Rapid technical advances have enabled multiparametric magnetic resonance imaging (mpMRI) combined with magnetic resonance (MR)–targeted biopsy to become valuable tools for early detection of clinically significant prostate cancer (PCa) while reducing overdiagnosis of indolent PCa [1–6]. There has been concern, however, that the widespread implementation and acceptance of mpMRI could be impaired by a lack of standardisation of image acquisition, interpretation and reporting guidance, and inter- and intraobserver variability that could result in poor clinical test performance in daily practise [7].
To expedite clinical evaluation and large-scale implementation of mpMRI, in May 2010 AdMeTech Foundation’s International Prostate MRI Working Group recommended development of standards of clinical performance by establishing a prostate imaging reporting and assessment system using BI-RADS (Breast Imaging and Reporting Archiving Data System) as a model. Dickinson et al [8] attempted to develop criteria for standardised acquisition and interpretation of mpMRI, but they noted that it was extremely difficult to define such criteria, even among experts in the field, and that reliable implementation into daily clinical practice remained problematic. To overcome these limitations, the European Society of Urogenital Radiology (ESUR) developed consensus-based guidelines for prostate mpMRI, including clinical indications, minimal and optimal imaging acquisition protocols, and a structured category assessment system known as the Prostate Imaging and Reporting and Data System (PI-RADS) version 1 (PI-RADS v1) [9].
Since its publication in 2012, the PI-RADS v1 system has achieved some acceptance, especially in Europe, and has been validated in prospective studies, randomised trials, and systematic analyses. A recent systematic review and meta-analysis [10] evaluating 14 published studies using PI-RADS v1 showed pooled sensitivity and specificity of 78% (95% confidence interval [CI], 72–89%) and 79% (95% CI: 68– 86%), respectively, for detecting significant PCa, demonstrating that mpMRI significantly changes the risk distribution of men with newly diagnosed PCa towards an increased prevalence of high-risk disease. Improved risk management with better identification of significant versus insignificant cancers may lead to more specific and individualised treatment options and less overtreatment of indolent disease.
For PI-RADS v1, it was not specified exactly how to combine the scores from each MRI sequence to derive an overall category assessment. This led to confusion in its application, and variable approaches were used. This contributed to the variability of PI-RADS v1 performance [10]. To improve this performance, Vaché et al [11] suggested refinement of the weighting given to each individual mpMRI parameter.
In early 2012, a joint steering committee of the American College of Radiology, ESUR, and AdMeTech Foundation agreed to collaborate on the development of an improved PI-RADS version 2 (PI-RADS v2). The PI-RADS v2 document was released online in December 2014 [12]. The specific aims were to establish guidelines for minimum acceptable technical parameters for prostate mpMRI, to simplify and standardise the terminology and content of mpMRI reports, to develop assessment categories that summarise the levels of suspicion or risk of having significant PCa, to reduce variability in imaging interpretations, to educate and enhance communication with referring clinicians, to enable standardised data collection for outcomes monitoring, and to facilitate quality assurance and research with the overall aims of improving patient outcomes. PI-RADS v2 is intended to be a “living” document that evolves as clinical experience and scientific validation data accrue.
The complete PI-RADS v2 document includes information regarding clinical considerations and technical specifications for mpMRI, normal anatomy and benign findings, guidelines and caveats for assessment and reporting of prostate mpMRI examinations, figures illustrating relevant findings on MR images, a diagram for mapping of findings, report templates, and a lexicon of terminology. An online atlas of findings and cases is also being developed as a learning and reference tool (http://www.acr.org/Quality-Safety/Resources/PIRADS). This paper provides a short description of PI-RADS v2. It provides discussion of some of the key differences and improvements compared with PI-RADS v1 (Table 1) and is focussed on the assessment criteria for detection and diagnosis of significant PCa on mpMRI examinations and clinical uses and limitations.
Table 1 –
Comparison of Prostate Imaging and Reporting and Data System versions 1 and 2
| PI-RADS v1 | PI-RADS v2 |
|---|---|
| A sum score of 3–15 (20 with MRSI) for T2W + DWI + DCE (+ MRSI) is suggested | 1–5 point dominant score: • For peripheral zone, DWI is dominant • For transition zone, T2W is dominant |
| Equal role for DCE (5-point scale) | Secondary role for DCE (positive or negative) |
| For DWI: ADC images are mandatory | For DWI: ADC and high b-value images (b value >1400) are mandatory |
| 27-Sector map | 39-Sector map |
| MRSI can be included | MRSI is not included |
| Size is not used for T2W + DWI | Size (>15 mm) is used for T2W + DWI to separate PI-RADS scores 4 and 5 |
ADC = apparent diffusion coefficient; DCE = dynamic contrast-enhanced imaging; DWI - diffusion weighted imaging; MRSI = magnetic resonance spectroscopic imaging; PI-RADS = Prostate Imaging and Reporting and Data System; T2W = T2-weighted imaging; v = version.
