Abstract
GM-CSF is a cytokine produced by T helper (Th) cells that plays an essential role in orchestrating neuroinflammation in experimental autoimmune encephalomyelitis, a rodent model of multiple sclerosis. Yet where and how Th cells acquire GM-CSF expression is unknown. In this study we identify mast cells in the meninges, tripartite tissues surrounding the brain and spinal cord, as important contributors to antigen-specific Th cell accumulation and GM-CSF expression. In the absence of mast cells, Th cells do not accumulate in the meninges nor produce GM-CSF. Mast cell-T cell co-culture experiments and selective mast cell reconstitution of the meninges of mast cell-deficient mice reveal that resident meningeal mast cells are an early source of caspase-1-dependent IL-1β that licenses Th cells to produce GM-CSF and become encephalitogenic. We also provide evidence of mast cell-T cell co-localization in the meninges and CNS of recently diagnosed acute MS patients indicating similar interactions may occur in human demyelinating disease.
Keywords: Experimental autoimmune, encephalomyelitis (EAE), Multiple sclerosis (MS), Meninges, Mast cells, GM-CSF, IL-1beta, Inflammasome, Caspase-1, T helper cells, T cell licensing, Myeloid cells
1. Introduction
Multiple sclerosis (MS) is an autoimmune demyelinating disease of the central nervous system (CNS) [1]. Immune-mediated damage to the myelin sheath and myelin-producing oligodendrocytes leads to focal demyelinating lesions, neuronal and axonal damage, and variable neurological deficits. Although the etiology is still unknown, it is generally accepted that MS is initiated by the migration of peripherally primed myelin-specific Th1 and Th17 cells into the immune specialized CNS [2]. However, studies in the mouse model of MS, experimental autoimmune encephalomyelitis (EAE), reveal that the transit of Th cells from priming sites in the peripheral lymphoid organs to the CNS parenchyma is not direct. Myelin-primed Th cells have been shown to localize in the lungs for 2–3 days after adoptive transfer where they undergo reprogramming and up-regulate genes that facilitate CNS homing and entry [3]. Th cell transit through the gut-associated lymphoid tissues and exposure to commensal bacteria has also been reported to promote the differentiation and expansion of Th17 cells in a spontaneous model of EAE [4]. These data have lead to the idea that newly primed autoreactive T cells are not inherently pathogenic, but must first be “licensed” in peripheral tissues through interactions with other resident immune cells as in the lung or with commensal bacteria in the gut [5].
Yet several features of the meninges, highly vascularized mast cell rich tissues that surround both the brain and spinal cord and enclose the cerebrospinal fluid (CSF), suggest they are more relevant sites for promoting T cell encephalitogenicity. In preclinical EAE, myelin-specific T cells interact with myelin-loaded resident antigen presenting cells (APCs) in the meninges days before autoreactive T cells are detected in the CNS [6,7]. In contrast, Th cell accumulation in the lungs is non-specific and lung-residing myelin-reactive Th cells first localize almost exclusively in the meninges and blood 48 h after a secondary transfer to naïve mice [3]. Resident mast cells in the meninges play an essential role in regulating early inflammation in EAE as well. Activated within a day of disease induction, mast cells express multiple pro-inflammatory mediators including IL-1β, a cytokine that promotes T cell production of GM-CSF and is essential for EAE development [8,9]. Mast cells also express TNF, which stimulates an early influx of neutrophils to the meninges and loss of blood brain barrier (BBB) integrity, resulting in large-scale immune cell infiltration into the CNS [8,10].
The reports of coincident early activation of mast cells and T cells in the meninges as well as the expression of IL-1β by meningeal mast cells [8] lead us to hypothesize that interactions between these cells has consequences for promoting T cell encephalitogenicity. There is substantial in vitro and in vivo data demonstrating that mast cell-T cell interactions occur and result in cross activation. Perhaps most relevant to EAE is the demonstration that mast cells co-localize with Th17 and regulatory T cells (Tregs) in the draining lymph nodes and CNS of mice with EAE [11]. Through the production of IL-6 and engagement of OX40L, mast cells counteract effector Th cell suppression by Tregs, thus amplifying the autoreactive response.
In this report, we demonstrate that in the absence of mast cells, Th cells do not accumulate in the meninges nor do they generate a robust GM-CSF response. Mast cell-T cell co-culture experiments and selective meningeal reconstitution of mast cell-deficient mice reveal that resident meningeal mast cells are an early source of caspase-1-dependent IL-1β, which in turn licenses Th cells to produce GM-CSF and become encephalitogenic. We also provide evidence of mast cell-T cell co-localization in the meninges in a subset of acute MS patients with prominent meningeal inflammation, suggesting that interactions between these cell types occur in vivo in human disease. These data have implications for developing meningeal mast cell targeted therapies that inhibit IL-1β production in early MS.
2. Methods
2.1. Mice
C57BL/6; WBB6F1-KitW/Wv and control WBB6; B6.129S2-Casp1tm1Flv/J and control C57BL/6NCr; and B6.PL-Thy1a/CyJ were purchased from Jackson Laboratory. The Il1b−/− mice were a kind gift from Dr. Thirumala-Devi Kanneganti. All mice were housed under specific pathogen free conditions in the Association for Assessment of Accreditation of Laboratory Animal Care approved facility at Northwestern University.
2.2. Active EAE induction
Four to eight week old mice were immunized with 100 μg MOG35–55 emulsified in Complete Freund’s Adjuvant and subject to blinded scoring as follows; 0: healthy, 1: flaccid tail, 2: ataxia, 3: full hind limb paralysis, 4: moribund, 5: death.
2.3. Generation of effector T cells
Ten to twelve days post immunization, the inguinal, axillary, and brachial lymph nodes were isolated from donor mice and homogenized. Isolated cells were cultured at 6 × 106 cells/mL for four days in 15% complete RPMI with 50 μg/mL of MOG35–55 or OVA323–339 supplemented with 10 ng/mL rmIL-23 (R & D Systems) and 12 ng/ml rmIL-12 (R & D Systems).
2.4. Adoptive transfer
Four × 106 T cell blasts were transferred intraperitoneally into recipient mice. No pertussis toxin was administered. Mice were scored as described above.
2.5. Generation of bonemarrow-derived mast cells (BMMCs) and meningeal reconstitution
BMMCs were generated as previously described [12]. Purity was determined by flow cytometry (>94% live cells co-expressing c-kit and FcεRIα). For reconstitution, 3–5 week old KitW/Wv mice were reconstituted intracranially at the cranial vertex with 1 × 106 BMMCs.
2.6. CNS and meningeal leukocyte isolation
Mice were perfused by injection of 30 mL PBS into the left ventricle. Brains and spinal cords were dissected out and incubated in serum free RPMI with 300 U/mL Type IV collagenase (Worthington Biochemical Corp.) at 37 °C for 90 min. The samples were then homogenized over metal screens and leukocytes were enriched on a 40% Percoll (VWR) gradient for flow cytometry analysis. The meninges were dissected from the calvarium under a light microscope. The meninges from 3 to 5 mice were pooled, digested with 1 mM EDTA (VWR) and 300 U/mL Type IV collagenase (Worthington Biochemical Corp.) at 37 °C for 30 min. The pooled samples were then homogenized over 70 μM cell strainers (MidSci) with plastic syringe plungers and processed for flow cytometry.
2.7. Flow cytometry
Cells were treated with FcBlock (anti-CD16/32-Biolegend) and stained with the indicated antibodies (eBioscience and Biolegend). For intracellular cytokine staining, cells were restimulated with 50 mg/mL MOG35–55 for 5 h with 3 μg/mL Brefeldin A (eBioscience) for the last 3 h. Cells were assayed for cytokine production using the Fixation & Permeabilization Kit (eBioscience).
