Abstract
Context
Patients with diabetes mellitus are at increased risk for bone fragility fracture secondary to multiple mechanisms. Hyperglycemia can induce true dilutional hyponatremia. Hyponatremia is associated with gait instability, osteoporosis, and increased falls and bone fractures, and studies suggest that compromised bone quality with hyponatremia may be independent of plasma osmolality. We performed a case-control study of patients with diabetes mellitus matched by median glycated hemoglobin (HbA1c) to assess whether hyponatremia was associated with increased risk of osteoporosis and/or fragility fracture.
Design
Osteoporosis (n = 823) and fragility fracture (n = 840) cases from the MedStar Health database were matched on age of first HbA1c ≥6.5%, sex, race, median HbA1c over an interval from first HbA1c ≥6.5% to the end of the encounter window, diabetic encounter window length, and type 1 vs type 2 diabetes mellitus with controls without osteoporosis (n = 823) and without fragility fractures (n = 840), respectively. Clinical variables, including coefficient of glucose variation and hyponatremia (defined as serum [Na+] <135 mmol/dL within 30 days of the end of the diabetic window), were included in a multivariate analysis.
Results
Multivariate conditional logistic regression models demonstrated that hyponatremia within 30 days of the outcome measure was independently associated with osteoporosis and fragility fractures (osteoporosis OR 3.09; 95% CI, 1.37 to 6.98; fracture OR, 6.41; 95% CI, 2.44 to 16.82).
Conclusions
Our analyses support the hypothesis that hyponatremia is an additional risk factor for osteoporosis and fragility fracture among patients with diabetes mellitus.
Keywords: fractures, hyponatremia, osteoporosis, sodium, diabetes mellitus
Low bone mineral density is one predicator of fragility fracture, although most fractures occur in individuals without osteoporosis [1]. Increasingly, it is recognized that having diabetes mellitus is a risk factor for fragility fracture [2, 3] with or without osteoporosis. Incidence of osteoporosis among patients with diabetes mellitus is not uniform and is incompletely understood [4, 5]. The risk of hip fracture in type 1 diabetes mellitus (T1DM) is significantly higher than is observed in type 2 diabetes mellitus (T2DM), although both are increased relative to risk among the normoglycemic population; and bone mineral density (BMD) is increased in T2DM relative to controls, whereas it is decreased in T1DM [6]. Despite this heterogeneity of densiometrically identified bone disease among patients with diabetes mellitus, common factors that may contribute to poor bone quality among all diabetic patients include effects of hyperglycemia on osteoblasts [7], osteoblastic precursors [8–10], osteocytes [11], and osteoclasts [12]; compromise of collagen strength caused by accumulation of advanced glycation end products in bone [13]; oxidative stress from glucose variability [14, 15]; and development of microvascular disease that damages bone vasculature [16]. Furthermore, gait disturbances observed among hyperglycemic patients [17] may be caused by compromised peripheral and central nervous systems [18, 19], as well as sarcopenia [20, 21], that collectively contribute to increased risk for falls [22, 23] and fractures [24].
Similarly, there is evidence that hyponatremia is a risk factor for fragility fracture with or without osteoporosis. Experimental and epidemiological studies associate hyponatremia with increased risk of both osteoporosis [25–27] and gait instability [28, 29] leading to increased falls [30, 31] and fractures [32, 33]. Analogous to the release of calcium from bone to maintain calcium homeostasis during calcium deficiency, studies suggest that sodium can be released from rich and mobilizable reservoirs in bone to maintain sodium homeostasis during relative sodium deficiency [34, 35]. Bone quality could thus be compromised at the expense of attempting to maintain normal serum sodium concentrations [Na+], although the mechanisms affecting this pathophysiology are inadequately understood. Hyponatremia may compromise bone quality directly and independently of plasma osmolality by activating osteoclast-mediated resorption and loss of bone through direct low-sodium sensing mechanisms [36], and/or by promoting differentiation of human mesenchymal stromal cells toward the adipogenic phenotype at the expense of osteogenesis [37]. Other studies have suggested that arginine vasopressin (AVP)—the hormone responsible for renal water conservation that is inappropriately elevated in relation to hypo-osmolality with hyponatremia in the syndrome of inappropriate antidiuretic hormone (SIADH)—may be responsible for affecting the release of sodium from bone through interaction with Avpr1α and Avpr2 receptors expressed in osteoblasts and osteoclasts [38]. The mechanisms underlying gait instability associated with hyponatremia are also under active investigation, and they may be caused by central [28] and/or peripheral [39] nervous system dysfunction. Importantly, and in support of the hypothesis that hyponatremia is causative of pathology and not just a marker of disease severity, both the negative effects of hyponatremia on bone quality [40] and gait instability [29, 41] may be reversible.
Cognizant that hyperglycemia can cause a true hyponatremia through osmotic translocation of water from the intracellular to the extracellular space [42], and that hyponatremia-induced bone resorption is independent of osmolality [36], we conducted a case-control study to ascertain whether hyponatremia is an additional independent risk factor for osteoporosis among patients with diabetes mellitus matched by median glycated hemoglobin (HbA1c) as an indicator of glycemic control. Because hyponatremia may contribute to increased risk for fracture among patients with diabetes mellitus through mechanisms not captured by densitometry—for example, by causing gait instability and increased falls—we also assessed for risk for fragility fracture independent of osteoporosis.
1. Materials and Methods
We conducted a matched case-control study within the MedStar Health institutions’ pooled patient electronic health records database. Methods of obtaining de-identified patient data using the Explore application on the Explorys platform have been described elsewhere [26, 43] and is briefly reviewed in an online repository [44]. This technology utilizes a server behind the firewall of participating MedStar Health institutions in the Maryland, Virginia, and greater Washington, DC, area to capture information from patients’ inpatient and outpatient records, including admissions, discharges, transfers, surgical procedures, and historical records. There were >3.6 million unique patient records in the MedStar Health database available for query at the time of the study. The duration of the patient records under investigation extends from electronic health record implementation in the MedStar system in 2002 to the beginning of the current study on 10 October 2016; however, data entered retrospective of electronic record implementation dates back as far as 1987 and was also included in the study. The study was approved by the Georgetown–Howard Universities Center for Clinical and Translational Sciences Institutional Review Board. The requirement for informed consent was waived in view of the de-identified nature of the analyses.
Patients were identified as having diabetes mellitus who had at least one HbA1c laboratory value ≥6.5%. From this pool of patients with diabetes mellitus, two groups of patients were selected as case subjects. The first group had at least one diagnosis of osteoporosis as defined by ICD-9 code 733 for osteoporosis. The second group had at least one diagnosis of fragility fracture as defined by ICD-9 codes for fracture of upper (810 to 819) or lower limb (820 to 829), pelvis (808), or vertebral column (805). Cases without matched controls on specified criteria (described below) were excluded. Patient cases and controls with no serum [Na+] in the database, with a diagnosis of heart failure, or with a creatinine clearance ≤30 mg/dL were excluded from the analysis.
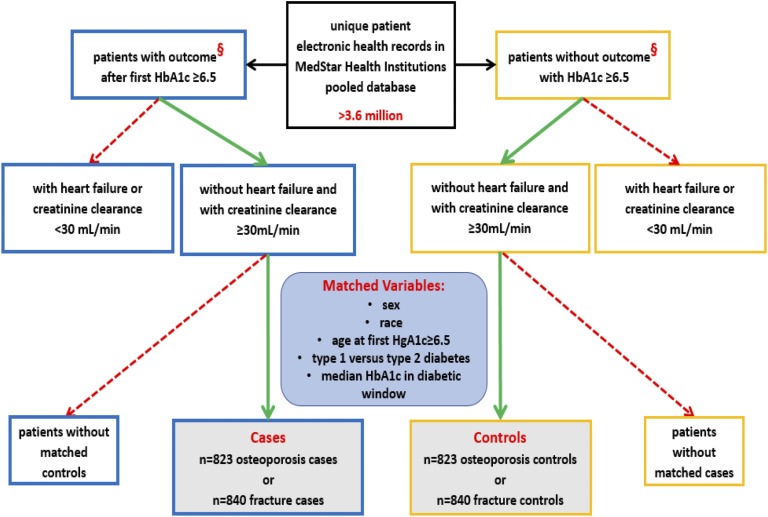
Two control groups from the patient pool with diabetes mellitus were selected. Each osteoporosis and fragility fracture case was matched separately on age at first HbA1c ≥6.5, duration of the diabetic encounter window, median HbA1c between first HbA1c ≥6.5 and the end of the encounter window, type 1 vs type 2 diabetes mellitus, sex, and race as specified by the categories listed in Table 1 and Table 2 with a control without patient record of osteoporosis or fragility fracture, respectively. Matching was performed with SAS 9.3 software using the Mayo Clinic gmatch general SAS macro. See Fig. 1 for a consort diagram depicting selection and matching processes for cases and controls for both the osteoporosis and fragility fracture studies.
