Abstract
Background:
Serrated colorectal lesions include hyperplastic polyps (HPs) and sessile serrated adenomas (SSAs). Optical biopsy could misclassify SSAs as unimportant if they resemble HPs.
Objective:
To explore the narrow-band imaging (NBI) features of SSAs. We hypothesized that SSAs resemble HPs under NBI.
Design:
Retrospective analysis of data from our prospective study of NBI in routine practice.
Setting:
Single specialty group.
Patients:
Patients undergoing colonoscopy.
Intervention:
Colonoscopy.
Main Outcome Measurements:
Polyp histology prediction by community gastroenterologists. Features of SSAs versus HPs and adenomas by using the Narrow-Band Imaging International Colorectal Endoscopic (NICE) Classification.
Results:
Among 2388 lesions, 141 were diagnosed on pathology as SSAs, 465 as HPs, and 1546 as adenomas. Each individual NICE feature of HPs was found in 38% to 42% of SSAs, 66% to 67% of HPs, and 15% to 20% of adenomas (P < .001 for each). Each individual NICE feature of adenomas was found in 57% to 62% of SSAs, 33% to 34% of HPs, and 80% to 84% of adenomas (P < .001 for each). Compared with HPs, SSAs were less likely (odds ratio [OR] 0.74; 95% confidence interval [CI], 0.69–0.79) and adenomas were even less likely (OR 0.62; 95% CI, 0.59–0.64) to have all 3 NICE features of HPs. SSAs >5 mm were more likely than smaller SSAs to have all 3 NICE features of adenomas. SSA location did not predict NBI features. Analyses restricted to high-confidence lesions showed similar results.
Limitations:
The endoscopists were not NBI experts.
Conclusion:
Community gastroenterologists observed a profile of NICE features among SSAs that was intermediate to the profiles observed for HPs and adenomas. These results require confirmation by NBI experts.
Relatively recently, sessile serrated adenomas (SSAs) have come to be appreciated as potentially premalignant colon lesions. SSAs belong to the group of serrated lesions that includes hyperplastic polyps (HPs) and traditional serrated adenomas. SSAs were first described in 19901 as a part of a group of histologically distinct variants of colorectal polypoid epithelial neoplasms. Since the initial description of SSAs, the criteria for pathologic diagnosis of SSAs have become more specific, including crypt serration, crypt dilation, and the presence of horizontal glands at the base.2–5 SSAs rarely have dysplasia, in contrast to traditional serrated adenomas.3,6 However, SSAs still can progress to cancer through the serrated neoplasia pathway and can lead to sporadic microsatellite instability with a high colon cancer rate.7–9 The endoscopic characteristics of SSAs have not been well-defined. Observational evidence suggests that SSAs usually are larger than HPs and are more frequently found in the right side of the colon.2,3,5,10,11
Real-time endoscopic assessment of colorectal polyp histology (optical biopsy12,13) could improve patient care14 and reduce costs.15,16 Accurate predictions could decrease resection rates of innocuous HPs as well as the need to submit all polyps for histopathology review, reducing costs and possibly adverse events. However, if SSAs were to resemble HPs under optical biopsy, SSAs could be misclassified as innocuous, adversely affecting decisions about resection or surveillance.
Narrow-band imaging (NBI) uses narrow-band light filters to highlight mucosal architecture and vasculature. The NBI International Colorectal Endoscopic (NICE) Classification describes criteria to distinguish adenomas from HPs based on color, vessels, and surface pattern.17 The accuracy of histology assessment by using NBI is greater for polyps assessed with high versus low confidence.12,16
Our objective was to explore the features of SSAs under NBI that were observed by community gastroenterologists in our recent prospective evaluation of real-time colorectal polyp optical biopsy with NBI.16 Because SSAs are serrated lesions like HPs, we hypothesized that most SSAs would resemble HPs under NBI. We report our retrospective analysis of data from our recent prospective study.
