Abstract
Eggs provide a rich source of nutrients for the developing embryo, making them a favoured food source for other organisms as well. Several defence mechanisms have evolved to protect the developing embryos against microbial threats. In this article, we elucidate the defence strategy of brown widow spider (Latrodectus geometricus) eggs against bacteria. Antibacterial activity was shown by inhibition of bacterial growth on agar plate, liquid culture and retarded biofilm formation. The defence strategy against bacterial invasion was demonstrated in the whole egg, whole egg extract, egg surface extract, eggshell and eggshell extract. The source and characteristics of this antibacterial activity are distinctive and stem in part from a dense layer of spheres covering the egg surface, likely originated from the oviposition fluid. The spheres are rich in low-molecular-weight proteins, yet their exact composition remains unknown. In this study, we demonstrate that the egg surface is hydrophobic, while the spheres are superhydrophilic. Egg surface roughness and hydrophobicity combined with its antibacterial chemical properties reduce the ability of bacteria to grow on the egg surface. Understanding the properties of these unique structures may contribute significantly to our knowledge of how nature deals with bacterial infections.
Keywords: spider, antibacterial, egg surface, spheres
1. Introduction
During the last few decades, most research dealing with antimicrobial characteristics of invertebrate eggs focused on marine invertebrates. Eggs of marine invertebrates appear to have broad-spectrum antimicrobial activity. Various types of molecules are known as antimicrobial agents in eggs of marine invertebrates, such as indole derivatives [1], free fatty acids [2], lysozymes [3] and glycoproteins [4]. Eggs of terrestrial invertebrates are often laid on or in the soil, where they are exposed to harmful soil microorganisms. Marchini et al. isolated an antibacterial peptide from the eggs of the Mediterranean fruit fly Ceratitis capitata [5]. Female ixodid ticks protect their eggs by covering them with a waxy substance, which contains at least two antimicrobial free fatty acids [6,7]. Lysozyme was isolated from eggs of the Mediterranean fruit fly [8], and the antimicrobial protein sapecin was found in eggs of flesh flies [9]. Yet, we are still far from understanding the overall strategies of antimicrobial defences of terrestrial arthropod eggs.
Bacterial contamination of spider eggs has not been documented in the literature, suggesting that there might be specific mechanisms to protect their eggs from pathogenic bacteria. For example, the eggs of the black widow spider, Latrodectus tredecimguttatus, contain toxic proteinaceous components, including the neurotoxic protein Latroeggtoxin-I [10], the sodium channel-inhibiting protein Latroeggtoxin-II [11], the insect-specific toxin Latroeggtoxin-III and the antibacterial peptide Latroeggtoxin-IV [12]. Intravenously injected extract of eggs of L. geometricus was also found to be toxic to mice [13], but it is not known if the eggs are toxic also to insects or microorganisms.
Spider eggs are usually physically protected by the egg-sac. The egg-sac consists of layers of silk that form a barrier between the embryos and the outer world, but other components inside the egg-sac may also be involved in protection. During construction of the egg-sac, the female first produces a silk platform. Standing beneath the disc, the female extrudes a viscous liquid into which the eggs are laid and adhere to one another and to the basal disc [14]. The egg mass is then covered by one or more layers of silk. The liquid that covers the eggs during oviposition dries onto the chorion and gives the egg surface a granular appearance [14,15].
Spider eggs are spherical and their chorion is covered by granules [16]. These granules or spheres, ranging in size from 0.14 to 10.8 µm diameter [17], are found on the chorion of many spider species [14,17–20] and differ in their shape, density, and distribution among different species [21,22]. Spherical structures on the egg chorion are typical not only to species of Araneae, but also to other arthropods and to several vertebrates. Spheres (1–10 µm diameter) have been found on reptile and avian eggs. The spheres can be composed of organic substances (in domestic hen, Gallus domesticus) or inorganic substances (in Phoenicopterus roseus, mallee fowl) [19]. Rough topography of eggshells was found in several arthropods, among them the mosquito Culex pipiens [23], the blow fly Lucilia cuprina [24] and the broad mite Polyphagotarsonemus latus [25]. The function of the eggshell spheres in most species is still unknown.
We focused here on the antibacterial defence mechanisms of eggs of the brown widow spider, Latrodectus geometricus Koch (Theridiidae). The brown widow spider has a cosmopolitan distribution [26]. It is commonly found in urban areas around homes. The egg-sac of a brown widow is silk-covered, spheroidal, pale yellow and with silk spikes on the surface, which are not present on egg-sacs of other Latrodectus species [27]. Brown widow females have the potential for high reproductive output. After a single mating, females can produce multiple clutches of approximately 120 eggs each, indicating an ability to store a large amount of viable sperm for long periods of time [28]. In the laboratory, L. geometricus females produce up to 29 eggs-sacs in a lifetime [28]. This reproductive ability contributes to the spider's successful invasion history [29] and makes them a favourite model animal for research.
In the present study, we demonstrate the broad-spectrum antibacterial activity of L. geometricus eggs and test the hypothesis that the eggshell and associated spherical granules play a significant role in the egg defence. Additionally, we analysed the eggshell composition and physical properties in order to understand its mechanism of antibacterial action.
2. Material and methods
2.1. Animal culture and collection of egg-sacs
Males and females of the brown widow spider, L. geometricus, were collected from urban gardens in Midreshet Ben-Gurion, Israel (30.8523° N, 34.7834° E), and brought to the laboratory in the Blaustein Institutes for Desert Research of Ben-Gurion University. Males and females were kept in plastic cups with a mesh lid. Males were fed ad libitum with Drosophila fruit flies. Adult females (about 50 at any given time) were fed weekly with three grasshopper nymphs (Schistocerca sp.). The offspring of field-caught females were raised in the laboratory and mated with non-sibs when they matured. Every few months, a few females were added from the field to prevent inbreeding in the laboratory culture. The egg-sacs from the laboratory-reared females were collected 1–10 days after oviposition.
Frozen egg-sacs (kept at −20°C) were used to obtain complete egg homogenate or eggshells. For all other experiments on whole eggs, we used eggs that were from freshly collected egg-sacs. In addition, eggshells were separated from frozen egg-sacs in which the young had hatched, but remained inside the egg-sac (spiderlings emerge from the egg-sac only after a first moult inside the sac [30]) The eggshells are opaque white and easily distinguishable from the yellowish, translucent exuviae, in which legs and other body parts are clearly visible. Eggs or eggshells were collected by tearing the egg-sac with forceps and shaking it above a Petri dish. Eggshells were counted under a microscope to collect them separately from the first instar exuviae.
2.2. Egg homogenization and extraction
400 mg of eggs, separated from egg-sacs on average 5 days following egg-laying, and before hatching, were homogenized in 4 ml of 5% acetic acid. Compound extraction was carried out for 5 h with gentle stirring in an ice-cold water bath. The supernatant, which was obtained by centrifugation at 20 000g for 20 min at 4°C, was dried by a speed-vacuum (CentriVap concentrator, Labconco) and resuspended in 150 µl saline (0.9% w/v NaCl) to obtain 5.09 mg ml−1 total protein concentration (Bradford).