Table 1.
Characteristics of Osteoporosis Study Subjects and Unadjusted ORs
| Osteoporosis (n = 823) | No Osteoporosis (n = 823) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Sex, no. (%) | ||||
| Female | 736 (89.4) | 736 (89.4) | ||
| Male | 87 (10.6) | 87 (10.6) | ||
| Race, no. (%) | ||||
| White | 336 (40.0) | 336 (40.0) | ||
| Black | 439 (52.3) | 439 (52.3) | ||
| Unknown/other | 65 (7.7) | 65 (7.7) | ||
| Diabetes mellitus classification, no. (%) | ||||
| Type 1 | 35 (4.3) | 35 (4.3) | ||
| Type 2 | 788 (95.7) | 788 (95.7) | ||
| Age at first HbA1c ≥6.5% in y, no. (%) | ||||
| ≥50 and <60 | 207 (25.2) | 203 (24.7) | ||
| ≥60 and <70 | 308 (37.4) | 312 (37.9) | ||
| ≥70 and <80 | 225 (27.3) | 233 (28.3) | ||
| ≥80 and <90 | 78 (9.5) | 71 (8.6) | ||
| ≥90 | 5 (0.6) | 4 (0.5) | ||
| Interval between first HbA1c ≥6.5% and last encounter in the database in mo, no. (%) | ||||
| <1 | 13 (1.6) | 7 (0.9) | ||
| ≥1 and <3 | 2 (0.2) | 5 (0.6) | ||
| ≥3 and <6 | 1 (0.1) | 5 (0.6) | ||
| ≥6 and <12 | 12 (1.5) | 3 (0.4) | ||
| ≥12 and <24 | 20 (2.4) | 15 (1.8) | ||
| ≥24 | 778 (94.2) | 788 (95.8) | ||
| Interval between first HbA1c ≥6.5% and outcome in mo, no. (%) | ||||
| <1 | 17 (2.1) | 14 (1.7) | ||
| ≥1 and <3 | 9 (1.1) | 12 (1.5) | ||
| ≥3 and <6 | 9 (1.1) | 9 (1.1) | ||
| ≥6 and <12 | 21 (2.6) | 21 (2.6) | ||
| ≥12 and <24 | 63 (7.7) | 64 (7.8) | ||
| ≥24 | 704 (85.5) | 703 (85.4) | ||
| Mean of median HbA1c (SD) | 7.11 (0.77) | 7.12 (0.77) | 0.74 (0.37–1.46) | 0.38 |
| Mean HbA1c (SD) | 7.22 (0.88) | 7.19 (0.79) | 1.39 (0.99–1.96) | 0.06 |
| BMI, mean (SD) | 30.35 (6.61) | 32.93 (7.65) | 0.95 (0.93–0.96) | <0.0001 |
| Coefficient of glucose variation, mean (SD) | 545 (677) | 615 (1071) | 1.00 (0.99–1.00) | 0.22 |
| Medication history, no. (%) | ||||
| Antiepileptic | 94 (11.4) | 97 (11.8) | 0.97 (0.71–1.31) | 0.8164 |
| Antipsychotic | 14 (1.7) | 10 (1.2) | 1.4 (0.622–3.152) | 0.4164 |
| Estrogen | 10 (1.2) | 15 (1.8) | 0.67 (0.30–1.48) | 0.3206 |
| Glucocorticoid | 149 (18.1) | 125 (15.2) | 1.26 (0.96–1.65) | 0.0984 |
| Insulin | 200 (24.3) | 215 (26.1) | 0.9 (0.71–1.14) | 0.3642 |
| Loop diuretic | 69 (8.4) | 77 (9.4) | 0.88 (0.62–1.25) | 0.4728 |
| Metformin | 339 (41) | 325 (40) | 1.08 (0.88–1.33) | 0.4600 |
| NSAID | 131 (15.9) | 169 (20.5) | 0.73 (0.57–0.94) | 0.0154 |
| Opiate | 130 (15.8) | 153 (18.6) | 0.82 (0.63–1.06) | 0.1325 |
| Progesterone | 6 (0.7) | 4 (0.5) | 1.5 (0.42–5.32) | 0.5299 |
| Proton pump inhibitor | 187 (22.7) | 229 (27.8) | 0.77 (0.62–0.96) | 0.02 |
| SSRI | 86 (10.5) | 93 (11.3) | 0.91 (0.66–1.25) | 0.5691 |
| Sulfonylurea | 173 (21) | 200 (24) | 0.80 (0.63–1.03) | 0.0900 |
| Thiazide | 271 (32.9) | 330 (40.1) | 0.72 (0.58–0.89) | 0.002 |
| Thiazolidinedione | 74 (9.0) | 79 (9.6) | 0.93 (0.66–1.30) | 0.6647 |
| Tricyclic antidepressant | 31 (3.8) | 26 (3.2) | 1.19 (0.71–2.01) | 0.5083 |
| Disease history, no. (%) | ||||
| Prior fracture | 110 (13) | 30 (4) | 4.07 (2.65–6.26) | <0.0001 |
| Liver | 56 (6.8) | 22 (2.7) | 2.7 (1.62–4.51) | 0.0001 |
| Pulmonary | 234 (28.4) | 160 (19.4) | 1.67 (1.32–2.12) | <0.0001 |
| Central nervous system | 191 (23.2) | 132 (16.0) | 1.58 (1.24–2.03) | 0.0003 |
| Malignancy | 25 (3.0) | 14 (1.7) | 1.79 (0.93–3.44) | 0.0824 |
| Acute kidney | 40 (4.9) | 14 (1.7) | 2.86 (1.56–5.25) | 0.0007 |
| Chronic kidney | 66 (8.0) | 37 (4.5) | 1.88 (1.23–2.87) | 0.0034 |
| Renal failure | 8 (1.0) | 4 (0.5) | 2 (0.60–6.64) | 0.2577 |
| Hypotension | 65 (7.9) | 33 (4.0) | 2.07 (1.34–3.20) | 0.0011 |
| Diabetic neuropathy | 77 (9.4) | 52 (6.3) | 1.54 (1.07–2.24) | 0.0218 |
| Diabetic ophthalmopathy | 43 (5.2) | 36 (4.4) | 1.23 (0.76–1.97) | 0.4002 |
| Diabetic peripheral circulatory | 9 (1.1) | 8 (1.0) | 1.14 (0.41–3.15) | 0.7964 |
| Hyponatremia | 49 (6.0) | 18 (2.2) | 3.21 (1.76–5.86) | 0.0001 |
| Behavioral history, no. (%) | ||||
| Tobacco use | 226 (27.5) | 164 (19.9) | 1.61 (1.26–2.06) | 0.0002 |
| Alcohol use | 99 (12.0) | 110 (13.4) | 0.87 (0.64–1.19) | 0.3864 |
Table 2.