PI-RADS v2 is not intended to be a comprehensive PCa diagnosis manual; it should be used in conjunction with other resources. Its intended clinical application is for the diagnostic evaluation and risk assessment of patients with suspected PCa prior to or after transrectal ultrasound (TRUS) biopsy. It has not been developed for detecting suspected recurrent PCa following therapy.
1. Technical considerations for image acquisition
The prostate mpMRI acquisition protocol should always include T2-weighted (T2W) and T1-weighted (T1W) sequences, diffusion weighted imaging (DWI), and dynamic contrast-enhanced imaging (DCE) sequences. Technologists performing the examination and/or supervising radiologists should undertake quality control of images. If image quality of a pulse sequence is compromised because of patient motion or another reason, measures should be taken to rectify the problem and, if possible, the sequence should be repeated.
Prostate mpMRI at both 1.5 and 3 T has become well established, and satisfactory technical results have been obtained at both magnetic field strengths; however, most members of the PI-RADS steering committee prefer, use, and recommend 3 T for prostate MRI. Performing mpMRI at magnetic field strengths <1.5 T is not advised. At this time, there is no consensus among experts concerning the potential benefits of the use of endorectal coils for cancer detection, and their use varies according to the clinical situation, local expertise, and available equipment. Taking these factors into consideration, the supervising radiologist should optimise imaging protocols to obtain the best and most consistent image quality possible on the MRI scanner used at the particular institution or centre.
1.1. T1-weighted images
T1W images are used primarily to determine the presence of postbiopsy haemorrhage within the prostate and seminal vesicles and to delineate the gland boundary. T1W images may also be useful for detection of nodal and skeletal metastases (the latter in the context of preliminary tumour staging when a highly suspicious prostatic lesion is detected, before biopsy confirmation).
1.2. T2-weighted images
T2W images are used to discern prostatic zonal anatomy; to assess abnormalities within the gland, especially in the transition zone (TZ); and to evaluate the patient for seminal vesicle invasion or extraprostatic extension. The quality of these images should be as high as possible because they are the key images for detecting significant cancers, especially in the TZ.
1.3. Diffusion weighted imaging
DWI reflects and measures the random motion of water molecules, the so-called Brownian motion, which becomes impeded focally when cancer is present. DWI is a key component of prostate mpMRI examinations, especially for detection of significant cancers in the peripheral zone (PZ). Diffusion weighted (DW) images are used to calculate apparent diffusion coefficient (ADC) maps (with monoexponential fitting of DW images acquired at b values ≤1000 s/mm2). High b-value images (≥1400 s/mm2) should be obtained (by direct acquisition or computed from the source DWI images) to facilitate detection of clinically significant PCa. It is now well established that the ADC value of a focal tumour is inversely correlated with the Gleason pattern: The lower the ADC value, the higher the Gleason pattern.
1.4. Dynamic contrast-enhanced imaging
DCE is the acquisition of rapidly obtained T1W images before, during, and after the intravenous bolus administration of a gadolinium-based contrast agent. Currently, the added value of DCE is not firmly established regarding tumour detection, with most published data showing that the added value of DCE over the combination of T2W and DWI is modest. Nevertheless, it is recommended that DCE should be included in all prostate mpMRI examinations to assist in the identification of some small, significant cancers; to assist in the diagnosis of nonmalignant causes of raised serum prostate-specific antigen (PSA), such as inflammation; and to provide additional information if DWI is technically limited. Although important, its role in determining PI-RADS v2 assessment categories is secondary to T2W and DWI. DCE serves primarily to help detect significant PCa and not to characterise it.
2. PI-RADS assessment
PI-RADS v2 uses a 5-point assessment scale indicating the likelihood that mpMRI findings correlate with the presence of clinically significant PCa at a particular anatomic location. Based on the current capabilities of mpMRI, clinically significant disease is defined as Gleason score >7 (including 3 + 4 with prominent but not predominant Gleason grade 4), and tumour volume >0.5 ml, and/or extraprostatic extension. PI-RADS assessment categories derived from mpMRI examinations relate to likely histopathologic findings only and do not incorporate other patient or cancer characteristics, such as PSA or clinical cancer risk categories, and they do not directly inform the choice of treatments available if PCa is diagnosed.