2.8. Quantitative real-time PCR analysis
RNA was isolated from co-cultured cells using the SV Total RNA Isolation System (Promega) and from the pooled meningeal samples using a hand held tissue homogenizer and the RNAeasy Fibrous Tissue Mini Kit (Qiagen). cDNA was generated using SuperScript III First-Strand Synthesis System (Invitrogen). Quantification was performed with iCycler iQ5 Real Time PCR Detection System (Bio-Rad) using PerfecTA SYBR Green SuperMix (BioRad). For each sample, the cycle number (Ct) at which the analysis threshold was reached, set in the linear range of the amplification curve (fluorescence = f[cycle]), was calculated. The relative expression of the cDNA template was calculated as the ratio of 2ΔCt for each transcript tested relative to a control housekeeping gene (Hprt). PCR primers are listed below.
| Gene | Forward | Reverse |
|---|---|---|
| Tpsab1 | CTGTTGGTGTTAGGAACCAGG | GTGCTGAATGCTTGTTTGCCA |
| Hdc | CGTGAATACTACCGAGCTAGAGG | ACTCGTTCAATGTCCCCAAAG |
| Tnf | GCCACCACGCTCTTCTGTCT | GGTCTGGGCCATAGAACTGATG |
| Il1b | GACGGCACACCCACCCT | AAACCGTTTTTCCATCTTCTTCTTT |
| Il17 | AGCTCATCCGAGTGGTCCAC | GCTTCCTGAGGCTGGATTCC |
| Csf2 | ATGCCTGTCACGTTGAATGAAG | GCGGGTCTGCACACATGTTA |
| Ifng | ACGTCATCCGAGTGGTCCAC | GCTTCCTGAGGCTGGATTCC |
2.9. Preparation and toluidine blue staining of meninges
Following perfusion, the calvarium (containing the meninges) was dissected and fixed in formalin for 24 h before transfer and storage in PBS. The calvarium was then stained with acidic toluidine blue for 15 s and rinsed three times in distilled water. The meninges were separated from the calvarium while submerged in distilled water and floated onto glass slides. After the tissue was dried and adhered to the slide, it was covered with a coat of clear nail polish. Mast cells are visualized as granulated purple chromatic cells under 10 × magnification with a Primo Plan ACHROMAT 10 ×/0.25 lens at room temperature with a Zeiss Primo Star light microscope using IC Capture 2.0 (The Imaging Source). The average number of mast cells in 18 1 mm × 1 mm squares in 6 different regions was calculated and expressed as number of mast cells per mm2.
2.10. Mast cell: T cell co-culture
Effector T cells were generated as described above. CD4+ T cells were isolated either with Dynabeads® FlowComp™ Mouse CD4 positive isolation beads (Invitrogen) or CD4 (L3T4) MicroBeads (Miltenyi). BMMCs were generated as described above and cultured with isolated CD4+ Th cells at a 1:1 ratio for 2–6 h at 37 °C with or without plate-bound anti-CD3 (2 μg/mL) and anti-CD28 (5 μg/mL). Following co-culture, T cells were separated from the BMMCs by bead separation. Recombinant mouse IL-1β (R &D) was used at 2, 10, and 50 ng/ml. Recombinant mouse IL-18 (R &D) was used at 2 and 50 ng/ml.
2.11. Histology
Formalin fixed, paraffin embedded, 5 mm thick sections from archival MS CNS tissue were analyzed for the presence of meningeal and parenchymal mast cells. Inclusion criteria (Supplemental Fig. 1) were: i) pathological diagnosis of confluent demyelinating disease consistent with MS; ii) presence of meninges; iii) autopsy performed within 3 years of symptom onset; and iv) sufficient tissue for additional histopathological analysis. Among 20 MS cases, 11 blocks from 11 cases (5 females, 6 males, median age: 36, range: 18–71 years) met the inclusion criteria. Sections were stained with hematoxylin-eosin, Luxol fast blue, and by immunohistochemistry with antibodies against proteolipid protein (Serotec, MC839G) and CNPase (Convance, SMI91) for myelin, CD3 (Serotec, MCA1477) for T cells, and tryptase (Abcam, ab2378) for mast cells. Demyelinating activity was classified according to previously published criteria [13].
2.12. Statistics
All statistics were performed with GraphPad Prism 6.0. Specific statistical tests are provided in each figure legend.
3. Results
3.1. Myelin specific Th cells accumulate in the meninges in early EAE
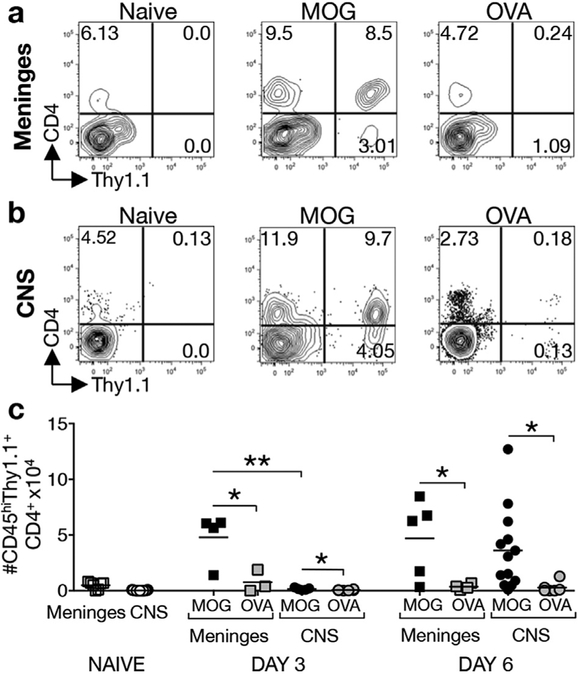
To study T cell-mast cell interactions in the meninges we utilized an adoptive transfer system that allows tracking of MOG-primed T cells. Thy1.1+ donor mice were immunized with MOG35–55 peptide. Ten days post immunization, lymph node cells were isolated and cultured for 4 days with MOG35–55 peptide under conditions that induce Th1 and Th17 cells. Four × 106 T cell blasts were then transferred to Thy1.2+ recipients. As a control, cells from OVA323–339-immunized Thy1.1+ mice were also expanded in vitro and transferred to Thy1.2+ recipients. Thy1.1+ cells were examined in the meninges (Fig. 1a,c) and the CNS (Fig. 1b,c) at days 3 and 6 post transfer. At both time points significantly more MOG-specific Th cells than OVA-specific Th cells were detected in the meninges and the CNS (Fig. 1c). This selective accumulation of MOG-specific Th cells likely reflects their interaction with myelin-bearing APCs in the meninges that serve to activate and retain MOG-specific T cells as previously reported [6,7,14].
Fig. 1. MOG35–55-primed, but not OVA323–339-primed, Th cells accumulate in the meninges and CNS early in EAE.
Four x106 MOG35–55- or OVA323–339-primed T cell blasts from Thy1.1+ donor mice were restimulated with peptide in vitro and transferred to congenic Thy1.2+ recipients. The frequency and numbers of Thy1.1+CD45+CD4+ cells in the meninges (a,c) and CNS (b,c) was determined 3 or 6 days post transfer. (a) Representative analysis of MOG35–55 or OVA323–339-primed CD4+Thy1.1+ T cells detected in the pooled meninges of Thy1.2+ recipients at day 6 post transfer. (b) Representative analysis of MOG35–55 or OVA323–339-primed CD4+Thy1.1+ T cells detected in the CNS (pooled brain and spinal cord) of Thy1.2+ recipients 6 days post transfer. Percentages of the CD45+/hi parent gate are shown. (c) Numbers of CD45+CD4+Thy1.1+ MOG35–55 or OVA323–339-primed T cells in the meninges and CNS at indicated days post T cell transfer. For meninges samples, each point represents the analysis of a pool of tissues from 3 to 5 mice and is expressed as numbers/mouse. CNS data points represent the analysis of individual mice. *p < 0.05 and **p < 0.01 by Student’s t-Test. 4 independent experiments.
Consistent with the idea that T cells traffic through the meninges prior to their entry into the CNS [14–16], it is notable that 30 times more MOG-specific Th cells are detected in the meninges compared to the CNS (4.81 × 104 ± 1.14 × 104 vs. 0.16 × 104 ± 0.05 × 104 respectively) at day 3 (Fig. 1c). However, this ratio changes by day 6, when similar numbers of Th cells are observed in the meninges and CNS (4.71 × 104 ± 1.56 × 104 vs. 3.63 × 104 ± 1.00 × 104 respectively).