METHODS
General study design
This was a retrospective analysis of data collected in our recent prospective study of NBI in routine clinical practice, which was designed to evaluate real-time optical biopsy of colon polyps by community-based gastroenterologists. During each study colonoscopy, up to 6 polyps were assessed. For the current study, we examined which NICE features the endoscopists reported during in vivo assessment of lesions later diagnosed on histopathology as SSAs, HPs, or adenomas. We performed a sensitivity analysis including only lesions that met the strict definition of high confidence, that is, lesions having 1 or more features associated with 1 histology (HP or adenoma) and no features associated with the other histology.12,16
Setting and patients
Our recent study included 14 gastroenterologists from a single-specialty practice in Ann Arbor, Michigan. Endoscopy suites were equipped with Evis Exera II CV-180 processors, CF-H180AL and PCF-H180AL colonoscopes (Olympus America, Center Valley, PA), and high-definition monitors. The institutional review boards of St. Joseph Mercy Hospital in Ann Arbor, Michigan and Stanford University approved the study.
The details of that study have been described previously.16 In brief, participating gastroenterologists first engaged in an ex vivo study phase with 3 self-administered components: a pre-test, a learning module that teaches the NICE classification, and a post-test. Then they engaged in an in vivo practice-based learning program that included real-time optical diagnosis with NBI, comparison to final pathology diagnosis, and confidential feedback on individual performance every 1 to 2 weeks. For each study polyp, participants recorded the location, size, morphology, confirmation of photograph acquisition under white light and NBI, individual NICE features observed, predicted histology by using NBI (hyperplastic lesion, adenoma, or other, with explanation), and the level of confidence. Histology was not predicted based on white light features alone. Each study polyp was resected and sent for pathology in an individual jar. Three community-based pathologists who were fellowship-trained and specialize in GI pathology interpreted all specimens. Criteria for the histopathologic diagnosis of SSA included basal crypt dilation, horizontal crypts, branched crypts, basal crypt serration, absent or subtle cytologic dysplasia, absence of a subepithelial collagen band, round-to-oval nuclear shape, and prominent cytoplasmic eosinophilia.18 Twelve of 13 participants identified adenomas with >90% accuracy at the end of the ex vivo study, and 3 of 12 participants did so with accuracy >90% in the in vivo study.16
All patients who were scheduled for colonoscopy at the single-specialty practice in Ann Arbor, Michigan, from March 2011 to March 2012 were eligible. Demographic characteristics of patients and procedure indications were recorded. Procedure indications were categorized as colorectal cancer screening or surveillance, bleeding and/or anemia, change in bowel habits, abdominal pain, and miscellaneous.
NICE features of lesions
The principal variables of interest were the NBI features of SSAs compared with HPs and adenomas used in the NICE classification. The NICE classification addresses color, vessels, and surface pattern. With the use of this system, a polyp is likely to be hyperplastic if it is the same color or lighter color than the background color, if there are no vessels or isolated lacy vessels, and if the surface pattern consists of dark or white spots of uniform size or if there is a homogeneous lack of pattern. A polyp is likely to be adenomatous if it is browner relative to the background color, if there are brown vessels surrounding white structures, and if the surface pattern consists of oval, tubular, or branched white structures surrounded by brown vessels.17 However, the features of HPs and adenomas are not mutually exclusive. Lesions displaying features of both HPs and adenomas were classified as low confidence.
Statistical analysis
Descriptive analyses are presented by using frequency tables and figures. Chi-square statistics were used to compare demographic characteristics of patients who had at least 1 SSA removed versus those who had none and to evaluate whether the likelihood of having each individual NICE feature of HPs, and alternatively adenomas, varied by lesion type.
To address our primary hypothesis, we explored whether a given polyp was described by the endoscopist as having exclusively the NICE features of adenomas or exclusively the NICE features of HPs. Logistic regression analyses with a random intercept for endoscopist were used to estimate the association between the outcome of NICE features (exclusively HP or adenoma features) and the variable of interest, lesion histopathology. For each outcome, we tested a composite null hypothesis at the 0.05 level of significance.
We used similar logistic regression techniques to relate characteristics of SSAs to the outcomes defined earlier. Characteristics of interest included size, location, and timing of the study as potential predictors. Size was defined as diminutive (<6 mm), small (6–9 mm), or large (≥10 mm); location was defined as rectosigmoid colon or proximal to the rectosigmoid colon; and endoscopist experience was dichotomized into “second half of the original study” versus “first half of the original study.” Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) are reported.
Because 32 statistical tests were conducted for secondary analyses, we adjusted for multiple comparisons by applying the Bonferroni correction, and we report adjusted P values.
