05). We found an inverse association of serum DKK1 with skeletal muscle density, independent of age, calf muscle area, and BMI (P < 0.05). Further, among persons without diabetes, we found no significant correlation of serum DKK1 and fasting insulin levels, fasting glucose levels, or HOMA-IR in unadjusted or age-adjusted models.
Table 2.
Association Between Serum DKK1 With Adiposity and Biomarkers of Glucose and Insulin Homeostasis
| Variable | Spearman Correlation Coefficient | P Value |
|---|---|---|
| BMI, kg/m2a | 0.20 | 0.011 |
| Waist circumference, cmb | 0.15 | 0.046 |
| DXA total body fat, kgb | 0.24 | 0.003 |
| DXA trunk fat, kgb | 0.21 | 0.009 |
| Calf muscle density, mg/cm3c | −0.25 | 0.002 |
| Fasting glucose, mg/dLd | −0.09 | 0.290 |
| Fasting insulin, μU/mLd | −0.13 | 0.130 |
| HOMA-IRd | −0.14 | 0.114 |
Adjusted for age.
Adjusted for age and height.
Adjusted for age, BMI, and calf muscle area.
Adjusted for age and BMI, in nondiabetic men only (n = 121).
We further performed multiple linear regression analyses of the variables that had a significant association with serum DKK1 (Fig. 1). Higher serum DKK1 was associated with significantly greater BMI, DXA-measured total body and trunk fat, and skeletal muscle density (all P < 0.04). For example, independent of age, each SD (1258 pg/mL) higher serum DKK1 was associated with a 3.0% greater BMI. Similarly, independent of age, BMI, and calf muscle area, each SD higher serum DKK1 was associated with 2.8% lower muscle density, indicating greater intramuscular adiposity. Results for association of serum DKK1 with waist circumference were of similar effect but not statistically significant (P = 0.09).
3. Discussion
This study found an association between serum DKK1 and general and central adiposity and skeletal muscle adiposity in humans. The main findings of this study are: that (i) serum DKK1 was positively associated with overall and central adiposity; (ii) serum DKK1 was inversely associated with skeletal muscle density, suggesting a positive relationship with intramuscular adiposity; and (iii) serum DKK1 was not associated with glucose, insulin, or HOMA-IR. These results suggest that serum DKK1 may be a biomarker for obesity.
The Wnt pathway inhibitor DKK1 has mostly been studied in the context of metabolic bone disease. The Wnt pathway promotes myogenesis and inhibits adipocyte differentiation during early human development [24]. Inhibition of the Wnt pathway has been hypothesized to hinder bone mineralization while encouraging preadipocyte differentiation into mature adipocytes [13]. Some studies suggest that Wnt inhibition might increase muscle fat content in murine models in vitro [25]. Lu et al. [14] have previously shown that DKK1 promotes differentiation and adipocytokine secretion in primary cultured human preadipocytes. These results from cell and animal models suggest that DKK1 may have the potential to affect body fat distribution in humans. However, the association of serum DKK1 with adiposity in a population-based human sample has not been tested until now. One human study has reported a reduction in DKK1 levels after exercise intervention in breast cancer survivors [26]; reduced DKK1 levels 3 months after bariatric surgery have also been reported in humans [27]. These studies help to indirectly support the possible connection between DKK1 levels and body fat.
The underlying mechanism by which DKK1 may contribute to increased adiposity is not clear. Laboratory experiments suggest that the Wnt pathway plays a critical role in adipocyte renewal and differentiation, and its inhibition may promote differentiation of multipotent stem cells to mature adipocytes [14]. In separate studies, inactivating LRP5 mutations have shown similar effects, which is the primary site of action of DKK1 [28], thus indirectly supporting the role of DKK1 in adipogenesis. Christodoulides et al. [15] presented evidence that DKK1 is secreted by human preadipocytes and DKK1 mRNA increases 6 hours after onset of human adipogenesis, followed by a progressive decline to undetectable levels once maturation is complete. However, the factors that regulate DKK1 production remain largely unknown and need further investigation. One hypothesis may be that DKK1 increases in response to the inflammatory stimuli seen in metabolic syndrome [29], and thus DKK1 may represent an early therapeutic target in this setting. In this context, our study adds valuable information to the existing knowledge regarding DKK1 and adiposity in humans.