3.2. Mast cells are activated by T cell transfer and regulate the accumulation of myelin-specific Th cells in the meninges
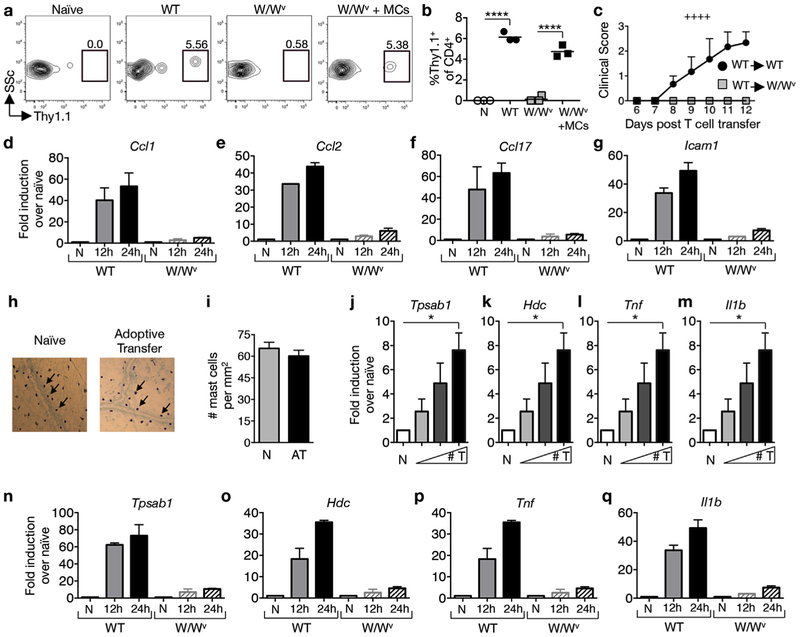
Many of the insights into mast cell contributions to EAE were gained in studies utilizing KitW/Wv mice, which exhibit an 80–90% reduction in c-kit signaling and lack mast cells [17]. We have previously shown that female KitW/Wv mice are protected from severe EAE and this protection is lost upon the systemic or local meningeal reconstitution of mast cells [10,12,18]. To determine whether mast cells influence T cell transit to the meninges, MOG35–55-primed T cells from Thy1.1+ wild type donors were transferred to either wild type or KitW/Wv Thy1.2+ recipients and their presence in the meninges was assessed 1 day post transfer. As shown in Fig. 2a,b, a discrete population of Thy1.1+ CD4+ T cells is observed in wild type recipients, whereas a much smaller population was detected in KitW/Wv recipients. Intracranial injection of mast cells, which selectively reconstitutes mast cells in the meninges and cervical lymph nodes of KitW/Wv mice [10], restores T cell influx to wild type levels (Fig. 2a,b). This deficit in early T cell accumulation in the meninges of KitW/Wv mice corresponds with the failure of these T cells to cause disease upon adoptive transfer (Fig. 2c).
Fig. 2.
Mast cells are activated by T cell transfer and regulate the accumulation of myelin-specific Th cells in the meninges. MOG35–55-primed T cells from Thy1.1+ mice were reactivated with MOG35–55 peptide in vitro for 4 days before transfer of 4 × 106 blasts to Thy1.2+ wild type (WT), mast cell-deficient KitW/Wv (W/Wv), or meningeal mast cell reconstituted KitW/Wv (W/Wv + MCs) recipients. (a) Representative flow cytometric analysis of Thy1.1+ CD4+ T cell infiltration in the meninges 24 h post transfer. Numbers represent percentage of CD45+CD4+ cells that are Thy1.1+.(b) Frequencies of Thy1.1+ T cells detected in the meninges of indicated recipients. Each data point represents the analysis of pooled meningeal tissue from 4 mice. ****p < 0.0001 by Student’s t-Test. (c) Clinical scores of WT and KitW/Wv recipients after adoptive transfer of 4 × 106 WT T cell blasts. ++++p < 0.0001 by two way ANOVA. 2 independent experiments using 4 mice each. (d–g) RT-PCR analyses of pooled meninges tissues from WT and W/Wv recipient mice at indicated time points. The expression of indicated genes was determined relative to Hprt and expressed as fold induction over naïve. n = 2 pooled samples of 5 mice each for each time point. 2 independent experiments. (h) Meningeal mast cells identified by toluidine blue staining and (i) mast cell numbers in naïve (N) and T cell recipient mice (AT) at 24 h post transfer, n = 9 mice. (j–m) RT-PCR analysis of pooled meninges samples as described in (d–g) 24 h after transfer of T cell blasts [0 (N), 2, 4, and 8 × 106] to wild type recipients. *p < 0.05 by Student’s t-Test. n = 4 per group, 2 independent experiments. (n–q) RT-PCR analyses of pooled meninges as described in (d–g). n = 2 pooled samples of 5 mice for each time point. 2 independent experiments. All values are expressed as mean ± SEM.
Early T cell accumulation in the meninges of wild type mice corresponds to a dramatic induction of Ccl1, Ccl2 and Ccl17 (Fig. 2d–f), genes that encode T cell-attracting chemokines, as early as 12 h post T cell transfer. The expression of these chemokines is reduced in KitW/Wv mice consistent with previous reports that they are expressed by mast cells [19,20]. We also detect c-kit-dependent expression of Icam1, encoding a molecule expressed by mast cells and other cells that mediates T cell activation through interaction with LFA-1 (Fig. 2g) [21].
The c-kit-dependent expression of chemokines and adhesion molecules indicates that T cell transfer alone can elicit mast cell gene expression in the absence of adjuvants commonly used in active disease induction. To further test this possibility, graded numbers of MOG35–55-primed T cells were transferred to wild type recipients. Mast cell numbers and the expression of mast cell-associated gene transcripts within the meninges were assessed 1 day post transfer. As shown in Fig. 2h and i, mast cell numbers do not increase upon T cell transfer at this time point. However, several inflammatory transcripts are significantly induced in a dose dependent manner with the highest expression in mice that received the most T cells. These include transcripts not expressed by T cells such as the mast cell-specific gene, Tpsab1, which encodes tryptase (Fig. 2j) and Hdc, which encodes histidine decarboxylase (Fig. 2k). Il1b and Tnf, which are expressed by multiple cell types including mast cells, T cells and macrophages, are also induced (Fig. 2l,m). Similar to chemokine genes, these transcripts are induced as early as 12 h post transfer and this expression is largely c-kit dependent (Fig. 2n–q).
3.3. Robust GM-CSF production by Th cells is acquired post priming in wild type but not KitW/Wv mice
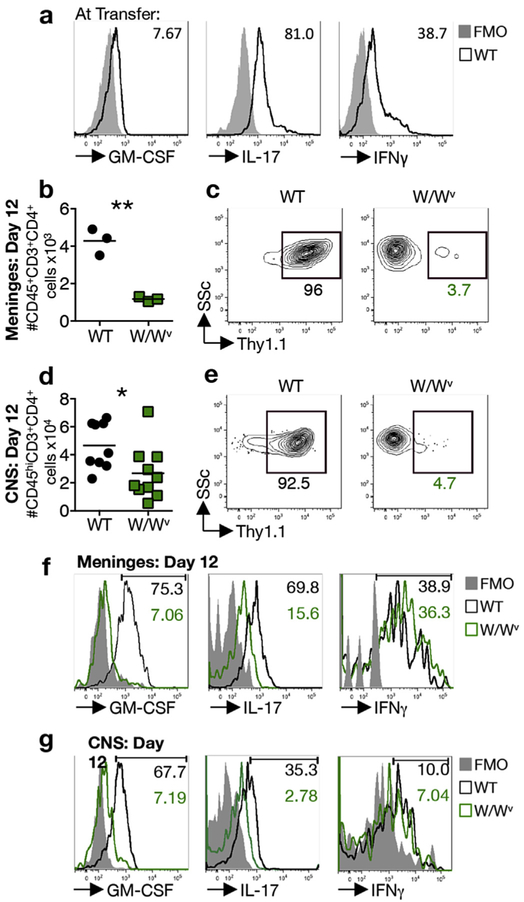
Myelin-specific Th cells accumulate in the meninges of disease-susceptible wild type but not disease-resistant mast cell-deficient recipients as early as day 1 (Fig. 2a–c). This observation suggests that T cell reactivation in the meninges enhances the expression of pathogenic cytokines such as GM-CSF, conferring encephalitogenicity. To test this possibility, T cells were primed in Thy1.1+ wild type mice and reactivated in vitro under conditions that do not favor robust GM-CSF production. T cell cytokine expression was compared prior to transfer and 12 days post transfer in both the meninges and CNS of Thy1.2+ wild type and KitW/Wv adoptive transfer recipients. Prior to transfer, T cell blasts express both IL-17 and IFNγ, but negligible GM-CSF (Fig. 3a). Upon transfer fewer Th cells are present in the meninges and CNS of KitW/Wv mice relative to wild type mice as expected (Fig. 3b,d) and only a small proportion of these are Thy1.1+ (Fig. 3c,e). Notably, the frequency of GM-CSF+ and IL-17+ T cells in both the meninges and CNS is dramatically reduced in KitW/Wv recipients, while the frequency of IFNγ-producing T cells does not differ (Fig. 3f, g). Although these experiments cannot distinguish whether the enhanced GM-CSF and IL-17 expression by T cells is acquired in the meninges or during transit to the meninges, they confirm that post-priming T cell licensing in the meninges is c-kit dependent.