All lesions that included an optical diagnosis based on NBI features were included in primary analyses. All analyses were performed initially for all lesions. Sensitivity analyses were then performed conditionally on only those lesions that met the strict definition of high confidence, as described earlier. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Patient demographic characteristics and procedure indications
Lesions were removed from 1675 patients undergoing colonoscopy. Among these, 129 patients had at least 1 SSA removed.
The median age of patients was 60.9 years and 58.0 years for patients who had at least 1 SSA (Table 1). Among all patients, 719 (42.9%) were women. A higher percentage of patients who had at least 1 SSA were women compared with patients who had no SSAs (51.9% vs 42.2%; P = .031) (Table 1). Colorectal cancer screening or surveillance were the indications in >80% of procedures, with a minority of procedures including bleeding and/or anemia, change in bowel habits, abdominal pain, and miscellaneous as the indication (Table 1).
TABLE 1.
Patient demographics and procedure indications
| All patients (n = 1675) | At least 1 SSA (n = 129) | No SSA (n = 1546) | P value | |
|---|---|---|---|---|
| Age, median (IQR), y | 60.9 (54.0–68.6) | 58.0 (51.7–68.2) | 61.0 (54.2–68.6) | .26 |
| Female, no. (%) | 719 (42.9) | 67 (51.9) | 652 (42.2) | .03 |
| Indication, no. (%) | ||||
| Surveillance | 694 (41.4) | 47 (36.4) | 647 (41.9) | .23 |
| CRC screening | 740 (44.2) | 62 (48.1) | 678 (43.9) | .36 |
| Bleeding and/or anemia | 178 (10.6) | 15 (11.6) | 163 (10.5) | .70 |
| Change in bowel habits | 102 (6.1) | 6 (4.7) | 96 (6.2) | .48 |
| Abdominal pain | 59 (3.5) | 8 (6.2) | 51 (3.3) | .09 |
| Miscellaneous | 57 (3.4) | 5 (3.9) | 52 (3.4) | .76 |
SSA, Sessile serrated adenoma; IQR, interquartile range; CRC, colorectal cancer.
Histopathologic diagnosis of lesions
In total, 2596 polyps were resected, and 2388 were assigned an optical diagnosis by the endoscopist based on NBI features. Of these, 141 were diagnosed on histopathology as SSAs, 465 as HPs, 1546 as adenomas, and the remainder as no histopathologic abnormality or miscellaneous diagnoses. Among lesions satisfying the definition of high confidence for NBI assessment, 115 were diagnosed on histopathology as SSAs, 418 as HPs, and 1384 as adenomas. No cytologic dysplasia was reported in any SSA.
NICE features: color, vessels, surface pattern
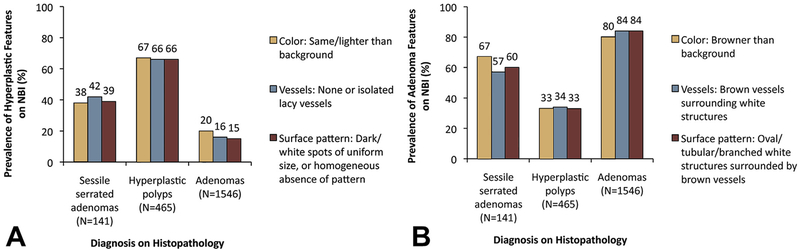
Among all lesions, each individual NICE feature of HPs was found in 38% to 42% of SSAs, 66% to 67% of HPs, and 15% to 20% of adenomas (P < .001 for each comparison between SSAs and HPs and between SSAs and adenomas) (Fig. 1A). In contrast, among all lesions, each individual NICE feature of adenomas was found in 57% to 67% of SSAs, 33% to 34% of HPs, and 80% to 84% of adenomas (P < .001 for each comparison between SSAs and HPs and between SSAs and adenomas) (Fig. 1B).
Figure 1.
A, Fractions of all lesions reported as having each individual Narrow-Band Imaging International Colorectal Endoscopic (NICE) Classification feature (color, vessels, surface pattern) characteristic of hyperplastic polyps. B, Fractions of all lesions reported as having each individual NICE classification feature characteristic of adenomas. P < .001 for each comparison between sessile serrated adenomas and hyperplastic polyps and between sessile serrated adenomas and adenomas. NBI, narrow-band imaging.
