An anti-ficolin-1 mAb is effective as therapy for autoimmunity
Keywords: autoimmune disease, CAWS, complement, FCN1, monoclonal antibody
Abstract
Previously, we reported that mRNA expression of ficolin-1 (FCN1), a component of the complement lectin pathway, is elevated in peripheral blood mononuclear cells of patients with vasculitis syndrome, and that FCN1-positive cells infiltrate into inflamed regions in patient specimens. In addition, we reported that the serum FCN1 concentration is elevated in patients with Kawasaki disease (KD), a pediatric vasculitis, but dramatically decreases after intravenous immunoglobulin (IVIG) treatment. Furthermore, we showed that FCN1 binds to IgG1 in a pull-down assay. These results suggested that removal of FCN1 may be a therapeutic mechanism of IVIG. In this study, we prepared anti-FCN1 monoclonal antibody (mAb) and examined its therapeutic potential in mice treated with Candida albicans water-soluble fraction (CAWS), which induces KD-like vasculitis in the coronary artery. Indeed, treatment with anti-FCN1 mAb decreased the histological score of vasculitis (P = 0.03). To investigate the role of FCN1, we assessed blood samples of patients with various autoimmune diseases and demonstrated that serum levels of FCN1 were elevated not only in patients with vasculitis, but also in those with rheumatoid arthritis. Additionally, FCN1-targeted treatment of a mouse model of arthritis [collagen antibody-induced arthritis (CAIA)] revealed that administration of anti-FCN1 mAb ameliorated symptoms of arthritis (P < 0.01). These results suggest that FCN1 is involved in the pathogenesis of autoimmune diseases, and that targeting FCN1 represents a promising strategy for treating these diseases.
Introduction
Vasculitis syndrome causes inflammation of blood vessel walls and their surrounding tissue in organs such as the kidney, skin, lung, peripheral nerves and brain, leading to blood flow disturbances and bleeding, which eventually results in organ damage and even death. Recently, Nishide et al. (1) reported that semaphorin 4D dysfunction is involved in the pathogenesis of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. However, the pathogenesis of this disease has not been fully elucidated. Glucocorticoids, immunosuppressants, intravenous immunoglobulin (IVIG) and monoclonal antibody (mAb) reagents such as tocilizumab [anti-interleukin (IL)-6 receptor], infliximab [anti-tumor necrosis factor (TNF)-α] or rituximab (anti-CD20) have clinical efficacy against these diseases, but treatment results are often unsatisfactory. For example, in Kawasaki disease (KD), a common vasculitis in children, IVIG therapy shortens the duration of fever and decreases the frequency of coronary artery abnormalities by 70–90% (2, 3), but fever persists in 10–15% of KD patients, and coronary aneurysm occurs in 2–5% (3–5). Therefore, additional therapies are required.
Previously, we conducted a comprehensive gene expression analysis of patients’ peripheral blood mononuclear cells, with the goal of identifying novel biomarkers for diagnosis and assessment of vasculitis syndrome. We observed elevated expression of ficolin-1 (FCN1), a complement lectin pathway protein, in patients with Takayasu arteritis (TA) (6) or microscopic polyangiitis (MPA) (7). Infiltration of FCN1-positive, CD68-positive immune cells was detected in inflamed regions of the aorta in TA patients and the glomeruli in MPA patients. Furthermore, serum FCN1 levels, which are elevated in patients with KD, decreased dramatically in patients with good response to treatment, but remained high in treatment-resistant patients. These findings suggested that FCN1 is involved in the pathogenesis of vasculitis syndrome (8).
Ficolins are classified as collectins (C-type lectins). C-type lectin is a type of carbohydrate-binding protein that requires calcium for binding. Ficolins recognize and bind to pathogens, resulting in their neutralization and opsonization, followed by activation of the complement lectin pathway (9). Members of the collectin family, including ficolins, mannan-binding lectin (10), surfactant protein (SP)-A and SP-D (11), recognize pathogen structures directly. Evolutionarily, the complement lectin pathway, which includes the ficolin family, is thought to be an ancestor of the classical complement pathway, and has a similar signal transduction cascade after activation of each specific serine protease (12). There are two ficolin homologs in mice, ficolin-a (Fcna) and ficolin-b (Fcnb). On the basis of chromosomal position, Fcnb is regarded as the ortholog of human FCN1 (13). FCN1 is thought to function locally in inflamed lesions following secretion from monocytes, macrophages and granulocytes (14). Recent work showed that FCN1 is also present in the serum, suggesting that it may play a role in systemic immunity (15). Most FCN1 released in response to an inflammatory stimulus such as N-formylmethionine-leucyl-phenylalanine (fMLP) binds to the outer membrane of the releasing cell (16). FCN1 is assembled on the cell surface via its fibrinogen-like domain, and accepts sialic acid as a ligand (16). The survival rate following infection with Streptococcus pneumoniae is markedly lower in Fcnb-deficient mice than in wild-type mice (17). Recently, Banda et al. (18) reported that Fcnb-knockout mice are resistant to collagen antibody-induced arthritis (CAIA), and that the complement system is involved in the mechanism. Together, these observations suggest that Fcnb plays an important role in host defense against bacterial infection, as well as in onset or exacerbation of autoimmune arthritis.
In this study, we prepared anti-FCN1 mAb and administered it to a mouse model of vasculitis induced by Candida albicans water-soluble fraction (CAWS). In addition, we assessed serum levels of FCN1 in patients with various autoimmune diseases and the relationship between levels of FCN1 and clinical markers. We also administered the anti-FCN1 mAb to a mouse model of arthritis. Because many other mAb reagents are commonly used in the clinical setting, e.g. anti-TNF, anti-IL-6, anti-IL-17 or anti-CD20, and these reagents are effective against various autoimmune diseases, we hypothesized that anti-FCN1 mAb administration is a promising candidate for novel immunological therapy against vasculitis and arthritis.
