TO THE EDITOR:
The National Action Plan for Combating Antibiotic-Resistant Bacteria set a goal of reducing inappropriate outpatient antibiotic use by 50% by 2020.1 A recent study2 estimated at least 30% of antibiotic prescriptions in ambulatory care settings in the United States during 2010–2011 were unnecessary. Inappropriate antibiotic prescribing also includes choosing an unnecessarily broad-spectrum antibiotic instead of an equally or more effective narrower-spectrum alternative. Otitis media (OM), sinusitis and pharyngitis collectively account for nearly one-third of all antibiotics prescribed in outpatient settings2 and professional guidelines recommend narrow-spectrum agents as first-line therapy for these conditions.2 Alternatives to first-line therapy are indicated in select circumstances, including for patients with penicillin allergy or recent treatment failure. The objective of this study was to measure the frequency with which first-line agents are prescribed for OM, sinusitis and pharyngitis.
METHODS
We identified antibiotic prescribing visits during 2010–2011 for OM (patients ≤19 years old only), sinusitis and pharyngitis using the National Ambulatory Medical Care Survey (NAMCS, which samples office-based physicians) and the National Hospital Ambulatory Medical Care Survey (NHAMCS, which samples hospital outpatient and emergency departments). The International Classification of Diseases, Ninth Revision, Clinical Modification codes were used to identify visits and assign diagnoses as described previously.2
National guidelines recommend first-line antibiotic therapy for each condition: amoxicillin or amoxicillin with clavulanate (alternative) for OM; amoxicillin or amoxicillin with clavulanate for sinusitis; and penicillin or amoxicillin for pharyngitis.2 First-line therapy represents the initial recommended antibiotics for treatment of patients without drug allergies, including alternative therapy indicated for specific situations, e.g., amoxicillin-clavulanate for OM with concurrent conjunctivitis. First-line therapy would not apply to treated patients returning for unplanned follow-up care with worsening symptoms suggesting treatment failure.
For each condition, the primary outcome was the percentage of visits that received first-line antibiotics (stratified by age: pediatric, ≤19, adult >19). All analyses were performed using Stata 12 (Stata Corp, College Station, TX).
RESULTS
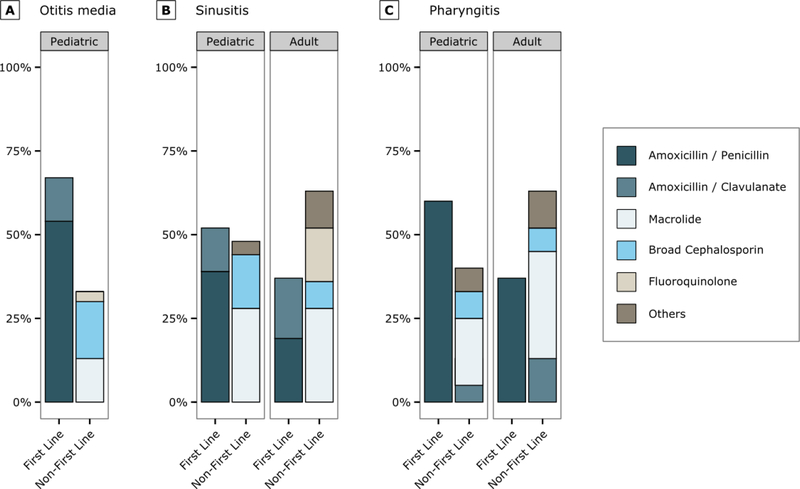
During 2010–2011, among visits where antibiotics were prescribed, physician prescribing of first-line antibiotics ranged from a low of 37% (95% CI: 32–43%) for adult patients with sinusitis and pharyngitis to a high of 67% (95% CI: 63–71%) for pediatric patients with OM (Figure). For all three conditions overall, use of first-line agents was 52% (95% CI: 49–55%). Physicians prescribed first line therapies more commonly to pediatric patients than to adults (p<0.001 for sinusitis and pharyngitis). The most common non-first-line antibiotic class prescribed was macrolides (Figure).
Figure.
Percentage of visits where antibiotics prescribed that are first line and non-first line for otitis media otitis media (A, first line: amoxicillin or amoxicillin/clavulanate), sinusitis (B, first line: amoxicillin or amoxicillin/clavulanate) and pharyngitis (C, amoxicillin or penicillin) during 2010–2011. Estimates based on 1705 sampled visits for otitis media, 463 for pediatric sinusitis, 1223 for adult sinusitis, 1006 for pediatric pharyngitis and 830 for adult pharyngitis.
Broad cephalosporin includes 2nd/3rd generation agents
Pediatric patients, ≤19 years old
COMMENT
Collectively, physicians prescribed first-line recommended antibiotics approximately half of the time during visits for OM, sinusitis and pharyngitis. Overuse of non-first line agents, especially macrolides, was higher for adults than children. Available evidence suggests that 10% of the population reports penicillin allergy3 and 10% of visits for sinusitis4 and OM result from failed first-line therapy5 suggesting that approximately 80% of visits for these diagnoses should be treated with first-line therapy.