Characteristics of Fracture Study Subjects and Unadjusted ORs
| Fracture (n = 840) | No Fracture (n = 840) | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Sex, no. (%) | ||||
| Female | 553 (65.8) | 553 (65.8) | ||
| Male | 284 (34.2) | 284 (34.2) | ||
| Race, no. (%) | ||||
| White | 284 (34.5) | 284 (34.5) | ||
| Black | 424 (51.5) | 424 (51.5) | ||
| Unknown/other | 115 (14.0) | 115 (14.0) | ||
| Diabetes mellitus classification, no. (%) | ||||
| Type 1 | 24 (2.9) | 24 (2.9) | ||
| Type 2 | 816 (97.1) | 816 (97.1) | ||
| Age at first HbA1c ≥6.5% in y, no. (%) | ||||
| ≥50 and <60 | 370 (44.1) | 362 (43.1) | ||
| ≥60 and <70 | 262 (31.2) | 270 (32.1) | ||
| ≥70 and <80 | 155 (18.5) | 157 (18.7) | ||
| ≥80 and <90 | 48 (5.7) | 47 (5.6) | ||
| ≥90 | 5 (0.6) | 4 (0.5) | ||
| Interval between first HbA1c ≥6.5% and last encounter in the database in mo, no. (%) | ||||
| <1 | 2 (0.2) | 5 (0.6) | ||
| ≥1 and <3 | 12 (1.4) | 8 (1.0) | ||
| ≥3 and <6 | 4 (0.5) | 4 (0.5) | ||
| ≥6 and <12 | 10 (1.2) | 9 (1.1) | ||
| ≥12 and <24 | 25 (3.0) | 14 (1.7) | ||
| ≥24 | 787 (93.7) | 800 (95.2) | ||
| Interval between first HbA1c ≥6.5% and outcome in mo, no. (%) | ||||
| <1 | 9 (1.1) | 10 (1.2) | ||
| ≥1 and <3 | 16 (1.9) | 14 (1.7) | ||
| ≥3 and <6 | 14 (1.7) | 14 (1.4) | ||
| ≥6 and <12 | 26 (3.1) | 27 (3.2) | ||
| ≥12 and <24 | 68 (8.1) | 69 (8.2) | ||
| ≥24 | 707 (84.2) | 706 (84.1) | ||
| Mean of median HbA1c (SD) | 7.32 (0.93) | 7.33 (0.93) | 0.73 (0.37–1.47) | 0.38 |
| Mean HbA1c (SD) | 7.42 (0.94) | 7.43 (1.00) | 0.95 (0.72–1.26) | 0.74 |
| BMI, mean (SD) | 31.40 (7.61) | 32.54 (7.45) | 0.97 (0.95–0.98) | <0.001 |
| Coefficient of glucose variation, mean (SD) | 439 (446) | 548 (648) | 0.99 (0.99–1.00) | <0.001 |
| Medication history, no. (%) | ||||
| Antiepileptic | 132 (15.7) | 102 (12.1) | 1.34 (1.02–1.76) | 0.0382 |
| Antipsychotic | 26 (3.1) | 15 (1.8) | 1.85 (0.94–3.63) | 0.075 |
| Estrogen | 14 (1.7) | 14 (1.7) | 1.00 (0.46–2.16) | 1 |
| Glucocorticoid | 160 (19.1) | 149 (17.7) | 1.10 (0.85–1.41) | 0.4806 |
| Insulin | 296 (35.2) | 294 (35.0) | 1.01 (0.82–1.25) | 0.9149 |
| Loop diuretic | 93 (11.1) | 90 (10.7) | 1.04 (0.76–1.41) | 0.8131 |
| Metformin | 354 (42) | 406 (48) | 0.77 (0.63–0.94) | 0.009 |
| NSAID | 184 (21.9) | 189 (22.5) | 0.97 (0.77–1.22) | 0.7663 |
| Opiate | 220 (26.2) | 176 (21.0) | 1.36 (1.08–1.72) | 0.0096 |
| Progesterone | 11 (1.3) | 9 (1.1) | 1.22 (0.51–2.95) | 0.6553 |
| Proton pump inhibitor | 230 (27.4) | 261 (31.1) | 0.83 (0.67–1.03) | 0.0937 |
| SSRI | 138 (16.4) | 108 (12.9) | 1.34 (1.02–1.77) | 0.0373 |
| Sulfonylurea | 221 (26) | 244 (29) | 0.86 (0.68–1.08) | 0.185 |
| Thiazide | 271 (32.3) | 313 (37.3) | 0.80 (0.65–0.98) | 0.0293 |
| Thiazolidinedione | 91 (9.6) | 102 (12.1) | 0.76 (0.55–1.04) | 0.0885 |
| Tricyclic antidepressant | 31 (3.7) | 34 (4.1) | 0.91 (0.55–1.50) | 0.701 |
| Disease history, no. (%) | ||||
| Osteoporosis | 105 (12.5) | 61 (7.3) | 1.92 (1.35–2.72) | 0.0003 |
| Liver | 66 (7.9) | 59 (7.0) | 1.13 (0.78–1.64) | 0.5105 |
| Pulmonary | 275 (32.7) | 179 (21.3) | 1.84 (1.46–2.30) | <0.0001 |
| Central nervous system | 238 (28.3) | 134 (16.0) | 2.07 (1.63–2.64) | <0.0001 |
| Malignancy | 27 (3.2) | 14 (1.7) | 2 (1.03–3.89) | 0.0413 |
| Acute kidney | 64 (7.6) | 31 (3.7) | 2.22 (1.41–3.50) | 0.0006 |
| Chronic kidney | 64 (7.6) | 36 (4.3) | 1.9 (1.23–2.94) | 0.0037 |
| Renal failure | 4 (0.5) | 4 (0.5) | 1 (0.25–4.00) | 1 |
| Hypotension | 62 (7.4) | 36 (4.3) | 1.84 (1.19–2.85) | 0.0063 |
| Diabetic neuropathy | 117 (13.9) | 70 (8.3) | 1.78 (1.30–2.45) | 0.0003 |
| Diabetic ophthalmopathy | 57 (6.8) | 39 (4.6) | 1.58 (1.01–2.48) | 0.046 |
| Diabetic peripheral circulatory | 13 (1.6) | 7 (0.8) | 1.86 (0.74–4.66) | 0.1867 |
| Hyponatremia | 58 (6.9) | 11 (1.3) | 8.83 (3.80–20.55) | <0.0001 |
| Behavioral history, no. (%) | ||||
| Tobacco use | 321 (38.2) | 224 (26.7) | 1.78 (1.43–2.22) | <0.0001 |
| Alcohol use | 167 (19.9) | 132 (15.7) | 1.39 (1.06–1.82) | 0.0175 |
Figure 1.
Consort diagram for osteoporosis and fragility fracture studies. The MedStar Health institutions’ pooled electronic records database was used to select patient records of interest. After applying predefined exclusion criteria, Mayo Clinic gmatch general SAS macro was used to match cases with osteoporosis with controls without osteoporosis and cases with fragility fracture with controls without fragility fracture, respectively. Outcome (§) was defined as diagnosis of osteoporosis or fragility fracture for the osteoporosis study or fragility fracture study, respectively.
A diabetic encounter window was defined for each osteoporosis and fracture case as the time between the date of the first HbA1c value ≥6.5% and the date of the first osteoporosis diagnosis or first fragility fracture diagnosis, respectively. Diabetic encounter windows for controls were defined by the encounter window of the matched cases. A hypothetical “time-to-event” date for each control was calculated by adding the duration of the respective case’s diabetic encounter window to the control’s first encounter date with an HbA1c value ≥6.5%. That is, the control diabetic encounter window was defined as the time between the date of the control’s first encounter with an HbA1c value ≥6.5% and a generated date representing the time to a hypothetical event (namely, an osteoporosis or fragility fracture diagnosis).
Case and control exposures to the clinical variables of interest were defined by the documentation of at least one disease diagnosis, drug prescription, or behavioral diagnostic code within the diabetic encounter window. Diagnostic codes for disease categories included in the analyses are provided in an online repository [44]. Case or control exposure to hyponatremia was defined as having at least one serum [Na+] measurement <135 mmol/L within 30 days prior to the end of the diabetic encounter window. Coefficient of glucose variation (percentage) for each case and control was calculated as the SD of glucose values within the diabetic window divided by mean glucose values within the diabetic window, multiplied by 100.
Descriptive statistics such as means and SDs were used for continuous variables and frequencies and percentages for categorical variables. Conditional logistic regression for matched case-control design was used to estimate the change in the risk of osteoporosis and fractures in the form of the OR, which measures the change in the odds of experiencing the outcome (osteoporosis or fragility fracture) given the categories of an exposure variable. Statistical significance was determined with a P value threshold of 0.05. Analyses were performed using SAS version 9.3 (SAS Institute) and Stata version 11 (StataCorp).
2. Results
Final analyses of both the osteoporosis and fragility fracture cases with matched controls included 3101 unique patient records. Records of 225 patients that were used as a case or control in the osteoporosis or fracture analysis were used as a case or control in the other respective analysis.