The PI-RADS v2 assessment categories are defined with the following scores:
1: Very low (clinically significant PCa is highly unlikely to be present)
2: Low (clinically significant PCa is unlikely to be present)
3: Intermediate (the presence of clinically PCa disease is equivocal)
4: High (clinically significant PCa is likely to be present)
5: Very high (clinically significant PCa is highly likely to be present)
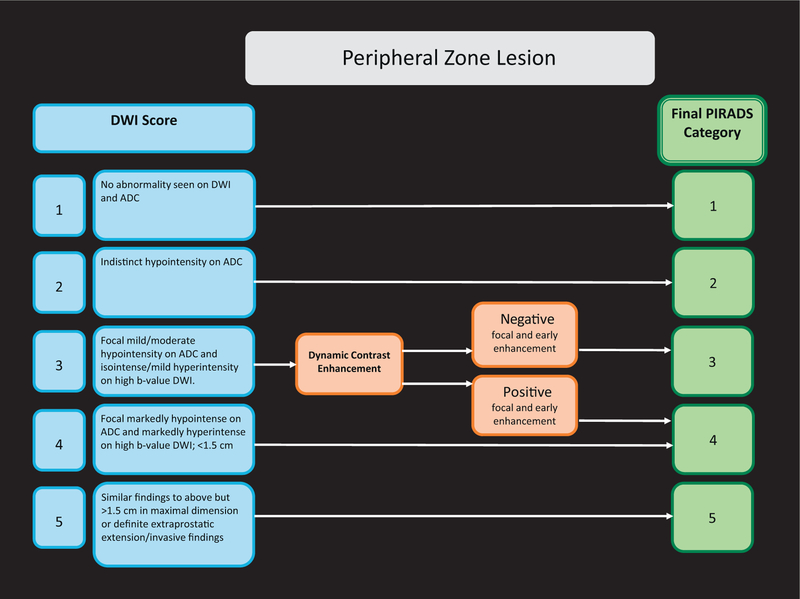
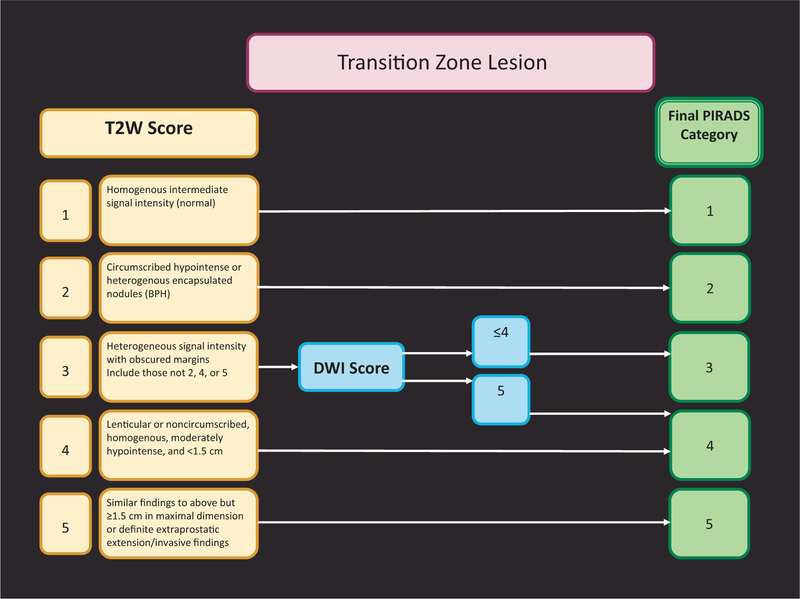
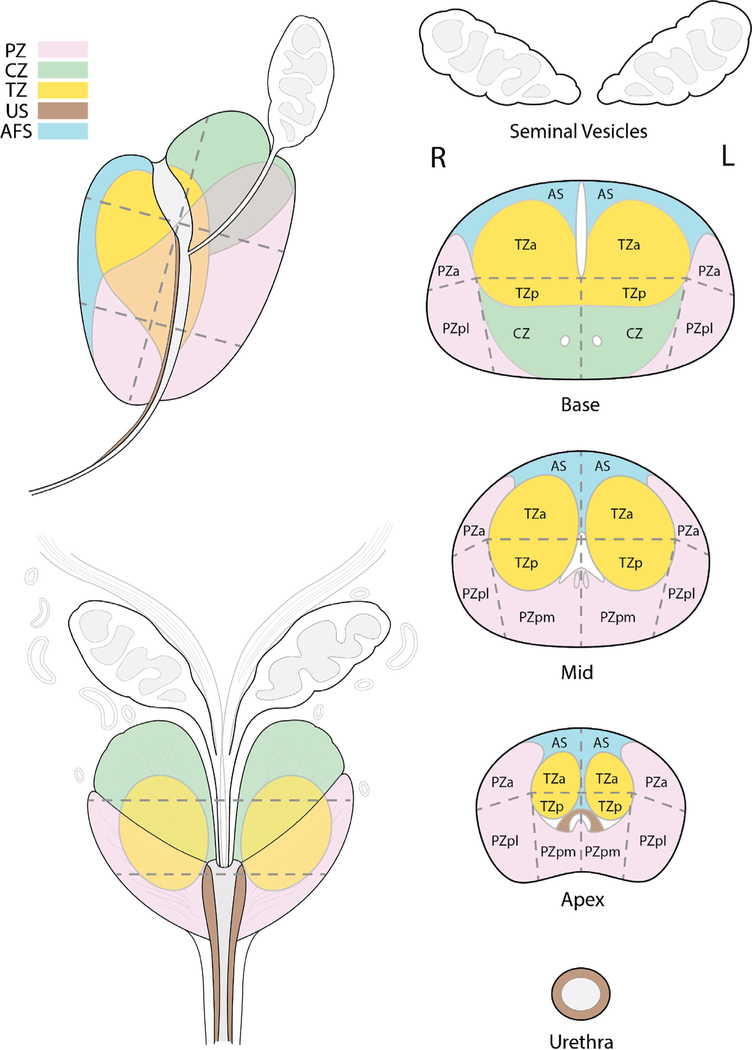
Important differences between PI-RADS v1 and PI-RADS v2 are presented in Table 1. Assignment of a PI-RADS assessment category for each lesion is based on the scoring of T2W, DWI, and DCE sequences performed sequentially according to zonal anatomy, as described in Figures 1 and 2. To localise each lesion, a 39-sector scheme was developed (Fig. 3). The scoring for T2W and DWI uses a 5-point scale; for DCE, a 2-point scale (positive or negative) is used. The latter is one of the major differences: In PI-RADS v2, contrast enhancement is either present or absent.
Fig. 1 –
PI-RADS v2 assessment for the peripheral zone.
ADC = apparent diffusion coefficient; DWI = diffusion weighted imaging.
Fig. 2 –
PI-RADS v2 assessment for the transition zone.