Fig. 3.
Robust GM-CSF production by Th cells is acquired post priming in wild type, but not KitW/Wv mice. (a) Representative analysis of cytokine production by MOG35–55 eprimed wild type donor T cell blasts prior to adoptive transfer. Numbers denote frequency of cytokine-expressing CD3+CD4+ blast cells and are representative of 3 independent experiments. (b) Accumulation of CD45+CD3+CD4+ T cells in the meninges at day 12 post transfer of 4 × 106 Thy1.1+ T blasts to wild type (WT) and KitW/Wv (W/Wv) recipients. Each data point represents analysis of meningeal tissue pooled from 3 mice and is expressed as average cell number per mouse. 3 independent experiments. **p < 0.01 by Student’s t-Test. (c) Representative analysis showing the frequency of Thy1.1+ T cells of the CD45+CD3+CD4+ population in the meninges of indicated recipients at day 12 post T cell transfer. (d) Accumulation of CD45hiCD3+CD4+ T cells in the CNS of indicated recipients at day 12 post T cell transfer. Each data point represents analysis of pooled brain and spinal cord tissue from one mouse, 3 independent experiments. *p < 0.05 by Student’s t-Test. (e) Representative analysis showing the frequency of Thy1.1+ T cells of the CD45hiCD3+CD4+ population in the CNS of indicated recipients. (f) Representative analysis of cytokine production by CD45+CD3+CD4+ Th cells in the meninges of pooled samples from 4 mice at day 12 post transfer of T cell blasts. Numbers denote frequency of cytokine expressing CD45+CD3+CD4+ cells in WT (black) and W/Wv (green) meninges. (g) Representative analysis of cytokine production by CD45hiCD3+CD4+ T cells in the CNS of indicated recipients at day 12 post transfer of T blasts. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Mast cell-derived IL-1β directly augments GM-CSF production by Th cells
T cell transfer elicits c-kit dependent Il1b expression in the meninges (Fig. 2m,q) and Th cells in the meninges produce a strong GM-CSF response (Fig. 3f). Thus we hypothesized that IL-1β expressed by meningeal mast cells drives Th cell GM-CSF expression. IL-1β-driven GM-CSF production by Th cells was previously shown to be dependent on caspase-1, an enzyme that cleaves pro-IL-1β, as well as pro-IL-18, to their active mature forms [22]. Although the cellular source of IL-1β was not identified in these experiments, T cells isolated from the draining lymph nodes of immunized Casp1−/− mice express significantly reduced GM-CSF compared to those from wild type mice.
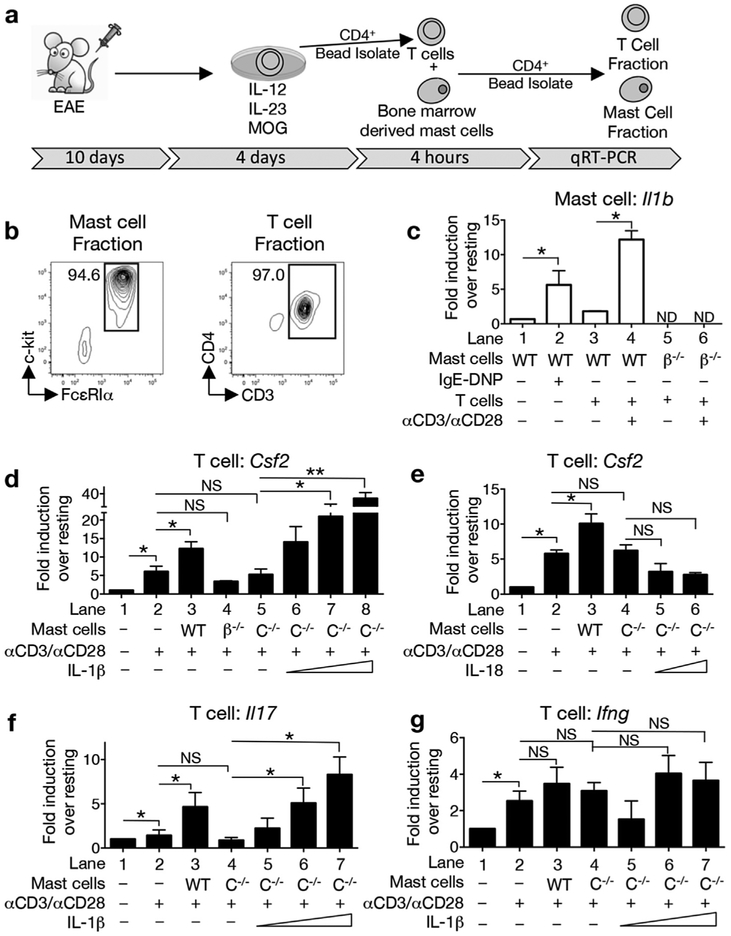
We first tested whether direct interactions between these cell populations result in cross-activation by establishing an in vitro mast cell: T cell co-culture system (Fig. 4a). T cells from MOG35–55-immunized mice were cultured under polarizing conditions to expand Th1 and Th17 cells. Purified CD4+ T cells, with or without anti-CD3/CD28 re-stimulation, were subsequently co-cultured with bone marrow-derived mast cells for 4 h. Upon re-isolation, the purity of the mast cell and Th cell fractions was greater than 94% (Fig. 4b). Cytokine gene expression by each individual population was evaluated by RT-PCR (Fig. 4c–g). As a control for inducible Il1b expression, wild type mast cells activated via cross-linking of the high affinity FcεR1 showed an average 6-fold increase in expression over unstimulated mast cells (Fig. 4c, lanes 1,2). The co-culture of anti-CD3/CD28-activated Th cells and wild type mast cells results in an approximately 12-fold increase in Il1b expression compared to co-cultures with resting Th cells (Fig. 4c, lanes 3, 4). As expected, neither Il1b−/− mast cells nor activated wild type Th cells express appreciable Il1b mRNA (Fig. 4c, lanes 5,6 and Supplemental Fig. 2a).
Fig. 4.
Mast cell-derived, caspase-1-dependent IL-1β directly augments GM-CSF production by Th cells. (a) Lymph node cells from MOG35–55- immunized donor mice were collected at day 10 post immunization and cultured with MOG35–55 peptide under Th1/Th17 polarizing conditions for 4 days. T cells were isolated using CD4+ magnetic beads and then co-cultured under various conditions with bone marrow-derived mast cells. After 4 h, CD4+ magnetic beads were used to separate the T and mast cell fractions, which were then assessed for gene expression by quantitative real time PCR. (b) Representative FACs analysis showing the purity of each fraction. The percentage of c-kit+FcεRIα+ or CD3+CD4+ cells is shown. Data is representative of 4 independent experiments. (c) Il1b expression by WT and Il1b −/−(β−/−) mast cells was analyzed in the presence or absence of Th cells. ND-not detected. (d, e) Csf2 induction by resting or reactivated (αCD3 and αCD28) Th cells in the absence of mast cells or in the presence of WT, β−/−, or Casp1−/−(C−/−) mast cells. (d) Exogenous IL-1β (2, 10, and 50 ng/ml) or (e) IL-18 (2 and 50 ng/ml) was added to the Casp1−/− mast cell: T cell co-cultures. (f,g) Induction of Il17 and Ifng by Th cells co-cultured with WT or C−/− mast cells. Gene expression is relative to Hprt and expressed as fold induction over resting cells. *p < 0.05 and **p < 0.01 by Student’s t-Test. n = 2 independent cultures for panel (e) and n = 4 for remaining panels (c,d,f,g). All values are expressed as mean ± SEM.
We next examined whether mast cells can directly elicit Csf2 expression by Th cells. As shown in Fig. 4d, lanes 1,2, activated Th cells show an average 6-fold increase in Csf2 expression over resting Th cells and expression is significantly increased by the addition of wild type mast cells (Fig. 4d, lane 3). Co-culture with Il1b−/− mast cells or with Casp1−/−mast cells, which cannot produce bioactive IL-1β or IL-18, does not induce increased Csf2 expression over anti-CD3/CD28 activation alone (Fig. 4d, compare lanes 2 and 4,5). GM-CSF production by mast cells was not detected in these experiments (Supplemental Fig. 2b).