Methods
Construction of anti-FCN1 mAb-producing hybridoma
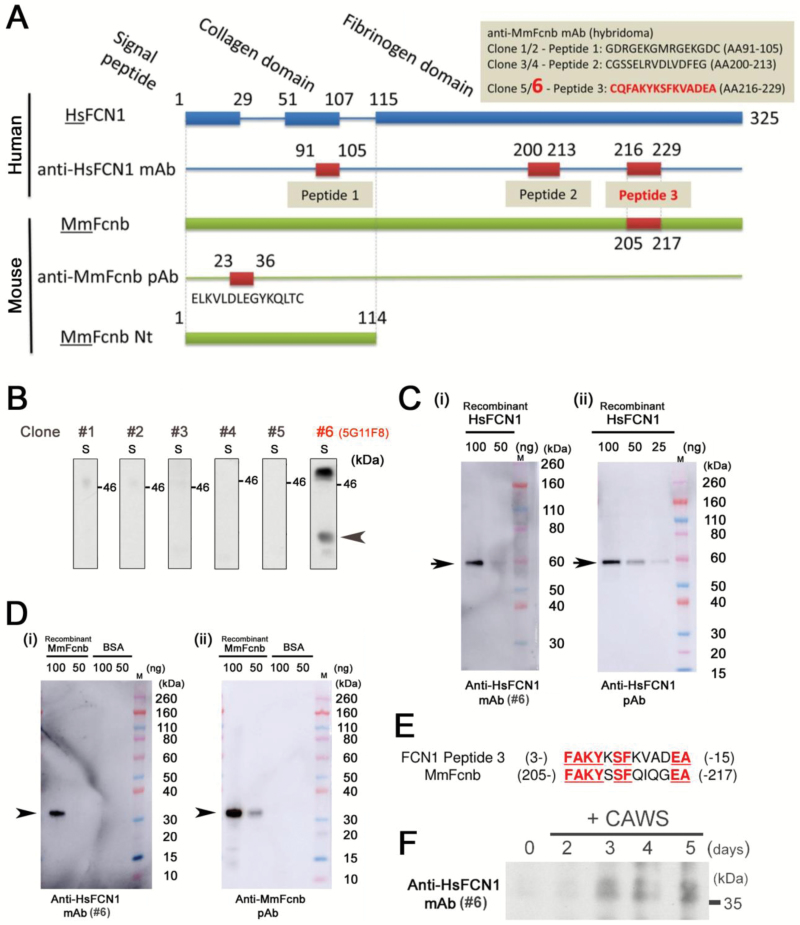
The amino acid sequence of FCN1 was examined using BLAST (https://blast.ncbi.nlm.nih.gov/), and three peptide sequences were selected: GDRGEKGMRGEKGDC (a.a. 91–105: peptide-1), CGSSELRVDLVDFEG (a.a. 200–213: peptide-2) and CQFAKYKSFKVADEA (a.a. 216–229: peptide-3) (Fig. 1A). Peptide-1 was derived from the collagen-like domain of FCN1, and peptides-2 and -3 were derived from the fibrinogen-like domain. The peptides were synthesized and used to immunize mice. Splenocytes were harvested and fused with myeloma cells. Two clones of hybridoma per peptide (six clones in total) were obtained (GenScript, Nanjing, China). The affinities of anti-FCN1 mAbs derived from the six clones were compared by western blotting to identify the clone with the highest affinity. The serum of a patient with vasculitis syndrome was subjected to 4–12% SDS-PAGE (Sodium dodecyl sulfate polyacrylamide gel-electrophoresis) (Thermo Fisher Scientific, Waltham, MA, USA) at 200 V for 50 min, and then transferred onto a PVDF membrane (Bio-Rad, Hercules, CA, USA). The membrane was blotted with anti-FCN1 mAb, followed by horseradish peroxidase (HRP)-conjugated anti-mouse IgG (NA931, GE Healthcare, Buckinghamshire, UK) as the secondary antibody. To investigate cross-reactivity between the anti-FCN1 mAb and mouse Fcnb, full-size recombinant human FCN1 protein with a Glutathione S-transferase (GST)-tag (H00002219-P01, Novus Biologicals, Littleton, CO, USA) and a truncated (Gln97~Ser254) form of recombinant mouse Fcnb protein with a His-tag and a T7-tag (Mus musculus Fcnb: MmFcnb) (RBP907Mu01, Cloud-Clone Corp, Katy, TX, USA) were purchased. Then, we subjected these recombinant proteins to SDS–PAGE under the same conditions described above and blotted with the anti-FCN1 mAb (#6), rabbit anti-FCN1 polyclonal antibody (pAb) (HPA001295, Sigma-Aldrich, Darmstadt, Germany) or rabbit anti-Fcnb pAb (prepared as described below), followed by HRP-conjugated anti-mouse IgG (GE Healthcare) or HRP-conjugated anti-rabbit IgG (NA934, GE Healthcare), respectively. Additionally, the serum of a vasculitis model mouse or an arthritis model mouse was subjected to SDS–PAGE under the same conditions and blotted with the anti-FCN1 mAb or the anti-Fcnb pAb, followed by HRP-conjugated anti-mouse IgG (GE Healthcare) or HRP-conjugated anti-rabbit IgG (GE Healthcare), respectively. Rabbit anti-Fcnb pAb was prepared as follows: BLAST was used to identify Fcnb-specific sequences, and a peptide (a.a. 23–40: ELKVLDLEGYKQLT) from the Fcnb protein was synthesized (Fig. 1A). A rabbit was immunized with the peptide to generate a pAb against Fcnb (GenScript).
Fig. 1.
Preparation of anti-FCN1 mAb-producing hybridoma. (A) Schematic representation of the positions and amino acid sequences of HsFCN1 peptides used for mouse immunization and the MmFcnb peptide used for rabbit immunization. Peptide-1 was derived from the collagen-like domain, and peptide-2 and peptide-3 from the fibrinogen-like domains of FCN1. The peptide ELKVLDLEGYKQLT corresponds to a.a. 23–36 of MmFcnb. (B) Affinity of each anti-FCN1 mAb clone was compared by western blotting using equal amounts of patient serum (S). Position of the putative band for HsFCN1 is indicated by an arrowhead. (C) Binding of anti-FCN1 mAb#6 (i) and commercially available anti-FCN1 pAb (ii) to recombinant GST-tagged full-size human FCN1 (HsFCN1). 100, 50 or 25 ng of recombinant HsFCN1 protein was loaded into each lane. Arrows indicate the band for recombinant HsFCN1 protein. M: size marker. (D) Binding of anti-FCN1 mAb#6 (i) and a homemade anti-MmFcnb pAb (ii) to recombinant truncated mouse Fcnb protein with His-tag and T7-tag. Arrowheads indicate the band for recombinant MmFcnb protein. BSA was also tested as a negative control. 100 or 50 ng of MmFcnb or BSA was loaded into each lane. M: size marker. (E) Comparison of amino acid sequences between human FCN1 peptide-3 (used to generate anti-FCN1 mAb#6) and mouse Fcnb. Matching sequences are highlighted in underlined red letters. (F) Western blotting with anti-FCN1 mAb#6 of equal amounts of mouse serum before (0 day) or after (2, 3, 4 and 5 weeks) treatment with CAWS. HsFCN1: Homo sapiens (human) FCN1; MmFcnb: Mus musculus (mouse) ficolin-b.
Anti-FCN1 mAb treatment for CAWS mouse, a model of vasculitis syndrome
CAWS was prepared from C. albicans strain NBRC1385 by conventional methods (19). Briefly, the culture was grown by agitation in 5-l C-limiting media at 400 rpm for 2 days at 27°C, with aeration at 5 l min−1. An equal volume of ethanol was added, and the mixture was allowed to stand undisturbed overnight, after which the precipitate was recovered. This fraction was dissolved in 250 mL distilled water, mixed with ethanol, and again allowed to stand undisturbed overnight. The precipitate was recovered and dried with acetone to obtain CAWS.