BPH = benign prostatic hyperplasia; DWI = diffusion weighted imaging; T2W = T2-weighted imaging.
Fig. 3 –
The 39-sector scheme. The sector map was adapted from Dickinson et al [8] and the European Society of Urogenital Radiology prostate magnetic resonance imaging guidelines 2012 [9]. It has been modified to represent adult prostate zone anatomy and uses 39 sectors. Reproduced with permission from the American College of Radiology [12].
a = anterior; AFS = anterior fibromuscular stroma; AS = anterior fibromuscular stroma; CZ = central zone; L = left; p = posterior; pl = lateral posterior; pm = medial posterior; PZ = peripheral zone; R = right; TZ = transition zone; US = urethral sphincter.
The most important difference between PI-RADS v1 and PI-RADS v2 is that to assign an overall PI-RADS v2 lesion assessment category, the scores from the T2W, DWI, and DCE sequences are not summated but rather are applied sequentially. The dominance of certain sequences (parameters) is used according to zonal anatomy. For the PZ, DWI is the primary determining sequence; therefore, for a detected PZ lesion, if the DWI score is 4 and the T2W score is 3, the PI- RADS assessment category should be 4. For the TZ, T2W is the primary determining sequence; if a detected TZ lesion has a T2W score of 4 and its DWI score is 2, the PI-RADS assessment category should be 4 (Figs. 1 and 2).