Because caspase-1 has a role in regulating the expression of bioactive IL-1β as well as IL-18 [23,24], we asked if mast cell-derived IL-1β alone is sufficient to increase Csf2 expression in Th cells. Casp1−/− mast cells and activated Th cells were co-cultured with increasing concentrations of either recombinant IL-1β or IL-18 (Fig. 4d,e). Addition of IL-1β (Fig. 4d, lanes 6–8) but not IL-18 (Fig. 4e, lanes 5,6) to the Casp1−/− mast cell: T cell co-culture restores the Th cell Csf2 response. We conclude that activated Th cells exert a direct effect on mast cells by stimulating the caspase-1 pathway to induce bioactive IL-1β expression. This in turn promotes increased T cell GM-CSF production.
Mast cell derived IL-1β also impacts Il17 expression. As shown in Fig. 4f, lanes 3,4, co-culture with wild type, but not Casp1−/− mast cells, induces increased Il17 expression. The addition of exogenous IL-1β, but not IL-18 (data not shown), restores this response to T cell-Casp1−/− mast cell co-cultures (Fig. 4f, lanes 5e7). Neither wild type nor Casp1−/− mast cells significantly affect the expression of Ifng by re-activated Th cells (Fig. 4g, lanes 3,4).
3.5. Caspase-1 expression by meningeal mast cells is sufficient for encephalitogenic Th cell generation
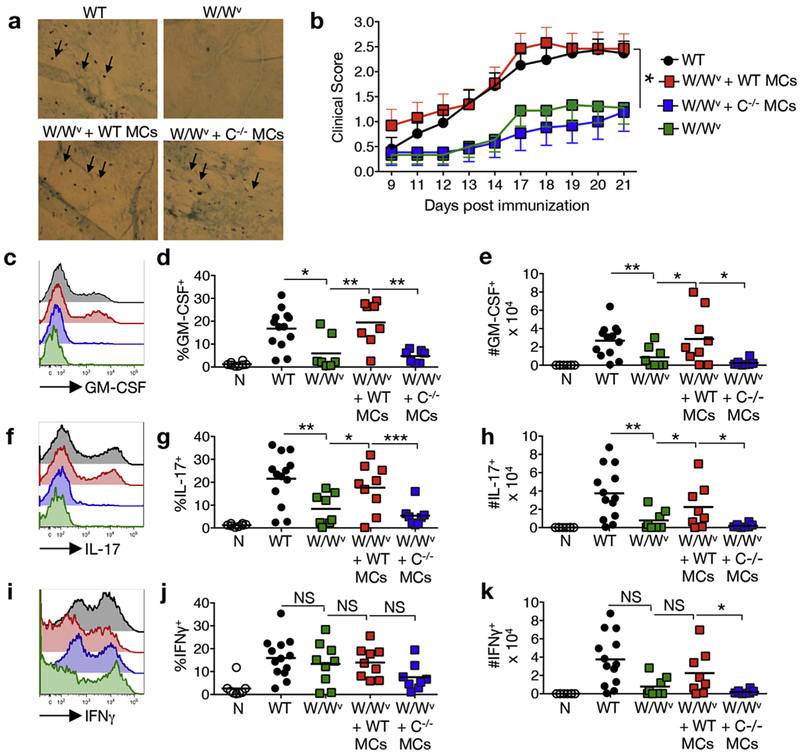
To determine if a meningeal mast cell-specific deficit in IL-1β production alters Th cell GM-CSF production and encephalitogenicity in vivo, KitW/Wv mice were selectively reconstituted with wild type or Casp1−/− mast cells by intracranial transfer as previously described [10] (Fig. 5a). Eight weeks post-reconstitution, EAE was induced by MOG35–55 peptide immunization and disease indices were compared (Fig. 5b and Table 1). KitW/Wv mice reconstituted with wild type mast cells show disease onset and severity similar to that of wild type controls. Both groups show significantly more severe disease than KitW/Wv mast cell deficient mice as previously reported [12]. However, reconstitution with Casp1−/− mast cells does not restore disease severity to wild type levels. In fact, the early disease course of the KitW/Wv + Casp1−/− mast cells is indistinguishable from that of the KitW/Wv non-reconstituted controls indicating that caspase-1 activity in meningeal mast cells is essential for severe disease.
Fig. 5.
Meningeal mast cell-derived caspase-1 is necessary for EAE development and Th cell encephalitogenicity in the CNS. The meninges of naïve KitW/Wv mice were selectively reconstituted with wild type (W/Wv + WT MCs) or Casp1−/−(W/Wv + C−/− MCs) bone marrow-derived mast cells. (a) Reconstitution was confirmed in a subset of mice by toluidine blue staining of the calvarial dura mater. Arrows denote toluidine blue-positive mast cells. (b) Eight weeks post reconstitution, EAE was induced by MOG35–55 peptide immunization and the disease course was compared to non-reconstituted controls. *p < 0.05 by 2-way ANOVA. n ≥ 12 for each group. 4 independent experiments. Data points are expressed as mean ± SEM. (c–k) Twelve days post immunization, CNS infiltrating leukocytes were isolated from a subset of the immunized mice and analyzed by flow cytometry. Representative analyses of GM-CSF (c), IL-17 (f), and IFNγ (i) production by CD45hiCD3+CD4+ cells are shown. FMO negative controls were used to set gates. Histogram colors correspond to experimental groups designated in (b). (d,g,j) Frequency and (e,h,k) numbers of CD45hi CD3+CD4+ cells that express cytokines *p < 0.05, **p < 0.01, and ***p < 0.001 by Student’s t-test. n ≥ 8 for each group, 3 independent experiments.
Table 1. Selective meningeal reconstitution of KitW/Wv mice with Casp1−/− mast cells fails to restore severe EAE.
The meninges of KitW/Wv (W/Wv) mice were selectively reconstituted with wild type (W/Wv + WT MCs) or Casp1 −/− (W/Wv + C−/−MCs) mast cells and disease indices were compared. Day of onset was determined in mice exhibiting clinical signs for ≥2 days. Peak score represents the average highest clinical score observed. Cumulative disease represents the sum of all clinical scores per mouse in each group over the observation period. Values are expressed as mean ± SEM.
| Group | Day of onset | Peak score | Cumulative disease, days 0–35 | Cumulative disease, days 0–21 | Cumulative disease, days 22–35 |
|---|---|---|---|---|---|
| WT | 12.91 ± 2.00 | 3.25 ± 0.13 | 37.79 ± 2.47 | 15.29 ± 1.63 | 22.50 ± 1.39 |
| W/Wv | 15.73 ± 1.09**++ | 1.82 ± 0.39***+ | 21.90 ±5.06**+ | 9.60 ± 2.378+ | 13.15 ± 2.76** |
| W/Wv + WT MCs | 12.17 ± 0.39 | 3.42 ± 0.32 | 41.25 ±6.125 | 18.67 ± 2.12 | 22.58 ± 4.10 |
| W/Wv + C−/− MCs | 16.75 ± l.84++ | 2.06 ± 0.50 | 24.31 ±6.76 | 8.19 ± 3.12+ | 16.13 ± 4.35 |
p < 0.01
p < 0.001 compared to WT by Student’s t-Test.
p < 0.05
p < 0.01 compared to W/Wv + WT MCs by Student’s t-Test. n ≥ 8 for all groups. 3 independent experiments.
The cytokine response of CNS-infiltrating Th cells was next assessed in these cohorts (Fig. 5cek). The frequency and number of GM-CSF-producing infiltrating Th cells is significantly reduced in the KitW/Wv + Casp1−/− mast cells cohort relative to both wild type mice and KitW/Wv mice reconstituted with wild type mast cells (Fig. 5c–e). IL-17 production is also diminished in Casp1−/− mast cell reconstituted KitW/Wv mice (Fig. 5f–h), supporting the co-culture data that indicates a role for mast-derived caspase-1 in Th cell IL-17 production. Although the frequency of IFNγ-producing Th cells was similar among all immunized mice (Fig. 5i–j), the number of IFNγ-producing Th cells is reduced in the KitW/Wv + Casp1−/− mast cells cohort compared to the wild type reconstituted KitW/Wv mice, perhaps suggesting an in vivo effect of IL-18, an IFNγ -inducing cytokine, that was not detected in the in vitro co-culture experiments (Fig. 5k). Taken together, the above data strongly support a critical role for caspase-1-dependent mast cell-derived IL-1β in encephalitogenic Th cell generation in EAE.
