Abstract
Context
The programmed cell death protein 1 (PD-1)/programmed cell death protein ligand 1 (PD-L1) pathway is a key regulator in T-cell activation and tolerance, limiting effector T-cell function in peripheral tissues. Atezolizumab, an anti–PD-L1 monoclonal antibody, is approved for treatment of some types of advanced cancer. Its main treatment-related adverse events are immune related, such as thyroid dysfunction and hypophysitis. Autoimmune endocrinopathy can occur as isolated manifestations; only a few cases of autoimmune polyendocrine syndromes have been reported thus far.
Case
We report a case of polyendocrine syndrome type 2, characterized by Addison disease (AD), type 1 diabetes mellitus (T1DM), accompanied by hypophysitis, in a patient treated with atezolizumab. Testing was positive for 21-hydroxylase and pituitary antibodies and negative for islet cells antibodies. HLA typing revealed DRB1*04 and DQB1*03 haplotypes, which are associated with increased susceptibility to T1DM and AD.
Conclusion
The type and severity of immune-related adverse events in polyendocrine syndrome type 2 are different and depend on the monoclonal antibody used. Although the numerous molecular mechanisms inducing autoimmune endocrine diseases are still unclear, a link exists between HLA haplotypes, gene variants involved in immune checkpoint molecule expression, and increased susceptibility to autoimmune endocrinopathies. Additional studies are needed to identify susceptible patients and adapt therapy to each patient.
Keywords: immune checkpoint inhibitors, diabetes mellitus, Addison disease, hypophysitis
Autoimmune polyendocrine syndrome type 2 (APS-2) is a polygenic disease in which HLA alleles and non-HLA genes determine the targeting of specific tissues by autoreactive T cells, leading to organ-specific autoimmunity. Aside from the genetic component, APS-2 is also influenced by environmental and endogenous factors, which act to break immune tolerance and initiate the autoimmune attack [1].
Therapeutic antibodies have been introduced in clinical practice to target key regulators of peripheral immune tolerance (namely, anti–CTLA-4, anti–PD-1, and anti–PD-L1) with the objective of activating the immune system against cancer cells. An undesirable but somewhat expected effect of immunotherapy is the triggering of autoimmune diseases. The prevalence of immune-related adverse events (irAEs) is related to the kind of immune checkpoint blockade used: Autoimmune thyroiditis is frequently found after patients are treated with anti–PD-1, whereas hypophysitis, which is considered a very rare condition, has been reported in up to 5% of patients receiving anti–CTLA-4 [2].
Type 1 diabetes mellitus (T1DM) and primary adrenal insufficiency are rare irAEs that can result in life-threatening diabetic ketoacidosis or adrenal crisis, respectively, if not diagnosed timely and managed properly. We describe a case of onset of APS-2 [an association of Addison disease (AD) and diabetes mellitus] accompanied by hypophysitis in a patient treated with the anti–PD-L1 atezolizumab.