2.3. Water extracts of eggs and eggshells
Egg surface extract was prepared by addition of 80 µl water (Milli Q water purification by Millipore unit) to 100 eggs with gentle shaking for 5 min. Eggshells collected as previously described were extracted with water (50 eggshells to 50 µl Milli Q water) or saline, with gentle shaking for 10 min. The egg surface extract and the eggshell extract were withdrawn and used for the antibacterial assays. The egg spheres were isolated by filtration with 0.45 µm syringe driven filter (Millex LH). The spheres were washed on the filter with 100 µl Milli Q water and resuspended in water before use.
2.4. Oviposition fluid harvest
Latrodectus geometricus females are capable of producing an egg-sac every week after copulation. Several females were mated and monitored for oviposition. Near the end of the egg-laying, and before egg-sac completion, the process was interrupted, the female was removed and the egg-sac with the partial silk covering was torn open with forceps. The oviposition fluid (approx. 20 µl) was collected by pipetting with 200 µl volume tips. The diameter of such a tip enabled fluid withdrawal, leaving the eggs intact. After collecting the fluid, the female was returned and completed the formation of the egg-sac. Oviposition fluid was kept at −20°C until used.
2.5. Antibacterial activity tests
2.5.1. Modified Kirby–Bauer diffusion susceptibility test
A modification of the Kirby–Bauer diffusion susceptibility test [31] was used to determine the bacterial susceptibility to the egg components of the brown widow spider. The following bacterial species were tested: Escherichia coli K12 (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 29213) and Morganella morganii. The latter strain was isolated directly from silk fibres taken from the egg-sacs as shown in electronic supplementary material, figure S2. It was purified by sub-culturing on LB media and was identified by sequencing its 16 s rRNA gene (HyLab Rehovot, Israel). The sequence was deposited in the gene bank under accession number MG489884. Briefly, the bacterial inoculums were placed in Luria-Bertani Broth, Miller (LB, Difco, MD, USA). Colonies were purified on the same media by repeated streaking of the different colonies on fresh agar. These strains were chosen so that both Gram-positive and Gram-negative bacteria were tested. A colony of each strain was suspended in 10 ml of LB and incubated overnight at optimal growth temperature (37°C). The culture was diluted to mid-log phase around an optical density (OD) of 0.5 at 600 nm. Samples for the susceptibility test were intact eggs, eggshells, egg-surface extract or egg homogenate extract. When intact eggs were tested for their antibacterial activity, for each bacterial culture, eggs from five egg-sacs were mixed and individual eggs were tested (n = 12 eggs for E. coli K12, n = 12 for P. aeruginosa, n = 12 for M. morganii and n = 51 for S. aureus). Soft agar LB plates (0.7% agar) were inoculated with the bacterial cultures and then the test samples (intact eggs, eggshells or 2 µl of extracts) were placed on the agar surface. Glass beads (Z250473-1PAK Sigma-Aldrich) of the same size as spider eggs (1 mm diameter) were sterilized and used as control. After incubation (30°C, 18 h) the inhibition zone was measured.
2.5.2. Bacterial susceptibility test in liquid media
The antibacterial test of egg homogenate was performed using the 96-well plate-based broth dilution method, also known as checkerboard assay [32]. Escherichia coli K12 inoculum (90 µl in LB) was added to each well of a sterile 96-well plate except for the blank wells. Brown widow egg homogenate (10 µl) was added to the 96-well plate with the LB medium. 90 µl of fresh LB broth with 10 µl saline were added to each blank well. Kanamycin (Sigma-Aldrich, 1 µg µl−1) was used as a positive control. 90 µl of bacterial inoculum in LB with 10 µl of saline was added to each of the negative control wells. In the solvent control 10 µl of acetic acid 5% were added to 90 µl of bacterial inoculum. The plates were covered with a plastic cover and then incubated at 37°C. Bacterial growth was monitored every 30 min by a plate-reader (SynergyMx, BioTek, Winooski, VT) at a wavelength of 600 nm.
2.5.3. Bacterial viability test
Gram-negative and Gram-positive bacterial strain viability was assessed with eggs and eggshells by single plate-serial dilution spotting (SP-SDS) test [33]. Escherichia coli K12 (ATCC 25922) and S. aureus (ATCC 29213) were used for this test. A colony of each strain was suspended in 10 ml of LB and incubated overnight at optimal growth temperature (37°C). The cultures were diluted to mid-log phase with an OD of 0.5 at 600 nm.
One hundred eggs were added to 200 µl bacterial suspension in each of the experiment wells of a sterile 96-well plate except for the blank wells, the negative and the positive control wells. Glass beads with similar diameter to the brown widow eggs (1 mm) were sterilized and used as a negative control. Kanamycin (Sigma-Aldrich, 1 µg µl−1) was used as a positive control. Five hours after incubation started, 10 µl aliquots were taken from each well and serially diluted (102–107). From each dilution 10 µl was taken and dropped over agar-gelled LB medium in six different sectors of 9 cm plates. The plates were then incubated at 30°C and the colonies were counted after 24 h.
The eggshells were tested in the viability test against E. coli K12. Twenty eggshells were added to 200 µl bacterial suspension in each of the experiment wells except for the blank wells, the negative and the positive control wells. Kanamycin (Sigma-Aldrich, 1 µg µl−1) was used as a positive control while wells that contained bacterial suspension without the addition of eggshells were used as control. The rest of the protocol was identical to the intact eggs viability test.
2.6. Bacterial adherence to egg surface in spraying assay
To examine the bacterial adherence to the egg surface, we used fluorescent microspheres (1 µm diameter, excitation 540 nm, emission 560 nm) with negatively charged surfaces (FluoSphere; Molecular probe, Eugene, OR) similar to most bacterial surfaces in nature [34]. FluoSpheres aerosol (109 FluoSpheres ml−1) was sprayed directly on the spider eggs using a Voyage aerosol nebulizer (Mefar). Sterilized glass slides were used as a positive control. FluoSpheres presence on eggs was examined by a fluorescence microscope (Zeiss AX10) after the spraying process. The same process was performed with fluorescently labelled Bacillus subtilis (strain 3610, 106 cells ml−1) cells expressing a red fluorescence protein [35]. Sterilized glass beads (1 mm diameter) were used as a positive control in this experiment.