A total of 823 osteoporosis cases were matched to 823 controls without osteoporosis. Of the 20,160 potential osteoporosis cases, 2500 were excluded because [Na+] values were unavailable; 6800 were excluded because the patient had a diagnosis of heart failure; and 3870 were excluded because of a creatinine clearance ≤30 mg/dL. Of the remaining 6990 osteoporosis cases, 5209 were excluded from the analysis because controls matching on all parameters were not obtained with the matching algorithm. Table 1 shows the characteristics of patients with and without osteoporosis. Osteoporosis cases and controls were predominantly female (89.4%) with T2DM (95.7%) and averaged 66.67 years of age (SD of 9.24) at first encounter with HbA1c ≥6.5. Of the serum [Na+] values for the osteoporosis cases and controls, 36.1% of the values were documented as acquired in the inpatient setting, 47.1% in the outpatient setting, 5.3% in the emergency room, and 11.5% unknown. Median HbA1c between cases (7.11%) and controls (7.12%) were not statistically different. Compared with patients without osteoporosis, patients with osteoporosis had a significantly lower body mass index (BMI; 32.93 vs 30.35). Unadjusted ORs indicate that being prescribed a nonsteroidal anti-inflammatory drug (NSAID), thiazide, or proton pump inhibitor was associated with a decreased risk of osteoporosis. Diagnosis of prior fracture, liver disease, pulmonary disease, central nervous system disease, acute or chronic kidney disease, hypotension, or diabetic neuropathy was associated with a higher risk of osteoporosis. Tobacco use or hyponatremia were also associated with increased risk of osteoporosis.
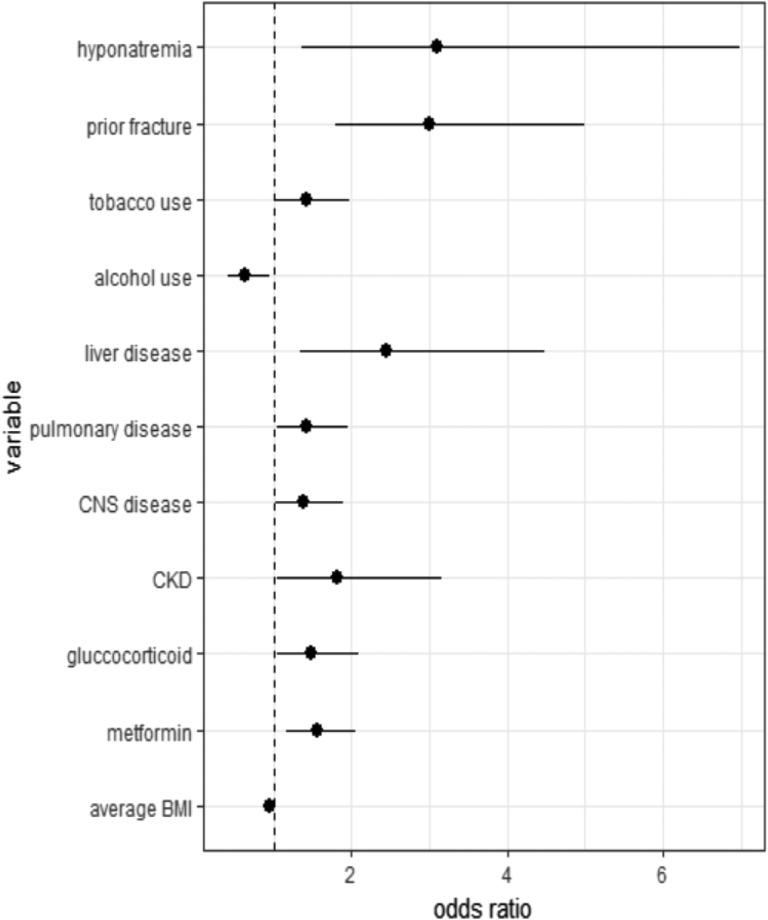
The results of conditional multivariate logistic regression analysis for the osteoporosis study are presented in Table 3. Figure 2 illustrates all statistically significant results. Hyponatremia, prior fracture, tobacco use, liver disease, pulmonary disease, central nervous system disease, chronic kidney disease, glucocorticoid use, or metformin use was associated with increased risk of osteoporosis. Higher average BMI or alcohol use was associated with a decreased risk of osteoporosis. The OR for osteoporosis associated with coefficient of glucose variation was not statistically significant. The OR associating hyponatremia with osteoporosis (OR, 3.09; CI, 1.37 to 6.98) was greater than any other variable analyzed.
Table 3.
Fully Adjusted ORs for Osteoporosis Study
| OR (95% CI) | P Value | |
|---|---|---|
| Antiepileptic | 0.84 (0.56–1.25) | 0.381 |
| Antipsychotic | 1.34 (0.45–3.97) | 0.602 |
| Estrogen | 0.83 (0.33–2.12) | 0.698 |
| Glucocorticoid | 1.49 (1.05–2.10) | 0.024 |
| Insulin | 0.73 (0.53–1.02) | 0.062 |
| Loop diuretic | 1.10 (0.69–1.77) | 0.692 |
| Metformin | 1.56 (1.17–2.06) | 0.002 |
| NSAID | 0.77 (0.55–1.07) | 0.124 |
| Opiate | 0.72 (0.49–1.04) | 0.076 |
| Progesterone | 1.98 (0.48–8.26) | 0.348 |
| Proton pump inhibitor | 0.78 (0.58–1.06) | 0.115 |
| SSRI | 0.92 (0.61–1.40) | 0.704 |
| Sulfonylurea | 0.84 (0.61–1.17) | 0.306 |
| Thiazide | 0.84 (0.63–1.11) | 0.210 |
| Thiazolidinedione | 1.08 (0.70–1.66) | 0.728 |
| Tricyclic antidepressant | 1.53 (0.80–2.91) | 0.204 |
| Prior fracture | 3.00 (1.80–5.00) | 0.000 |
| Liver | 2.45 (1.34–4.48) | 0.003 |
| Pulmonary | 1.42 (1.04–1.95) | 0.030 |
| Central nervous system | 1.39 (1.02–1.89) | 0.037 |
| Malignancy | 1.32 (0.57–3.05) | 0.521 |
| Acute kidney | 1.09 (0.50–2.39) | 0.825 |
| Chronic kidney | 1.82 (1.05–3.16) | 0.032 |
| Renal failure | 2.92 (0.62–13.73) | 0.174 |
| Hypotension | 1.25 (0.72–2.17) | 0.432 |
| Diabetic neuropathy | 1.54 (0.95–2.49) | 0.081 |
| Diabetic ophthalmopathy | 0.99 (0.55–1.78) | 0.978 |
| Diabetic peripheral circulatory | 1.15 (0.31–4.32) | 0.834 |
| Tobacco use | 1.42 (1.03–1.97) | 0.034 |
| Alcohol use | 0.63 (0.42–0.95) | 0.025 |
| BMI, average | 0.95 (0.93–0.97) | 0.000 |
| Coefficient of glucose variation | 1.00 (0.99–1.00) | 0.870 |
| Hyponatremia | 3.09 (1.37–6.98) | 0.007 |
Figure 2.
Fully adjusted ORs for variables in the osteoporosis study that reached statistical significance of P < 0.05 in the multivariate conditional logistic model. Osteoporosis study ORs included the following: hyponatremia 3.08 (1.37 to 6.98); prior fracture 3.00 (1.80 to 5.00); tobacco use 1.42 (1.03 to 1.97); alcohol use 0.63 (0.42 to 0.94); liver disease 2.45 (1.34 to 4.48); pulmonary disease 1.42 (1.04 to 1.95); central nervous system (CNS) disease 1.39 (1.02 to 1.89); chronic kidney disease (CKD) 1.82 (1.05 to 3.16); glucocorticoid 1.49 (1.05 to 2.10); metformin 1.56 (1.17 to 2.06); average BMI 0.95 (0.93 to 0.97).