When T2W and DWI are of adequate diagnostic quality, DCE plays a minor role in determining PI-RADS assessment category; however, DCE has a supporting role in the indeterminate category 3 PZ lesions. Absence of early enhancement within a lesion usually adds little information, and diffuse enhancement not localised to a specific T2W or DWI abnormality can be seen in the setting of inflammation, high-grade prostatic intraepithelial neoplasia, atypical small acinar proliferation, after biopsy, or with a sparse Gleason 3 + 3 tumour with an inflammatory focus. Moreover, DCE does not contribute to the overall assessment category when the findings suggest a low (PI-RADS 1 or 2) or high (PI-RADS 4 or 5) likelihood of clinically significant PCa in the PZ. When a PZ lesion has a DWI score of 3, a positive DCE increases the likelihood that the finding corresponds to a clinically significant PCa and thus upgrades the assessment category to PI-RADS 4 (Fig. 1). Likewise, when a TZ lesion has a T2W score of 3, a DWI score of 5 upgrades the assessment category to PI-RADS 4 (Fig. 2).
Finally, because larger tumours have an increased chance of being significant, for PI-RADS v2, a size criterion for T2WI and DWI was introduced. Based on the findings of Wolters at al [13], a cut-off of 1.5 cm was proposed to separate a score of 4 from 5 in both the PZ and the TZ.
3. Reporting mpMRI
The following clinical information should be available to radiologists at the time of MRI reporting: recent serum PSA level and PSA history; date and results of prostate biopsy, including number of cores, locations, and Gleason scores of positive biopsies (with percentage of core involvement and/or core length); and other relevant clinical history, including ethnicity, family history, digital rectal examination (DRE) findings, and prior prostate therapy.
The report should include a measurement of prostate gland volume. It can be combined with PSA to calculate PSA density.
3.1. Mapping
All suspicious intraprostatic lesions seen on mpMRI should be assigned to their zonal location, either PZ (including the central zone [CZ]) or TZ on the sector map, and assigned a PI-RADS overall assessment category. Because the CZ, like the TZ, often shows hypointensity on T2W and ADC and high signal on the high b-value images, it can mimic significant PCa; therefore, in PI-RADS v2, this zone is separately indicated in the 39-sector scheme (Fig. 3). Findings with a PI-RADS assessment category of 3, 4, or 5 should be assigned on the sector map (Fig. 3), and the index (dominant) lesion should be identified. The index lesion is the one with the highest PI-RADS assessment category or, alternatively, the largest lesion if there is more than one with the same category. If there are more than four suspicious findings, then only the four with the highest PI- RADS assessment categories should be reported. Reporting of additional or definitely benign findings is optional but may be helpful as landmarks to guide biopsy or to track lesions on subsequent examinations. If a suspicious finding extends beyond the boundaries of one sector, all neighbouring involved sectors should be indicated on the sector map as a single lesion.
3.2. Measurement of lesions
With current techniques, mpMRI has been shown to underestimate tumour size, volume, and extent, especially for Gleason grade 3 disease. Nonetheless, the following measurement rules are recommended. The minimum requirement is to report the single largest dimension of a suspicious lesion on an axial image. If the largest dimension of a suspicious lesion is on sagittal and/or coronal images, this measurement and imaging plane should be reported.
PZ lesions should be measured on ADC maps. TZ lesions should be measured on T2W images. If lesion measurements are difficult or compromised on ADC maps (for PZ) or T2W (for TZ), then measurement should be made on sequences that show the lesion outline the best. The image number or series and sequence used for measurement should be indicated.
3.3. Sector map
The recommended sector map used in PI-RADS v2 was adapted from Dickinson et al [8] and the ESUR prostate MRI guidelines 2012 [9]. It has been modified to represent adult prostate zone anatomy and uses 39 sectors (Fig. 3).
4. How to use PI-RADS v2 clinically
Assignment of the PI-RADS v2 overall assessment category is based on mpMRI findings only. For directing patient management, including the need and strategy for biopsy, the results of mpMRI with PI-RADS assessment should always be combined with clinical factors like serum PSA kinetics, family history, DRE findings, and previous biopsy results. Targeted MR biopsy should be considered for PI-RADS assessment category 4 or 5 lesions but not for PI-RADS 1 or 2; however, this approach may not be always appropriate. In the face of high suspicion and a “negative” MRI with no category PI-RADS ≥3 lesions, systematic biopsy may be appropriate. Alternatively, if it is felt that the assessment underestimates the presence of a significant PCa, image, and interpretation quality should be carefully evaluated.
For PI-RADS 3 assessments, other clinical factors become increasingly important. If there is low clinical suspicion of significant PCa (eg, PSA density <0.15), then a repeat mpMRI in 9–12 mo can be considered. In contrast, if there is high clinical suspicion of significant PCa (eg, PSA density >0.20), a sextant biopsy, in addition to targeted cores, should be considered to rule out an MRI-invisible significant PCa [14].