Skeletal muscle adiposity has been linked to increased risk for diabetes in persons of African ancestry [3]. However, the mechanisms driving increased muscle fat are not yet clear. Although the direct relationship between other Wnt inhibitors, such as sclerostin, and T2D has been previously explored [30, 31], no such studies have been performed for serum DKK1. In our study, we found no significant correlation between serum DKK1 and fasting serum glucose level or insulin levels or HOMA-IR among persons without diabetes. The absence of an association may be due to the smaller number of the nondiabetic individuals in our sample (n = 121). More important, a potential link between serum DKK1 and T2D could be indirectly driven through central and skeletal muscle adiposity, which are important risk factors for T2D. Alternatively, serum DKK1 could represent an early biomarker for T2D in a high-risk population, even before its clinical development, and further longitudinal studies are needed to explore this hypothesis. Larger studies are thus warranted to further evaluate the potential association between DKK1 and measures of glucose homeostasis, insulin resistance, and overt diabetes.
Our study has several potential limitations, including a relatively small sample size, which is a subset of a larger cohort. However, we found no significant differences in participant characteristics between our subsample and the entire cohort, which suggests that our subsample is a good representation of the total study sample. The small sample size still provides only limited power to detect significant associations. For example, our sample of 121 men without diabetes provides only 33% power to find a correlation coefficient of 0.14 as being statistically significant. In this context, the lack of significant associations between serum DKK1 and factors affecting glucose homeostasis should be cautiously interpreted. The cross-sectional nature of our study prevents us from drawing any causal links between serum DKK1 and adiposity measures. Further, our findings may not apply to younger men, younger or older women, or other racial/ethnic groups. Nonetheless, African-ancestry populations are at the epicenter of the obesity and diabetes epidemic and in much need of further exploration of novel risk factors that can explain their high burden of diabetes; our study addresses this important knowledge gap.
Finally, there may be potential limitations to using DKK1 as a biomarker because of its large inter- and intraindividual variation over time, as demonstrated in a recent study examining 24-hour levels of DKK1 in healthy patient samples [32]. We do not expect this to be a major issue in our study because all samples were collected under the same conditions (in the morning following an overnight fast). Nonetheless, before DKK1 can be reliably used as a biomarker, additional studies are required to more clearly characterize the diurnal release and metabolism of DKK1 as well as other factors contributing to its serum levels.
In summary, our study demonstrates that serum DKK1 is positively associated with overall, central, and skeletal muscle adiposity in humans. These findings suggest that serum DKK1 may be a potential biomarker for obesity. Our study thus adds to the growing evidence for a role of Wnt modulators in human body composition and may be important in unraveling the mechanisms driving the racial/ethnic disparity in T2D risk.
Acknowledgments
Financial Support: This research was supported by National Institutes of Health (NIH) grant R01AR049747(J.M.Z.); NIH grant R01DK097084 (I.M.); NIH grant R01-AG033618(J.M.Z.); NIH grant T32 DK007252 (H.A.); Endocrine Fellows Foundation Grant (H.A.), and NIH Grant K01-HL125658 (A.L.K.).