A total number of 840 fragility fracture cases were matched to 840 controls without fragility fracture. Of the 20,810 potential fragility fracture cases, 6300 were excluded because [Na+] values were unavailable; 5570 were excluded because the patient had a diagnosis of heart failure; and 3520 were excluded because of a creatinine clearance ≤30 mg/dL. Of the remaining 5420 fragility fracture cases, 4514 were excluded from the analysis because controls matching on all parameters were not obtained with the matching algorithm. Table 2 shows the characteristics of patients with and without fragility fracture. Fragility fracture cases and controls were predominantly female (65.8%) with T2DM (97.1%) and averaged 62.76 years of age (SD of 9.76) at first encounter with HbA1c ≥6.5. Of the serum [Na+] values for the fragility fracture cases and controls, 46.4% of the values were documented as acquired in the inpatient setting, 37.3% in the outpatient setting, 7.5% in the emergency room, and 8.8% unknown. Median HbA1cs between cases (7.32%) and controls (7.33%) were not statistically different. Compared with patients without fragility fracture, patients with fragility fracture had a significantly lower BMI (32.54 vs 31.40). Unadjusted ORs indicate that being prescribed an opiate, an antiepileptic, or a selective serotonin reuptake inhibitor (SSRI); using tobacco or alcohol; or having a diagnosis of osteoporosis, pulmonary disease, central nervous system disease, malignancy, acute or chronic kidney disease, hypotension, diabetic neuropathy, or diabetic ophthalmopathy was associated with an increased risk of fragility fracture. Hyponatremia was associated with an increased risk of fragility fracture. Thiazide use was associated with a decreased risk of fragility fracture.
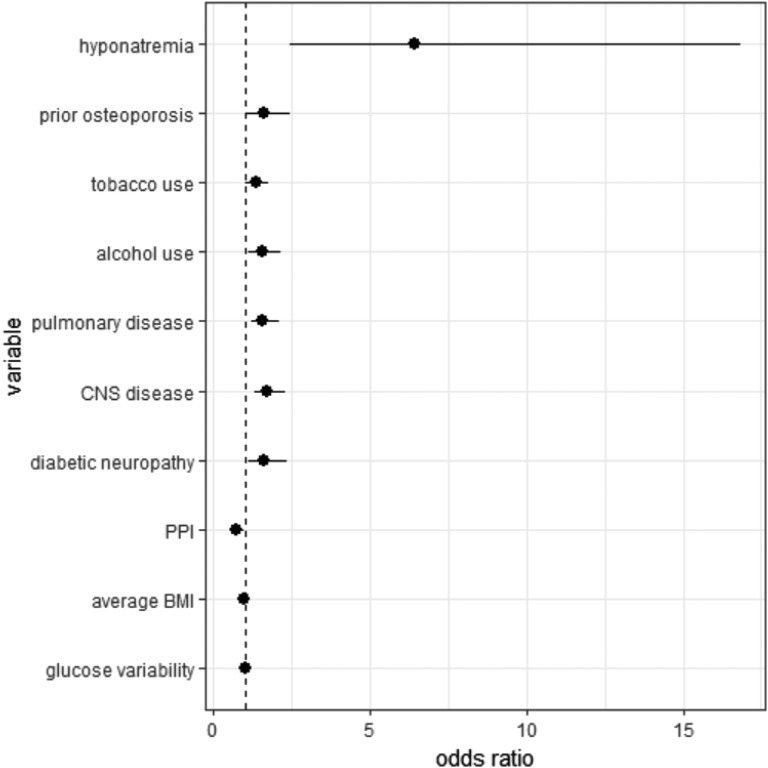
The results of the conditional multivariate logistic regression models for fragility fracture are presented in Table 4. Figure 3 illustrates all statistically significant results. Hyponatremia, prior osteoporosis, tobacco or alcohol use, pulmonary disease, central nervous system disease, or diabetic neuropathy was associated with increased risk of fragility fracture. Higher BMI or proton pump inhibitor use was associated with decreased risk of fragility fracture. The OR for fracture associated with coefficient for glucose variation was 1.01 with a 95% CI of 1.00 to 1.02. The OR associating hyponatremia with fragility fracture (OR, 6.41; 95% CI, 2.44 to 16.82) was greater than any other variable analyzed.
Table 4.
Fully Adjusted ORs for Fracture Study
| OR (95% CI) | P Value | |
|---|---|---|
| Antiepileptic | 1.10 (0.77–1.56) | 0.594 |
| Antipsychotic | 1.44 (0.65–3.17) | 0.371 |
| Estrogen | 1.11 (0.44 –2.77) | 0.829 |
| Glucocorticoid | 0.86 (0.62–1.19) | 0.359 |
| Insulin | 0.82 (0.61–1.10) | 0.192 |
| Loop diuretic | 1.04 (0.70–1.52) | 0.861 |
| Metformin | 0.79 (0.61–1.02) | 0.074 |
| NSAID | 1.01 (0.76–1.35) | 0.935 |
| Opiate | 1.27 (0.92–1.76) | 0.149 |
| Progesterone | 1.28 (0.42–3.93) | 0.665 |
| Proton pump inhibitor | 0.70 (0.53–0.94) | 0.016 |
| SSRI | 1.17 (0.83–1.65) | 0.359 |
| Sulfonylurea | 0.87 (0.65–1.16) | 0.329 |
| Thiazide | 0.85 (0.65–1.09) | 0.202 |
| Thiazolidinedione | 0.77 (0.52–1.14) | 0.195 |
| Tricyclic antidepressant | 0.76 (0.40–1.42) | 0.390 |
| Osteoporosis | 1.61 (1.07–2.42) | 0.022 |
| Liver | 0.79 (0.51–1.24) | 0.306 |
| Pulmonary | 1.57 (1.19–2.07) | 0.001 |
| Central nervous system | 1.71 (1.28–2.27) | 0.000 |
| Malignancy | 1.17 (0.54–2.57) | 0.689 |
| Acute kidney | 1.15 (0.64–2.07) | 0.638 |
| Chronic kidney | 1.38 (0.79–2.42) | 0.256 |
| Renal Failure | 0.45 (0.07–2.73) | 0.388 |
| Hypotension | 1.16 (0.69–1.93) | 0.573 |
| Diabetic neuropathy | 1.61 (1.10–2.36) | 0.014 |
| Diabetic ophthalmopathy | 1.30 (0.77–2.19) | 0.324 |
| Diabetic peripheral circulatory | 1.67 (0.54–5.09) | 0.367 |
| Tobacco use | 1.35 (1.04–1.77) | 0.026 |
| Alcohol use | 1.53 (1.09–2.13) | 0.012 |
| BMI, average | 0.98 (0.96–0.99) | 0.009 |
| Coefficient of glucose variation | 1.01 (1.00–1.02) | 0.015 |
| Hyponatremia | 6.41 (2.44–16.82) | 0.000 |
Figure 3.
Fully adjusted ORs of variables in the fragility fracture study that reached statistical significance of P < 0.05 in the multivariate conditional logistic model. Fragility fracture study ORs included the following: hyponatremia 6.41 (2.44 to 16.82); prior osteoporosis 1.61 (1.07 to 2.42); tobacco use 1.35 (1.04 to 1.77); alcohol use 1.53 (1.10 to 2.13); pulmonary disease 1.57 (1.19 to 2.07); central nervous system (CNS) disease 1.71 (1.28 to 2.27); diabetic neuropathy 1.61 (1.10 to 2.36); proton pump inhibitor (PPI) 0.70 (0.53 to 0.94); average BMI 0.98 (0.96 to 0.99); glucose variability (coefficient of glucose variation) 1.00 (1.00 to 1.02).
3. Discussion
Our data suggest that, independent of glycemic indices, hyponatremia among persons with diabetes mellitus is associated with increased risk of osteoporosis and fragility fracture. Interestingly, the OR of fracture with hyponatremia (OR, 6.41; 95% CI, 2.44 to 16.82) was double in magnitude to the OR of osteoporosis with hyponatremia (OR, 3.09; 95% CI, 1.37 to 6.98). Furthermore, hyponatremia was a greater risk factor for fracture than osteoporosis in our multivariate analysis, suggesting that hyponatremia may incur risk for fracture both by compromising bone quality as measured by densitometry (osteoporosis) and by a second, additive mechanism. This second mechanism likely is gait instability caused by hyponatremia [28, 29] that contributes to increased mechanical falls [30, 31] and fractures [32, 45].