If biopsy of PI-RADS 4 or 5 lesions does not yield a high percentage of clinically significant PCa, the quality of the mpMRI, the PI-RADS assessment, and the biopsy technique itself should be re-evaluated critically because published data show that significant PCa is detected in 86% and 93% of PI-RADS 4 and 5 lesions, respectively, using the PI-RADS v1 system [5,15–17]. Definitive, documented, and appropriate explanatory histopathology should be obtained from all PI- RADS 4 and 5 lesions.
5. Limitations of PI-RADS v2 and suggested future developments
It is important that radiologists be properly trained to use PI-RADS v2. Case conferences and multidisciplinary meetings with histopathologic correlation are helpful for calibrating the accuracy of PI-RADS category assessments. Even for experienced radiologists, it is a challenge to start using the PI-RADS v2 assessment system. Because the radiologist has experience with his or her own “homemade” subjective scoring assessment, that system may initially be more accurate than the new PI-RADS v2 system that the radiologist must learn to use. In this respect, adequate training and experience matter. Recently, Muller et al found only moderate interreader agreement (κ = 0.46) [18] using PI-RADS v2, but this can be explained by the extremely divergent reader experience. Two readers were expert radiologists with many years of experience interpreting prostate mpMRI exams, but the third reader was a research medical doctor with limited experience with mpMRI, especially using PI-RADS v2. Even with this extreme difference in reader experience, PI-RADS v2 showed moderate interreader agreement [18]. Furthermore, the overall PI-RADS v2 assessment resulted in better estimation of the risk of significant PCa, and PI-RADS v2 scores were concordant with pathology results in both PZ and TZ (area under the curve of 0.86 and 0.87, respectively). This underscores the intrinsic robustness of the PI-RADS v2 assessment technique but also emphasises the needs for specific PI-RADS v2 training, documentation of observer variability according to reader experience, and further data on intra- and interobserver variability. In another study in which adequate training was performed—consisting of >2 wk of intensive personalised teaching of both technicians and expert readers followed by PI-RADS supervised reading with >300 mpMRI scans—κ statistics showed substantial agreement (0.77), with 92% agreement for PI-RADS v1 categories 1–3 versus 4 and 5 [3].
Because the dominant factors for PI-RADS v2 assessment are T2W for the TZ and DWI for the PZ, identification of the zonal location of the lesion is vital. Areas in which this may be especially problematic include the interface of the CZ, the intraprostatic seminal vesicles and PZ at the base of the gland, and the interface of the anterior horn of the PZ with TZ and the anterior fibromuscular stroma. The anterior-apical region is another problematic region requiring special attention.
The ability to reliably detect and characterise clinically significant PCa in the TZ depends on the more subjective T2W anatomic criteria. Benign prostatic hyperplasia (BPH) is intrinsically heterogeneous, including ill-defined structures and those that are highly cellular and vascular. This is why tumour detection in the TZ is less accurate compared with the PZ; the normal PZ has a more homogenous appearance. Although T2W images are dominant in the TZ, any lesion with low ADC and high signal intensity on high b-value images should be regarded with caution and should be carefully evaluated and biopsied if necessary. In clinical practice, visually bright foci in the TZ on high b-value DWI help draw the radiologist’s attention to a potential lesion and trigger more detailed analysis of this area using the rest of the MR data set. In this sense, initial localisation of the suspicious region using the b-value >1400 images can be useful in raising one’s confidence regarding the presence of a lesion, even if the final PI-RADS category is largely determined by T2W imaging.
Compared with PI-RADS v1, the role of DCE in PI-RADS v2 is limited, and future studies will show whether this sequence can be omitted. Although it is advised that a PZ lesion with a DWI score of 3 should be upgraded when DCE is positive (Fig. 1), one should be aware that focal prostatitis also frequently enhances and thus may create a false-positive result.
In the same vein, it should be noted for TZ lesions with a score of 3 on T2W images that a DWI score of 5 upgrades the final PI-RADS assessment (Fig. 2). The cut-off was placed at 5 for this upgrade because BPH nodules can resemble tumours (being hypercellular or proliferative on histology). When such lesions are <1.5 cm, there is less confidence about their nature and perhaps less need for immediate sampling, but follow-up of these smaller lesions is recommended nevertheless. Validation studies using targeted systematic biopsy and whole-mount prostate histopathology for documenting these specific phenomena are needed to refine future versions of PI-RADS.