2.7. Microtitre plate biofilm production assay
Morganella morganii, P. aeruginosa PO1 and Klebsiella oxytoca [36] were grown in LB overnight. The cultures were diluted with fresh LB medium to get an OD of 1 at a wavelength of 600 nm. Fresh LB medium (135 µl) was transferred with the diluted bacterial culture (15 µl) into the experiment wells of a 96-well plate (Waltman, transparent polystyrene, flat bottom). Fifty eggshells that were separated previously from empty egg-sacs after emergence of the young were added to the experiment wells. Wells without eggshells served as a negative control while kanamycin (Sigma-Aldrich, 1 µg µl−1) was used as a positive control. 135 µl of fresh LB with 15 µl saline were added to each blank well. This procedure was performed for each of the bacterial strains. The plate was covered and incubated for 18 h at 37°C. Quantitative crystal violet analysis of biofilm formation was performed using Djordjevic et al. assay [37]. The level (OD) of crystal violet present in the distaining solution was measured at 595 nm by a spectrophotometer (Infinite M200, Tecan).
2.8. Scanning electron microscopy
Spider eggs and eggshells were examined using a scanning electron microscopy (SEM) instrument (Quanta 200, FEI) without dehydration and fixation. The samples were attached to the microscope stub using double-sided adhesive, dried and coated by a 20 nm gold layer before observation.
2.9. Transmission electron microscopy
Primary fixation of intact eggs was done with modified Karnovsky's fixative (2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M cacodylate buffer pH 7.2) for 30 min at 37°C. After two washes in the same buffer, the samples were post-fixed with 1% osmium tetroxide at 4°C for 30 min followed by repeated washing with the same buffer. After fixation, samples were dehydrated in graded ethanol series and propylene oxide, followed by a gradual embedding in Araldite 502 (Electron Microscopy Sciences, Fort Washington, PA, USA). In the final, full-strength Araldite, the samples were subjected to mild vacuum (400 mbar) for 1 h at room temperature followed by an overnight polymerization at 60°C. The resin blocks were carefully trimmed to expose the underlying aragonite. The aragonite was dissolved by immersing the blocks in 0.32% HCl for 48 h. The blocks then were rinsed in water and dried thoroughly. The blocks were immersed in Araldite for 3 h in the same vacuum conditions and polymerized overnight at 60°C. Sections of various thicknesses (60–90 nm) were cut using a Leica ultracut UCT microtome (Leica Microsystems, Nussloch, Germany) and picked up on 300 mesh copper grids. The sections were contrasted by uranyl acetate and lead citrate.
Sections were observed using a JEM-1230 transmission electron microscopy (TEM) instrument (JEOL Ltd, Tokyo, Japan) operated at 80 kV. Electron micrographs were taken using TemCam-F214 (Tietz Video & Image Processing Systems (TVIPS) Gauting, Germany).
2.10. MALDI analysis and SDS-PAGE protein separation
Spheres released with water from the egg surface, and oviposition fluid, were mixed with 2,5-dihydroxybenzoic acid matrix and spotted on a matrix-assisted laser desorption ionization (MALDI) target. Mass spectrometry (MS) was performed with an Autoflex speed MALDI TOF/TOF instrument and peaks-picking was performed with BioTools analysis software (Bruker Daltonics, Bremen Germany). Water-released spheres, oviposition fluid and egg homogenate proteins were further mixed with denaturation sample buffer and resolved on 12% sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE; (Mini-PROTEAN electrophoresis system, Bio-Rad). Proteins were visualized with colloidal Coomassie blue staining.
2.11. Water contact angle
The water contact angles of the eggs, and of a glass slide coated with egg spheres, were measured in order to determine the degree of wettability of the egg surface. The eggs were attached to a microscope slide using double-sided adhesive while the egg spheres, which were extracted in deionized water, were dried on a glass slide. To measure the contact angle of the spheres we used a modified measurement protocol for bacterial cell surface contact angle [38]. A glass slide was used as substrate instead of cellulose triacetate filter. The glass slide was covered homogeneously with the eggs spheres. A bare glass slide was used as control. Water contact angles were measured with a contact angle analyser system (OCA 20, DataPhysics Instruments GmbH, Germany). For egg measurement, the droplet volume was 0.2 µl while a drop of 1 µl was used for the egg spheres measurement. All measurements were carried out at 25°C.
2.12. Hydrophobicity
The hydrophobicity of the eggs was examined by the MATH (microbial adhesion to hydrocarbons) test assay [39]. n-Hexadecane, which is completely apolar, was added to a mixture of eggs and deionized water following a brief period of mixing. The phases were allowed to separate for 15 min and then they were analysed for their adherence either to n-hexadecane phase or to the water phase.
2.13. Zeta potential
Egg surface spheres are released when eggs come in contact with water. To measure the polarity of egg surface, 100 eggs were mixed with 1 ml of Millipore water for 10 min. The egg-surface water extract containing the spheres was analysed by a Zetasizer Nano ZS (Malvern, UK) to measure the zeta potential value.
3. Results
3.1. Intact Latrodectus geometricus eggs inhibit bacterial growth
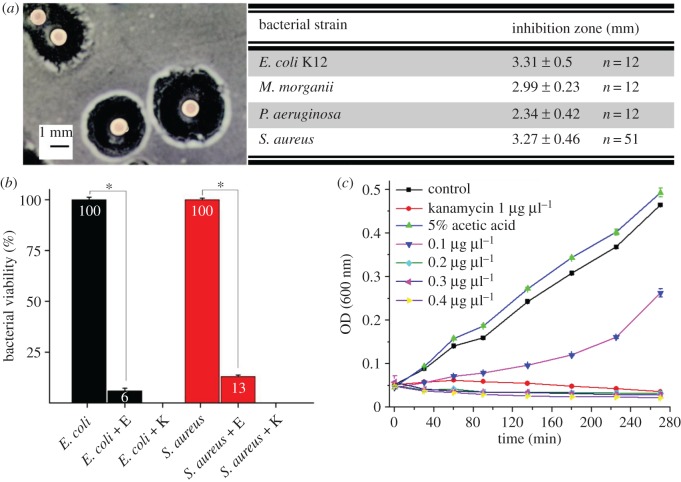
In the modified Bauer diffusion test, the brown widow eggs showed a clear inhibition zone against the Gram-negative strains, E. coli K12, P. aeruginosa and M. morganii, as well as the Gram-positive strain, S. aureus (figure 1a). The eggs were left on the agar plates with the bacteria and finally hatched successfully. To demonstrate whether this wide-range activity is bacteriostatic or bactericidal, the viability of Gram-negative and Gram-positive strains was estimated by SP-SDS test. Bacterial viability of E. coli and S. aureus decreased significantly (t = 9.4, d.f. = 18, p < 0.001 and t = 11.5, d.f. = 6, p < 0.0001 respectively) when eggs were incubated with the bacterial culture (figure 1b). The antibacterial activity of egg homogenate extract was determined against E. coli K12 using the liquid growth inhibition assay. Addition of 10 µl of the egg homogenate extract to the bacterial suspension exhibited a strong inhibition effect (figure 1c) that was comparable to the effect of 1.0 µg µl−1 kanamycin.
Figure 1.