Using the CultiLife culture bag system (Takara Bio, Kusatsu, Japan), fed-batch cultures of anti-FCN1 mAb hybridoma were grown for 4 weeks in a 5% CO2 incubator. Cells were cultured in Dulbecco's Modified Eagle's Medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France) and 100 U l−1 penicillin-streptomycin (Thermo Fisher Scientific). Antibodies were purified using Ab-Capcher ExTra (ProteNova, Takamatsu, Japan). Buffer was exchanged to PBS(−), and the concentration of the mAb was determined using Amicon Ultra-15 30K centrifugal filter units (Sigma-Aldrich) and CBB staining (Nacalai Tesque). To avoid an IVIG-like effect at higher doses of IgG, the dose of anti-FCN1 mAb administered to mice was lower than that of IVIG (2 g kg−1). We decided to administer a dose of 200 mg kg−1, higher than that typically used for other mAb therapies in clinical settings [e.g. 8 mg kg−1 for anti-IL-6 receptor mAb (tocilizumab); 3–10 mg kg−1 for anti-TNF mAb (infliximab)]. Mouse IgG2ba isotype-control antibody was obtained from BioXcell (C1.18.4, West Lebanon, NH, USA).
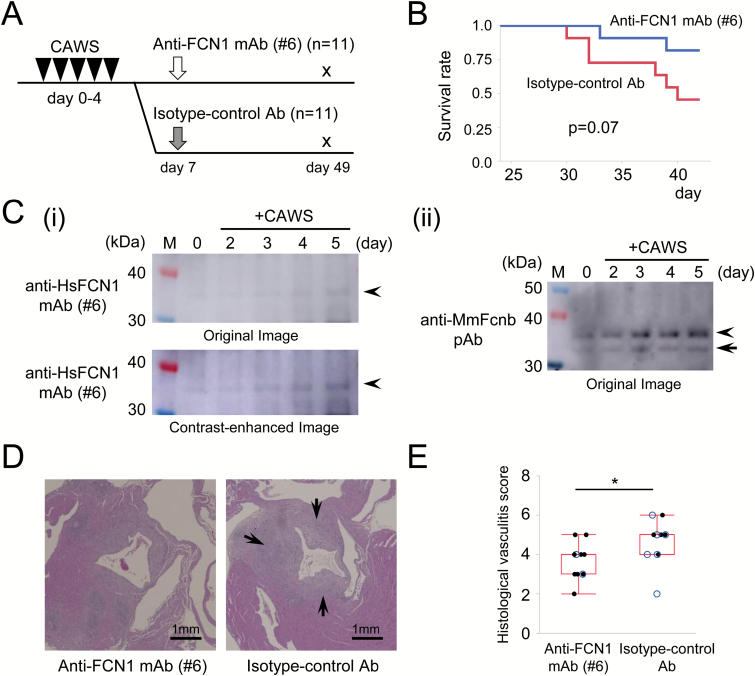
Male DBA/2 mice (n = 22) were obtained from Japan SLC (Hamamatsu, Shizuoka, Japan) and raised in a specific pathogen-free (SPF) environment. Mice were divided into two groups: anti-FCN1 mAb (n = 11) and isotype-control antibody (n = 11). CAWS (1 mg per body) was administered intra-peritoneally daily from day 0 to day 4. Anti-FCN1 mAb or isotype-control antibody was administered intravenously on day 7. The protocol of the experiment is illustrated schematically in Fig. 2(A). Body weight measurements and blood samples were obtained on days 0, 7, 9, 14, 21, 28, 35, 42 and 49. On day 49, the mice were sacrificed. When a mouse died or was euthanized due to weakness, samples were taken at that time. Hearts were fixed with 10% formalin neutral buffer solution (Wako Pure Chemical, Osaka, Japan). Following paraffin embedding, cross-sections were cut out at the aortic valve level, stained with hematoxylin–eosin (HE) and examined microscopically. All animal experiments were performed at Unitech (Chiba, Japan). The experimental protocol was approved by the facility’s animal experiments committee. The histological vasculitis score was calculated using the following criteria: the area around the aorta was divided into three parts; one region of inflammatory cell infiltration was counted as one point, and one surrounding blood vessel with inflammatory cell infiltration was counted as one point; and the sum of points was regarded as the histological vasculitis score. Levels of serum cytokines, including IL-1β, IL-4, IL-6, IL-10, IL-12p40, IL-17A, interferon-γ (IFN-γ), TNF, granulocyte macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) were measured with Cytokine Bead Array kits (Becton Dickinson, Franklin Lakes, NJ, USA).
Fig. 2.
Treatment of CAWS mouse, a model of vasculitis, with anti-FCN1 mAb. (A) Experimental protocol. Black triangles indicate intra-peritoneal injection of CAWS (1 mg per day, 5 consecutive days from day 0 to day 4). White arrow indicates anti-FCN1 mAb administration, and gray arrow indicates isotype-control antibody administration via intravenous injection (4 mg, day 7, respectively). Mice were sacrificed on day 49. (B) Comparison of survival by the Kaplan–Meier method. Difference in survival rate was examined by log-rank test. (C) Western blotting with anti-HsFCN1 mAb#6 (i) or anti-MmFcnb pAb (ii) of equal amounts of mouse serum before (0 day) or after (2, 3, 4 and 5 days) treatment with CAWS to detect a band for MmFcnb (arrowheads). Arrows denote the putative bands for degradation products of MmFcnb protein. Both original (top panel) and contrast-enhanced (bottom panel) images are presented in parallel to highlight the putative MmFcnb band in mouse serum (i). M: lane for size marker. (D) Representative histological images of aortitis in mice treated with the anti-FCN1 mAb (left panel) or isotype-control antibody (right panel). Arrow indicates cellular infiltration and wall thickness of aorta. Scale bar, 1 mm. (E) Comparison of the histological vasculitis score. Death during the observational period is indicated by blue circles. Differences in vasculitis score were examined by the Wilcoxon rank-sum test. HsFCN1: Homo sapiens (human) FCN1; MmFcnb: Mus Musculus (mouse) ficolin-b; *P < 0.05.
Patients’ blood samples and ethical considerations
Blood samples and clinical information were obtained from patients with autoimmune diseases who were treated at Osaka University Hospital between July 2012 and December 2015. Blood samples were also obtained from healthy individuals. All participants were given written explanations and gave their consent for the use of their clinical information and blood samples for research. This research was approved by the ethics committee of Osaka University Hospital and Research Institute for Microbial Diseases, and experiments were conducted in accordance with the Helsinki Declaration. Participants were classified as patients with vasculitis syndrome [granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), MPA or TA], rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Behçet’s disease (BD) or myositis (polymyositis or dermatomyositis). Vasculitis syndrome, RA, SLE and BD were classified according to the corresponding criteria (20–23). Blood samples were left standing for over 30 min and centrifuged at 1500 × g for 30 min. Sera were stored at −80°C until use.