Among all lesions, all 3 NICE features of HPs were found in 43 SSAs (31%), 283 HPs (61%), and 191 adenomas (12%) (adjusted P < .001 for SSAs vs HPs and SSAs vs adenomas). In contrast, all 3 NICE features of adenomas were found in 70 SSAs (50%), 133 HPs (29%), and 1185 adenomas (77%) (adjusted P < .001 for SSAs vs HPs and SSAs vs adenomas). Addressing our primary outcome, compared with HPs, SSAs were less likely (OR 0.74; 95% CI, 0.69–0.79) and adenomas were even less likely (OR 0.62; 95% CI, 0.59–0.64) to have exclusively the 3 NICE features of HPs (P value for composite null hypothesis < .001). Conversely, compared with adenomas, SSAs were less likely (OR 0.78; 95% CI, 0.73–0.84) and HPs were even less likely (OR 0.62; 95% CI, 0.59–0.65) to have exclusively the 3 NICE features of adenomas (P value for composite null hypothesis < .001).
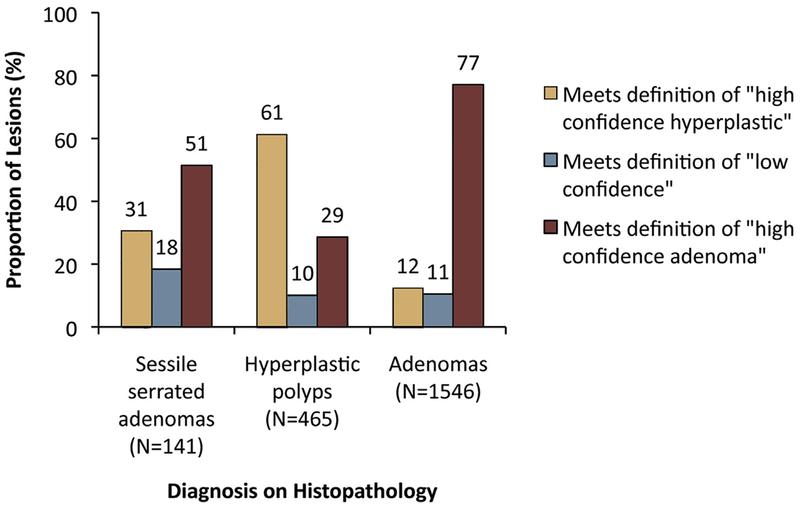
When lesions were categorized as fulfilling the criteria for high-confidence HPs or adenomas, or low confidence, SSAs demonstrated a broader spectrum of features than HPs and adenomas, including a higher proportion of lesions with mixed features (low confidence) (Fig. 2).
Figure 2.
Fractions of lesions fulfilling the criteria for high confidence hyperplastic polyps or adenomas (defined as having 1 or more features associated with 1 histology and no features associated with the other) or low confidence (defined as having features of both histologies). Sessile serrated adenomas demonstrated a broader spectrum of features than did hyperplastic polyps or adenomas.
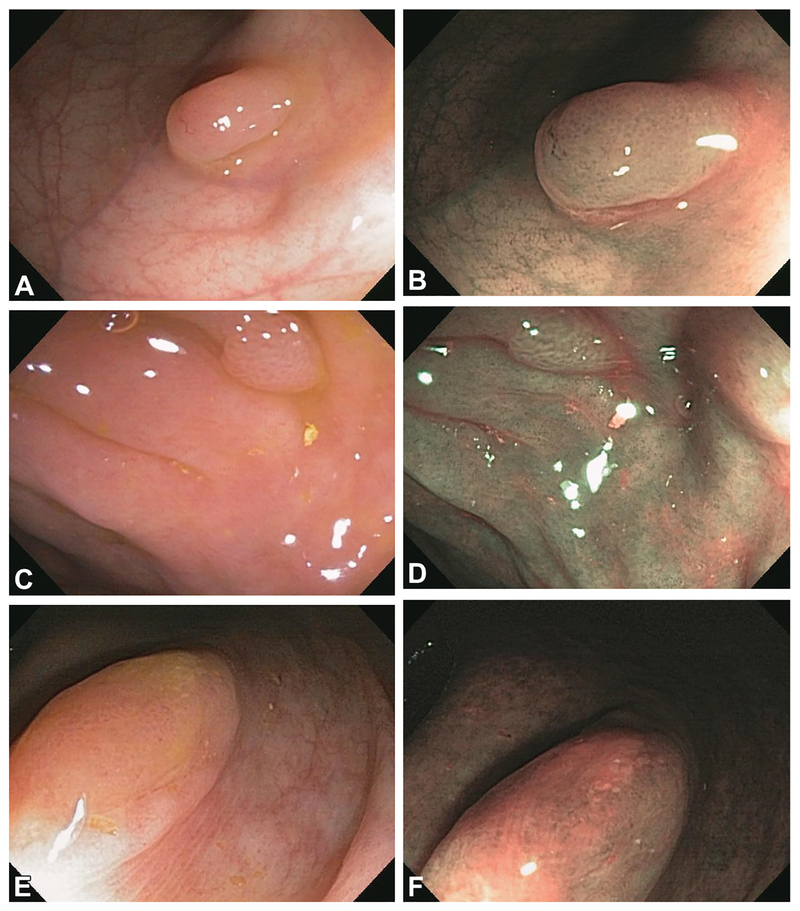
Figure 3 shows examples under white light and NBI of an HP, adenoma, and SSA assessed and removed in our original study.
