Abstract
OBJECTIVES
We conducted a pilot study for a large definitive clinical trial evaluating the impact of ranolazine in women with angina, evidence of myocardial ischemia, and no obstructive coronary artery disease (CAD).
BACKGROUND
Women with angina, evidence of myocardial ischemia, but no obstructive CAD frequently have microvascular coronary dysfunction. The impact of ranolazine in this patient group is unknown.
METHODS
A pilot randomized, double-blind, placebo-controlled, crossover trial was conducted in 20 women with angina, no obstructive CAD, and ≥10% ischemic myocardium on adenosine stress cardiac magnetic resonance (CMR) imaging. Participants were assigned to ranolazine or placebo for 4 weeks separated by a 2-week washout. The Seattle Angina Questionnaire and CMR were evaluated after each treatment. Invasive coronary flow reserve (CFR) was available in patients who underwent clinically indicated coronary reactivity testing. CMR data analysis included the percentage of ischemic myocardium and quantitative myocardial perfusion reserve index (MPRI).
RESULTS
The mean age of subjects was 57 ± 11 years. Compared with placebo, patients on ranolazine had significantly higher (better) Seattle Angina Questionnaire scores, including physical functioning (p = 0.046), angina stability (p = 0.008), and quality of life (p = 0.021). There was a trend toward a higher (better) CMR mid-ventricular MPRI (2.4 [2.0 minimum, 2.8 maximum] vs. 2.1 [1.7 minimum, 2.5 maximum], p = 0.074) on ranolazine. Among women with coronary reactivity testing (n = 13), those with CFR ≤3.0 had a significantly improved MPRI on ranolazine versus placebo compared to women with CFR >3.0 (∆ in MPRI 0.48 vs. –0.82, p = 0.04).
CONCLUSIONS
In women with angina, evidence of ischemia, and no obstructive CAD, this pilot randomized, controlled trial revealed that ranolazine improves angina. Myocardial ischemia may also improve, particularly among women with low CFR. These data document approach feasibility and provide outcome variability estimates for planning a definitive large clinical trial to evaluate the role of ranolazine in women with microvascular coronary dysfunction. (Microvascular Coronary Disease In Women: Impact Of Ranolazine; NCT00570089).
Keywords: angina, ischemic heart disease, ranolazine, women
Women with angina, evidence of ischemia by stress testing, and no obstructive coronary artery disease (CAD) by angiography frequently have microvascular coronary dysfunction (MCD), which carries an adverse prognosis for cardiovascular events including myocardial infarction, stroke, heart failure, and sudden cardiac death (1–3). Patients with MCD use more healthcare resources and have higher healthcare costs similar to those for obstructive CAD; there are an estimated 2 to 3 million women in the United States with MCD. Persistent angina despite treatment with nitrates, beta-blockers, and calcium channel blockers in this patient population is a therapeutic challenge. Ranolazine is a newer U.S. Food and Drug Administration–approved medication for treatment of angina, and several clinical trials have demonstrated the safety and efficacy of ranolazine as an antianginal agent (4–10). Studies to date have not clearly demonstrated in a controlled fashion whether the anti-anginal effect of ranolazine is due to an anti-ischemic mechanism.
Previous work in patients with cardiac syndrome X has demonstrated that adenosine stress perfusion cardiac magnetic resonance (CMR) is often abnormal with a circumferential subendocardial hypoperfusion pattern (11). Additional work suggests that stress CMR can differentiate ischemia due to hemodynamically significant stenosis in a major epicardial artery versus nonsegmental etiologies based on the pattern of hypoperfusion (12,13).
We conducted a pilot study of ranolazine in women with angina, evidence of myocardial ischemia by CMR, and no obstructive CAD to document feasibility of this approach and to collect data on outcome variability for the purpose of planning a large definitive clinical trial.
METHODS
Patient population.
Inclusion criteria included women with signs and symptoms of myocardial ischemia (chest pain and abnormal routine stress testing) and no obstructive CAD (<50% epicardial coronary stenosis in all epicardial coronary arteries) on clinically indicated coronary angiography, who had an abnormal adenosine stress CMR (≥10% ischemic myocardium) within the previous 12 months were enrolled. No intercurrent cardiac events occurred be-tween the qualifying CMR scan and trial enrollment and completion. Exclusion criteria were: 1) contraindications to withholding nitrates, calcium channel agents, and alpha and beta-adrenergic blockers for 24 h before testing; 2) contraindications to CMR including implantable cardioverter-defibrillators, pacemakers, and severe claustrophobia; 3) hepatic insufficiency, prolonged QT, renal failure; 4) use of drugs that inhibit CYP3A such as diltiazem, verapamil, ketoconazole, macrolides, and HIV protease inhibitors; 5) women younger than 18 years of age (3 women of child-bearing age were enrolled), pregnant, or breastfeeding; 6) women taking drugs that prolong the QT interval; and 7) life expectancy <6 months. The Institutional Review Board at Cedars-Sinai Medical Center approved the study, and all subjects gave written informed consent before study participation.