Measurement of FCN1 level in patients with various autoimmune diseases
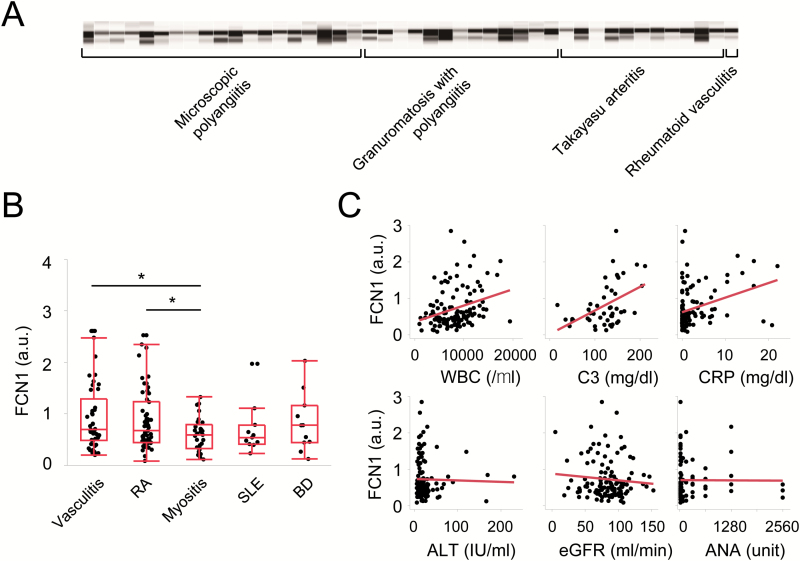
An automated simple western immunoassay system (WES, ProteinSimple, Minneapolis, MN, USA) was used to measure serum FCN1 levels. Serum samples were diluted 1:40 with reducing buffer, and then heated for 5 min at 96°C. Capillaries were blotted with 1:100 rabbit anti-FCN1 pAb (Sigma-Aldrich), followed by 1:100 HRP-conjugated anti-rabbit IgG (GE Healthcare). Fluorescence intensity was analyzed with Compass software v2.7.1 (ProteinSimple), and then converted to a conventional gel-like diagram. In our previous report (8), two bands for FCN1 were observed in the sera of KD patients using anti-FCN1 antibodies; both bands were considered to be derived from FCN1 proteins because their intensities were significantly weakened by IVIG treatment. Thus, we also consider the two bands observed in some autoimmune disease patients (as shown in Fig. 3A) to be derived from FCN1 proteins. The appearance of the two bands in some patients may be due to degradation or post-translational modification; the specific cause will be investigated in future experiments. In Fig. 3(B), we combined the intensity of the two bands, considering them both as derived from FCN1. Serum from a healthy volunteer was used as the standard. Relative fluorescence intensity was calculated by dividing the fluorescence intensity of each sample by that of the standard, and the value was expressed in arbitrary units (a.u.).
Fig. 3.
Serum FCN1 levels in patients with autoimmune diseases. (A) Determination of serum FCN1 concentrations using the automated simple western immunoassay system. Fluorescence intensities were originally obtained as quantitative values, and then converted to a conventional gel-like diagram. (B) Serum FCN1 concentrations in patients with several autoimmune diseases. Values are expressed in arbitrary units (a.u.). Differences were evaluated by the Wilcoxon rank-sum test. (C) Laboratory values that correlated positively with serum FCN1 concentrations (top panels) or did not correlate with serum FCN1 concentrations (bottom panels). WBC: white blood cell count; C3: complement factor C3; CRP: C-reactive protein; ANA: anti-nuclear antibody; *P < 0.05.
Anti-FCN1 mAb treatment for CAIA mouse, a model of RA
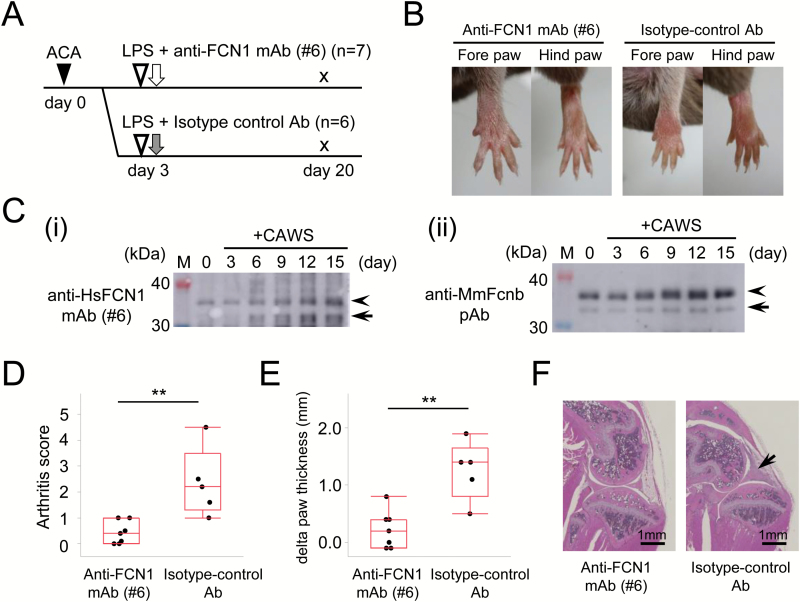
To assess the efficacy of anti-FCN1 mAb on RA, we conducted another treatment experiment using CAIA mice, an experimental model of RA. The experimental protocol was approved by the facility’s animal experiments committee. Seven-week-old male DBA/1J mice (n = 13) were purchased from Japan SLC and habituated for 1 week. Mice were divided into two groups: anti-FCN1 mAb (n = 7) or isotype-control antibody (n = 6). On day 0, 1.5 mg of anti-type II collagen antibody cocktail (#53040, Chondrex, Redmond, WA, USA) was administered intra-peritoneally. On day 3, 50 μg of lipopolysaccharide (Chondrex) was administered intra-peritoneally, followed by intra-peritoneal injection of 200 μg of anti-FCN1 mAb or isotype-control antibody (Fig. 4A). Mice were housed in an SPF environment. The arthritis score was calculated based on the appearance of each joint: 0 for no joint swelling; 0.1 for swelling on each finger joint; 0.5 for mild swelling of wrist or ankle; and 1.0 for severe swelling of wrist or ankle (24). In addition, the thickness of the limb paw was measured with a caliper. On day 20, the mice were sacrificed. Left hind limb samples were fixed with 10% formalin neutral buffer solution (Wako Pure Chemical) and decalcified by the Plank–Rychlo procedure. Sections were made from knee joints in the sagittal direction, stained with HE and examined microscopically.