1. Case Report
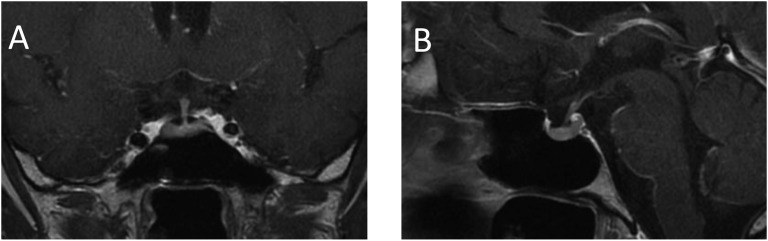
A 60-year-old man was diagnosed with unresectable metastatic lung adenocarcinoma at age 52 years and treated with 37 cycles of chemotherapy with cisplatin and pemetrexed. In April 2018, because of tumor progression, he received a second-line treatment with atezolizumab as part of a phase III/IV, single-arm, multicenter study. He had no comorbidities or a family history of autoimmune or endocrine diseases. Baseline thyroid function assessment, and serum blood glucose and electrolyte levels were unremarkable. Atezolizumab was prescribed at a dosage of 1200-mg every 3 weeks. After the two initial doses, hyperglycemia developed, requiring basal-bolus insulin therapy. After the fourth dose, the patient was admitted to our Endocrinology and Metabolic Diseases Unit because of severe weakness, nausea, abdominal pain, thirst, and dizziness. Laboratory tests revealed severe hyperglycemia with diabetic ketoacidosis, hyperkalemia, and hyponatremia. Pituitary function was tested and found normal (Table 1). After 3 days of insulin infusion and IV fluids, the patient returned to basal-bolus insulin therapy, achieving fair glycemic control. Further assessment showed undetectable serum C-peptide levels and slightly elevated HbA1c. Because of persistence of mild hyperkalemia and hyponatremia, although ketoacidosis was corrected, we assessed the ACTH-adrenal axis, documenting low morning ACTH and cortisol, low urinary free cortisol level, absent response of cortisol to ACTH, and no response of cortisol and ACTH to corticotropin-releasing factor. Serum electrolyte levels normalized after hydrocortisone and fludrocortisone replacement therapy was started. Pituitary function evaluation was suggestive for hypogonadotropic hypogonadism, whereas IGF-1, prolactin, and TSH levels were all normal (Table 1). No adrenal secondary lesions were detected on an abdominal CT scan; sellar MRI with gadolinium contrast showed a normal-sized pituitary gland with regular enhancement and no secondary lesions in the hypothalamic-pituitary region (Fig. 1). After reporting the adverse event to the trial sponsors and competent regulatory body, atezolizumab was withdrawn. Three months after discontinuation of the drug, tumor progression was documented on a CT scan.
Table 1.
Laboratory and Hormonal Test Results Before (Wk 0) and During Atezolizumab Therapy (Wk 3–11)a
| Test | Wk | Reference Range | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 11 | 12 | 13 | ||
| Glycemia | 96 | 95 | 149 | 189 | 549 | 180 | 220 | 74–109 mg/dL |
| C-peptide | 0.7 | 0.0 | >1 ng/mL | |||||
| Sodium | 138 | 141 | 133 | 132 | 121 | 132 | 133 | 135–145 mEq/L |
| Potassium | 3.98 | 4.26 | 5.18 | 5.69 | 7.91 | 5.8 | 4.5 | 3.5–5.1 mEq/L |
| Bicarbonate | 25 | 21 | 15 | 26 | 22–30 mmol/L | |||
| Calcium | 9.2 | 8.6–10.2 mg/dL | ||||||
| Creatinine | 1.1 | 0.7–1.2 mg/dL | ||||||
| eGFR | 95 | >90 mL/min | ||||||
| PTH | 26 | 8–40 ng/L | ||||||
| IGF-1 | 157.2 | 164.8 | 49–193 ng/mL | |||||
| PRL | 17.4 | 11.14 | 2–25 ng/mL | |||||
| LH | 6.4 | 1.1 | 1.2 | 1.4–12.7 mUI/mL | ||||
| FSH | 21.4 | 1.3 | 1.3 | 1.3–19.5 mUI/mL | ||||
| Testosterone | 1.5 | 3.2 | 1.75–7.8 μg/L | |||||
| SHBG | 89.7 | 90.1 | 13.0–89.5 nmol/L | |||||
| ACTH | 18 | 21 | 4 | 5 | <50 ng/L | |||
| Cortisol | 8.2 | 6.1 | <0.4 | <0.4 | 6.7–22.6 μg/dL | |||
| Cortisol peak after ACTH test | 0.4 | >18.0 μg/L | ||||||
| Renin | 350.6 | 4.4–46.1 mIU/mL | ||||||
| Aldosterone | 1.2 ng/dL | 2.2–35.3 ng/dL | ||||||
| TSH | 0.98 | 0.76 | 0.69 | 0.72 | 0.60 | 0.40–4.00 μU/mL | ||
| fT4 | 1.35 | 0.96 | 1.21 | 1.24 | 0.83 | 0.7–1.7 ng/dL | ||
| AbTg | <0.1 | <1.0 | <1.0 | <30.0 UI/mL | ||||
| AbTPO | <0.1 | <1.0 | <1.0 | <10.0 UI/mL | ||||
| Anti–TSH-R Ab | 0.11 | < 1.50 UI/L | ||||||
| Anti–21-OH Ab | 89.33 | <0.40 U/mL | ||||||
| Anti–IA2 Ab | 0.0 | 0.0 | <1.0 UI/mL | |||||
| Anti–GAD Ab | 0.0 | 0.0 | <1.0 UI/mL | |||||
| Anti–pituitary Ab | Positive | Negative | ||||||
Abbreviations: Ab, antibody; AbTg, Ab anti-thyroglobulin; AbTPO, Ab anti-thyroperoxydase; eGFR, estimated glomerular filtration rate; fT4, free thyroxine; PRL, prolactin.