Study design.
Eligible women had baseline screening 12-lead electrocardiogram and blood chemistry for creatinine and glomerular filtration rate. The study was a double-blind, placebo-controlled, crossover design in which treatment order to ranolazine and placebo was randomly assigned (Fig. 1). After enrollment in the study, participants were randomly assigned to ranolazine or placebo for 4 weeks followed by a 2-week washout. Participants then crossed over to the alternative study medication for the second 4-week treatment period. The participant’s usual antianginal medication regimen was continued unchanged throughout study duration. Women randomized to ranolazine received 500 mg orally twice daily for 2 weeks, and the dose was increased to 1,000 mg twice daily for an additional 2 weeks if tolerated. All patients tol-erated the 500-mg dosing, whereas 2 patients did not tolerate the 1,000-mg dosing because of GI side effects, and their dose was reduced. All participants completed baseline demographic and health history questionnaires, including the Seattle Angina Questionnaire (SAQ) and Duke Activity Status Index (DASI). The SAQ and DASI were administered and CMR was performed at the end of each 4-week treatment period. The SAQ is a self-administered, 19-item questionnaire that is well validated and sensitive to clinical changes in stable coronary heart disease patients (14). The DASI is a 12-item self-administered questionnaire that has also been validated as a measure of functional capacity (15).
Figure 1. Study Design Flow Diagram.
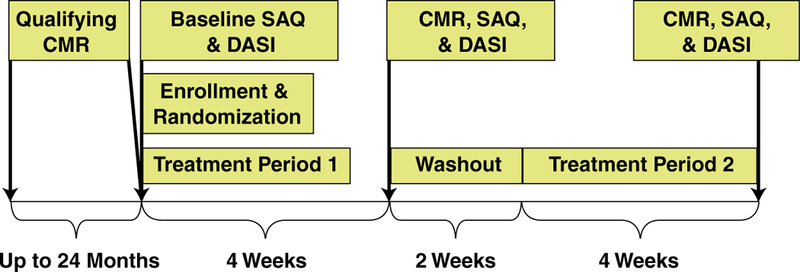
Treatment periods 1 and 2: randomized to sequence of ranolazine first followed by crossover to placebo or vice versa. CMR = cardiac magnetic resonance; DASI = Duke Activity Score Index; SAQ = Seattle Angina Questionnaire.
Cardiac magnetic resonance.
All CMR studies were performed with a 1.5-T magnet (Siemens Sonata, Erlangen, Germany) with electrocardiogram gating and a phased array coil using a highly standardized protocol with 0.05 mmol/kg gadolinium first-pass perfusion 3 slice stress, followed by rest first-pass perfusion. Left ventricular ejection fraction and wall motion were evaluated using cine imaging, which was performed in left ventricular short-axis slices from the base to the apex. Delayed enhancement imaging was performed to evaluate for scar. The dose of adenosine stress (140 μg/kg –1/min–1 over 5 min) was consistent for all studies. All patients tolerated adenosine stress. CMR was performed approximately 4 h after the morning dose of study drug, at the same time of day with identical fasting state, adenosine and gadolinium dosing infusion protocols, and magnet settings. A re-presentative case example of a subject CMR on placebo and on ranolazine is shown in Figure 2.
Figure 2. Case Example.