Fig. 4.
Treatment of CAIA mouse, a model of RA, with anti-FCN1 mAb. (A) Experimental protocol. Black triangle indicates intra-peritoneal injection of anti-type II collagen antibody cocktail (1.5 mg, day 0). White triangles indicate lipopolysaccharide (LPS) administration (50 µg). White arrow indicates anti-FCN1 mAb administration, and gray arrow indicates isotype-control antibody administration, both by intra-peritoneal injection (200 µg, day 3, respectively). Mice were sacrificed on day 20. (B) Representative appearances of limbs of mice treated with anti-FCN1 mAb (left panels) or isotype-control antibody (right panels). (C) Western blotting with anti-HsFCN1 mAb#6 (i) or anti-MmFcnb pAb (ii) of equal amounts of mouse serum before (0 day) or after (3, 6, 9, 12 and 15 days) treatment with CAIA to detect a band for MmFcnb (arrowheads). Arrows denote the putative bands for degradation products of MmFcnb protein. M: lane for size marker. (D) Comparison of arthritis score. (E) Comparison of the change from baseline in total paw thickness of four limbs. The differences were examined by the Wilcoxon rank-sum test. (F) Representative histological images of the knee joint in mice treated with the anti-FCN1 mAb (left panel) or isotype-control antibody (right panel). Arrow indicates cellular infiltration and swelling of the synovium. Scale bar, 1 mm. ACA: anti-collagen antibody cocktail; HsFCN1: Homo sapiens (human) FCN1; MmFcnb: Mus Musculus (mouse) ficolin-b; **P < 0.01.
Statistical analysis
JMP version 12 (SAS Institute, Cary, NC, USA) was used for statistical analysis. The significance of differences for continuous values between groups was assessed by the Wilcoxon rank-sum test. Correlations between FCN1 concentrations and clinical laboratory results were determined by Pearson’s correlation coefficient. Differences in survival were analyzed by the log-rank test. Statistical significance was set at P < 0.05.
Results
Preparation of anti-FCN1 mAb
Because the serum level of FCN1 is dramatically elevated in KD patients, and administration of IVIG decreases the serum FCN1 level (8), we surmised that the anti-FCN1 mAb may also be useful as treatment for KD patients. Hence, we prepared anti-FCN1 mAb by immunizing mice with three peptides derived from the FCN1 protein (Fig. 1A). Two clones per peptide (a total of six clones) of anti-FCN1 mAb-producing hybridoma were prepared. To investigate the affinity of the anti-FCN1 mAb derived from each clone, we analyzed a KD patient’s serum by western blotting. We found that anti-FCN1 mAb#6 (5G11F8), prepared from peptide-3, had the strongest affinity for the FCN1 protein in serum (arrowhead in Fig. 1B). We confirmed that this anti-FCN1 mAb#6 recognized full-size recombinant human HsFCN1 (Fig. 1C-i) with sensitivity similar to that of commercially available rabbit anti-HsFCN1 pAb (Fig. 1C-ii). The band for GST-tagged recombinant HsFCN1 (arrow) migrated slower (~60 kDa) than HsFCN1 (~37 kDa) due to the extra molecular weight of GST-tag. Notably, anti-FCN1 mAb#6 also recognized recombinant mouse MmFcnb protein (Fig. 1D-i). This cross-reactivity is probably because the amino acid sequence of HsFCN1 peptide-3, which was used to generate anti-FCN1 mAb#6, is highly similar to the corresponding region of MmFcnb (Fig. 1E). The band for recombinant mouse MmFcnb protein with the His-tag and the T7-tag migrated (arrowhead) slightly faster than non-tagged full-size Fcnb because this commercially available Fcnb is a truncated form of Fcnb protein. We confirmed that this single band is derived from specific recognition of MmFcnb protein by the fact that this band is not detected when bovine serum albumin was used as a negative control (rightmost two lanes in Fig. 1D). Because a commercially available anti-MmFcn antibody recognized both MmFcna and MmFcnb, we prepared a rabbit anti-MmFcnb pAb using the peptide ELKVLDLEGYKQLT (Fig. 1A). This peptide is located at the N-terminal portion of MmFcnb, but is not present in MmFcna (bottom panel in Fig. 1D). To determine whether anti-FCN1 mAb#6 could also recognize MmFcnb in mouse serum, we performed a small-scale mouse experiment in which CAWS was intravenously injected, serum was collected at the indicated time after CAWS treatment and western blotting was performed. Indeed, anti-FCN1 mAb#6 revealed that MmFcnb was up-regulated after CAWS treatment (Fig. 1F). These results encouraged us to use anti-FCN1 mAb#6 and anti-MmFcnb pAb for subsequent experiments in mouse models of autoimmune diseases.
Anti-FCN1 mAb treatment of the CAWS mouse, a model of vasculitis syndrome
CAWS is used to establish a mouse model of vasculitis syndrome (25). Vasculitis can be induced in a highly reproducible manner by simple intra-peritoneal administration of CAWS in DBA/2 mice. The main components of CAWS are alpha-mannan and beta-glucan (26), and pathogenicity is associated with alpha-mannan. Due to the similarity of this model to KD in terms of disease progression and lesion distribution, the CAWS model is frequently used for in vivo experiments. Anti-FCN1 mAb#6 (see Fig. 1B) was administered to CAWS mice, a model of vasculitis (Fig. 2A). Survival (life, death or euthanasia due to weakness), body weight over time, histological findings and serum cytokine levels were compared between the anti-FCN1 mAb and isotype-control antibody groups.
No death occurred until day 30 in either treatment group, and there was no statistically significant difference in body weight at day 0 (P = 1.0) or day 35 (P = 0.27). Thereafter, the mice began to die. At day 49, 2 out of 11 (18%) mice in the anti-FCN1 mAb group died (no euthanasia due to weakness), whereas 6 out of 11 (55%) animals in the isotype-control antibody group died (including three that underwent euthanasia due to weakness) as shown in Fig. 2(B) (P = 0.07). Western blotting using anti-HsFCN1 mAb#6 or anti-MmFcnb pAb confirmed that the Fcnb level in the mouse serum gradually increased at 2, 3, 4 and 5 days after CAWS treatment (Fig. 2C). Typical histological images of aortitis are shown in Fig. 2(D). Cellular infiltration in the vessel wall and wall thickness were more severe in the isotype-control antibody group (right panel), but milder in the anti-FCN1 mAb group (left panel). The histological vasculitis score was significantly lower in the anti-FCN1 mAb group than in the isotype-control antibody group, as shown in Fig. 2(E) (P = 0.03). Serum concentrations of cytokines during treatment are shown in the Supplementary Figure. Levels of inflammatory cytokines such as IL-1β, IL-6, IFN-γ and G-CSF increased in both groups. On the other hand, the levels of IL-4, IL-10, IL-12p40, IL-17A, TNF and GM-CSF tended to be lower in the anti-FCN1 mAb group. Specifically, bimodality was observed in IL-1β, IL-4, IL-10, IL-17A, TNF, G-CSF and GM-CSF levels during the acute phase around days 9–14 and the subacute phase around days 35–42.