Intact L. geometricus eggs inhibit bacterial growth. (a) Inhibition zones measured in Gram-negative strains, E. coli K12, P. aeruginosa and M. morganii, and Gram-positive strain, S. aureus, in the modified Kirby–Bauer diffusion test. Figure on left shows E. coli inhibition zone. The results represent the inhibition zone (average and s.d.) of eggs from five different egg clutches for each of the bacterial species. (b) Viability of E. coli and S. aureus decreased when eggs (noted as E) were incubated with bacterial culture. Kanamycin (1 µg µl−1, noted as K) was used as positive control. Asterisks indicate significant difference (p < 0.001). (c) Liquid E. coli growth inhibition assay with 5% acetic acid egg homogenate at increasing concentrations. Kanamycin (1 µg µl−1) was used for positive control and saline and acetic acid (5%) solution served as negative control. (Online version in colour.)
3.2. Egg surface is covered with spheres
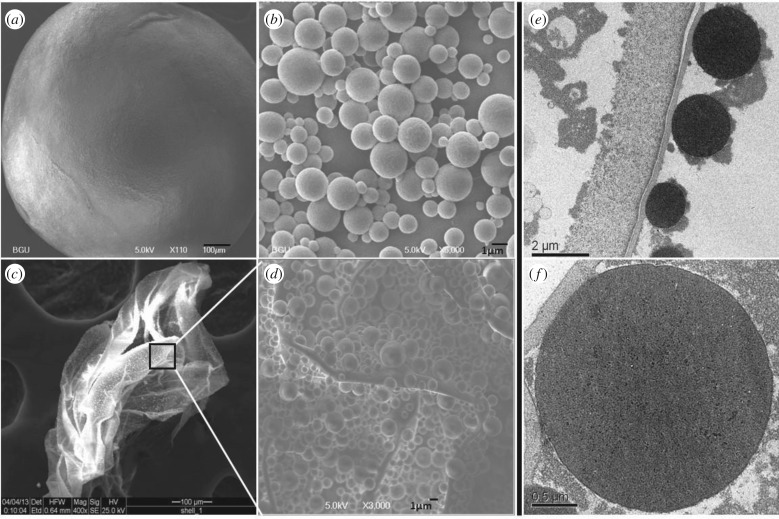
Since antibacterial activity was seen in the unbroken, whole L. geometricus eggs, the question whether the antibacterial activity comes from the internal or the external egg components was addressed. We first examined the ultrastructure of the egg surface using SEM and TEM. Latrodectus geometricus eggs are spherical, approximately 1 mm in diameter (figure 2a), and coated with a dense layer of spheroidal granules (figure 2b) that adhere to the egg chorion. The diameter of these spheres was not uniform and extended between 0.4 and 3.6 µm (figure 2b). The dense spheroidal layer was seen also on the empty eggshells after hatching of spiderlings (figure 2c,d). TEM analysis of the egg surface showed electron-dense pattern staining of spheres (figure 2e,f). The differential staining of the spheres and their density could be clearly distinguished from the granular material surrounding them, which suggest that they differ in composition (figure 2e).
Figure 2.
Latrodectus geometricus egg surface is covered with spheres. Egg structure visualized by SEM (a–d) and TEM (e,f). (a) Entire egg, (b) close-up of spheres, (c) eggshell, (d) eggshell surface showing spheres. TEM images of a section of the egg showing the surface (e) and an enlargement of a sphere (f).
3.3. Egg surface limits bacterial adhesion and growth
After SEM examination of the eggs and eggshells, the role of the egg surface containing the spheres was examined. FluoSpheres aerosol was sprayed directly on the spider eggs to examine their attachment to the egg surface. After spraying, fluorescent microscope analysis of the RFP labelled cells (excitation 546/12; beam splitter 560; emission 607/80) showed that negatively charged FluoSpheres were attached to the egg surface and to the glass slide that was used as control. When we used fluorescent Bacillus subtilis in this experiment, bacterial adherence was seen (electronic supplementary material, figure S1).
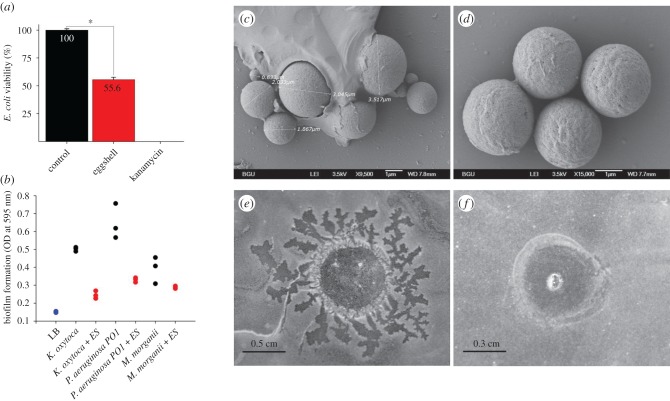
When 20 eggshells were added to an E. coli culture, in the SP-SDS viability test, bacterial growth was reduced by 45% compared to the control (t = 2.6, d.f. = 5, p < 0.05, figure 3a). Moreover, when 50 eggshells were added to each of three replicates of K. oxytoca, P. aeruginosa PO1 and M. morganii cultures, biofilm formation of these bacterial strains was reduced (22–33% of the control for K. oxytoca, 31–40% for P. aeruginosa, and 44–92% for M. morganii; figure 3b). The effect of the addition of eggshells was significant overall (two-way ANOVA, treatment effect: F1,12 = 85.44, p < 0.0001). Biofilm formation was significantly lower for K. oxytoca and P. aeruginosa in the presence of eggshells (Tukey post hoc comparisons: p = 0.0007 and p = 0.0002, respectively), but not in the case of M. morganii (p = 0.23).
Figure 3.
The eggshells and saline extract of the eggshells inhibit biofilm formation and bacterial survival. (a) The viability of E. coli culture in the presence of 20 eggshells or 1 µg µl−1 kanamycin compared to control. Bacterial culture without the addition of eggshells was used as negative control. (b) Biofilm production of K. oxytoca, P. aeruginosa and M. morganii inoculums with or without 50 eggshells (ES). SEM analysis of water spheres extract encased by organic material (c) or not (d). Saline extract of eggshells (2 µl) was tested for its bacterial growth inhibition capability against M. morganii (e) and S. aureus (f). (Online version in colour.)
A clear inhibition zone was seen around the eggshells when they were placed on LB plates with bacteria that were grown from an internal silk fibre of the egg-sac (electronic supplementary material, figure S2). To elucidate the source of the egg inhibitory components, spheres of eggshells were rapidly released with water after 5 min incubation period. The water extract appears as a cloudy, opaque, solution indicating that the eggshell spheres do not dissolve in water. The samples undergo evaporation before gold sputtering for SEM. Upon evaporation of the water, the spheres that were recovered retained the same shape (figure 3c,d). The material coating the spheres in figure 3c is likely an organic residue left after evaporation of the water (electronic supplementary material, figure S4). Moreover, examining 2 µl of eggshell saline extract by the Kirby–Bauer test revealed a clear inhibitory effect on the bacteria M. morganii and S. aureus (figure 3e,f respectively; see also the controls in electronic supplementary material, figure S5).