We recognize that the rationale linking pathology associated with hyponatremia to skeletal and nervous system physiology in diabetes mellitus is circumstantial, as investigations to date have not been undertaken with simultaneous consideration of both sodium and glucose levels. Inarguably, hyperglycemia is directly toxic to bone, although there is recent evidence that suggests that there are additional mechanisms independent of absolute glucose level that cause increased bone resorption markers in patients with hyperglycemia [46–48]. There is also evidence that rapid changes in glucose level (with concomitant rapid changes in absolute serum sodium) can cause toxicity to the nervous system that is similar in character to the pathology seen among patients with rapidly corrected serum sodium levels in hypo-osmolar hyponatremia [49, 50]. As serum [Na+] levels are dependent on glucose levels, the principal challenge of the current study was to demonstrate that hyponatremia among patients with diabetes mellitus could be associated with osteoporosis and fragility fracture independent of the degree of hyperglycemia.
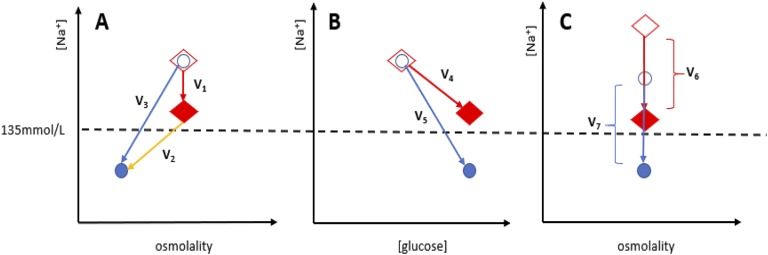
The possibility that patients with diabetes mellitus can have comparable glycemic control but disparate serum [Na+] levels made our case-control study possible. There are three potential mechanisms by which patients with diabetes mellitus could have comparable glycemic control but lower serum [Na+] levels than do their matched controls. Figure 4 illustrates these potential mechanisms. First, as depicted in Fig. 4A, patients could be both hyperglycemic and have an additional disorder of water homeostasis (such as nonosmotic secretion of AVP caused by a medication) that induces a hypo-osmolar hyponatremia. Second, as depicted in Fig. 4B, some patients could have physiology that depresses serum [Na+] levels lower than would be expected for a given degree of hyperglycemia. Such a phenomenon has been seen in at least one epidemiological study in diabetic patients [51, 52]. Third, as depicted in Fig. 4C, some patients could have low-normal baseline serum [Na+] levels when euglycemic. With the same delta changes in serum glucose and subsequently the same delta changes in serum [Na+] caused by translocation of fluid from the intracellular space to the extracellular space, patients with lower baseline serum [Na+] levels when euglycemic would also have lower serum [Na+] levels when hyperglycemic compared with patients with high baseline serum [Na+] levels. Although our study design limits our ability to distinguish which of these mechanisms were at play in our study, experimental data do suggest that the third mechanism of isotonic hyponatremia could contribute to increased risk of osteoporosis and fracture, as discussed below.
Figure 4.
Potential mechanisms by which cases with diabetes mellitus could have comparable glycemic control but lower serum [Na+] levels than do their matched controls. For each model, diamonds represent controls, and circles represent cases. Shapes without fill represent cases or controls before exposure to the forces causing [Na+] depression. Solid-filled shapes represent cases or controls after exposure to the forces depressing serum [Na+] levels. (A) Patients could be both hyperglycemic and have an additional disorder of water homeostasis (such as nonosmotic secretion of AVP caused by a medication) that induces a hypo-osmolar hyponatremia. V1 represents the iso-osmolar component of [Na+] depression caused by the translocation of fluid from the intracellular space to the extracellular space. V2 represents the component of [Na+] depression caused by nonosmotic forces, such as exposure to inappropriately high levels of AVP in SIADH, which cause a hypo-osmolar hyponatremia. V3 represents the combined forces (V1 + V2) that depress the [Na+] level of the case lower than the sodium level of the control. (B) Some patients could have physiology that depresses serum [Na+] levels lower than would be expected for a given degree of hyperglycemia. Although V4 and V5 represent forces that cause an equal change in serum glucose concentration [glucose], the case serum [Na+] is depressed by a force with a greater slope than the control. (C) Some patients could have low-normal baseline serum [Na+] levels when euglycemic. With the same delta changes in serum glucose and subsequently the same delta changes in serum [Na+] caused by translocation of fluid from the intracellular space to the extracellular space (V6= V7), patients with lower baseline serum [Na+] levels when euglycemic (a lower homeostatic [Na+] level) would also have lower serum [Na+] levels when hyperglycemic compared with patients with high baseline serum [Na+] levels.
Frequent hyperglycemia is thought to contribute to increased risk of bone fragility fractures through multiple mechanisms [5]. We introduce the provocative hypothesis that the osmotic property of glucose, which induces dilutional hyponatremia by affecting translocation of water from the intracellular to the extracellular space, may also contribute to the pathophysiology underpinning increased fracture risk among patients with diabetes mellitus. We speculate that hyperglycemia-induced hyponatremia stimulates biologic processes that facilitate release of rich sodium reservoirs from bone to maintain sodium and water homeostasis at the expense of bone quality [35]. Models suggest that impaired nerve conduction [39] and gait stability [28] may be directly related to lowering of [Na+]. Furthermore, hyperglycemia-induced hyponatremia could compound fracture risk by precipitating or worsening gait instability, leading to increased falls and fracture risk [53].
Previous studies in experimental animals have indicated that sustained chronic hyponatremia is associated with marked bone loss in association with increased osteoclast numbers in bone [25]. Subsequent in vitro studies confirmed the effect of low extracellular [Na+] to stimulate both osteoclastogenesis and osteoclast resorbing activity. Although in vivo studies cannot differentiate between the effects caused by hyponatremia from those caused by hypo-osmolality, in vitro studies in which the osmolality of low [Na+] culture medium was corrected to normo-osmolality by addition of mannitol clearly demonstrated that the osteoclast activation was driven by low extracellular [Na+] and not by low osmolality [36].
The well-known effects of hyperglycemia to lower serum [Na+] are often disregarded as being of little or unclear clinical significance, because the resulting hyponatremia is isotonic rather than hypotonic. For example, two epidemiological studies addressing the risk of fracture with hyponatremia were designed to exclude hyperglycemia-induced hyponatremia as a potential confounder [26, 54]. However, to dismiss hyperglycemia-induced hyponatremia ignores the potential effects of low [Na+] on cells independent of changes in osmolality. The finding that osteoclastogenesis and osteoclast activity are driven predominantly by low extracellular [Na+] rather than low osmolality raised the possibility that hyperglycemia-induced hyponatremia may be of pathological significance, rather than just a manifestation of osmotic homeostasis. The current study was crafted to test these hypotheses formulated in response to data gleaned from experimental studies.
Our study was limited by its retrospective character and missing data. We used coefficient of glucose variation as a parameter of glucose variability. Coefficient of glucose variation was designed to be used for continuous glucose monitoring data when many interval glucose values are available. In contrast, we had limited glucose values, sometimes measured at long intervals from each other in the patient record. BMI data were also not available for all cases and controls. Furthermore, we had no direct measure of serum osmolality, and thus we could not discern when serum sodium values were depressed secondary to translocation of water (an isotonic hyponatremia) or secondary to other circumstances, such as diuretic use or SIADH (hypo-osmolar hyponatremia). Additionally, by study design, we were unable to address how hyponatremia severity or duration modulated risk for osteoporosis or fragility fracture. That is, severe serum sodium depression among cases compared with controls matched by glycemic indices would likely not be secondary to translocational hyponatremia, which usually induces more moderate serum sodium depression. Furthermore, most patients with diabetes do not have persistently decreased serum sodium levels. Rather, their sodium levels fluctuate with their degree of glycemic control. Consequently, “duration” of hyponatremia cannot be defined by persistently low sodium levels.