Examinations were repeated at wk 12 and 13 during insulin and glucocorticoids replacement therapy.
Figure 1.
MRI with gadolinium contrast showing a normal-sized pituitary and stalk and normal enhancement. (A) Coronal image; (B) sagittal image.
A. Organ-Specific Antibodies and HLA Typing
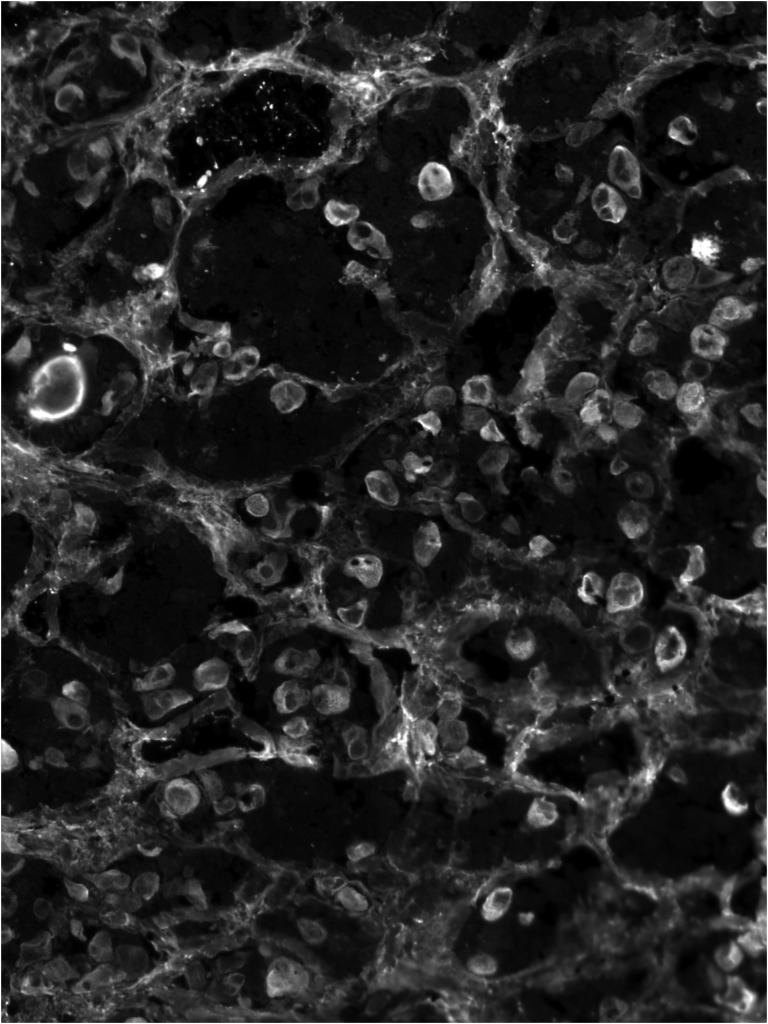
Tests for insulin cell antibodies (tyrosine phosphatase-based islet antigen 2 antibodies and glutamic acid decarboxylase antibody) were negative, whereas tests for 21-hydroxylase antibodies were positive. Pituitary antibodies, tested by immunofluorescence using human pituitary substrate, as previously described [3, 4], were present (Fig. 2). Double immunofluorescence staining showed that these antibodies recognized TSH-secreting cells but not ACTH-, LH/FSH-, or GH-secreting cells.