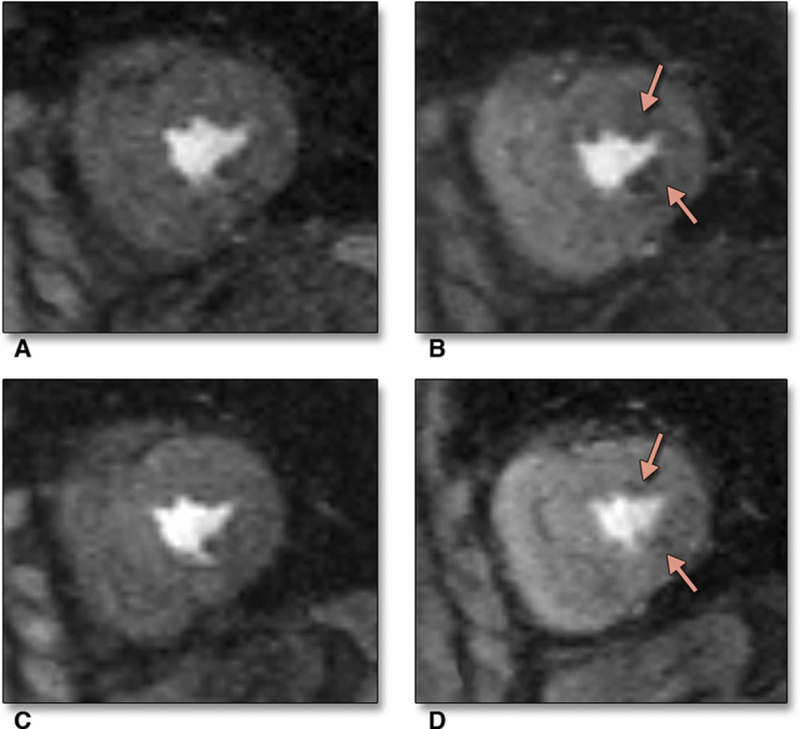
Cardiac magnetic resonance images acquired during rest (A) and stress (B) on placebo, depicting first-pass subendocardial hypoperfusion in the anterolateral and inferolateral walls (arrows) at the mid-ventricular level. After treatment with ranolazine, rest (C) and stress (D) CMR images show significant improvement in the areas of hypoperfusion (arrows) at the mid-ventricular level.
The presence and percentage of myocardial hypoperfusion were defined semiquantitatively by 2 experienced readers (D.B. and L.T.) who were blinded to the clinical status of the patients using the National Heart, Lung and Blood Institute (NHLBI)– sponsored Women’s Ischemia Syndrome Evaluation (WISE) CMR core laboratory protocol. Studies were analyzed using a visual semiquantitative 5-point, 16-segment American Heart Association scoring system (14). For visual interpretation, first and second studies were considered side by side, with the reader blinded to treatment assignment for the 2 stress perfusion studies. Scores in the basal mid-axis and distal short-axis slices were summed to obtain a summed rest score, a summed stress score, and a summed difference score (14). The percentage of ischemic myocardium was defined as the sum of the scores divided by 64, which is a product of the 16 segments analyzed and the worst uptake score of 4 (16).
First-pass perfusion images were also analyzed using CAAS MRV CMR analysis software version 3.3 (Pie Medical Imaging B.V., Maastricht, the Netherlands). Epicardial and endocardial contours of the left ventricular myocardium for 3 short-axis slices (basal, mid, and apical) were determined by the software and manually corrected if needed to acquire intensity over time curves, which were used to measure the myocardial perfusion reserve index (MPRI). The whole myocardial, subendocardial, and subepicardial MPRI were calculated as the ratio of stress/rest relative perfusion up-slope, corrected for left ventricular cavity up-slope. The subendocardial and subepicardial layers were automatically defined by the software as the inner and outer 50% of the wall thickness between the contoured myocardium. These methods using quantitative up-slope curves used in this study are reproducible techniques with high interstudy and observer reproducibility (17,18).
Invasive CFR testing.
Clinically indicated invasive coronary reactivity testing (CRT) using the NHLBI– sponsored WISE protocol to measure coronary microvascular and macrovascular endothelium-dependent and nonendothelium-dependent function (3,19,20) was available in a majority subset (n = 13). Specifically, coronary arterial flow reserve (CFR) was measured by graded intracoronary adenosine injections of 18 μg and 36 μg to create maximal hyperemia, and flow in the proximal left anterior descending was measured with a Doppler flow wire (FloWire, Volcano, San Diego, California). Graded doses of intracoronary acetylcholine were used to test endothelial function and determine coronary blood flow; intracoronary nitroglycerin was used to assess smooth muscle function.
Statistical analysis.
Randomized treatment comparisons were made according to intention-to-treat principles using nonparametric statistics. Comparing the ranolazine and placebo visits, we used the Friedman test of k-related samples or the Wilcoxon signed-rank test for comparing 2 related samples. In some cases, a profile plot was used to compare changes in CMR measurements (placebo/ranolazine). A trend analysis was applied using the orthogonal trend contrast coefficients. A mixed-effects model was used to analyze for carryover effect, which tests for influence of one treatment over another. All tests of hypotheses were 2 sided with a type I error rate of 0.05, and p value <0.05 was considered statistically significant. All statistical analyses were done using SAS software (version 9.1, SAS Institute Inc., Cary, North Carolina).
RESULTS
Patient characteristics.