Disclosure Summary: E.E.K. serves as a principal investigator on a clinical trial for Regeneron Pharmaceuticals. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- DKK1
Dickkopf-related protein 1
- DXA
dual-energy x-ray absorptiometry
- HOMA-IR
insulin resistance estimated by homeostasis model assessment
- pQCT
peripheral quantitative CT
- T2D
type 2 diabetes
References and Notes
- 1. Hartz AJ, Rupley DC Jr, Kalkhoff RD, Rimm AA. Relationship of obesity to diabetes: influence of obesity level and body fat distribution. Prev Med. 1983;12(2):351–357. [DOI] [PubMed] [Google Scholar]
- 2. Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283(17):2253–2259. [DOI] [PubMed] [Google Scholar]
- 5. Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288(14):1723–1727. [DOI] [PubMed] [Google Scholar]
- 6. Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263–1268. [DOI] [PubMed] [Google Scholar]
- 7. Haffner SM, D’Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE, Bergman RN Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742–748. [DOI] [PubMed] [Google Scholar]
- 8. Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290(2):199–206. [DOI] [PubMed] [Google Scholar]
- 9. Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr. 2010;91(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yim J-E, Heshka S, Albu JB, Heymsfield S, Gallagher D Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol. 2008;104(3):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Wheeler VW, Kuller LH, Faulkner KA, Zmuda JM Osteoporotic Fractures in Men Research GroupTobago Health Studies Research Group . Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009;94(8):2735–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab. 2009;20(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu H, Li X, Mu P, Qian B, Jiang W, Zeng L. Dickkopf-1 promotes the differentiation and adipocytokines secretion via canonical Wnt signaling pathway in primary cultured human preadipocytes. Obes Res Clin Pract. 2016;10(4):454–464. [DOI] [PubMed] [Google Scholar]
- 15. Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O’Rahilly S, Sethi JK, Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci. 2006;119(Pt 12):2613–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hill DD, Cauley JA, Sheu Y, Bunker CH, Patrick AL, Baker CE, Beckles GLA, Wheeler VW, Zmuda JMet al. Correlates of bone mineral density in men of African ancestry: the Tobago bone health study. Osteoporos Int. 2008;19(2):227–234. [DOI] [PubMed] [Google Scholar]
- 17. Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR; Look AHEAD Adipose Research Group . Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring). 2006;14(1):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–110. [DOI] [PubMed] [Google Scholar]
- 19. Miljkovic I, Kuipers AL, Cvejkus R, Bunker CH, Patrick AL, Gordon CL, Zmuda JM. Myosteatosis increases with aging and is associated with incident diabetes in African ancestry men. Obesity (Silver Spring). 2016;24(2):476–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simonsick EM, Maffeo CE, Rogers SK, Skinner EA, Davis D, Guralnik JM, Fried LP. Methodology and feasibility of a home-based examination in disabled older women: the Women’s Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52(5):M264–M274. [DOI] [PubMed] [Google Scholar]
- 21.DKK100 C. RRID:AB_2756393.
- 22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 23. Miljkovic-Gacic I, Ferrell RE, Patrick AL, Kammerer CM, Bunker CH. Estimates of African, European and Native American ancestry in Afro-Caribbean men on the island of Tobago. Hum Hered. 2005;60(3):129–133. [DOI] [PubMed] [Google Scholar]
- 24. Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1-2):1–7. [DOI] [PubMed] [Google Scholar]
- 25. Vertino AM, Taylor-Jones JM, Longo KA, Bearden ED, Lane TF, McGehee RE, Jr, MacDougald OA, Peterson CA. Wnt10b deficiency promotes coexpression of myogenic and adipogenic programs in myoblasts. Mol Biol Cell. 2005;16(4):2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim TH, Chang JS, Park K-S, Park J, Kim N, Lee JI, Kong ID. Effects of exercise training on circulating levels of Dickkpof-1 and secreted frizzled-related protein-1 in breast cancer survivors: A pilot single-blind randomized controlled trial. PLoS One. 2017;12(2):e0171771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muschitz C, Kocijan R, Marterer C, Nia AR, Muschitz GK, Resch H, Pietschmann P. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100(3):891–901. [DOI] [PubMed] [Google Scholar]
- 28. Qiu W, Andersen TE, Bollerslev J, Mandrup S, Abdallah BM, Kassem M. Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. J Bone Miner Res. 2007;22(11):1720–1731. [DOI] [PubMed] [Google Scholar]
- 29. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415–1428. [DOI] [PubMed] [Google Scholar]
- 30. García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, Muñoz-Torres M. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(1):234–241. [DOI] [PubMed] [Google Scholar]
- 31. Yu OH, Richards B, Berger C, Josse RG, Leslie WD, Goltzman D, Kaiser SM, Kovacs CS, Davison KS. The association between sclerostin and incident type 2 diabetes risk: a cohort study. Clin Endocrinol (Oxf). 2017;86(4):520–525. [DOI] [PubMed] [Google Scholar]
- 32. van der Spoel E, Oei N, Cachucho R, Roelfsema F, Berbée JFP, Blauw GJ, Pijl H, Appelman-Dijkstra NM, van Heemst D. The 24-hour serum profiles of bone markers in healthy older men and women. Bone. 2018;120:61–69. [DOI] [PubMed] [Google Scholar]