Figure 2.
Immunofluorescence cytosolic diffuse staining pattern produced by pituitary antibodies.
In our case, detection of pituitary antibodies was done using patient’s serum at a 1:10 dilution and an anti-human antibody (Alexa 488, catalog no. 709-546-149; Jackson ImmunoResearch) [5] at a 1:400 dilution to reveal antibody binding. As a second step, for double immunofluorescence, the following antibodies were used: anti-TSH (catalog no. hBeta TSH; A.F. Parlow National Hormone and Peptide Program) [6] at a 1:10 dilution; anti-LH (catalog no. AFP-55881789; A.F. Parlow National Hormone and Peptide Program) [7] at a 1:10 dilution; anti-FSH (catalog no. AFP-891891; A.F. Parlow National Hormone and Peptide Program) [8] at a 1:50 dilution; anti-ACTH (catalog no. 701293; Thermo Fisher Scientific) [9] at a 1:100 dilution; and anti-GH (catalog no. 374266; Santa Cruz Biotechnology) [10] at a 1:100 dilution. To reveal antibody binding Alexa 488 (catalog no. 150077; Abcam) [11] or Alexa 594 (catalog no. 115-586-144; Jackson ImmunoResearch) [12] were used at a 1:400 dilution.
HLA typing revealed DRB1*04 and DQB1*03 haplotypes. These were compatible with increased susceptibility to T1DM and AD.
2. Discussion
We report a unique case of an APS characterized by rapid onset of diabetic ketoacidosis, AD, and hypophysitis during anticancer immunotherapy with atezolizumab, an anti–PD-L1 antibody. T1DM is a rare irAE that often manifests a progression through diabetic ketoacidosis. Only 45 cases have been reported so far, to our knowledge: the majority with anti–PD-1 and only six with anti–PD-L1 therapy. In keeping with the current literature, manifestation of diabetes in the patient we report on here was rapid and independent of the dose of anti–PD-L1. Diabetic ketoacidosis was described in 71% of cases so far reported and, remarkably, in almost all (five of six) of patients treated with anti–PD-L1 therapy [2]. Therefore, the term “fulminant diabetes mellitus,” used to describe dramatic clinical presentation in patients receiving immunotherapy [13], well applies to this case.
The PD-1/PD-L1 pathway is a key regulator in T-cell activation and tolerance [14], though many other immune effects remain to be elucidated. A mouse model reported in 2003 [15] lends an initial insight into T1DM onset in patients receiving immunotherapy. The study was the first to show that PD-1 and PD-L1 blockade can rapidly precipitate T1DM in NOD mice. PD-L1 was expressed in the inflamed β cells of the mice, suggesting a mechanism of downregulation of lymphocyte function at the site of inflammation by parenchymal cells themselves [15]. In humans, few studies have reported a low expression of PD-1 on activated T cells in patients with T1DM [16, 17]. These observations suggest that an imbalance between activated and resting T cells might promote autoimmunity by a mechanism similar to that of PD-1 blockade therapy [18].
The case we report was characterized by an insidious presentation of hypophysitis with reduced ACTH and gonadotropin secretion. Hypoadrenalism was mixed (i.e., both primary and secondary) and presented with hyponatremia and hyperkalemia that initially went unnoticed due to concomitant ketoacidosis. A strong positivity for pituitary antibodies was evident on immunofluorescence, although MRI did not indicate signs of pituitary inflammation, as described in ∼25% of patients who develop hypophysitis secondary to immunotherapy [2].