The baseline demographic and clinical characteristic data for the 20 women are shown in Table 1. Despite current antianginal therapy, all patients were symptomatic with angina at the time of study enrollment; 95% had typical angina in addition to 45% with shortness of breath, 30% with palpitations, and 15% with nausea. Between the qualifying CMR and study entry (mean 10.2 ± 8.5 months), most patients had been started on beta-blocker, angiotensin-converting enzyme inhibitor, and statin therapy, as clinically indicated. The ranolazine/placebo interventions were well tolerated with 100% study completion and no dropouts.
Table 1.
Baseline Demographic and Clinical Variables (n = 20)
| Age, yrs, | 57 ± 11 |
| Body mass index, kg/m2 | |
| Mean ± SD | 25.6 ± 3.8 |
| >30 kg/m2 | 3 (15) |
| Race (non-Caucasian) | 4 (20) |
| Tobacco use | |
| Current | 0 (0) |
| Former | 10 (50) |
| Never | 10 (50) |
| Hypertension | 10 (50) |
| Hyperlipidemia | 12 (60) |
| Family history of premature coronary artery disease |
14 (70) |
| Coronary reactivity testing | 13 (65) |
| Baseline cardiac magnetic resonance | |
| Percentage of ischemic myocardium | 21.9 (16.8, 31.3) |
| Summed difference score | 20.3 (15.6, 33.6) |
| Global MPRI | 1.4 (1.2, 1.9) |
| Mid-ventricular MPRI | 1.4 (1.2, 2.1) |
| Symptoms | |
| Typical angina | 19 (95) |
| Shortness of breath | 9 (45) |
| Palpitations | 6 (30) |
| Nausea | 3 (15) |
| Left ventricular ejection fraction, % | 71 ± 6 |
| Beta-blockers | 14 (70) |
| Calcium channel blockers | 4 (20) |
| Angiotensin-converting enzyme inhibitors | 9 (45) |
| Angiotensin receptor blockers | 3 (15) |
| Nitrates | 9 (45) |
Values are mean ± SD, n (%), or median (minimum, maximum).
MPRI = myocardial perfusion reserve index.
Angina results.
Three of 5 SAQ subscale scores were higher (better) on ranolazine compared with placebo, including significantly higher physical functioning, angina stability, and quality of life (Table 2). Treatment satisfaction trended lower on ranolazine (p = 0.058) (Table 2). There was no significant entry or exit group differences in the DASI on ranolazine compared with placebo (8.6 [3.7 minimum, 11.5 maximum] METS vs. 8.9 [5.4 minimum, 12.1 maximum] METS, p = 0.47). We found no carryover effect for SAQ subscales or CMR imaging variables described in the following.
Table 2.
SAQ Scores on Ranolazine Versus Placebo
| Ranolazine | Placebo | Treatment Effect (p Value) |
|
|---|---|---|---|
| Physical functioning | 91.7 (79.2, 97.9) | 83.3 (66.6, 97.2) | 0.046 |
| Angina stability | 75.0 (50.0, 100.0) | 50.0 (25.0, 75.0) | 0.008 |
| Angina frequency | 80.0 (50.0, 100.0) | 75.0 (60.0, 87.5) | 0.197 |
| Treatment satisfaction | 87.5 (75.0, 100.0) | 93.8 (75.0, 00.0) | 0.058 |
| Quality of life | 75.0 (60.4, 83.3) | 66.7 (58.3, 75.0) | 0.021 |
Values are median (minimum, maximum). A higher SAQ score is better in each domain. The bold type indicates statistically significant p Values.
SAQ = Seattle Angina Questionnaire.
CMR results.
We did not observe rest perfusion abnormalities in any subjects. Specifically, only the stress images had significant hypoperfusion abnormalities, which is reflected in the percentage of ischemic myocardium, summed difference scores, and MPRI scores reported in Tables 1 and 3. Compared with placebo, treatment with ranolazine resulted in group differences uniformly in the hypothesized direction by CMR; however, none were statistically significant (Table 3). Notably, there was a trend of higher mid-ventricular MPRI, an area of highest CMR measurement reproducibility, on ranolazine compared with placebo that approached statistical significance (Table 3).
Table 3.