3.4. The egg is hydrophobic while egg spheres are superhydrophilic and positively charged
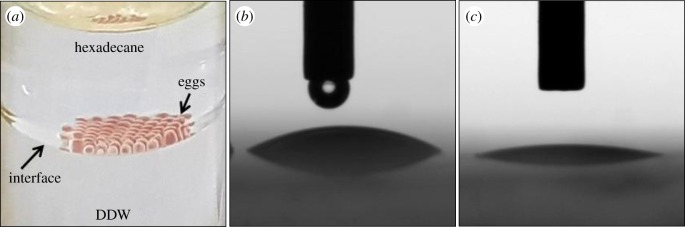
The spheres revealed by microscopic examination formed a rough surface on the spider eggs (figure 2). To determine whether the observed microscopic roughness affects the hydrophobicity and wettability, bacterial adhesion to the egg surface and water contact angle (CA) measurements were performed. Due to the spherical shape of an egg and its small diameter (1 mm), CA measurements of water droplets that are also spherical are difficult. Consequently, we used the simple, rapid MATH test assay to determine whether the egg surface nature is hydrophobic or hydrophilic. The MATH test showed that eggs that remained near the interphase adhered to the hydrophobic n-hexadecane phase (figure 4a). However, the water CA of the egg spheres, released with water from eggs and dried on glass slide, was found to be less than 10° (figure 4c), compared to CA of 34.7° when a water drop was placed on a glass slide without spheres (figure 4b). This low CA indicates superhydrophilicity of the spheres.
Figure 4.
Eggs are hydrophobic while egg spheres are superhydrophilic. (a) MATH test of entire, untreated eggs showing eggs adhered to the hydrophobic n-hexadecane phase. (b) Water contact angle measured when a water drop was placed on a glass slide or (c) on egg spheres that were dried on a glass slide. (Online version in colour.)
Zeta potential of the deionized water extract of eggs exhibited a value of +4.895 mV at 25°C. The low zeta potential value indicates that the particles (spheres) are not stable in solution and tend to aggregate.
3.5. Oviposition fluid and eggshell water extract contain proteinaceous spheres
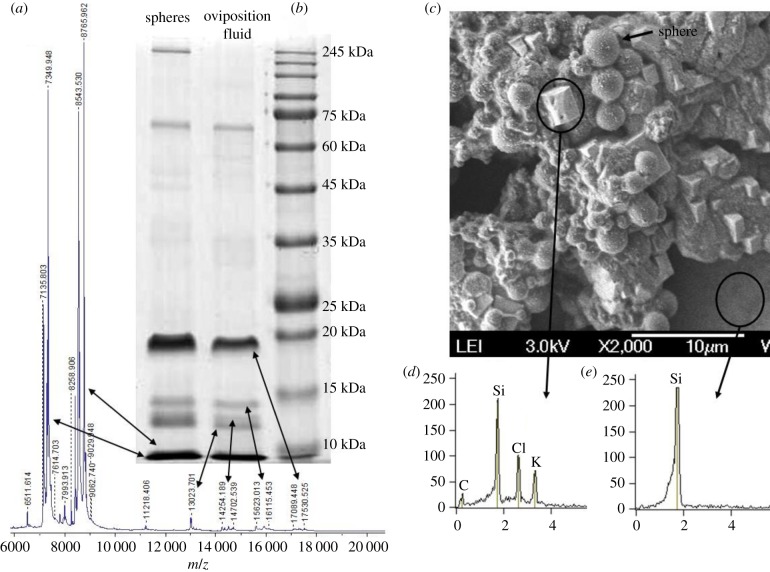
SEM analysis indicated that the most abundant component of the oviposition fluid was the spheres, which were identical in shape and diameter to the egg-surface spheres (figure 5c). The dried oviposition fluid also contained salt crystals; upon analysis, these crystals appeared to be KCl (figure 5d). MALDI-MS analysis indicated that the m/z component of the oviposition fluid was identical to the m/z components of the egg-surface water extract. The most abundant masses are the approximately 7300, approximately 8550, approximately 8700 m/z and the masses at the range of approximately 14 500, approximately 15 600 and approximately 17 000 m/z (figure 5a). The MALDI-MS results were in line with the SDS-PAGE results. The most abundant Coomassie blue stained bands were the approximately 7, approximately 8, approximately 14, approximately 15 and approximately 17 kDa (figure 5b). Interestingly, the SDS-PAGE profile of the oviposition fluid was almost identical to the profile of the filter purified spheres released from egg surface (figure 5b) and to the profile of the egg surface crude extract (electronic supplementary material, figure S3).
Figure 5.
Oviposition fluid is similar in composition to eggshell water extract. (a) MALDI-MS analysis of the oviposition fluid. (b) Coomassie-blue-stained SDS-PAGE of oviposition fluid. Arrows show the similarity of the m/z masses of the MALDI-MS analysis to the SDS-PAGE protein profile. (c) SEM image of oviposition fluid dried on a silica chip. (d) The white crystals present in the oviposition fluid were identified by SEM composition analysis as KCl crystals. (e) SEM analysis of the silica chip surface indicates that the origin of the KCl crystals is the oviposition fluid. (Online version in colour.)
4. Discussion
Intact eggs placed on the surface of an inoculated Petri dish were capable of protecting the developing embryo. Our results demonstrate that the antibacterial activity of the intact egg is performed by both internal (egg homogenate) and external (eggshell) components. We showed potent inhibition of egg homogenate against Gram-positive and Gram-negative bacteria: 10 µl of egg homogenate caused total inhibition of E. coli growth, comparable to that of the positive control kanamycin (1 μg µl−1). These results indicate that the overall antibacterial activity of the eggs is not specific against either Gram-negative or Gram-positive strains, but is generally antibacterial. More specific antibacterial activity of egg homogenate was observed in some marine and terrestrial arthropods. For example, egg homogenate of the marine spiny starfish Marthasterias glacialis inhibited the growth of the coastal water strains of Vibrio alginolyticus and Vibrio vulnificus (89.6% and 78% growth inhibition, respectively), while pathogenic strains as Salmonella sp. and E. coli were not affected (1.2% and 7.2% growth inhibition) [3]. Egg homogenate of the tick Rhipicephalus (Boophilus) microplus exhibited activity against the Gram-positive bacterium M. luteus; however no activity was detected against the Gram-negative bacterium E. coli [40]. By contrast, brown widow eggs showed similar potent activity against all bacterial strains tested.