Hypophysitis secondary to PD-L1 blockade is a very uncommon irAE; it is reported to date in <0.1% of treated patients [2] and is not yet well defined. Hypophysitis secondary to CTLA-4 blockade has higher prevalence (3.2%) and was well characterized in studies showing CTLA-4 antigen expression in pituitary cells [4]. The administration of CTLA-4–blocking antibodies to patients with high levels of CTLA-4 antigen in the pituitary can trigger hypophysitis through type IV (T-cell dependent, typical of autoimmune diseases) and type II (IgG dependent) immune mechanisms [19]. We cannot exclude in this case a similar pathogenesis whereby PD-L1 is expressed on pituitary cells, acts as the target autoantigen, and initiates the immune response. Nonetheless, the latter seems unlikely to account for the effect of atezolizumab, because it contains a modified Fc region designed to limit antibody- and complement-dependent cytotoxicity.
Primary adrenal insufficiency in the patient in this report was confirmed by positivity of 21-hydroxylase and, ex juvantibus, by normalization of hyperkalemia with fludrocortisone. AD is an extremely infrequent irAE and, to our knowledge, no cases have been so far reported after anti–PD-L1 [2]. Of note, some reports claim PD-L1 gene variants are associated with risk of AD developing [20].
As in spontaneous APSs, organ-specific autoantibodies often accompany the autoimmune manifestations in patients receiving immunotherapy, although their predictive role and time course is uncertain [2]. The patient we report on showed clear positivity for 21-hydroxylase and for pituitary antibodies, but not for islet cells antibodies. In keeping with other reports, double immunofluorescence staining showed that pituitary antibodies clearly recognized TSH-secreting cells [4] with a not-yet-functional defect of the pituitary-thyroid axis in this patient.
In conclusion, we report the case of a patient with genetic susceptibility(i.e., DRB1*04 and DQB1*03 HLA haplotypes) for T1DM and AD, and in whom the clinical onset of APS was triggered by immunotherapy with anti–PD-L1 blockade at a relatively old age. In patients treated with anti–PD-L1 therapy, blockade onset of APS, although not reported previously, to our knowledge (Table 2), should be kept in mind particularly with respect to the potential sudden development of life-threatening conditions such as diabetic ketoacidosis and adrenal crisis. The distinct functional profiles and antigen targeting of immune checkpoint inhibitors should be taken into account and treatment tailored to the patient to exploit these pathways to enhance immune responses against tumors and minimize irAEs.
Table 2.
| Reference | Tumor Type | Checkpoint Inhibitor | Presentation |
|---|---|---|---|
| Marchand et al. [21] | Pulmonary pleomorphic carcinoma | Nivolumab | DM and hypophysitis |
| Humayun and Poole [22] | Melanoma | Pembrolizumab | DM and hypophysitis |
| Tsiogka et al. [23] | Melanoma | Ipilimumab | DM and hypophysitis |
| Gauci et al. [24] | Melanoma | Nivolumab | DM and thyroid disease |
| Hofmann et al. [25] | Melanoma | Nivolumab | DM and thyroid disease |
| Li et al. [26] | Lung SCC | Nivolumab | DM and thyroid disease |
| Lowe et al. [27] | Melanoma | Nivolumab | DM and thyroid disease |
| Hughes et al. [28] | Melanoma | Nivolumab | DM and thyroid disease |
| Hansen et al. [29] | Melanoma | Pembrolizumab | DM and thyroid disease |
| Gaudy et al. [30] | Melanoma | Pembrolizumab | DM and thyroid disease |
| Hughes et al. [28] | Melanoma | Pembrolizumab | DM and thyroid disease |
| Kong et al. [31] | Lung SCC | Pembrolizumab | DM and thyroid disease |
| Alhusseini and Samantray [32] | Lung adenocarcinoma | Pembrolizumab | DM and thyroid disease |
| Min and Ibrahim [33] | Melanoma | Ipilimumab | AD and hypophysitis |
| Yang et al. [34] | RCC | Ipilimumab | AD and hypophysitis |
| Paepegaey et al. [35] | Melanoma | Pembrolizumab | AD and thyroid disease |
Abbreviations: DM, diabetes mellitus; RCC, renal cell carcinoma; SCC, squamous cell carcinoma.