Visual and Quantitative CMR Defects on Ranolazine versus Placebo
| Ranolazine | Placebo | Treatment Effect (p Value) |
|
|---|---|---|---|
| Percentage of ischemic myocardium | 11.7(8.0, 19.3) | 16.0 (8.6, 22.7) | 0.64 |
| Summed difference score | 10.2 (3.5, 15.2) | 14.8 (5.5, 19.5) | 0.82 |
| MPRI | |||
| Global | 2.1 (2.2, 2.4) | 1.9 (1.7, 2.5) | 0.66 |
| Mid-ventricular | 2.4 (2.0, 2.8) | 2.1 (1.7, 2.5) | 0.074 |
| Subendocardial (whole) | 2.0 (1.7, 2.2) | 1.8 (1.5, 2.3) | 0.66 |
| Subendocardial (mid-ventricular) | 2.1 (1.7, 2.5) | 1.9 (1.5, 2.3) | 1.0 |
| Subepicardial (whole) | 2.3 (2.1, 2.6) | 2.0 (1.8, 2.5) | 0.18 |
| Subepicardial (mid-ventricular) | 2.6 (2.2, 3.0) | 2.2 (1.9, 2.5) | 0.18 |
Values are median (minimum, maximum). For summed difference score, lower is better.
MPRI = myocardial perfusion reserve index (higher is better perfusion reserve).
We next stratified the results according to baseline (qualifying) CMR abnormality. Women with a baseline MPRI ≤1.5 (lower perfusion reserve) had a trend toward higher MPRI on ranolazine compared with placebo (p = 0.2), whereas those women with an MPRI >1.5 did not (p = 0.55) (Fig. 3). Similar trends were observed for the visual qualifying summed difference score (data not shown).
Figure 3. MPRI on Ranolazine Versus Placebo According to Qualifying MPRI.
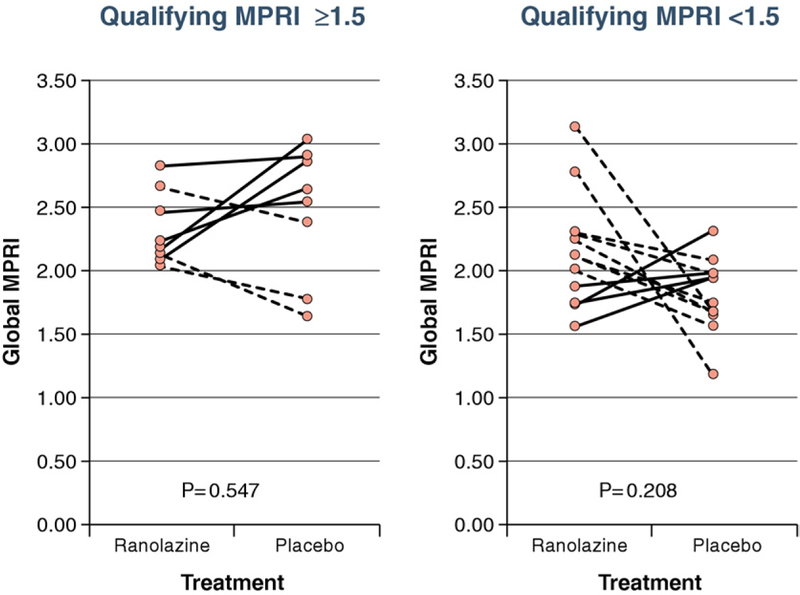
Solid lines indicate subjects who had a higher myocardial perfusion reserve index (MPRI) on ranolazine than on placebo (n = 9), and dashed lines indicate subjects who did not (n = 11).
We next evaluated CMR results stratified by SAQ angina response, defined as a change in SAQ angina frequency of >0 (placebo/ranolazine). This analysis demonstrated that among angina responders, MPRI trended toward being higher on ranolazine than on placebo (2.4 vs. 2.0, treatment effect p = 0.059); there were no group differences when stratified by the other SAQ scores.
Subgroup analysis of the 13 women who underwent clinically indicated invasive CRT demonstrated that the subgroup with lower CFR ( <3.0) had significant MPRI improvement on ranolazine compared to women with CFR >3.0 (Δ in MPRI placebo/ranolazine: 0.48 vs. –0.82, p = 0.04) (Fig. 4).
Figure 4. MPRI Change (Placebo/Ranolazine) According to CFR in the Subset of Women With Coronary Reactivity Testing (n = 13).
