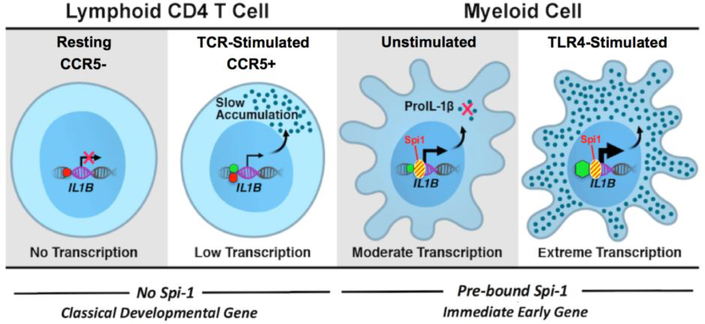
Figure 5. Monocytes and T cells have distinct mechanisms for IL1B gene regulation.
LPS-treated monocytes have high levels of active monovalent H3K4me3 ( ) but lack inhibitory H3K27me3 (
) but lack inhibitory H3K27me3 ( ) epigenetic histone modifications at the IL1B promoter. These modifications, along with activated NF-κB and C/EBPβ [9], drive a vigorous Pol II engagement with extremely high transcriptional activity (
) epigenetic histone modifications at the IL1B promoter. These modifications, along with activated NF-κB and C/EBPβ [9], drive a vigorous Pol II engagement with extremely high transcriptional activity ( ) and correspondingly high levels of cytoplasmic proIL-1β protein (
) and correspondingly high levels of cytoplasmic proIL-1β protein ( ). Unstimulated monocytes contain lower levels of monovalent H3K4me3 at the IL1B promoter and are deficient in inhibitory marks, resulting in weak Pol II engagement and extremely low transcription levels compared to stimulated monocytes. Accumulation of proIL-1β in these cells is inhibited due to a translational blockade (
). Unstimulated monocytes contain lower levels of monovalent H3K4me3 at the IL1B promoter and are deficient in inhibitory marks, resulting in weak Pol II engagement and extremely low transcription levels compared to stimulated monocytes. Accumulation of proIL-1β in these cells is inhibited due to a translational blockade ( ). The myeloid competence factor, Spi1 (
). The myeloid competence factor, Spi1 ( ), is constitutively bound at a constant level to the IL1B promoter in these cells [9]. In contrast to the immediate-early LPS responsive IL1B gene in monocytes, slowly-activated developmentally-regulated genes possess bivalent promoters with both active H3K4me3 and inhibitory H3K27me3 epigenetic histone modifications. The IL1B promoter in lymphoid CCR5+ CD4 T cells contains such a bivalent H3K4me3+/H3K27me3+ mark, resulting in decreased Pol II engagement and significantly lower (~25‑ times) transcription activity, as compared to resting monocytes. Interestingly, despite such a low level of transcription, intracellular proIL-1β accumulates in CCR5+ CD4 T cells in amounts higher than unstimulated monocytes. CCR5- CD4 T cells exclusively contain the inhibitory H3K27me3, which does not support active IL1B transcription until after the engagement of the T cell receptor (TCR).
), is constitutively bound at a constant level to the IL1B promoter in these cells [9]. In contrast to the immediate-early LPS responsive IL1B gene in monocytes, slowly-activated developmentally-regulated genes possess bivalent promoters with both active H3K4me3 and inhibitory H3K27me3 epigenetic histone modifications. The IL1B promoter in lymphoid CCR5+ CD4 T cells contains such a bivalent H3K4me3+/H3K27me3+ mark, resulting in decreased Pol II engagement and significantly lower (~25‑ times) transcription activity, as compared to resting monocytes. Interestingly, despite such a low level of transcription, intracellular proIL-1β accumulates in CCR5+ CD4 T cells in amounts higher than unstimulated monocytes. CCR5- CD4 T cells exclusively contain the inhibitory H3K27me3, which does not support active IL1B transcription until after the engagement of the T cell receptor (TCR).