Pembrolizumab and nivolumab.
Ipilimumab.
No cases were described during anti-PD-L1 therapy [2].
Acknowledgments
We thank Dr. Patrizio Caturegli, Dr. Fabiana Pani, and Dr. Elena Sabini, Department of Pathology, Johns Hopkins University, Baltimore, Maryland, for pituitary antibodies testing.
Written informed consent was obtained from the patient for publication of this case report.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AD
Addison disease
- irAE
immune-related adverse event
- PD-1
programmed cell death protein 1
- PD-L1
programmed cell death protein ligand 1
- T1DM
type 1 diabetes mellitus
References and Notes
- 1. Husebye ES, Anderson MS, Kämpe O. Autoimmune polyendocrine syndromes. N Engl J Med. 2018;378(12):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ricciuti A, De Remigis A, Landek-Salgado MA, De Vincentiis L, Guaraldi F, Lupi I, Iwama S, Wand GS, Salvatori R, Caturegli P. Detection of pituitary antibodies by immunofluorescence: approach and results in patients with pituitary diseases. J Clin Endocrinol Metab. 2014;99(5):1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iwama S, De Remigis A, Callahan MK, Slovin SF, Wolchok JD, Caturegli P. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6(230):230ra45. [DOI] [PubMed] [Google Scholar]
- 5.RRID: AB_2340569.
- 6.RRID: AB_2756403.
- 7.RRID: AB_2756402.
- 8.RRID: AB_2314008.
- 9.RRID: AB_2532462.
- 10.RRID: AB_10989917.
- 11.RRID: AB_2630356.
- 12.RRID: AB_2338897.
- 13. Okamoto M, Okamoto M, Gotoh K, Masaki T, Ozeki Y, Ando H, Anai M, Sato A, Yoshida Y, Ueda S, Kakuma T, Shibata H. Fulminant type 1 diabetes mellitus with anti-programmed cell death-1 therapy. J Diabetes Investig. 2016;7(6):915–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol. 2001;22(5):265–268. [DOI] [PubMed] [Google Scholar]
- 15. Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, Yamazaki T, Azuma M, Iwai H, Khoury SJ, Auchincloss H Jr, Sayegh MH. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsutsumi Y, Jie X, Ihara K, Nomura A, Kanemitsu S, Takada H, Hara T. Phenotypic and genetic analyses of T-cell-mediated immunoregulation in patients with type 1 diabetes. Diabet Med. 2006;23(10):1145–1150. [DOI] [PubMed] [Google Scholar]
- 17. Fujisawa R, Haseda F, Tsutsumi C, Hiromine Y, Noso S, Kawabata Y, Mitsui S, Terasaki J, Ikegami H, Imagawa A, Hanafusa T. Low programmed cell death-1 (PD-1) expression in peripheral CD4(+) T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol. 2015;180(3):452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clotman K, Janssens K, Specenier P, Weets I, De Block CEM. Programmed cell death-1 (PD-1) inhibitor induced type 1 diabetes mellitus: mini-review. J Clin Endocrinol Metab. 2018;103(9):3144–3154. [DOI] [PubMed] [Google Scholar]
- 19. Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, Lupi I. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225–3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell AL, Cordell HJ, Soemedi R, Owen K, Skinningsrud B, Wolff AB, Ericksen M, Undlien D, Husebye E, Pearce SH. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison’s disease and Graves’ disease susceptibility. J Clin Endocrinol Metab. 2009;94(12):5139–5145. [DOI] [PubMed] [Google Scholar]
- 21. Marchand L, Paulus V, Fabien N, Pérol M, Thivolet C, Vouillarmet J, Saintigny P. Nivolumab-induced acute diabetes mellitus and hypophysitis in a patient with advanced pulmonary pleomorphic carcinoma with a prolonged tumor response. J Thorac Oncol. 2017;12(11):e182–e184. [DOI] [PubMed] [Google Scholar]