Figure 3.
Examples of histopathologically confirmed hyperplastic polyps under white light (A) and NBI (B), a histopathologically confirmed adenoma under white light (C) and NBI (D), and a histopathologically confirmed sessile serrated adenoma under white light (E) and NBI (F) assessed and removed in our original prospective study. The endoscopist who removed the sessile serrated adenoma reported seeing in it the Narrow-Band Imaging International Colorectal Endoscopic Classification features of hyperplastic polyp for color (same or lighter than the background) and of adenoma for vessels (brown vessels surrounding white structures) and surface pattern (oval, tubular, or branched white structures surrounded by brown vessels). NBI, narrow-band imaging.
Sensitivity analysis: lesions assessed with high confidence
When the analysis was restricted to only the lesions that satisfied the definition of high confidence, slightly lower fractions of both SSAs and adenomas had the individual NICE features of HPs, and slightly higher fractions had the individual NICE features of adenomas. Among these high-confidence lesions, all 3 NICE features of HPs were found in 43 SSAs (37%), 283 HPs (68%), and 190 adenomas (14%) (P < .001 for SSAs vs HPs and SSAs vs adenomas). In contrast, all 3 NICE features of adenomas were found in 70 SSAs (61%), 133 HPs (32%), and 1184 adenomas (86%) (P < .001 for SSAs vs HPs and SSAs vs adenomas). For these high-confidence lesions, compared with HPs, SSAs were less likely (OR 0.74; 95% CI, 0.68–0.80) and adenomas were even less likely (OR 0.59; 95% CI, 0.56–0.61) to have exclusively the 3 NICE features of HPs (P value for composite null hypothesis < .001). Conversely, for these high-confidence lesions, compared with adenomas, SSAs were less likely (OR 0.79; 95% CI, 0.74–0.85) and HPs were even less likely (OR 0.59; 95% CI, 0.56–0.61) to have exclusively the 3 NICE features of adenomas (P value for composite null hypothesis < .001).
Lesion size and location and endoscopist experience
The fractions of SSAs with the individual NICE features of HPs, the individual NICE features of adenomas, all 3 NICE features of HPs exclusively, and all 3 NICE features of adenomas exclusively—stratified by size, location in the colorectum, and endoscopist experience—are shown in Table 2.
TABLE 2.