PI-RADS v2 uses size criteria with a threshold of 1.5 cm to separate PI-RADS 4 and 5 lesions on T2W and DW images. In practise, a focal lesion on DWI <1.5 cm that is most likely to represent significant PCa by virtue of extraprostatic extension or invasive behaviour should be assigned to the PI-RADS 5 category. Regardless, because biopsy is recommended for both PI-RADS 4 and 5 lesions, the management of such lesions is unlikely to be altered. Again, further validation of this 1.5-cm cut-off is required.
Another limitation of PI-RADS v2 is that the evaluation of ADC maps and high b-value images is subjective despite the numerical nature of ADC (unit: ×10−3 mm2/s). The definitions of markedly hypointense signals on ADC maps and markedly hyperintense signals on high b-value images remain subjective but understood nevertheless by experienced radiologists. It would be helpful if threshold values could be assigned for ADC values for insignificant and significant PCa and for benign pathologies including prostatitis. The problem of choosing cut-off values relates to the fact that ADC values depend on the choice of b values for DW images used for calculations (hence the recommendation to use only b values <1000 s/mm2), but it also depends on the diffusion time achieved on diffusion sequences (which is highly dependent on scanner specifications) and on a variety of other technical factors. A solution would be for each institution to determine its own ADC cut- off value based on biopsy and prostatectomy results. To enable comparisons between imaging systems, ADC measurements can also be calibrated using biological tissues with low variance in diffusion properties (eg, brain) or test objects made of bioequivalent materials or ice-water phantoms [19,20].
Although mpMRI is an accurate technique for detecting significant PCa, it misses significant PCa at a low percentage (6–25%). Lesions missed are usually invasive PCa inter- mixed with normal tissue or low-grade or mucinous PCa; however, tumour volume of missed PCa is usually low (<0.5 ml), and only 7–14% have >20% Gleason grade ≤4 components [6,16,21–23]. The rate at which this occurs is low for PI-RADS 1 and 2 lesions and higher for PI-RADS 3 lesions. The exact prevalence of all PCa and significant PCa for each PI-RADS v2 category has yet to be determined, and investigators are encouraged to re-evaluate their historical archives and to perform prospective studies to document the prevalence of missed significant disease according to PI- RADS v2 assessment categories.
As already noted, PI-RADS v2 needs to be tested and validated in different clinical scenarios. Initially, data are required on test performance (rates of detection of clinically significant and insignificant PCa) in first- or repeat-biopsy patient subpopulations. Validation should be undertaken using MR-targeted biopsy, systematic biopsy (saturation or template techniques), and whole-mount histopathology. Comparison with the current standard of systematic TRUS biopsy should also be undertaken. The need for targeted biopsy alone or in combination with systematic TRUS biopsy should be assessed. Long-term follow-up data are also needed before we can conclude that PI-RADS v2 is effective in directing patient management and for improving outcomes of patients with suspected PCa. The role of PI- RADS v2 assessments in directing the selection and monitoring of patients undergoing active surveillance would be interesting. It is anticipated that as evidence continues to accrue in the field of mpMRI and for MRI- targeted in-bore or out-of-bore biopsies and image-guided focal therapy interventions, specific recommendations and/or algorithms regarding need for biopsy and management will be included in future versions of PI-RADS. PI- RADS v2 is the next step in prostate mpMRI standardisation, helping to objectively improve the detection and localisation of significant PCa. Consequently, its use in clinical practise is highly recommended.
The PI-RADS steering committee strongly supports the continued development of promising MRI methodologies for assessment of PCa and local staging using novel and/or advanced research tools not included in PI-RADS v2. Consideration will be given to incorporating them into future versions of PI-RADS as relevant data and experience become available.
Acknowledgment statement:
The authors are grateful to the other members of the ACR Joint PI-RADS Steering Committee of the American College of Radiology, AdMeTech Foundation’s International Prostate MRI Working Group, and the Prostate MRI subcommittee of the European Society of Urogenital Radiology for developing PI-RADS v2, in particular, Mythreyi Chatfield of the American College of Radiology.
Funding support: The research and development of AdMeTech Foundation’s International Prostate MRI Working Group were made possible by a grant awarded and managed by the U.S. Army Medical Research and Materiel Command (USAMRMC) and Telemedicine and Advanced Technologies Research Center (TATRC) at Fort Detrick, Maryland, under contract numbers W81XWH-09-0552 and W81XWH-11-1-0077.