- 22. Humayun M, Poole R. A case of multiple immune toxicities from ipilimumab and pembrolizumab treatment. Hormones (Athens). 2016;15(2):303–306. [DOI] [PubMed] [Google Scholar]
- 23. Tsiogka A, Jansky G, Bauer JW, Koelblinger P. Fulminant type 1 diabetes after adjuvant ipilimumab therapy in cutaneous melanoma. Melanoma Res. 2017;27(5):524–525. [DOI] [PubMed] [Google Scholar]
- 24. Gauci ML, Laly P, Vidal-Trecan T, Baroudjian B, Gottlieb J, Madjlessi-Ezra N, Da Meda L, Madelaine-Chambrin I, Bagot M, Basset-Seguin N, Pages C, Mourah S, Boudou P, Lebbé C, Gautier JF. Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: A case report and literature review. Cancer Immunol Immunother. 2017;66(11):1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofmann L, Forschner A, Loquai C, Goldinger SM, Zimmer L, Ugurel S, Schmidgen MI, Gutzmer R, Utikal JS, Göppner D, Hassel JC, Meier F, Tietze JK, Thomas I, Weishaupt C, Leverkus M, Wahl R, Dietrich U, Garbe C, Kirchberger MC, Eigentler T, Berking C, Gesierich A, Krackhardt AM, Schadendorf D, Schuler G, Dummer R, Heinzerling LM. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:190–209. [DOI] [PubMed] [Google Scholar]
- 26. Li L, Masood A, Bari S, Yavuz S, Grosbach AB. Autoimmune diabetes and thyroiditis complicating treatment with nivolumab. Case Rep Oncol. 2017;10(1):230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lowe JR, Perry DJ, Salama AK, Mathews CE, Moss LG, Hanks BA. Genetic risk analysis of a patient with fulminant autoimmune type 1 diabetes mellitus secondary to combination ipilimumab and nivolumab immunotherapy. J Immunother Cancer. 2016;4:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hughes J, Vudattu N, Sznol M, Gettinger S, Kluger H, Lupsa B, Herold KC. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38(4):e55–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hansen E, Sahasrabudhe D, Sievert L.. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: Presentation, management and outcome. Cancer Immunol Immunother. 2016;65(6):765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gaudy C, Clevy C, Monestier S, Dubois N, Préau Y, Mallet S, Richard MA, Grob JJ, Valéro R, Béliard S. Anti-PD1 pembrolizumab can induce exceptional fulminant type 1 diabetes. Diabetes Care. 2015;38(11):e182–183. [DOI] [PubMed] [Google Scholar]
- 31. Kong SH, Lee SY, Yang YS, Kim TM, Kwak SH. Anti-programmed cell death 1 therapy triggering diabetic ketoacidosis and fulminant type 1 diabetes. Acta Diabetol. 2016;53(5):853–856. [DOI] [PubMed] [Google Scholar]
- 32. Alhusseini M, Samantray J. Autoimmune diabetes superimposed on type 2 diabetes in a patient initiated on immunotherapy for lung cancer. Diabetes Metab. 2017;43(1):86–88. [DOI] [PubMed] [Google Scholar]
- 33. Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;1(3):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis S, Lowy I, White DE, Rosenberg SA. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30(8):825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paepegaey AC, Lheure C, Ratour C, Lethielleux G, Clerc J, Bertherat J, Kramkimel N, Groussin L. Polyendocrinopathy resulting from pembrolizumab in a patient with a malignant melanoma. J Endocrine Soc .2017;1(6):646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]