3.6. Evidence of meningeal mast cell-T cell co-localization in a subset of acute MS patients
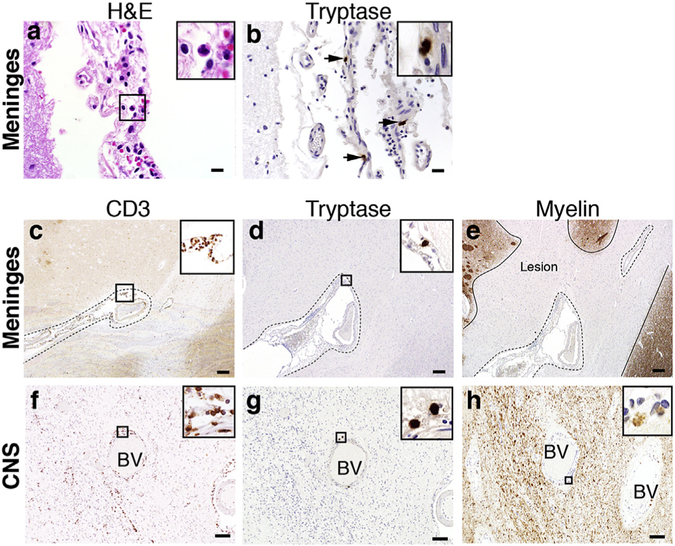
Although mast cells are also normal residents in the meninges and CNS of humans, they are somewhat rare and difficult to image under homeostatic conditions [25–28]. In MS, most studies have focused on the identification of meningeal mast cells from tissues of patients with late/progressive disease [29–32]. Yet there is a paucity of data that addresses the presence of mast cells and T cells in the meninges during early disease. We examined meningeal tissues from a small cohort of acute MS patients that were within 2–3 years of diagnosis. Of 11 cases that showed frank meningeal inflammation as previously defined [13] (Fig. 6a and Supplemental Fig. 1), four of these (36.4%) were positive for mast cell tryptase staining (Fig. 6b). All were positive for CD3+ T cells. We observed meningeal mast cell-T cell co-localization topographically associated with subpial cortical demyelination (Fig. 6c–e). Mast cells were also present in white matter parenchymal lesions of differing demyelinating stages (early active, inactive, and remyelinated), often in close proximity to infiltrating T cells [data not shown]. As shown in (Fig. 6f–h), perivascular mast cells and T cells co-localized within an early active MS lesion, characterized by myelin-laden macrophages. These observations suggest that, as in EAE, there is the potential for similar mast cell-T cell crosstalk in the meninges of MS patients.
Fig. 6.
Mast cell-T cell co-localization in the meninges and CNS of acute MS patients. (a) H&E staining shows brain-associated meningeal granulocyte infiltration in an acute MS autopsy case. (b) Immunohistochemistry analysis of tryptase positive mast cells (arrows) present within the meninges of the same case. (c) Immunohistochemistry analysis of CD3+ T cells identified in the meninges (designated by dashed outline) of a brain sulcus of an acute MS autopsy case. (d) Immunohistochemistry analysis of tryptase positive mast cells on a consecutive section of (c) reveals mast cells and T cells co-localized in the meninges. (e) Myelin PLP staining on a consecutive section of (c,d), shows subpial cortical demyelination associated with meningeal inflammation. Lesion borders are designated by a solid line. (f) Immunohistochemistry analysis of infiltrating perivascular CD3+ T cells identified in the CNS parenchyma of an acute MS autopsy case. BV = blood vessel. (g) Immunohistochemistry analysis of perivascular tryptase positive mast cells identified in a consecutive section of (f) shows mast cell-T cell co-localization within an active demyelinating lesion. (h) Myelin CNPase staining of consecutive sections of (f,g) within the CNS parenchyma shows myelin debris within macrophages suggesting early demyelinating activity. Enlarged views shown in panel insets. Scale bars in A–B: 20 μm, C–E: 200 μm, F–H: 100 μm.
4. Discussion
The precise mechanisms that lead to oligodendrocyte and neuronal damage in EAE and MS are still unclear. However, it is now generally accepted that CNS-infiltrating Th cells are not the direct effectors of CNS pathology. Rather Th cells orchestrate a local and damaging inflammatory response mediated largely by myeloid cells [33]. T cell-derived GM-CSF, which in this setting acts predominantly on CCR2+ monocytes, is critical for this myeloid cell recruitment and activation in EAE [9,34,35]. Based on this data, together with the finding that GM-CSF-deficient (Csf2−/−) Th cells fail to transfer EAE, current models equate Th cell encephalitogenicity with GM-CSF expression [9,33–36]. In MS, the frequency of GM-CSF-producing Th cells corresponds with more severe disease suggesting a similar mechanism exists in humans [37].
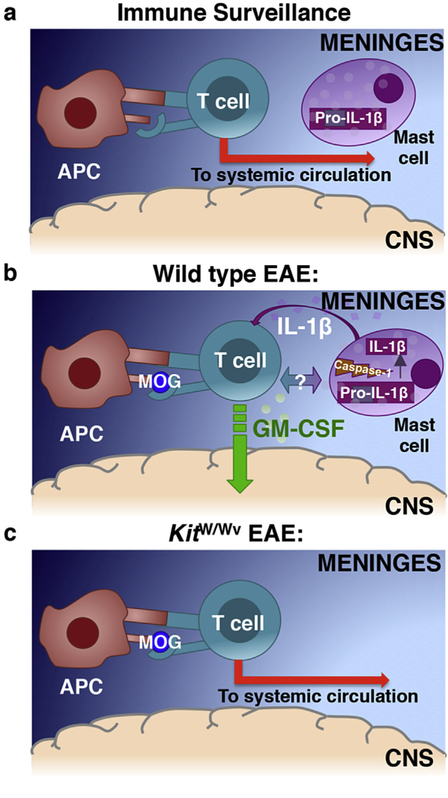
Our studies implicate the relatively large resident mast cell population in the meninges as promoters of T cell encephalitogenicity and thus establish the meninges as critical sites of T cell licensing. Although once considered solely as physical protection for the CNS, the meninges are now appreciated as locations of immune activity in both health and disease. Similar to immune border sites such as the skin, gut and lungs, mast cells, DCs, macrophages and innate lymphoid cells are normal residents [38,39]. Thus, it has been proposed that the meninges provide a first line of defense from infections that threaten the CNS (Fig. 7a). However, we and others have shown that the meninges are sites of pathological immune responses in EAE and MS (reviewed in Ref. [39]). This concept is embodied in the “two wave model,” which proposes that peripherally-primed myelin specific Th cells gain access to the immune specialized CNS by first accessing the meninges [15,40]. In a first wave, primed myelin-specific Th cells enter the meninges where they encounter resident myelin-loaded APCs and are retained and reactivated. The resulting sustained inflammation in the meninges is thought to promote a second wave of pathologic immune cell infiltration into the CNS parenchyma [6,13,39]. In an extension of this model, we propose that mast cell activation is a critical component of the first wave. Mast cells release T cell-attracting chemokines, amplifying T cell influx to the meninges, and IL-1β, which drives GM-CSF production by Th cells (Fig. 7b). GM-CSF- producing Th cells enter the CNS in the second wave where they elicit the recruitment and survival of inflammatory monocytes.
Fig. 7.
A Model: Meningeal mast cell derived, inflammasome-dependent, IL-1β is necessary for T cell encephalitogenicity and infiltration into the CNS. (a) During physiological immune surveillance, T cells transit through the meninges. Under steady-state conditions, the surveying T cells do not interact with resident antigen presenting cells in the meninges and instead re-enter systemic circulation. (b) In EAE/MS, autoreactive transiting T cells interact with cognate antigen presenting cells. Activated T cells also interact with and activate mast cells resulting in caspase-1-dependent cleavage of pro-IL-1β to its mature form. Upon release, mast cell-derived IL-1β licenses T cells to produce GM-CSF and become encephalitogenic. (c) In the absence of meningeal mast cells (KitW/Wv mice), mast cell: T cell interactions are nonexistent, resulting in decreased GM-CSF production by transiting T cells, less efficient CNS-entry of T cells and other cells and reduced clinical disease severity.