Measurements of serum FCN1 concentration by automated simple western assay
We wondered whether an elevated serum level of FCN1 would also be observed in other autoimmune disease patients. To address this issue, we examined a total of 152 patients: 46 with vasculitis syndrome (including 20 MPA, 12 GPA, 9 TA, 4 EGPA and 1 rheumatoid vasculitis), 55 with RA, 11 with SLE, 11 with BD and 29 with myositis (19 dermatomyositis and 10 polymyositis). Patient characteristics are shown in Table 1. Notably, serum levels of FCN1 were significantly higher in patients with vasculitis syndrome or RA than in patients with myositis (Fig. 3B). By contrast, serum FCN1 levels were not significantly higher in patients with SLE, BD or myositis than in those with other diseases. There were significant positive correlations between serum concentrations of FCN1 and levels of representative inflammatory markers such as white blood cell count (correlation coefficient: r = 0.39), complement factor C3 (r = 0.52), complement factor C4 (r = 0.52), C-reactive protein (r = 0.43), serum amyloid A (r = 0.67) and fibrinogen (r = 0.38) (representative results are shown in top panels of Fig. 3C). There were no significant correlations between serum concentrations of FCN1 and liver function (alanine aminotransferase: ALT, r = −0.05), renal function (estimated glomerular filtration rate: eGFR, r = −0.11), titer of rheumatoid factor (r = −0.06) or titer of anti-nuclear antibody (r = −0.04) (bottom panels of Fig. 3C, and for all results, see Table 2). Additionally, levels of FCN1 were positively correlated with the titer of C-ANCA (r = 0.40) and P-ANCA (r = 0.29), which are indicators for disease activity of ANCA-associated vasculitis. These results suggest that serum levels of FCN1 were elevated in patients with vasculitis syndrome or RA in accordance with the severity of inflammation. Therefore, we investigated the efficacy of anti-FCN1 mAb#6 in a mouse model of RA.
Table 1.
Characteristics of participants
| Vasculitis | RA | SLE | BD | Myositis | |
|---|---|---|---|---|---|
| N | 46 | 55 | 11 | 11 | 29 |
| Age (years) | 64 [52, 72] | 57 [44, 70] | 33 [31, 36] | 39 [33, 51] | 59 [40, 68] |
| Female (%) | 54% | 87% | 100% | 55% | 62% |
| Disease duration (months) | 12 [2, 33] | 86 [24, 162] | 129 [7, 183] | 33 [7, 68] | 10 [2, 47] |
| GC use (%) | 59% | 58% | 82% | 18% | 52% |
| IS use (%) | 11% | 87% | 36% | 18% | 28% |
| WBC (per μl) | 9990 [8360, 12505] | 7840 [5340, 10290] | 5970 [3790, 8210] | 5300 [4460, 8680] | 7160 [4070, 11680] |
| CRP (mg dl−1) | 0.98 [0.19, 6.71] | 0.81 [0.06, 2.52] | 0.15 [0.10, 0.48] | 0.05 [0.04, 0.80] | 0.20 [0.04, 0.63] |
Continuous values are expressed as median value and interquartile range. GC: glucocorticoid; IS: immunosuppressant; WBC: white blood cell count; CRP: C-reactive protein.
Table 2.
Correlations between levels of FCN1 and clinical markers
| Category | Clinical marker | n | r | 95% CI | P-value |
|---|---|---|---|---|---|
| Inflammation | White blood cell count | 115 | 0.39 | 0.22, 0.53 | <0.001 |
| ESR | 72 | 0.28 | 0.05, 0.48 | 0.017 | |
| C-reactive protein | 115 | 0.43 | 0.27, 0.57 | <0.001 | |
| Immunoglobulin G | 59 | −0.01 | −0.27, 0.25 | 0.93 | |
| Serum amyloid A | 31 | 0.67 | 0.41, 0.83 | <0.001 | |
| Liver function | ALT | 114 | −0.05 | −0.23, 0.13 | 0.13 |
| Renal function | eGFR | 113 | −0.11 | −0.29, 0.08 | 0.26 |
| Creatinine | 114 | 0.20 | 0.02, 0.37 | 0.034 | |
| Complement | Complement factor C3 | 48 | 0.52 | 0.28, 0.70 | <0.001 |
| Complement factor C4 | 47 | 0.52 | 0.27, 0.70 | <0.001 | |
| CH50 | 31 | 0.30 | −0.06, 0.59 | 0.10 | |
| Autoantibody | Rheumatoid factor | 50 | −0.06 | −0.34, 0.22 | 0.66 |
| Anti-nuclear antibody | 89 | −0.04 | −0.24, 0.17 | 0.74 | |
| C-ANCA | 51 | 0.40 | 0.14, 0.61 | 0.0039 | |
| P-ANCA | 56 | 0.29 | 0.03, 0.51 | 0.031 | |
| ACPA | 70 | 0.25 | 0.01, 0.45 | 0.040 | |
| Anti-dsDNA antibody | 32 | −0.16 | −0.49, 0.20 | 0.37 | |
| Coagulation | Fibrinogen | 25 | 0.38 | −0.02, 0.67 | 0.065 |
| d-dimer | 40 | 0.09 | −0.23, 0.39 | 0.58 | |
| Others | Albumin | 26 | −0.16 | −0.51, 0.25 | 0.45 |
| Ferritin | 43 | −0.02 | −0.32, 0.28 | 0.87 | |
| Immune complex | 27 | −0.30 | −0.61 0.09 | 0.13 | |
| MMP3 | 66 | −0.12 | −0.36, 0.12 | 0.32 |
n: number; r: Pearson’s correlation coefficient; CI: confidence interval; ESR: erythrocyte sedimentation rate; CH50: 50% hemolytic activity of complement; ACPA: anti-cyclic citrullinated peptide antibody; dsDNA: double-stranded DNA; MMP3: matrix metalloproteinase 3.