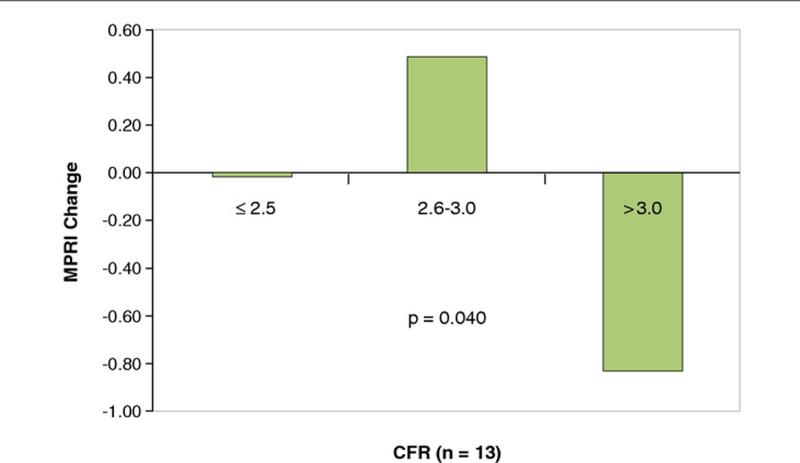
Women with lower coronary flow reserve (CFR) (≤2.5, n = 5) and (2.6 to 3.0, n = 6) had a significantly greater myocardial perfusion reserve index (MPRI) change (placebo/ranolazine) compared to women with CFR >3.0 (n = 2) (p = 0.04). A higher MPRI number indicates greater perfusion.
DISCUSSION
To our knowledge, this is the first controlled trial to report an improvement in SAQ angina with ranolazine compared with placebo in women with signs and symptoms of myocardial ischemia but no obstructive CAD. In this pilot trial, we also demonstrated a possible anti-ischemic role of ranolazine as a mechanism of action for its antianginal effects. These data document approach feasibility and provide outcome variability estimates for planning a definitive large clinical trial to evaluate the role of ranolazine in women with MCD.
Evidence of both ischemia and angina predict adverse cardiovascular events in women with and without obstructive CAD (1,2,21–24). Ranolazine has been established as an effective antianginal in patients with obstructive CAD, and there is evidence that it improves ischemia based on electrocardiographic stress testing (4). A recent open-label, nonrandomized study suggested that ranolazine treatment is associated with improvement in automated quantitative analysis of single-photon emission computed tomography images (25). Our results are consistent with and extend these observations in a randomized, controlled trial setting using quantitative estimates of myocardial perfusion imaging and detailed measurement of the improvements in the burden of angina for women with evidence of MCD.
Sex differences in angina exist and likely affect antianginal therapy. The CARISA (Combination Assessment of Ranolazine in Stable Angina) study demonstrated that addition of ranolazine in women had similar reductions in angina frequency and nitroglycerin use as in men, although less improvement in exercise testing–related angina (8). Analysis from the MERLIN–TIMI 36 (Metabolic Efficiency with Ranolazine for Less Ischemia in Non– ST-Elevation Acute Coronary Syndromes– Thrombolysis In Myocardial Infarction 36) study showed that women had less obstructive CAD compared with men, and women had a 29% reduction in recurrent ischemia on ranolazine compared with placebo, a change that was not evident in men (26). Given these previous findings and the hemodynamic tolerability profile of ranolazine, we hypothesized that women with evidence of MCD would demonstrate angina improvement on therapy.
Our current study demonstrates that women with angina, evidence of ischemia, and no obstructive CAD have significant benefit from ranolazine compared with placebo in the objective measure of SAQ angina. We report that 3 of 5 SAQ measures were significantly improved with ranolazine therapy. Treatment satisfaction trended in a negative direction on ranolazine; this may have been due to the play of chance, patient expectation that ranolazine would provide a complete relief of angina, or, alternatively, could be related to side effects of ranolazine, although all subjects completed the study with no dropouts. Of note, subjects continued their routine medications while taking ranolazine.
Trials addressing the use of antianginal medications in women are critical due to the growing burden of ischemic heart disease; more than one-half of the 8.9 million patients in the United States are women with chronic stable angina. Chronic stable angina is not benign and not only portends significant morbidity and mortality (22,26), it also leads to poor quality of life (27). Although obstructive CAD is a prominent cause of ischemic heart disease, women have a 2-fold increase in normal coronaries (no obstructive disease visible by traditional angiography) compared with men in the setting of acute coronary syndrome, non–ST-segment elevation and ST-segment elevation myocardial infarction (19).
Women with angina often require multiple medications for symptom control (19). Pharmacological therapy for chronic stable angina for symptom relief has centered on beta-blockers, calcium channel blockers, and nitrates (28); these agents reduce myocardial oxygen demand by reducing inotropy and chronotropy, preload, and blood pressure. Although effective in general, many patients experience breakthrough and refractory angina despite treatment, and other times, these agents are not tolerated because of their hemodynamic side effects. Ranolazine, a piperazine derivative, is believed to work on the ischemic myocardium via inhibition of late inward Na+ current, which interrupts calcium (Ca2+ ) overload in ischemic myocytes. Increased Ca2+ overload in the ischemic myocardium has been linked to left ventricular diastolic dysfunction. Studies have shown that ranolazine improves left ventricular diastolic function in ischemic heart disease patients (29,30). As an antianginal agent, ranolazine is particularly promising because it does not have significant effects on heart rate and blood pressure.