The eggshell provides physical protection of the embryo from the external environment. We hypothesized that the eggshell may have an additional role in preventing bacterial colonization on the egg surface, and thus we tested the role of the eggshells in the prevention of bacterial contamination. Eggshells showed clear inhibition against bacteria that grew out of a fibre taken from the internal silk of the egg-sac and placed on the agar. Biofilm formation of P. aeruginosa PO1 and K. oxytoca cultures was significantly reduced to 27% and 36% respectively. When eggshells were added to E. coli cultures, the viability of the bacterial cells decreased to 56% compared to the control. These results support our hypothesis that the eggshells play a significant role in protecting the egg against bacterial invasion. The inhibition of biofilm development may occur due to diffusible factors released from eggshells, which may act to reduce the number of living bacterial cells on the eggshell and thus reduce biofilm formation. We propose that due to their cationic nature, the released spheres form electrostatic interactions with the bacteria in solution, which may contribute to lower the bacterial adhesion to the wells. Egg wax from the cattle tick Rhipicephalus (Boophilus) microplus [41,42] can also inhibit P. aeruginosa PA14 biofilm, suggesting that arthropod eggs have diverse ways of inhibiting the development of biofilms on their surface.
Buffkin et al. [43] demonstrated that in the black widow spider L. tredecimguttatus, toxins were contained in the eggs themselves and not in the egg shells. Three toxic proteins (of 23.8, 28.7 and 36.0 kDa) were isolated from the egg homogenate in addition to a broad-spectrum antibacterial peptide with a molecular weight of 3.6 kDa [10–12,44]. In L. geometricus eggs, SDS-PAGE tests and MS analysis of the antibacterial eggshell water extract showed that the most abundant Coomassie blue-stained bands were the approximately 7, approximately 8, approximately 14, approximately 15 and approximately 17 kDa. It is not yet clear which chemical components in the egg are responsible for the antibacterial activity. It is possible that some of the toxins found in L. tredecimguttatus eggs also occur in eggs of the congeneric L. geometricus. This issue should be examined further.
Hydrophilicity/hydrophobicity or wettability of surfaces is known to affect bacterial adhesion [45]. Both superhydrophobic surfaces, with water CA > 150° and negative zeta potential, and superhydrophilic surfaces, with water CA ≤ 10° and negative zeta potential, were shown to have self-cleaning properties [46,47]. Surface wettability is influenced by microscale and nanoscale surface topography. Surface roughness induces superhydrophobicity and low adhesion of bacteria to the surface [45]. Brush-turkey eggshells, for example, are coated with nanospheres made of calcium phosphate [48], which create a superhydrophobic, rough topography that limits bacterial adhesion and as such, contributes to the antimicrobial defence system of the eggs [49]. According to the Cassie–Baxter model, when a water droplet is placed in contact with a rough surface, the liquid may not penetrate fully into the surface texture, but it forms a solid–liquid–air interface due to the trapped air in the microstructures [50,51]. Spider eggs are rough due to the coating of egg spheres over the chorion and sphere sizes and densities are variable over the surface [17,22]. In the MATH test, the spider eggs were found in the hydrophobic phase. We suggest that pockets of air are trapped between the spheres, causing the egg surface to be hydrophobic. While the egg surface was found to be hydrophobic, isolated egg spheres were superhydrophilic, since their measured water CA was less than 10°.
Although the spheres had a positive zeta potential and could attract negatively charged bacteria [51], we suggest that the rough hydrophobic topography might reduce the ability of bacteria to adhere to the egg surface. Bacterial adherence to the surface was tested by a massive challenge of artificial bacterial aerosol or FluoSpheres aerosol. The test revealed that negatively charged bacteria and FluoSpheres could adhere to the egg surface as well as to glass beads of similar size as the egg. The aerosol test needs further experimentation and analysis to arrive at a conclusion regarding the function of the spheres. Nevertheless, the demonstrated activity of the intact eggshell and water extract of the eggshell against bacteria suggests that the antibacterial effect is due to biochemical agents located on the egg surface. Biochemical analysis of the spheres revealed five prominent Coomassie blue stained bands, indicating that the spheres are mainly made up of a combination of low-weight proteins. Although we think that the spheres are an important factor in protecting the eggs from microorganisms, it is not yet clear whether the active component is one of the eggshell proteins or a non-proteinaceous substance.
Further studies are needed to elucidate the complete structure and nature of the spheres. The origin of the egg spheres is also not yet clear. Morishita et al. [52] observed a material resembling the egg spheres in the oviducts and uterus of the brown recluse spider, Loxosceles intermedia, and investigated its histochemical properties. They suggested that this material coats the oocyte on its way out. The egg spheres appear on the egg surface when the liquid surrounding the eggs is absorbed during egg laying [15]. Thus, we hypothesized that the spheres should be found in the oviposition liquid. To test this hypothesis, the oviposition liquid was isolated from the egg-sac immediately after laying and examined with SEM. The liquid contained a mixture of salt crystals that were identified as KCl crystals, spheres, and additional unknown materials. In addition, MS analysis and SDS-PAGE tests of the oviposition liquid revealed high similarity of the m/z masses to the purified egg spheres and to the egg surface crude extract. This supports our hypothesis that the egg spheres appear in the oviposition liquid and become attached to the egg surface soon after egg laying.
In conclusion, the eggs of L. geometricus are protected from bacterial threats due to both chemical and physical properties of the eggshells. Distinct spheroid structures, made of low molecular weight proteins, were found to envelope each egg. These spheres are restricted to the chorion of the eggs and are found in the oviposition fluid as well. The combined mechanisms associated with the surface of the brown widow spider egg appear to be a distinctive model of antibacterial defence mechanisms in animals.
Supplementary Material
Acknowledgements
We thank Simy Weil for the valuable discussion and suggestions, Iris Musli for maintaining the spider culture facility and Rivka Yehuda for the help with protein separation.
Data accessibility
All data are provided in full in the results section of this paper. Additional supporting data of this study are provided as electronic supplementary material accompanying this paper.
Authors' contributions
V.M. conceived of the study, carried out the laboratory work and data analysis, participated in the design of the study and drafted the manuscript; Z.R. designed the bacterial work and antibacterial analysis; Y.L. designed and coordinated the work on spiders and egg collection; I.K. designed and coordinated the biochemical study, carried out the statistical analyses and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
We received no funding for this study.