While there are likely other cellular sources of IL-1β in the meninges that contribute to an ongoing Th cell GM-CSF response, including CCR2+ monocytes [35], we contend that mast cells are an early and essential source in the first wave of EAE. In the absence of IL-1β-producing mast cells in mast cell deficient KitW/Wv mice or in KitW/Wv mice reconstituted with Casp1−/− mast cells, GM-CSF production by CNS-infiltrating T cells and disease severity is profoundly attenuated (Fig. 5b–e and 7c). The attenuated disease course of KitW/Wv mice or KitW/Wv mice reconstituted with Casp1−/− mast cells aligns with the results of previous studies. Th cells that are unable to respond to IL-1β (Il1r−/−) have similar deficits in GM-CSF production and pathogenicity [22]. Furthermore, Il1b−/−, Il1r1−/−, Casp1−/−, Asc−/− and Nlrp3−/− mice, which have deficiencies in IL-1β signaling or in components of the inflammasome that regulates IL-1β processing, are protected from EAE [22,41–44].
Caspase-1-independent production of IL-1β by Th17 cells has recently been shown to be relevant in the second wave of T cell CNS infiltration [45]. While T cell-intrinsic IL-1β affects the survival and proliferation of these cells, it exerts only a modest effect on GM-CSF production. These data raise the intriguing possibility that distinct IL-1β sources provide unique functional influence in disease.
We have demonstrated that there is bidirectional activation of mast cells and T cells in early EAE. Mast cells exert their influence on T cells through Il1b expression, but what is less clear is how T cells affect mast cell inflammasome activity. Our adoptive transfer experiments show that T cell transfer alone is sufficient for mast cell activation. However whether this requires direct cell-cell interactions or is mediated by soluble factors released by T cells such as ATP has not been determined. Class II MHC expression can be induced in mast cells, which confers the ability to present antigen to T cells [46,47]. This suggests that T cells may cross-activate mast cells that are serving as APCs. Studies showing that direct interactions between LFA-1/ICAM-1 and OX40/O40L trigger both mast cell and T cell activation [11,21,48] as well as our observation of mast cell-T cell co-localization in the inflamed meninges of MS patients provide evidence for a contact-dependent mechanism. Ongoing studies are investigating these questions.
Aberrant inflammasome activation is associated with several other CNS inflammatory conditions including stroke, Alzheimer’s disease, and traumatic brain injury [49]. In MS, the elevated expression of IL-1β and caspase-1 by peripheral blood monocytes correlates with increased severity and progression of disease [50–54]. Elevated levels of IL-1β are also reported in the CSF of MS patients before clinical relapses and CASP1 expression is detected in MS plaques [55–58]. Finally, there is evidence that meningeal inflammation is often pronounced in disease and precedes the formation of CNS demyelinating lesions in some patients [13,59]. Thus, our data suggest new therapeutic possibilities that target meningeal mast cells and mast cell-derived IL-1β in MS and other myeloid cell-driven CNS inflammatory diseases. Importantly, because the meninges have less restrictive vasculature than the CNS, the need for modulating therapies to cross the BBB is eliminated [reviewed in Ref. [39]]. There is clinical evidence to support this type of therapeutic approach. Although not its primary mechanism of action, the FDA-approved MS drug teriflunomide (Aubagio) induces mast cell apoptosis through its effects on c-kit [60]. Another non-specific c-kit inhibitor, imatinib mesylate (Gleevec), reduces EAE severity [61]. Based on their efficacy in other autoimmune diseases, therapies that interfere with IL-1β signaling may also be of use in MS patients [62]. For example, a large-scale retrospective analysis of rheumatoid arthritis patients who received anakinra, an IL-1R antagonist, revealed an odds ratio of 0.80 (95% CI 0.29 to 2.24) for developing a demyelinating disorder in this cohort [63]. Finally, a recent report demonstrated the effectiveness of an NLRP3 inhibitor in reducing circulating IL-1β and ameliorating EAE [64].
5. Conclusions
Our study extends the growing body of evidence implicating IL-1β in EAE/MS pathophysiology by identifying a mechanism for IL-1β pathogenicity and defining mast cells as a critical source of this cytokine in early disease.
Supplementary Material
Acknowledgements
We thank Blayne Sayed for designing the mast cell-T cell co-culture system, Kelly Foy for assistance with mast cell quantification, Mark Ebel for assistance with RT-PCR analyses and Thirumala-Devi Kanneganti for the kind gift of the Il1b−/− mice. Grant support: NMSS RG 4684A5/1 (M.A.B), NIH F31 NS084691 (A.E.R.), NIH F31 NS068031 (M.W.-C.), Department of Immunology T32 (5 T32 AI 7047–33) and a grant from the Mayo Clinic Center for MS and Autoimmune Neurology (CMSAN) (M.W.-C.).
Footnotes
Conflict of interest
All authors declare that they have no conflict of interest.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jaut.2016.06.015.
References
- [1].Hauser SL, Oksenberg JR, The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration, Neuron 52 (2006) 61–76. [DOI] [PubMed] [Google Scholar]
- [2].Nylander A, Hafler DA, Multiple sclerosis J Clin. Investig 122 (2012) 1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schlager C, Lodygin D, et al. , T cells become licensed in the lung to enter the central nervous system, Nature 488 (2012) 675–679. [DOI] [PubMed] [Google Scholar]
- [4].Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, et al. , Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination, Nature 479 (2011) 538–541. [DOI] [PubMed] [Google Scholar]
- [5].Ransohoff RM, Immunology: licensed in the lungs, Nature 488 (2012) 595–596. [DOI] [PubMed] [Google Scholar]
- [6].Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, et al. , Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis, Ann. Neurol 65 (2009) 457–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brown DA, Sawchenko PE, Time course and distribution of inflammatory and neurodegenerative events suggest structural bases for the pathogenesis of experimental autoimmune encephalomyelitis, J. Comp. Neurol 502 (2007) 236–260. [DOI] [PubMed] [Google Scholar]
- [8].Christy AL, Walker ME, Hessner MJ, Brown MA, Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE, J. Autoimmun 42 (2012) 50–61. [DOI] [PubMed] [Google Scholar]
- [9].El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. , The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF, Nat. Immunol 12 (2011) 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sayed BA, Christy AL, Walker ME, Brown MA, Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J. Immunol 184 (2010) 6891–6900. [DOI] [PubMed] [Google Scholar]
- [11].Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. , Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation, Blood 114 (2009) 2639–2648. [DOI] [PubMed] [Google Scholar]
- [12].Secor VH, Secor WE, Gutekunst CA, Brown MA, Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis, J. Exp. Med 191 (2000) 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lucchinetti CF, Popescu BFG, Bunyan RF, Moll NM, Roemer SF, Lassmann H, et al. , Inflammatory cortical demyelination in early multiple sclerosis, N. Engl. J. Med 365 (2011) 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Axtell RC, Steinman L, Gaining entry to an uninflamed brain, Nat. Immunol 10 (2009) 453–455. [DOI] [PubMed] [Google Scholar]
- [15].Ransohoff RM, Immunology: in the beginning, Nature 462 (2009) 41–42. [DOI] [PubMed] [Google Scholar]
- [16].Engelhardt B, Ransohoff RM, Capture, crawl, cross: the T cell code to breach the blood-brain barriers, Trends Immunol. 33 (2012) 579–589. [DOI] [PubMed] [Google Scholar]
- [17].Galli SJ, Kitamura Y, Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo, Am. J. Pathol 127 (1987) 191–198. [PMC free article] [PubMed] [Google Scholar]
- [18].Sayed BA, Walker ME, Brown MA, Cutting edge: mast cells regulate disease severity in a relapsing-remitting model of multiple sclerosis, J. Immunol 186 (2011) 3294–3298. [DOI] [PubMed] [Google Scholar]