Invasive CRT can be performed for a definitive assessment of MCD in women with persistent angina with no obstructive CAD (2,3,20). Current mechanistic pathways for MCD include endothelial and smooth muscle dysfunction due to oxidative and/or inflammatory stress (31,32). Both abnormal coronary vascular response to acetylcholine (i.e., failure to dilate) and abnormal CFR predict adverse cardiovascular outcomes in women (3,20). Previous data indicate that invasive measures of coronary reactivity correlate with CMR-measured perfusion reserve (33,34). In our study, 13 of the 20 women had undergone CRT, and ranolazine resulted in a significant improvement in myocardial perfusion reserve in women with a baseline CFR of <3.0. These data suggest that a large, definitive clinical trial of ranolazine in this population should target subjects with low myocardial perfusion reserve.
Study limitations.
Our pilot study, by design, included a small number of subjects for demonstration of approach feasibility and determination of study outcome variability for sample size calculation. Our study is therefore limited with regard to definitive statements regarding the role of ranolazine as anti-ischemic treatment of symptomatic women with evidence of ischemia but no obstructive CAD. We minimized the influence of a carryover effect by interjecting a 2-week washout period. However, the potential for influence on certain aspects of our study design remain plausible including patient familiarity with the testing protocol, which remains unaccounted for in our data analysis. Also, although a majority of the enrolled women had invasive CRT, this did not include all subjects. The strengths of our study include the use of a validated measure, the SAQ, and core laboratory myocardial perfusion evaluation. A potential limitation may be the visual segmental scoring range of 0 to 4 for the semiquantitative myocardial perfusion, which may exceed the visually detectable grades of hypoperfusion; however, we also used a quantitative myocardial perfusion reserve analysis. A final limitation is that although SAQ measures chest pain and shortness of breath to evaluate angina, many women with ischemia experience anginal equivalents such as fatigue, indigestion, and weakness.
CONCLUSIONS
In women with angina, evidence of myocardial ischemia, and no obstructive CAD, the data from this pilot study suggest a role for ranolazine in improving angina and ischemic myocardium. Our data support the feasibility of our approach and provide outcome variability data with which to plan a larger, definitive, randomized trial comparing the effectiveness of ranolazine as an antianginal and anti-ischemic therapy in women with MCD.
Acknowledgments
Dr. Slivka is currently at the Kaiser Permanente Medical Group. This work was supported by an unrestricted research grant from CV Therapeutics/Gilead and by contracts from the National Heart, Lung and Blood Institute, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164; a GCRC grant MO1-RR00425 from the National Center for Research Resources; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Denville, New Jersey, the Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles. Gregory Thomas, MD, MPH, served as Guest Editor for this article.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CFR
coronary flow reserve
- CMR
cardiac magnetic resonance
- CRT
coronary reactivity testing
- DASI
Duke Activity Status Index
- MCD
microvascular coronary dysfunction
- MPRI
myocardial perfusion reserve index
- SAQ
Seattle Angina Questionnaire
Footnotes
The authors have reported that they have no relationships to disclose.