Features of sessile serrated adenomas stratified by size, location in the colorectum, and endoscopist experience
| Stratified by size* no (%) |
Stratified by location no (%) |
Stratified by time during clinical study no (%) |
|||||
|---|---|---|---|---|---|---|---|
| Diminutive | Small | Large | Rctosigmoid colon | Proximal to rectosigmoid colon | 1st Half | 2nd Half | |
| 71 (51) | 48 (34) | 21 (15) | 25 (18) | 116 (82) | 37 (26) | 104 (74) | |
| NBI features of hyperplastic polyps | |||||||
| Color: same/lighter than background | 35 (49) | 16 (33) | 2 (10) | 13 (52) | 41 (35) | 20 (54) | 34 (33) |
| Vessels: none or isolated lacy vessels | 38 (54) | 17 (35) | 3 (14) | 13 (52) | 46 (40) | 21 (57) | 38 (37) |
| Surface pattern: dark/white spots of uniform size or homogenous absence of pattern | 36 (51) | 15 (31) | 3 (14) | 12 (48) | 43 (37) | 21 (57) | 34 (33) |
| NBI features of adenomas | |||||||
| Color: browner than background | 36 (51) | 32 (67) | 19 (91) | 12 (48) | 75 (65) | 17 (46) | 70 (67) |
| Vessels: brown vessels surrounding white structures | 33 (44) | 30 (63) | 17 (81) | 12 (48) | 68 (59) | 16 (43) | 64 (62) |
| Surface pattern: oval/tubular/branched white structures surrounded by brown vessels | 35 (49) | 32 (67) | 17 (81) | 13 (52) | 71 (61) | 16 (43) | 68 (65) |
| Polyps with all 3 NBI features of hyperplastic polyps | 27 (38) | 13 (27) | 2 (9) | 11 (44) | 32 (28) | 19 (51) | 24 (23) |
| Polyps with all 3 NBI features of adenomas | 25 (35) | 28 (58) | 17 (81) | 11 (44) | 59 (51) | 14 (38) | 56 (54) |
NBI, Narrow-band imaging.
One polyp was missing size.
Compared with diminutive SSAs, large (adjusted OR 0.78; 95% CI, 0.62–0.98) but not small (adjusted OR 0.91; 95% CI, 0.77–1.07) SSAs were less likely to have exclusively the 3 NICE features of HPs. Compared with diminutive SSAs, small (adjusted OR 1.21; 95% CI, 1.02–1.43) and large (adjusted OR 1.53; 95% CI, 1.20–1.94) SSAs were both more likely to have exclusively the 3 NICE features of adenomas.
Compared with SSAs found in the rectosigmoid colon, SSAs found proximal to the rectosigmoid colon were not more or less likely to have exclusively the 3 NICE features of HPs (adjusted OR 0.90; 95% CI, 0.74–1.10) or the 3 NICE features of adenomas (adjusted OR 0.97; 95% CI,0.79–1.19).
Compared with SSAs assessed during the first half of the clinical study, SSAs assessed during the second half of the clinical study were less likely to have exclusively the 3 NICE features of HPs (adjusted OR 0.76; 95% CI, 0.65–0.90), but they were not more or less likely to have exclusively the 3 NICE features of adenomas (adjusted OR 1.08, 95% CI,0.91–1.28).
The results of sensitivity analyses restricted to high-confidence lesions were very similar to the results presented earlier.
DISCUSSION
In this study, we explored the NBI features that were observed by community gastroenterologists in 141 SSAs compared with the NBI features observed in HPs and adenomas in our recent prospective trial of NBI in routine clinical practice. Because SSAs are serrated lesions like HPs, we hypothesized that most SSAs would resemble HPs under NBI. In contrast with our hypothesis, community gastroenterologists observed a profile of NICE features among SSAs that was intermediate to the profiles observed for HPs and adenomas. If these results are confirmed by endoscopists who have demonstrated high levels of accuracy in predicting the histology of HPs and adenomas based on NBI features (NBI experts), they could have implications for the implementation of widespread real-time endoscopic assessment of the histology of colorectal polyps.
SSAs are being increasingly recognized as having clinical significance. Although the histopathologic criteria for the diagnosis of SSAs have been increasingly well-characterized since they were initially described in 1990,2–5 the endoscopic characteristics of SSAs have not been well-defined. Based on observational studies, SSAs have variably been described as larger polyps that are found on the right side of the colon.2,3,5,10,11 Few studies have described the endoscopic appearance of SSAs when advanced imaging technologies were used. In a study that used chromoendoscopy, the surface patterns of 52 serrated adenomas were characterized as hyperplastic (small crypt openings) in 7 (41%), cerebriform (tortuous and cerebriform structures) in 4 (24%), and a combination of the two in 6 (35%).19 Those authors noted that inspection at colonoscopy might be insufficient to differentiate HPs from serrated adenomas, given that it was difficult to differentiate the hyperplastic pattern of serrated adenomas from the HPs themselves.19 In a more recent study that used NBI in patients with hyperplastic polyposis syndrome, the profiles of Kudo pit patterns and vascular pattern intensity observed in SSAs were intermediate to the patterns observed in HPs and adenomas but were closer to those of HPs than adenomas.20 In a recent study investigating the NBI characteristics of 50 SSAs ex vivo, in photographs and in patients with serrated polyposis, 4 experts suggested that cloud-like surface, indistinct borders, irregular shape, and dark spots inside the crypts were NBI features associated with SSAs.21 Our study differs from this recent study in that we studied prospective, real time, in vivo histology assessment by community gastroenterologists who used the NICE classification. In conjunction with our results, the observations of Hazewinkel et al21 suggest that features other than those included in the NICE classification might be necessary for accurate optical diagnosis of SSAs.