References
- 1.Benkendorff K, Bremner JB, Davis AR. 2001. Indole derivatives from the egg masses of Muricid molluscs. Molecules 6, 70–78. ( 10.3390/60100070) [DOI] [Google Scholar]
- 2.Benkendorff K, Davis AR, Rogers CN, Bremner JB. 2005. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 316, 29–44. ( 10.1016/j.jembe.2004.10.001) [DOI] [Google Scholar]
- 3.Stabili L, Pagliara P. 1994. Antibacterial protection in Marthasterias glacialis eggs—characterization of lysozyme-like activity. Comp. Biochem. Physiol. B 109, 709–713. ( 10.1016/0305-0491(94)90134-1) [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki M, Ohye H, Kisugi J, Kamiya H. 1990. Bacteriostatic and cytolytic activity of purple fluid from the sea hare. Dev. Comp. Immunol. 14, 379–383. ( 10.1016/0145-305X(90)90030-I) [DOI] [PubMed] [Google Scholar]
- 5.Marchini D, Marri L, Rosetto M, Manetti AGO, Dallai R. 1997. Presence of antibacterial peptides on the laid egg chorion of the medfly Ceratitis capitata. Biochem. Biophys. Res. Commun. 240, 657–663. ( 10.1006/bbrc.1997.7694) [DOI] [PubMed] [Google Scholar]
- 6.Arrieta MC, Leskiw BK, Kaufman WR. 2006. Antimicrobial activity in the egg wax of the African cattle tick Amblyomma hebraeum (Acari: Ixodidae). Exp. Appl. Acarol. 39, 297–313. ( 10.1007/s10493-006-9014-5) [DOI] [PubMed] [Google Scholar]
- 7.Yu ZJ, Thomson ELS, Liu JZ, Dennis JJ, Jacobs RL, Kaufman WR. 2012. Antimicrobial activity in the egg wax of the tick Amblyomma hebraeum (Acari: Ixodidae) is associated with free fatty acids C16:1 and C18:2. Exp. Appl. Acarol. 58, 453–470. ( 10.1007/s10493-012-9586-1) [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Sousa JM, Gavilanes JG, Municio AM, Perez-Aranda A, Rodriguez R. 1977. Lysozyme from the insect Ceratitis capitata eggs. Eur. J. Biochem. 72, 25–33. ( 10.1111/j.1432-1033.1977.tb11220.x) [DOI] [PubMed] [Google Scholar]
- 9.Komano H, Homma K, Natori S. 1991. Involvement of sapecin in embryonic cell proliferation of Sarcophaga peregrina (flesh fly). FEBS Lett. 289, 167–170. ( 10.1016/0014-5793(91)81061-C) [DOI] [PubMed] [Google Scholar]
- 10.Li J, Yan Y, Wang J, Guo T, Hu W, Duan Z, Wang X, Liang S. 2013. Purification and partial characterization of a novel neurotoxic protein from eggs of black widow spiders (Latrodectus tredecimguttatus). J. Biochem. Mol. Toxicol. 27, 337–342. ( 10.1002/jbt.21493) [DOI] [PubMed] [Google Scholar]
- 11.Li J, Yan Y, Yu H, Peng X, Zhang Y, Hu W, Duan Z, Wang X, Liang S. 2014. Isolation and identification of a sodium channel-inhibiting protein from eggs of black widow spiders. Int. J. Biol. Macromol. 65, 115–120. ( 10.1016/j.ijbiomac.2014.01.004) [DOI] [PubMed] [Google Scholar]
- 12.Lei Q, Yu H, Peng X, Yan S, Wang J, Yan Y, Wang X. 2015. Isolation and preliminary characterization of proteinaceous toxins with insecticidal and antibacterial activities from black widow spider (L. tredecimguttatus) eggs. Toxins (Basel) 7, 886–899. ( 10.3390/toxins7030886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt JO, Vetter RS, Howe AK. 2017. Egg toxicity in diverse spider taxa. J. Arachnol. 45, 209–212. ( 10.1636/JoA-17-009.1) [DOI] [Google Scholar]
- 14.Conti E, Costa G, Marletta A, Viscuso R, Vitale DGM. 2015. The chorion of eggs in a Namibian Ariadna species (Araneae: Segestriidae): morphological and SEM analyses. J. Arachnol. 43, 224–227. ( 10.1636/M14-72) [DOI] [Google Scholar]
- 15.Foelix RF. 2011. Biology of spiders, 3rd edn Oxford, UK: Oxford University Press. [Google Scholar]
- 16.Ehn A. 1963. The embryonic development of the spider Torania variata Poc. (Sparassidae). Zool. Bidr. Upps. 36, 37–47. [Google Scholar]
- 17.Humphreys WF. 1983. The surface of spiders’ eggs. J. Zool. 200, 303–316. ( 10.1111/j.1469-7998.1983.tb02312.x) [DOI] [Google Scholar]
- 18.Michalik P, Reiher W, Tintelnot-Suhm M, Coyle FA, Alberti G. 2005. Female genital system of the folding-trapdoor spider Antrodiaetus unicolor (Hentz, 1842) (Antrodiaetidae, Araneae): ultrastructural study of form and function with notes on reproductive biology of spiders. J. Morphol. 263, 284–309. ( 10.1002/jmor.10309) [DOI] [PubMed] [Google Scholar]
- 19.Humphreys WF. 1987. The accoutrements of spiders’ eggs (Araneae) with an exploration of their functional importance. Zool. J. Linn. Soc. 89, 171–201. ( 10.1111/j.1096-3642.1987.tb00654.x) [DOI] [Google Scholar]
- 20.Berendonck B, Greven H. 2005. Genital structures in the entelegyne widow spider Latrodectus revivensis (Arachnida; Araneae; Theridiidae) indicate a low ability for cryptic female choice by sperm manipulation. J. Morphol. 263, 118–132. ( 10.1002/jmor.10296) [DOI] [PubMed] [Google Scholar]
- 21.Grim JN, Slobodchikoff CN. 1982. Spider egg chorion sphere size and density. Ann. Entomol. Soc. Am. 75, 330–334. ( 10.1093/aesa/75.3.330) [DOI] [Google Scholar]
- 22.Grim JN, Slobodchikoff CN. 1978. Chorion surface-features of some spider eggs. Pan-Pac. Entomol. 54, 319–322. [Google Scholar]
- 23.Hinton HE. 1967. Respiratory system of egg-shell of common housefly. J. Insect Physiol. 13, 647–651. ( 10.1016/0022-1910(67)90115-1) [DOI] [Google Scholar]
- 24.Sukontason KL, Piangjai S, Bunchu N, Chaiwong T, Sripakdee D, Boonsriwong W, Vogtsberger RC, Sukontason K. 2006. Surface ultrastructure of the puparia of the blow fly, Lucilia cuprina (Diptera: Calliphoridae), and flesh fly, Liosarcophaga dux (Diptera: Sarcophagidae). Parasitol. Res. 98, 482–487. ( 10.1007/s00436-005-0102-y) [DOI] [PubMed] [Google Scholar]
- 25.Baker RA. 2012. ‘Plastrons and adhesive organs’—the functional morphology of surface structures in the broad mite, Polyphagotarsonemus latus (Banks, 1904). Zeszyty Naukowe Acta Biologica Uniwersytet Szczeciński 19, 89–96. [Google Scholar]
- 26.Howell WM, Jenkins RL. 2004. Spiders of the eastern United States: a photographic guide. Boston, MA: Pearson Education. [Google Scholar]