REFERENCES
- 1.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:2993–9. [DOI] [PubMed] [Google Scholar]
- 2.Pepine CJ. Ischemic heart disease in women. J Am Coll Cardiol 2006;47 3 Suppl:S1–3. [DOI] [PubMed] [Google Scholar]
- 3.von Mering GO, Arant CB, Wessel TR, et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 2004;109:722–5. [DOI] [PubMed] [Google Scholar]
- 4.Chaitman BR. Ranolazine for the treatment of chronic angina and potential use in other cardiovascular conditions. Circulation 2006;113:2462–72. [DOI] [PubMed] [Google Scholar]
- 5.Chaitman BR, Pepine CJ, Parker JO, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA 2004;291:309–16. [DOI] [PubMed] [Google Scholar]
- 6.Chaitman BR, Skettino SL, Parker JO, et al. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol 2004;43:1375–82. [DOI] [PubMed] [Google Scholar]
- 7.Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J 2006;27:42–8. [DOI] [PubMed] [Google Scholar]
- 8.Wenger NK, Chaitman B, Vetrovec GW. Gender comparison of efficacy and safety of ranolazine for chronic angina pectoris in four randomized clinical trials. Am J Cardiol 2007;99:11–8. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol 2005; 95:311–6. [DOI] [PubMed] [Google Scholar]
- 10.Stone PH, Gratsiansky NA, Blokhin A, Huang IZ, Meng L. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol 2006;48:566–75. [DOI] [PubMed] [Google Scholar]
- 11.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002; 346:1948–53. [DOI] [PubMed] [Google Scholar]
- 12.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol 2008;51:466–72. [DOI] [PubMed] [Google Scholar]
- 13.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 2008;29:480–9. [DOI] [PubMed] [Google Scholar]
- 14.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333–41. [DOI] [PubMed] [Google Scholar]
- 15.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief selfadministered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 1989;64:651–4. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. J Nucl Cardiol 2002;9:240–5. [DOI] [PubMed] [Google Scholar]
- 17.Chih S, Macdonald PS, Feneley MP, Law M, Graham RM, McCrohon JA. Reproducibility of adenosine stress cardiovascular magnetic resonance in multi-vessel symptomatic coronary artery disease. J Cardiovasc Magn Reson 2010;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkington AG, Gatehouse PD, Ablitt NA, Yang GZ, Firmin DN, Pennell DJ. Interstudy reproducibility of quantitative perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005;7:815–22. [DOI] [PubMed] [Google Scholar]
- 19.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA 2005; 293:477–84. [DOI] [PubMed] [Google Scholar]
- 20.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 2006; 295:1404–11. [DOI] [PubMed] [Google Scholar]
- 23.Holubkov R, Laskey WK, Haviland A, et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am Heart J 2002;144:826–33. [DOI] [PubMed] [Google Scholar]
- 24.Pepine CJ. Ischemic heart disease in women: facts and wishful thinking. J Am Coll Cardiol 2004;43:1727–30. [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman R, Belardinelli L, Blackburn B, Heo J, Iskandrian AE. A study of the effects of ranolazine using automated quantitative analysis of serial myocardial perfusion images. J Am Coll Cardiol Img 2009;2:1301–9. [DOI] [PubMed] [Google Scholar]
- 26.Mega JL, Hochman JS, Scirica BM, et al. Clinical features and outcomes of women with unstable ischemic heart disease: observations from metabolic efficiency with ranolazine for less ischemia in non-ST-elevation acute coronary syndromes-thrombolysis in myocardial infarction 36 (MERLINTIMI 36). Circulation 2010;121:1809–17. [DOI] [PubMed] [Google Scholar]
- 27.Olson MB, Kelsey SF, Matthews K, et al. Symptoms, myocardial ischaemia and quality of life in women: results from the NHLBI-sponsored WISE Study. Eur Heart J 2003;24:1506–14. [DOI] [PubMed] [Google Scholar]
- 28.Fraker TD Jr., Fihn SD, Gibbons RJ, et al. 2007. chronic angina focused update of the ACC/AHA 2002 Guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines Writing Group to Develop the Focused Update of the 2002 Guidelines for the Management of Patients With Chronic Stable Angina. J Am Coll Cardiol 2007;50:2264–74. [DOI] [PubMed] [Google Scholar]
- 29.Cocco G, Rousseau MF, Bouvy T, et al. Effects of a new metabolic modulator, ranolazine, on exercise tolerance in angina pectoris patients treated with beta-blocker or diltiazem. J Cardiovasc Pharmacol 1992;20:131–8. [PubMed] [Google Scholar]
- 30.Hayashida W, van Eyll C, Rousseau MF, Pouleur H. Effects of ranolazine on left ventricular regional diastolic function in patients with ischemic heart disease. Cardiovasc Drugs Ther 1994;8:741–7. [DOI] [PubMed] [Google Scholar]
- 31.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 2010;121:2317–25. [DOI] [PubMed] [Google Scholar]
- 32.Lerman A, Sopko G. Women and cardiovascular heart disease: clinical implications from the Women’s Ischemia Syndrome Evaluation (WISE) Study. Are we smarter? J Am Coll Cardiol 2006;47 Suppl:S59 – 62. [DOI] [PubMed] [Google Scholar]
- 33.Barmeyer AA, Stork A, Muellerleile K, et al. Comparison of quantitative coronary angiography and first-pass perfusion magnetic resonance imaging for the detection of an impaired coronary perfusion in nonsevere coronary stenosis. J Magn Reson Imaging 2008; 27:1005–11. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim T, Nekolla SG, Schreiber K, et al. Assessment of coronary flow reserve: comparison between contrast-enhanced magnetic resonance imaging and positron emission tomography. J Am Coll Cardiol 2002;39:864–70. [DOI] [PubMed] [Google Scholar]