This study has limitations. It was not possible to confirm the presence of allergy or previous treatment history, factors that influence appropriate antibiotic selection. These data are from 2010–11, but are the most recent complete data available from NAMCS/NHAMCS and we do not have reason to believe practice patterns have substantially changed.
This study provides evidence of substantial overuse of non-first-line antibiotics for three of the most common conditions in ambulatory care that collectively account for > 40 million antibiotic prescriptions annually.2 These findings indicate that the problem of inappropriate antibiotic prescribing includes not only prescriptions that are unnecessary altogether, but also selection of inappropriate agents.2 As a result, stewardship interventions should address both antibiotic overuse and inappropriate antibiotic selection to improve patient safety and healthcare quality. Implementation of stewardship strategies is a key component to meeting the National Action Plan goal of reducing inappropriate antibiotic use by 50% in outpatient settings.1
Acknowledgements:
The authors would like to thank Tia Carter, MS, Allan Coukell, BSc Pharm, and Elizabeth Jungman, JD, MPH, of The Pew Charitable Trusts for their assistance in convening author meetings. The above named individuals did not receive any compensation for their role in the study.
Funding: This project was made possible through a partnership with the Centers for Disease Control and Prevention (CDC) Foundation. Support for this project was provided by Pew Charitable Trusts.
Role of the Sponsor/Funder statement: CDC participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The Pew Charitable Trusts participated in the interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The Pew Charitable Trusts sponsored in-person and telephone author meetings and supported some author travel to in-person meetings.
Conflict of Interests/Financial Disclosures:
Dr. Hersh has received funding from the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, Pfizer/Joint Commission, and Merck.
Footnotes
Access to data: Dr. Hersh and Mr. Shapiro had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Outpatient Antibiotic Use Target-Setting Workgroup Collaborators: Monina Bartoces, PhD, Centers for Disease Control and Prevention, Atlanta, GA; Eva A. Enns PhD, Division of Health Policy & Management, University of Minnesota, Minneapolis, MN; Thomas M. File Jr MD, Summa Health System and Northeast Ohio Medical University, Akron, OH; Jonathan A. Finkelstein, MD MPH, Boston Children’s Hospital and Harvard Medical School, Boston, MA; Jeffrey S. Gerber, MD PhD, Children’s Hospital of Philadelphia and University of Pennsylvania School of Medicine, Philadelphia, PA; David Y. Hyun, MD, The Pew Charitable Trusts, Washington, DC; Jeffrey A. Linder MD MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA; Ruth Lynfield, MD, Minnesota Department of Health, St. Paul, MN; David J. Margolis, MD PhD, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA; Larissa S. May, MD MSPH, Department of Emergency Medicine, University of California-Davis, Sacramento, CA; Daniel Merenstein, MD, Department of Family Medicine, Georgetown University Medical Center, Washington, DC; Joshua P. Metlay, MD PhD, Division of General Internal Medicine, Massachusetts General Hospital, Boston, MA; Jason G. Newland, MD MEd, Division of Pediatric Infectious Diseases, Washington University School of Medicine, St. Louis, MO; Jay F. Piccirillo, MD, Department of Otolaryngology-Head and Neck Surgery, Washington University School of Medicine, St. Louis, MO; Rebecca M. Roberts, MS, Centers for Disease Control and Prevention, Atlanta, GA; Guillermo Sanchez MPH, PA-C, Centers for Disease Control and Prevention, Atlanta, GA; Katie J. Suda, PharmD, MS, Department of Veterans Affairs, University of Illinois at Chicago, Chicago, IL; Ann Thomas, MD MPH, Oregon Public Health Division, Portland, OR; Teri Moser Woo, PhD, Pacific Lutheran University, Tacoma, WA; Rachel M. Zetts, The Pew Charitable Trusts, Washington, DC;
Contributor Information
Adam L. Hersh, Pediatric Infectious Diseases, University of Utah, Salt Lake City, UT.
Katherine E. Fleming-Dutra, Centers for Disease Control and Prevention, Atlanta, GA.
Daniel J. Shapiro, School of Medicine, University of California, San Francisco, San Francisco, CA.
David Y. Hyun, The Pew Charitable Trusts, Washington, DC.
Lauri A. Hicks, Centers for Disease Control and Prevention, Atlanta, GA.
References
- 1.The White House. National action plan for compating antibiotic resistant bacteria. https://www.whitehouse.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed August 3, 2015.
- 2.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010–2011. JAMA. 2016;315(17):1864–1873. [DOI] [PubMed] [Google Scholar]
- 3.Joint Task Force on Practice P, American Academy of Allergy A, Immunology, et al. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259–273. [DOI] [PubMed] [Google Scholar]
- 4.Piccirillo JF, Mager DE, Frisse ME, Brophy RH, Goggin A. Impact of first-line vs second-line antibiotics for the treatment of acute uncomplicated sinusitis. JAMA. 2001;286(15):1849–1856. [DOI] [PubMed] [Google Scholar]
- 5.Capra AM, Lieu TA, Black SB, Shinefield HR, Martin KE, Klein JO. Costs of otitis media in a managed care population. Pediatr Infect Dis J. 2000;19(4):354–355. [DOI] [PubMed] [Google Scholar]