Anti-FCN1 mAb treatment of the CAIA mouse, a model of RA
The CAIA mouse is a model of RA. In strict terms, it differs from human RA, e.g., in terms of the cytokine profile (27). However, it is a model that reproducibly develops arthritis following administration of a single anti-type II collagen antibody cocktail and lipopolysaccharide, both of which are commercially available (28). Arthritis developed from about day 6 in our experiments. Figure 4(B) shows typical images of fore and hind limbs in mice treated with the anti-FCN1 mAb (left panels) or isotype-control antibody (right panels). Western blotting using anti-HsFCN1 mAb#6 or anti-MmFcnb pAb (Fig. 4C) confirmed that the Fcnb level in the mouse serum gradually increased at 3, 6, 9, 12 and 15 days after CAIA treatment (arrow and arrowhead in Fig. 4C). One mouse in the isotype-control antibody group died on day 6 with unknown cause, and was excluded from the analysis. The arthritis score at day 8 is shown in Fig. 4(D). The change from baseline in total paw thickness of all four limbs is shown in Fig. 4(E). The arthritis score and total paw thickness were significantly lower in the anti-FCN1 mAb group than in the isotype-control antibody group (P = 0.0069 and P = 0.0071, respectively). Typical histological images of the knee joint are shown in Fig. 4(F). In this figure, cellular infiltration in the synovium is apparent in a mouse treated with isotype-control antibody (right panel), but not a mouse treated with anti-FCN1 mAb (left panel).
Discussion
Previously, we reported that in patients with KD, serum levels of FCN1 decrease dramatically in response to IVIG treatment, indicating that the binding of FCN1 to IgG is a mechanism of IVIG treatment (8). We conducted this study to investigate whether treatment targeting FCN1 could be therapeutically effective against vasculitis, as well as whether FCN1 is involved in the pathogenesis of other autoimmune diseases. We found that treatment by an anti-FCN1 mAb decreased the severity of disease in a model of KD vasculitis. Next, we assessed blood samples of patients with other autoimmune diseases. Serum concentrations of FCN1 were significantly higher in patients with vasculitis syndrome or RA than in those with autoimmune myositis. Serum concentrations of FCN1 were positively correlated with representative clinical inflammatory markers such as C-reactive protein, white blood cell count or complement factor C3, but poorly correlated with liver function, renal function or titers of autoantibodies such as rheumatoid factor or anti-nuclear antibody. Interestingly, titers of autoantibodies such as C-ANCA or P-ANCA, which reflect the disease activity of vasculitis, were correlated with serum levels of FCN1. Finally, we demonstrated that treatment with anti-FCN1 mAb decreased the severity of arthritis in the CAIA model.
In the vasculitis model, as shown in Fig. 2(B), the difference in survival rate was not statistically significant, but the difference at day 49 was 37% (82% in the anti-FCN1 mAb group versus 45% in the isotype-control antibody group), which is not a trivial difference. Increasing injections of anti-FCN1 mAb may improve the result. Further study with more participants or with increasing injections of the treatment antibody is needed to confirm the effect. In the arthritis model, as shown in Fig. 4(B), (D) and (E), the severity of arthritis was significantly reduced in the anti-FCN1 mAb group. Therefore, FCN1 is not only a serum marker that reflects the degree of inflammation, but may also be involved in the pathogenesis of these diseases.
Gout et al. (29) introduced the point mutation G221F into FCN1, which inhibited binding to 9-O-acetylated sialic acid. The anti-FCN1 mAb used in our experiments was raised against a peptide corresponding to a.a. 216–229, which encompasses G221. This amino acid sequence may be important for promoting inflammation through binding to 9-O-acetylated sialic acid, and thus for the observed effects in this study. This time, we could not clarify whether anti-FCN1 mAb inhibits the binding of FCN1 to 9-O-acetylated sialic acid, and this hypothesis should be investigated in further experiment. It is possible that the mechanism of the anti-inflammatory effect of anti-FCN1 mAb is different from that of IVIG, which accelerates the clearance of serum FCN1. Banda et al. (18) showed that Fcnb-deficient mice are resistant to CAIA, and also demonstrated the involvement of the complement pathway in this arthritis model. Their research shows that Fcnb is important for the development of CAIA, and their results are consistent with our observations in anti-FCN1 mAb-treated mice, suggesting that treatment targeting FCN1 in autoimmune diseases has clinical potential. Several reports have described the relationship between FCN1 and CD43 (leukosialin). CD43 is present on the cell membrane of almost all blood cells, and its electrical and physical barriers inhibit interactions such as antigen presentation to T cells (30). Rørvig et al. (16) reported that 90% of FCN1 released by an inflammatory stimulus such as fMLP binds to the cell membrane of the releasing cell. Moreno-Amaral et al. (31) reported that FCN1 binds to and bridges CD43 on the cell membrane. It is possible that localization of CD43 exposes a portion of the cell membrane not covered by CD43, facilitating cell–cell interactions and promoting an immune response. Seveau et al. (32) reported that external localization of CD43 by anti-CD43 mAb causes changes in cell morphology, suggesting that binding of FCN1 to the cell membrane may introduce an activation signal to the interior of the cell. Inhibiting the binding of FCN1 to CD43 with anti-FCN1 mAb may suppress the inflammatory cascade. Another hypothesis is that FCN1 binds to inflammatory cells, which may be removed by antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) after exposure to an anti-FCN1 mAb.
This study demonstrates the involvement of FCN1 in autoimmune diseases and raises the possibility of applying FCN1-targeting agents to the treatment of these diseases. Recently, several reagents that target complement molecules have been developed. Eculizumab, a mAb against complement protein C5, is currently used to treat patients with paroxysmal nocturnal hemoglobinuria. Avacopan, a C5 receptor inhibitor, is in clinical trials for treatment of patients with ANCA-associated vasculitis and C3-related nephritis. In other words, we have entered the era of complement-targeted treatments.
In summary, we showed here that serum FCN1 level is elevated in vasculitis syndrome and correlates with the degree of inflammation. Treatment with an anti-FCN1 mAb ameliorated disease severity in two models of autoimmune diseases. Accordingly, we postulate that FCN1 is a promising therapeutic target for autoimmune diseases. Further studies are needed to confirm the anti-inflammatory effect of anti-FCN1 mAb and the underlying mechanism, and to apply anti-FCN1 therapy in a clinical setting.
Funding
This work was supported by the research program ‘Hub for Predictive and Preventive Precision Medicine Driven by Big Data’, conducted by RIKEN, the Japan Science and Technology Agency and Osaka University.
Supplementary Material
Acknowledgements
We thank Yorika Kato for technical assistance with some of the biochemical analyses, and Yoshinobu Matsuura for advice on mouse experiments. T.H. and H.N. designed the study. M.K., T.H. and H.N. wrote the manuscript. N.N.-M. and N.O. provided CAWS for use in mouse experiments. M.K., K.O. and N.Y. performed biochemical experiments. M.K., T.H. and A.K. performed analysis of patients’ serum samples and clinical information.
Conflicts of interest statement: the authors declared no conflicts of interest.