In our study, the profile of NBI features observed in SSAs by community gastroenterologists, when the NICE classification was used, was intermediate to the profiles observed in HPs and adenomas (Figs. 1A and 1B). The results of our sensitivity analysis restricted to lesions assessed with high confidence must be interpreted with caution. Overall, these results were similar to the results including all polyps. By definition, however, SSAs assessed with high confidence exclude those in which features of both HPs and adenomas were observed. Thus, this analysis minimizes, by design, any mixed features present in SSAs as a whole.
If most SSAs were to resemble HPs under NBI, as we hypothesized initially, SSAs could be mistaken for HPs during colonoscopy with optical biopsy and subsequently managed inappropriately. If management were guided only by high-confidence optical biopsies, then any lesion with mixed features would be resected and sent for histopathology. Therefore, SSAs with mixed features would not be misclassified solely based on NBI appearance. What matters, then, is the fraction of diminutive SSAs that would be assessed with high confidence, but incorrectly, as HPs with a “resect and discard” or a “diagnose and decide” strategy. Our results suggest that this fraction might be approximately one-third (Table 2). Future studies, including studies in which NBI assessment is performed by endoscopists who have demonstrated high levels of accuracy in performing optical biopsy with NBI and in which the histopathologic diagnosis of SSA is confirmed by more than 1 expert pathologist, are required to improve the estimate of the fraction of SSAs that are at risk of being assessed with NBI as innocuous HPs.
The SSAs resected in our study were more frequently assessed as diminutive, as opposed to small or large, than in some prior studies. SSA size affected the prevalence of NICE features of adenomas observed in the SSAs, with larger lesions more likely to resemble adenomas than smaller lesions. The SSAs resected in our study were predominantly located in the area proximal to the rectosigmoid colon when compared with the rectosigmoid area, which is similar to what has been seen in prior studies. Among SSAs, location was not a predictor for displaying the NBI features of either HPs or adenomas. Under a policy in which any proximal polyp is resected regardless of NBI features, then all proximal SSAs would be removed. However, misclassification of proximal SSAs as HPs could lead to surveillance recommendations that do not adhere with current guidelines.
In this study, among all patients with at least 1 lesion removed at colonoscopy, at least 1 SSA was found in 9.3% of women and in 6.5% of men (Table 1). Previous studies have demonstrated no difference in SSA predominance by sex10 or a slight predominance for women.22 Based on the available evidence, it may be reasonable for endoscopists to be particularly alerted to look for SSAs in women.
Our study has limitations. First, the gastroenterologists participating in our original study were not experts in NBI but were undergoing training in NBI for optical biopsy of polyps. However, it is notable that they usually observed the NICE features of HPs in histopathologically confirmed HPs and the NICE features of adenomas in histopathologically confirmed adenomas (Figs. 1A and 1B), particularly in high-confidence lesions. Thus, their observations for SSAs deserve careful consideration. We attempted to control for possible learning by examining the polyps discovered during the first versus the second half of the study but found no effect. Second, our study’s 3 pathologists were fellowship-trained and specialize in GI pathology, but each lesion underwent only 1 histopathologic interpretation in the course of usual clinical care. It is recognized that there can be interobserver disagreement between pathologists. Anecdotally, endoscopists who have developed refined skills in optical biopsy tend to have the opinion that the NBI features of SSAs indeed resemble those of HPs and that dark spots that might reflect open pits in SSAs might be misinterpreted by endoscopists less experienced with NBI as the NICE features of adenomas. Therefore, our results must be interpreted with caution and should be considered as hypothesis-generating only.