One strength of the current study is that we controlled for many parameters of hyperglycemia. That is, our cases and controls were matched on a categorization of hyperglycemia etiology (i.e., T1DM vs T2DM), a parameter of hyperglycemia duration (i.e., diabetic window), and a measure of hyperglycemia severity (i.e., median HbA1c). Oxidative stress caused by glucose variability is also proposed as a mechanism of hyperglycemia toxicity, and in a recent study, bone cortical area was inversely associated with glycemic variability as measured by coefficient of glucose variation in patients with T1DM [55]. Recognizing that our matching did not account for glucose variability or glucose excursions, we included coefficient of glucose variation as a variable in our multivariate analysis. Coefficient of glucose variation was not strongly associated with osteoporosis or fracture in the current study, nor did the inclusion of coefficient of glucose variation in the multivariate analysis significantly affect the OR for osteoporosis or fragility fraction with hyponatremia. We do not suggest that our analysis refutes the putative role that glycemic variability contributes to fracture risk; rather, our results suggest that cases and controls were well matched on glycemic parameters and had similar glucose variability.
Another strength of this study is that we control for multiple confounders by including clinical factors associated with risk for osteoporosis and fragility fracture in the multivariate analysis. For example, thiazide diuretic was included in our analysis because it may decrease fracture risk by creating a positive calcium balance or increase fracture risk by causing postural hypotension or hyponatremia. Although hyponatremia was a significant risk factor for osteoporosis and fragility fracture, neither thiazide diuretic nor hypotension was statistically significant for either outcome in our analysis, which suggests that hypotension caused by thiazide diuretic was not a driver for increased risk for fracture in our population.
Although our study is primarily hypothesis generating, there are potential clinical implications that derive from the results. First, they reaffirm that hyperglycemia-induced hyponatremia is a true hyponatremia caused by translocation of water from the intracellular to the extracellular fluid, not an artifactual pseudohyponatremia, which is a frequent misinterpretation [54]. Second, they challenge the prevailing concept that hyperglycemia-induced hyponatremia is of no clinical significance, because hyponatremia was associated with increased risk of osteoporosis and fractures independent of well-matched indices of glycemic control. When matched for glycemic indices, our data suggest that hyponatremia itself is the main driver of osteoporosis and fracture, and the overall effect of glucose on risk for osteoporosis or fragility fracture needs further investigation with controlled trials.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health/National Institute on Aging Grant R01 AG053506 (to J.G.V.) and by National Center for Advancing Translational Sciences Grants UL1-TR001409, KL2-TR001432, and TL1-TR001431 (to J.G.V.).
Disclosure Summary: J.G.V. has received research grant support and noncontinuing medical education–related fees from Otsuka Pharmaceutical, as well as noncontinuing medical education–related fees from Corcept Therapeutics and Ferring Pharmaceuticals. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AVP
arginine vasopressin
- BMD
bone mineral density
- BMI
body mass index
- NSAID
nonsteroidal anti-inflammatory drug
- SIADH
syndrome of inappropriate antidiuretic hormone
- SSRI
selective serotonin reuptake inhibitor
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
Contributor Information
Rachel L Usala, Email: rlu4@georgetown.edu.
Joseph G Verbalis, Email: verbalis@georgetown.edu.
References and Notes
- 1. Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, Burckhardt P, Cooper C, Christiansen C, Cummings S, Eisman JA, Fujiwara S, Glüer C, Goltzman D, Hans D, Krieg MA, La Croix A, McCloskey E, Mellstrom D, Melton LJ III, Pols H, Reeve J, Sanders K, Schott AM, Silman A, Torgerson D, van Staa T, Watts NB, Yoshimura N. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–1046. [DOI] [PubMed] [Google Scholar]
- 2. Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care. 2015;38(10):1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int. 2005;16(12):1713–1720. [DOI] [PubMed] [Google Scholar]
- 4. Sellmeyer DE, Civitelli R, Hofbauer LC, Khosla S, Lecka-Czernik B, Schwartz AV. Skeletal metabolism, fracture risk, and fracture outcomes in type 1 and type 2 diabetes. Diabetes. 2016;65(7):1757–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Napoli N, Chandran M, Pierroz DD, Abrahamsen B, Schwartz AV, Ferrari SL; IOF Bone and Diabetes Working Group . Mechanisms of diabetes mellitus-induced bone fragility. Nat Rev Endocrinol. 2017;13(4):208–219. [DOI] [PubMed] [Google Scholar]
- 6. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int. 2007;18(4):427–444. [DOI] [PubMed] [Google Scholar]
- 7. García-Hernández A, Arzate H, Gil-Chavarría I, Rojo R, Moreno-Fierros L. High glucose concentrations alter the biomineralization process in human osteoblastic cells. Bone. 2012;50(1):276–288. [DOI] [PubMed] [Google Scholar]
- 8. Rinker TE, Hammoudi TM, Kemp ML, Lu H, Temenoff JS. Interactions between mesenchymal stem cells, adipocytes, and osteoblasts in a 3D tri-culture model of hyperglycemic conditions in the bone marrow microenvironment. Integr Biol. 2014;6(3):324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao YF, Zeng DL, Xia LG, Zhang SM, Xu LY, Jiang XQ, Zhang FQ. Osteogenic potential of bone marrow stromal cells derived from streptozotocin-induced diabetic rats. Int J Mol Med. 2013;31(3):614–620. [DOI] [PubMed] [Google Scholar]
- 10. Ehnert S, Freude T, Ihle C, Mayer L, Braun B, Graeser J, Flesch I, Stöckle U, Nussler AK, Pscherer S. Factors circulating in the blood of type 2 diabetes mellitus patients affect osteoblast maturation—description of a novel in vitro model. Exp Cell Res. 2015;332(2):247–258. [DOI] [PubMed] [Google Scholar]
- 11. Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461(2):193–199. [DOI] [PubMed] [Google Scholar]
- 12. Catalfamo DL, Britten TM, Storch DL, Calderon NL, Sorenson HL, Wallet SM. Hyperglycemia induced and intrinsic alterations in type 2 diabetes-derived osteoclast function. Oral Dis. 2013;19(3):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto M, Sugimoto T. Advanced glycation end products, diabetes, and bone strength. Curr Osteoporos Rep. 2016;14(6):320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ohara M, Nagaike H, Goto S, Fukase A, Tanabe Y, Tomoyasu M, Yamamoto T, Hayashi T, Fukui T, Hirano T. Improvements of ambient hyperglycemia and glycemic variability are associated with reduction in oxidative stress for patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;139:253–261. [DOI] [PubMed] [Google Scholar]
- 15. Bacevic M, Brkovic B, Albert A, Rompen E, Radermecker RP, Lambert F. Does oxidative stress play a role in altered characteristics of diabetic bone? A systematic review. Calcif Tissue Int. 2017;101(6):553–563. [DOI] [PubMed] [Google Scholar]
- 16. Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res. 2015;30(12):2188–2199. [DOI] [PubMed] [Google Scholar]
- 17. Maksimovic A, Hanewinckel R, Verlinden VJ, Ligthart S, Hofman A, Franco OH, van Doorn PA, Tiemeier H, Dehghan A, Ikram MA. Gait characteristics in older adults with diabetes and impaired fasting glucose: the Rotterdam Study. J Diabetes Complications. 2016;30(1):61–66. [DOI] [PubMed] [Google Scholar]
- 18. D’Silva LJ, Lin J, Staecker H, Whitney SL, Kluding PM. Impact of diabetic complications on balance and falls: contribution of the vestibular system. Phys Ther. 2016;96(3):400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brach JS, Talkowski JB, Strotmeyer ES, Newman AB. Diabetes mellitus and gait dysfunction: possible explanatory factors. Phys Ther. 2008;88(11):1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, de Rekeneire N, Harris TB, Schwartz AV, Tylavsky FA, Cho YW, Newman AB; Health, Aging, and Body Composition Study . Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6):1507–1512. [DOI] [PubMed] [Google Scholar]