Footnotes
Conflicts of interest: The authors have nothing to disclose.
References
- [1].Fütterer jj, Briganti A, De Visschere P, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging?. A systematic review of the literature. Eur Urol 2015;68:1045–53. [DOI] [PubMed] [Google Scholar]
- [2].Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68: 438–50. [DOI] [PubMed] [Google Scholar]
- [3].Pokorny MR, de Rooij M, Duncan E, et al. Prospective study of diagnostic accuracy comparing prostate cancer detection by trans-rectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies. Eur Urol 2014;66:22–9. [DOI] [PubMed] [Google Scholar]
- [4].Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015;33: 17.e1–7. [DOI] [PubMed] [Google Scholar]
- [5].Delongchamps NB, Peyromaure M, Schull, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol 2013;189:493–9. [DOI] [PubMed] [Google Scholar]
- [6].Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313: 390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heidenreich A Consensus criteria for the use of magnetic resonance imaging in the diagnosis and staging of prostate cancer: not ready for routine use. Eur Urol 2011;59:495–7. [DOI] [PubMed] [Google Scholar]
- [8].Dickinson L, Ahmed HU, Allen C, et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 2011;59:477–94. 10.1016/j.eururo.2010.12.009, Epub 2010 Dec 21. PMID: 21195536. [DOI] [PubMed] [Google Scholar]
- [9].Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hamoen EH, de Rooij M, Witjes JA, Barentsz JO, Rovers MM. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol 2015;67: 1112–21. [DOI] [PubMed] [Google Scholar]
- [11].Vaché T1, Bratan F, Me`ge-Lechevallier F, Roche S, Rabilloud M, Rouvie`re O. Characterization of prostate lesions as benign or malignant at multiparametric MR imaging: comparison of three scoring systems in patients treated with radical prostatectomy. Radiology 2014;272:446–55. [DOI] [PubMed] [Google Scholar]
- [12].PI-RADS™ Prostate Imaging and Reporting and Data System: 2015, version 2. American College of Radiology Web site. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS%20V2.pdf.
- [13].Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol 2011;185: 121–5. [DOI] [PubMed] [Google Scholar]
- [14].Radtke JP, Kuru TH, Boxler S, et al. Comparative analysis of trans-perineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol 2015;193:87–94. [DOI] [PubMed] [Google Scholar]
- [15].Renard-Penna R, Mozer P, Cornud F, et al. Prostate Imaging Reporting and Data System and Likert scoring system: multiparametric MR imaging validation study to screen patients for initial biopsy. Radiology 2015;275:458–68. [DOI] [PubMed] [Google Scholar]
- [16].Portalez D, Mozer P, Cornud F, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol 2012;62: 986–96. [DOI] [PubMed] [Google Scholar]
- [17].Delongchamps NB, Lefe`vre A, Bouazza N, Beuvon F, Legman P, Cornud F. Detection of significant prostate cancer with magnetic resonance targeted biopsies–should transrectal ultrasound-magnetic resonance imaging fusion guided biopsies alone be a standard of care? J Urol 2015;193:1198–204. [DOI] [PubMed] [Google Scholar]
- [18].Muller BG, Shih JH, Sankineni S, et al. Prostate cancer: interobserver agreement and accuracy with the revised Prostate Imaging Reporting and Data System at multiparametric MR imaging. Radiology 2015:142818. [DOI] [PMC free article] [PubMed]
- [19].Malyarenko DI, Newitt D, J Wilmes L, et al. Demonstration of nonlinearity bias in the measurement of the apparent diffusion coefficient in multicenter trials. Magn Reson Med In press. 10.1002/mrm.25754 [DOI] [PMC free article] [PubMed]
- [20].Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 2009;11:102–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abd-Alazeez M, Ahmed HU, Arya M, et al. The accuracy of multiparametric MRI in men with negative biopsy and elevated PSA level–can it rule out clinically significant prostate cancer? Urol Oncol 2014;32:45.e17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abd-Alazeez M, Kirkham A, Ahmed HU, et al. Performance of multiparametric MRI in men at risk of prostate cancer before the first biopsy: a paired validating cohort study using template prostate mapping biopsies as the reference standard. Prostate Cancer Prostatic Dis 2014;17:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arumainayagam N1, Ahmed HU, Moore CM, et al. Multiparametric MR imaging for detection of clinically significant prostate cancer: a validation cohort study with transperineal template prostate mapping as the reference standard. Radiology 2013;268: 761–9. [DOI] [PubMed] [Google Scholar]