In conclusion, community gastroenterologists observed a profile of NICE features among SSAs that was intermediate to the profiles observed for HPs and adenomas. These results are not consistent with our hypothesis that most SSAs would resemble HPs. Clinically, the relevant question is what fraction of diminutive SSAs might be assessed with NBI with high confidence, but incorrectly, as HPs. Our results suggest that this fraction might be approximately one-third. These results should prompt future studies with rigorous research protocols for histopathologic diagnosis of SSAs and optical biopsy of SSAs by NBI experts, designed to improve the estimate of the fraction of SSAs that are at risk of being assessed with NBI as innocuous HPs.
Take-home Message.
Community gastroenterologists who were undergoing training in narrow-band imaging (NBI) observed a profile of features among sessile serrated adenomas (SSAs) that was intermediate to the profiles of hyperplastic polyps (HPs) and adenomas. Approximately one-third of diminutive SSAs were assessed with high confidence, but incorrectly, as HPs.
Future studies with rigorous research protocols for histopathologic diagnosis of SSAs and with optical biopsy of SSAs by NBI experts are required to further clarify the NBI features of SSAs.
Acknowledgments
DISCLOSURE: Grant support for U.L. was provided by the Division of Gastroenterology, Stanford University School of Medicine. No other financial relationships relevant to this publication were disclosed.
Abbreviations:
- HP
hyperplastic polyp
- NBI
narrow-band imaging
- NICE
NBI International Colorectal Endoscopic (classification)
- SSA
sessile serrated adenoma
REFERENCES
- 1.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas: a distinct form of colorectal neoplasia. Am J Surg Pathol 1990;14:524–37. [DOI] [PubMed] [Google Scholar]
- 2.Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology 2005;47: 32–40. [DOI] [PubMed] [Google Scholar]
- 3.Torlakovic E, Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol 2003;27:65–81. [DOI] [PubMed] [Google Scholar]
- 4.East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am 2008;37:25–46. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–29; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol 2008;32:21–9. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien MJ, Yang S, Clebanoff JL, et al. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol 2004;28:423–34. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol 2006;30: 1491–501. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg DW, Yang S, Pleau DC, et al. Mutations in BRAF and KRAS differentially distinguish serrated versus non-serrated hyperplastic aberrant crypt foci in humans. Cancer Res 2007;67:3551–4. [DOI] [PubMed] [Google Scholar]
- 10.Gurudu SR, Heigh RI, De Petris G, et al. Sessile serrated adenomas: demographic, endoscopic and pathological characteristics. World J Gastroenterol 2010;16:3402–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol 2011;106:229–40; [DOI] [PubMed] [Google Scholar]
- 12.Rex DK. Narrow-band imaging without optical magnification for histo-logic analysis of colorectal polyps. Gastroenterology 2009;136:1174–81. [DOI] [PubMed] [Google Scholar]
- 13.Ignjatovic A, East JE, Suzuki N, et al. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol 2009;10:1171–8. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Kahi C, O’Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011;73:419–22. [DOI] [PubMed] [Google Scholar]
- 15.Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol 2010;8:865–9; [DOI] [PubMed] [Google Scholar]
- 16.Ladabaum U, Fioritto A, Mitani A, et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology 2013;144:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oba S, Tanaka S, Sano Y, et al. Current status of narrow-band imaging magnifying colonoscopy for colorectal neoplasia in Japan. Digestion 2011;83:167–72. [DOI] [PubMed] [Google Scholar]
- 18.Li SC, Burgart L. Histopathology of serrated adenoma, its variants, and differentiation from conventional adenomatous and hyperplastic polyps. Arch Pathol Lab Med 2007;131:440–5. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto T, Mizuno M, Shimizu M, et al. Serrated adenoma of the colorectum: colonoscopic and histologic features. Gastrointest Endosc 1999;49:736–42. [DOI] [PubMed] [Google Scholar]
- 20.Boparai KS, van den Broek FJ, van Eeden S, et al. Hyperplastic polyposis syndrome: a pilot study for the differentiation of polyps by using high-resolution endoscopy, autofluorescence imaging, and narrow-band imaging. Gastrointest Endosc 2009;70:947–55. [DOI] [PubMed] [Google Scholar]
- 21.Hazewinkel Y, López-Cerón M, East JE, et al. Endoscopic features of sessile serrated adenomas: validation by international experts using high-resolution white-light endoscopy and narrow-band imaging. Gastrointest Endosc 2013;77:916–24. [DOI] [PubMed] [Google Scholar]
- 22.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 2010;63: 681–6. [DOI] [PubMed] [Google Scholar]