References
- 1. Nishide M. Nojima S. Ito D., et al. 2017. Semaphorin 4D inhibits neutrophil activation and is involved in the pathogenesis of neutrophil-mediated autoimmune vasculitis. Ann. Rheum. Dis. 76:1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furusho K. Kamiya T. Nakano H., et al. 1984. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 2:1055. [DOI] [PubMed] [Google Scholar]
- 3. Newburger J. W. Takahashi M. Burns J. C., et al. 1986. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 315:341. [DOI] [PubMed] [Google Scholar]
- 4. Beiser A. S. Takahashi M. Baker A. L. Sundel R. P. and Newburger J. W. 1998. A predictive instrument for coronary artery aneurysms in Kawasaki disease. US Multicenter Kawasaki Disease Study Group. Am. J. Cardiol. 81:1116. [DOI] [PubMed] [Google Scholar]
- 5. Sundel R. P. 2015. Kawasaki disease. Rheum. Dis. Clin. North Am. 41:63. [DOI] [PubMed] [Google Scholar]
- 6. Okuzaki D., Kobayashi S., Sakuri M., et al. 2012. Ficolin 1 expression is elevated in the peripheral blood mononuclear cells of Takayasu’s vasculitis patients. J. Mol. Biomark. Diagn. 2:125. [Google Scholar]
- 7. Muso E. Okuzaki D. Kobayashi S., et al. 2013. Ficolin-1 is up-regulated in leukocytes and glomeruli from microscopic polyangiitis patients. Autoimmunity 46:513. [DOI] [PubMed] [Google Scholar]
- 8. Okuzaki D. Ota K. Takatsuki S. I., et al. 2017. FCN1 (M-ficolin), which directly associates with immunoglobulin G1, is a molecular target of intravenous immunoglobulin therapy for Kawasaki disease. Sci. Rep. 7:11334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holmskov U. Thiel S. and Jensenius J. C. 2003. Collections and ficolins: humoral lectins of the innate immune defense. Annu. Rev. Immunol. 21:547. [DOI] [PubMed] [Google Scholar]
- 10. Turner M. W. 2004. The role of mannose-binding lectin in health and disease. Neth. J. Med. 62:4. [DOI] [PubMed] [Google Scholar]
- 11. Eggleton P. and Reid K. B. 1999. Lung surfactant proteins involved in innate immunity. Curr. Opin. Immunol. 11:28. [DOI] [PubMed] [Google Scholar]
- 12. Bohlson S. S. O’Conner S. D. Hulsebus H. J. Ho M. M. and Fraser D. A. 2014. Complement, C1q, and C1q-related molecules regulate macrophage polarization. Front. Immunol. 5:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Endo Y. Liu Y. Kanno K. Takahashi M. Matsushita M. and Fujita T. 2004. Identification of the mouse H-ficolin gene as a pseudogene and orthology between mouse ficolins A/B and human L-/M-ficolins. Genomics 84:737. [DOI] [PubMed] [Google Scholar]
- 14. Liu Y. Endo Y. Iwaki D., et al. 2005. Human M-ficolin is a secretory protein that activates the lectin complement pathway. J. Immunol. 175:3150. [DOI] [PubMed] [Google Scholar]
- 15. Honoré C. Rørvig S. Munthe-Fog L., et al. 2008. The innate pattern recognition molecule Ficolin-1 is secreted by monocytes/macrophages and is circulating in human plasma. Mol. Immunol. 45:2782. [DOI] [PubMed] [Google Scholar]
- 16. Rørvig S. Honore C. Larsson L. I., et al. 2009. Ficolin-1 is present in a highly mobilizable subset of human neutrophil granules and associates with the cell surface after stimulation with fMLP. J. Leukoc. Biol. 86:1439. [DOI] [PubMed] [Google Scholar]
- 17. Endo Y. Takahashi M. Iwaki D., et al. 2012. Mice deficient in ficolin, a lectin complement pathway recognition molecule, are susceptible to Streptococcus pneumoniae infection. J. Immunol. 189:5860. [DOI] [PubMed] [Google Scholar]
- 18. Banda N. K. Acharya S. Scheinman R. I., et al. 2017. Deconstructing the lectin pathway in the pathogenesis of experimental inflammatory arthritis: essential role of the lectin ficolin b and mannose-binding protein-associated serine protease 2. J. Immunol. 199:1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagi-Miura N. Okuzaki D. Torigata K., et al. 2013. CAWS administration increases the expression of interferon γ and complement factors that lead to severe vasculitis in DBA/2 mice. BMC Immunol. 14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jennette J. C., Falk R. J., Bacon P. A., et al. 2013. 2012 Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 65:1. [DOI] [PubMed] [Google Scholar]
- 21. Aletaha D. Neogi T. Silman A. J., et al. 2010. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 62:2569. [DOI] [PubMed] [Google Scholar]
- 22. Petri M. Orbai A. M. Alarcón G. S., et al. 2012. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64:2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Japanese Ministry of Health, Labor and Welfare Diagnostic criteria for Behçet’s disease. 2010. [Google Scholar]
- 24. Yoshitomi H. Sakaguchi N. Kobayashi K., et al. 2005. A role for fungal β-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohno N. 2003. Chemistry and biology of angiitis inducer, Candida albicans water-soluble mannoprotein-beta-glucan complex (CAWS). Microbiol. Immunol. 47:479. [DOI] [PubMed] [Google Scholar]
- 26. Uchiyama M. Ohno N. Miura N. N., et al. 1999. Chemical and immunochemical characterization of limulus factor G-activating substance of Candida spp. FEMS Immunol. Med. Microbiol. 24:411. [DOI] [PubMed] [Google Scholar]
- 27. Kagari T. Doi H. and Shimozato T. 2002. The importance of IL-1β and TNF-α, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J. Immunol. 169:1459. [DOI] [PubMed] [Google Scholar]
- 28. Hutamekalin P. Saito T. Yamaki K., et al. 2009. Collagen antibody-induced arthritis in mice: development of a new arthritogenic 5-clone cocktail of monoclonal anti-type II collagen antibodies. J. Immunol. Methods 343:49. [DOI] [PubMed] [Google Scholar]
- 29. Gout E. Garlatti V. Smith D. F., et al. 2010. Carbohydrate recognition properties of human ficolins: glycan array screening reveals the sialic acid binding specificity of M-ficolin. J. Biol. Chem. 285:6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostberg J. R. Barth R. K. and Frelinger J. G. 1998. The Roman god Janus: a paradigm for the function of CD43. Immunol. Today 19:546. [DOI] [PubMed] [Google Scholar]
- 31. Moreno-Amaral A. N. Gout E. Danella-Polli C., et al. 2012. M-ficolin and leukosialin (CD43): new partners in neutrophil adhesion. J. Leukoc. Biol. 91:469. [DOI] [PubMed] [Google Scholar]
- 32. Seveau S. Lopez S. Lesavre P. Guichard J. Cramer E. M. and Halbwachs-Mecarelli L. 1997. Leukosialin (CD43, sialophorin) redistribution in uropods of polarized neutrophils is induced by CD43 cross-linking by antibodies, by colchicine or by chemotactic peptides. J. Cell Sci. 110:1465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.