- 21. Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, Zuliani G, Ferrucci L. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35(8):1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwartz AV, Hillier TA, Sellmeyer DE, Resnick HE, Gregg E, Ensrud KE, Schreiner PJ, Margolis KL, Cauley JA, Nevitt MC, Black DM, Cummings SR. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25(10):1749–1754. [DOI] [PubMed] [Google Scholar]
- 23. Vinik AI, Vinik EJ, Colberg SR, Morrison S. Falls risk in older adults with type 2 diabetes. Clin Geriatr Med. 2015;31(1):89–99, viii. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, Jamal SA, Black DM, Cummings SR; Study of Osteoporotic Features Research Group . Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32–38. [DOI] [PubMed] [Google Scholar]
- 25. Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, Resnick HE. Hyponatremia-induced osteoporosis. J Bone Miner Res. 2010;25(3):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Usala RL, Fernandez SJ, Mete M, Cowen L, Shara NM, Barsony J, Verbalis JG. Hyponatremia is associated with increased osteoporosis and bone fractures in a large US health system population. J Clin Endocrinol Metab. 2015;100(8):3021–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Upala S, Sanguankeo A. Association between hyponatremia, osteoporosis, and fracture: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(4):1880–1886. [DOI] [PubMed] [Google Scholar]
- 28. Fujisawa H, Sugimura Y, Takagi H, Mizoguchi H, Takeuchi H, Izumida H, Nakashima K, Ochiai H, Takeuchi S, Kiyota A, Fukumoto K, Iwama S, Takagishi Y, Hayashi Y, Arima H, Komatsu Y, Murata Y, Oiso Y. Chronic hyponatremia causes neurologic and psychologic impairments. J Am Soc Nephrol. 2016;27(3):766–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1–71.e8. [DOI] [PubMed] [Google Scholar]
- 30. Rittenhouse KJ, To T, Rogers A, Wu D, Horst M, Edavettal M, Miller JA, Rogers FB. Hyponatremia as a fall predictor in a geriatric trauma population. Injury. 2015;46(1):119–123. [DOI] [PubMed] [Google Scholar]
- 31. Tachi T, Yokoi T, Goto C, Umeda M, Noguchi Y, Yasuda M, Minamitani M, Mizui T, Tsuchiya T, Teramachi H. Hyponatremia and hypokalemia as risk factors for falls. Eur J Clin Nutr. 2015;69(2):205–210. [DOI] [PubMed] [Google Scholar]
- 32. Hoorn EJ, Rivadeneira F, van Meurs JB, Ziere G, Stricker BH, Hofman A, Pols HA, Zietse R, Uitterlinden AG, Zillikens MC. Mild hyponatremia as a risk factor for fractures: the Rotterdam Study. J Bone Miner Res. 2011;26(8):1822–1828. [DOI] [PubMed] [Google Scholar]
- 33. Jamal SA, Arampatzis S, Harrison SL, Bucur RC, Ensrud K, Orwoll ES, Bauer DC. Hyponatremia and fractures: findings from the MrOS study. J Bone Miner Res. 2015;30(6):970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergstrom WH, Wallace WM. Bone as a sodium and potassium reservoir. J Clin Invest. 1954;33(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Usala RL, Verbalis JG. Disorders of water and sodium homeostasis and bone. Curr Opinion in Endocrine and Metabolic Res. 2018;3:83–92. [Google Scholar]
- 36. Barsony J, Sugimura Y, Verbalis JG. Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem. 2011;286(12):10864–10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fibbi B, Benvenuti S, Giuliani C, Deledda C, Luciani P, Monici M, Mazzanti B, Ballerini C, Peri A. Low extracellular sodium promotes adipogenic commitment of human mesenchymal stromal cells: a novel mechanism for chronic hyponatremia-induced bone loss. Endocrine. 2016;52(1):73–85. [DOI] [PubMed] [Google Scholar]
- 38. Tamma R, Sun L, Cuscito C, Lu P, Corcelli M, Li J, Colaianni G, Moonga SS, Di Benedetto A, Grano M, Colucci S, Yuen T, New MI, Zallone A, Zaidi M. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia [published correction appears in Proc Natl Acad Sci USA. 2014;111(38):14002]. Proc Natl Acad Sci USA. 2013;110(46):18644–18649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vandergheynst F, Gombeir Y, Bellante F, Perrotta G, Remiche G, Mélot C, Mavroudakis N, Decaux G. Impact of hyponatremia on nerve conduction and muscle strength. Eur J Clin Invest. 2016;46(4):328–333. [DOI] [PubMed] [Google Scholar]
- 40. Sejling AS, Thorsteinsson AL, Pedersen-Bjergaard U, Eiken P. Recovery from SIADH-associated osteoporosis: a case report. J Clin Endocrinol Metab. 2014;99(10):3527–3530. [DOI] [PubMed] [Google Scholar]
- 41. Verbalis JG, Ellison H, Hobart M, Krasa H, Ouyang J, Czerwiec FS; Investigation of the Neurocognitive Impact of Sodium Improvement in Geriatric Hyponatremia: Efficacy and Safety of Tolvaptan (INSIGHT) Investigators . Tolvaptan and neurocognitive function in mild to moderate chronic hyponatremia: a randomized trial (INSIGHT). Am J Kidney Dis. 2016;67(6):893–901. [DOI] [PubMed] [Google Scholar]
- 42. Katz MA. Hyperglycemia-induced hyponatremia—calculation of expected serum sodium depression. N Engl J Med. 1973;289(16):843–844. [DOI] [PubMed] [Google Scholar]
- 43. Kaelber DC, Foster W, Gilder J, Love TE, Jain AK. Patient characteristics associated with venous thromboembolic events: a cohort study using pooled electronic health record data. J Am Med Inform Assoc. 2012;19(6):965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Usala RL, Fernandez SJ, Mete M, Shara NM, Verbalis JG. Data from: Hyponatremia is associated with increased osteoporosis and bone fractures in diabetics with matched glycemic control. DigitalGeorgetown. http://hdl.handle.net/10822/1052819. Deposited 4 December 2018.
- 45. Corona G, Norello D, Parenti G, Sforza A, Maggi M, Peri A. Hyponatremia, falls and bone fractures: a systematic review and meta-analysis. Clin Endocrinol (Oxf). 2018;89(4):505–513. [DOI] [PubMed] [Google Scholar]
- 46. Christensen MB, Lund A, Calanna S, Jørgensen NR, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab. 2018;103(1):288–294. [DOI] [PubMed] [Google Scholar]
- 47. Maagensen H, Junker AE, Jørgensen NR, Gluud LL, Knop FK, Vilsbøll T. Bone turnover markers in patients with nonalcoholic fatty liver disease and/or type 2 diabetes during oral glucose and isoglycemic intravenous glucose. J Clin Endocrinol Metab. 2018;103(5):2042–2049. [DOI] [PubMed] [Google Scholar]
- 48. Westberg-Rasmussen S, Starup-Linde J, Hermansen K, Holst JJ, Hartmann B, Vestergaard P, Gregersen S. Differential impact of glucose administered intravenously or orally on bone turnover markers in healthy male subjects. Bone. 2017;97:261–266. [DOI] [PubMed] [Google Scholar]
- 49. Talluri S, Charumathi R, Khan M, Kissell K. Atypical presentation of central pontine myelinolysis in hyperglycemia. Endocrinol Diabetes Metab Case Rep. 2017;2017:17-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pliquett RU, Noll A, Ibe R, Katz A, Ackmann C, Schreiber A, Girndt M. Hyperglycemia-related central pontine demyelinization after a binge-eating attack in a patient with type-2 diabetes: a case report. BMC Endocr Disord. 2018;18(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Carlotti AP, St George-Hyslop C, Guerguerian AM, Bohn D, Kamel KS, Halperin M. Occult risk factor for the development of cerebral edema in children with diabetic ketoacidosis: possible role for stomach emptying. Pediatr Diabetes. 2009;10(8):522–533. [DOI] [PubMed] [Google Scholar]
- 52. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126(3):256–263. [DOI] [PubMed] [Google Scholar]
- 53. Sattar L, Renneboog B, Decaux G. Hyperglycemia induces attention and gait deficits in diabetic mellitus patients. Acta Diabetol. 2017;54(10):953–959. [DOI] [PubMed] [Google Scholar]
- 54. Ayus JC, Fuentes NA, Negri AL, Moritz ML, Giunta DH, Kalantar-Zadeh K, Nigwekar SU, Thadhani RI, Go AS, De Quiros FG. Mild prolonged chronic hyponatremia and risk of hip fracture in the elderly. Nephrol Dial Transplant. 2016;31(10):1662–1669. [DOI] [PubMed] [Google Scholar]
- 55. Verroken C, Pieters W, Beddeleem L, Goemaere S, Zmierczak HG, Shadid S, Kaufman JM, Lapauw B. Cortical bone size deficit in adult patients with type 1 diabetes mellitus. J Clin Endocrinol Metab. 2017;102(8):2887–2895. [DOI] [PubMed] [Google Scholar]