- 27.Abalos JW. 1962. The egg-sac in the identification of species of Latrodectus (black-widow spiders). Psyche 69, 268–270. ( 10.1155/1962/36967) [DOI] [Google Scholar]
- 28.Bouillon A. 1957. Les fonctions du cocon chez l'araignée Latrodectus geometricus C. Koch. Léopoldville: Éditions de l'Université. [Google Scholar]
- 29.Vetter RS, Vincent LS, Danielsen DW, Reinker KI, Clarke DE, Itnyre AA, Kabashima JN, Rust MK. 2012. The prevalence of brown widow and black widow spiders (Araneae: Theridiidae) in urban southern California. J. Med. Entomol. 49, 947–951. ( 10.1603/ME11285) [DOI] [PubMed] [Google Scholar]
- 30.Downes MF. 1986. Postembryonic development of Latrodectus hasselti Thorell (Araneae, Theridiidae). J. Arachnol. 14, 293–301. [Google Scholar]
- 31.Bauer AW, Kirby WM, Sherris JC, Turck M. 1966. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496. ( 10.1093/ajcp/45.4_ts.493) [DOI] [PubMed] [Google Scholar]
- 32.Lorian V. 1991. Antibiotics in laboratory medicine, 3rd edn Baltimore, MD: Williams & Wilkins. [Google Scholar]
- 33.Thomas P, Sekhar AC, Upreti R, Mujawar MM, Pasha SS. 2015. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol. Rep. (Amst) 8, 45–55. ( 10.1016/j.btre.2015.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zvikelsky O, Weisbrod N. 2006. Impact of particle size on colloid transport in discrete fractures. Water Resour. Res. 42, W12S08 ( 10.1029/2006WR004873) [DOI] [Google Scholar]
- 35.Ariel G, Rabani A, Benisty S, Partridge JD, Harshey RM, Be'er A. 2015. Swarming bacteria migrate by Levy Walk. Nat. Commun. 6, 8396 ( 10.1038/ncomms9396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lutskiy MY, Avneri-Katz S, Zhu N, Itsko M, Ronen Z, Arnusch CJ, Kasher R. 2015. A microbiology-based assay for quantification of bacterial early stage biofilm formation on reverse-osmosis and nanofiltration membranes. Sep. Purif. Technol. 141, 214–220. ( 10.1016/j.seppur.2014.12.003) [DOI] [Google Scholar]
- 37.Djordjevic D, Wiedmann M, McLandsborough LA. 2002. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl. Environ. Microbiol. 68, 2950–2958. ( 10.1128/AEM.68.6.2950-2958.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busscher HJ, Weerkamp AH, van der Mei HC, Van Pelt AW, de Jong HP, Arends J. 1984. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl. Environ. Microbiol. 48, 980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg M, Rosenberg E. 1985. Bacterial adherence at the hydrocarbon-water interface. Oil Petrochem. Pollut. 2, 155–162. ( 10.1016/S0143-7127(85)90178-4) [DOI] [Google Scholar]
- 40.Esteves E, Fogaca AC, Maldonado R, Silva FD, Manso PPA, Pelajo-Machado M, Valle D, Daffre S. 2009. Antimicrobial activity in the tick Rhipicephalus (Boophilus) microplus eggs: cellular localization and temporal expression of microplusin during oogenesis and embryogenesis. Dev. Comp. Immunol. 33, 913–919. ( 10.1016/j.dci.2009.02.009) [DOI] [PubMed] [Google Scholar]
- 41.Zimmer KR, Macedo AJ, Nicastro GG, Baldini RL, Termignoni C. 2013. Egg wax from the cattle tick Rhipicephalus (Boophilus) microplus inhibits Pseudomonas aeruginosa biofilm. Ticks Tick Borne Dis. 4, 366–376. ( 10.1016/j.ttbdis.2013.01.005) [DOI] [PubMed] [Google Scholar]
- 42.Zimmer KR, Seixas A, Conceicao JM, Zvoboda DA, Barros MP, Tasca T, Macedo AJ, Termignoni C. 2013. Cattle tick-associated bacteria exert anti-biofilm and anti-Tritrichomonas foetus activities. Vet. Microbiol. 164, 171–176. ( 10.1016/j.vetmic.2013.01.029) [DOI] [PubMed] [Google Scholar]
- 43.Buffkin DC, Russell FE, Deshmukh A. 1971. Preliminary studies on the toxicity of black widow spider eggs. Toxicon 9, 393–402. ( 10.1016/0041-0101(71)90138-3) [DOI] [PubMed] [Google Scholar]
- 44.Yan S, Wang X. 2015. Recent advances in research on widow spider venoms and toxins. Toxins (Basel) 7, 5055–5067. ( 10.3390/toxins7124862) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bixler GD, Bhushan B. 2014. Rice- and butterfly-wing effect inspired self-cleaning and low drag micro/nanopatterned surfaces in water, oil, and air flow. Nanoscale 6, 76–96. ( 10.1039/c3nr04755e) [DOI] [PubMed] [Google Scholar]
- 46.Nishimoto S, Bhushan B. 2013. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 3, 671–690. ( 10.1039/C2RA21260A) [DOI] [Google Scholar]
- 47.Yuan Y, Hays MP, Hardwidge PR, Kim J. 2017. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 7, 14 254–14 261. ( 10.1039/C7RA01571B) [DOI] [Google Scholar]
- 48.Board RG. 1982. Properties of avian egg-shells and their adaptive value. Biol. Rev. 57, 1–28. ( 10.1111/j.1469-185X.1982.tb00362.x) [DOI] [Google Scholar]
- 49.D'Alba L, Jones DN, Badawy HT, Eliason CM, Shawkey MD. 2014. Antimicrobial properties of a nanostructured eggshell from a compost-nesting bird. J. Exp. Biol. 217, 1116–1121. ( 10.1242/jeb.098343) [DOI] [PubMed] [Google Scholar]
- 50.Liu JL, Feng XQ, Wang GF, Yu SW. 2007. Mechanisms of superhydrophobicity on hydrophilic substrates. J. Phys. Condens. Matter 19, 356002 ( 10.1088/0953-8984/19/35/356002) [DOI] [Google Scholar]
- 51.Choi W, Tuteja A, Mabry JM, Cohen RE, Mckinley GH. 2009. A modified Cassie–Baxter relationship to explain contact angle hysteresis and anisotropy on non-wetting textured surfaces. J. Colloid Interface Sci. 339, 208–216. ( 10.1016/j.jcis.2009.07.027) [DOI] [PubMed] [Google Scholar]
- 52.Morishita R, Aparecida Ferreira S, Santiago Filha A, Ditzel Faraco C. 2003. Studies on oogenesis and oviposition in the brown spider Loxosceles intermedia (Araneae: Sicariidae). Anat. Rec. A 273A, 575–582. ( 10.1002/ar.a.10062) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided in full in the results section of this paper. Additional supporting data of this study are provided as electronic supplementary material accompanying this paper.