- [19].Gombert M, Dieu-Nosjean MC, Winterberg F, Bunemann E, Kubitza RC, Da Cunha L, et al. , CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation, J. Immunol 174 (2005) 5082–5091. [DOI] [PubMed] [Google Scholar]
- [20].Oliveira SH, Lukacs NW, Stem cell factor and igE-stimulated murine mast cells produce chemokines (CCL2, CCL17, CCL22) and express chemokine receptors, Inflamm. Res 50 (2001) 168–174. [DOI] [PubMed] [Google Scholar]
- [21].Inamura N, Mekori YA, Bhattacharyya SP, Bianchine PJ, Metcalfe DD, Induction and enhancement of Fc(epsilon)RI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation, J. Immunol 160 (1998) 4026–4033. [PubMed] [Google Scholar]
- [22].Lukens JR, Barr MJ, Chaplin DD, Chi H, Kanneganti TD, Inflammasome-derived IL-1beta regulates the production of GM-CSF by CD4(+) T cells and gammadelta T cells, J. Immunol 188 (2012) 3107–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. , Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production, Nature 386 (1997) 619–623. [DOI] [PubMed] [Google Scholar]
- [24].Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. , Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme, Science 275 (1997) 206–209. [DOI] [PubMed] [Google Scholar]
- [25].Dropp JJ, Mast cells in mammalian brain, Acta Anat. (Basel) 94 (1976) 1–21. [DOI] [PubMed] [Google Scholar]
- [26].Edvinsson L, Cervos-Navarro J, Larsson LI, Owman C, Ronnberg AL, Regional distribution of mast cells containing histamine, dopamine, or 5-hydroxytryptamine in the mammalian brain, Neurology 27 (1977) 878–883. [DOI] [PubMed] [Google Scholar]
- [27].Goldschmidt RC, Hough LB, Glick SD, Padawer J, Mast cells in rat thalamus: nuclear localization, sex difference and left-right asymmetry, Brain Res. 323 (1984) 209–217. [DOI] [PubMed] [Google Scholar]
- [28].Panula P, Yang HY, Costa E, Histamine-containing neurons in the rat hypothalamus, Proc. Natl. Acad. Sci. U. S. A 81 (1984) 2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S, The mast cells of the multiple sclerosis brain, J. Neuroimmunol 70 (1996) 131–138. [DOI] [PubMed] [Google Scholar]
- [30].Kruger PG, Mast cells and multiple sclerosis: a quantitative analysis, Neuropathol. Appl. Neurobiol 27 (2001) 275–280. [DOI] [PubMed] [Google Scholar]
- [31].Kruger PG, Bo L, Myhr KM, Karlsen AE, Taule A, Nyland HI, et al. , Mast cells and multiple sclerosis: a light and electron microscopic study of mast cells in multiple sclerosis emphasizing staining procedures, Acta Neurol. Scand 81 (1990) 31–36. [DOI] [PubMed] [Google Scholar]
- [32].Toms R, Weiner HL, Johnson D, Identification of IgE-positive cells and mast cells in frozen sections of multiple sclerosis brains, J. Neuroimmunol 30 (1990) 169–177. [DOI] [PubMed] [Google Scholar]
- [33].Croxford AL, Spath S, Becher B, GM-CSF in neuroinflammation: licensing myeloid cells for tissue damage, Trends Immunol. 36 (2015) 651–662. [DOI] [PubMed] [Google Scholar]
- [34].Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, et al. , RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation, Nat. Immunol 12 (2011) 560–567. [DOI] [PubMed] [Google Scholar]
- [35].Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, et al. , The cytokine GM-CSF drives the inflammatory signature of CCR2(+) monocytes and licenses autoimmunity, Immunity 43 (2015) 502–514. [DOI] [PubMed] [Google Scholar]
- [36].Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN, GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis, J. Immunol 178 (2007) 39–48. [DOI] [PubMed] [Google Scholar]
- [37].Hartmann FJ, Khademi M, Aram J, Ammann S, Kockum I, Constantinescu C, et al. , Multiple sclerosis-associated IL2RA polymorphism controls GM-CSF production in human TH cells, Nat. Commun 5 (2014) 5056. [DOI] [PubMed] [Google Scholar]
- [38].Shechter R, London A, Schwartz M, Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates, Nat. Rev. Immunol 13 (2013) 206–218. [DOI] [PubMed] [Google Scholar]
- [39].Russi AE, Brown MA, The meninges: new therapeutic targets for multiple sclerosis, Transl. Res 165 (2015) 255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, et al. , C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE, Nat. Immunol 10 (2009) 514–523. [DOI] [PubMed] [Google Scholar]
- [41].Furlan R, Martino G, Galbiati F, Poliani PL, Smiroldo S, Bergami A, et al. , Caspase-1 regulates the inflammatory process leading to autoimmune demyelination, J. Immunol 163 (1999) 2403–2409. [PubMed] [Google Scholar]
- [42].Gris D, Ye Z, Iocca HA, Wen H, Craven RR, Gris P, et al. , NLRP3 plays a critical role in the development of experimental autoimmune encephalomyelitis by mediating Th1 and Th17 responses, J. Immunol 185 (2010) 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y, Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis, Int. Immunol 18 (2006) 399–407. [DOI] [PubMed] [Google Scholar]
- [44].Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, et al. , Interferon-beta therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome, Sci. Signal 5 (2012) ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Martin BN, Wang C, Zhang CJ, Kang Z, Gulen MF, Zepp JA, et al. , T cell-intrinsic ASC critically promotes T17-mediated experimental autoimmune encephalomyelitis, Nat. Immunol 17 (2016) 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kambayashi T, Allenspach EJ, Chang JT, Zou T, Shoag JE, Reiner SL, et al. , Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation, J. Immunol 182 (2009) 4686–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kambayashi T, Baranski JD, Baker RG, Zou T, Allenspach EJ, Shoag JE, et al. , Indirect involvement of allergen-captured mast cells in antigen presentation, Blood 111 (2008) 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. , CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction, Immunity 29 (2008) 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Freeman LC, Ting JP, The pathogenic role of the inflammasome in neuro-degenerative diseases, J. Neurochem 136 (2016) 29–38. [DOI] [PubMed] [Google Scholar]
- [50].Balashov KE, Rottman JB, Weiner HL, Hancock WW, CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions, Proc. Natl. Acad. Sci. U. S. A 96 (1999) 6873–6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Karni A, Koldzic DN, Bharanidharan P, Khoury SJ, Weiner HL, IL-18 is linked to raised IFN-gamma in multiple sclerosis and is induced by activated CD4(+) T cells via CD40-CD40 ligand interactions, J. Neuroimmunol 125 (2002) 134–140. [DOI] [PubMed] [Google Scholar]
- [52].Losy J, Niezgoda A, IL-18 in patients with multiple sclerosis, Acta Neurol. Scand 104 (2001) 171–173. [DOI] [PubMed] [Google Scholar]
- [53].Mann CL, Davies MB, Stevenson VL, Leary SM, Boggild MD, Ko Ko C, et al. , Interleukin 1 genotypes in multiple sclerosis and relationship to disease severity, J. Neuroimmunol 129 (2002) 197–204. [DOI] [PubMed] [Google Scholar]
- [54].Peelen E, Damoiseaux J, Muris AH, Knippenberg S, Smolders J, Hupperts R, et al. , Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis, Mol. Immunol 63 (2015) 521–529. [DOI] [PubMed] [Google Scholar]
- [55].de Jong BA, Huizinga TW, Bollen EL, Uitdehaag BM, Bosma GP, van Buchem MA, et al. , Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis, J. Neuroimmunol 126 (2002) 172–179. [DOI] [PubMed] [Google Scholar]
- [56].Feakes R, Sawcer S, Broadley S, Coraddu F, Roxburgh R, Gray J, et al. , Interleukin 1 receptor antagonist (IL-1ra) in multiple sclerosis,J. Neuroimmunol 105 (2000) 96–101. [DOI] [PubMed] [Google Scholar]
- [57].Furlan R, Filippi M, Bergami A, Rocca MA, Martinelli V, Poliani PL, et al. , Peripheral levels of caspase-1 mRNA correlate with disease activity in patients with multiple sclerosis; a preliminary study, J. Neurol. Neurosurg. Psychiatry 67 (1999) 785–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ming X, Li W, Maeda Y, Blumberg B, Raval S, Cook SD, et al. , Caspase-1 expression in multiple sclerosis plaques and cultured glial cells, J. Neurol. Sci 197 (2002) 9–18. [DOI] [PubMed] [Google Scholar]
- [59].Popescu BF, Lucchinetti CF, Meningeal and cortical grey matter pathology in multiple sclerosis, BMC Neurol. 12 (2012) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sawamukai N, Saito K, Yamaoka K, Nakayamada S, Ra C, Tanaka Y, Leflunomide inhibits PDK1/Akt pathway and induces apoptosis of human mast cells, J. Immunol 179 (2007) 6479–6484. [DOI] [PubMed] [Google Scholar]
- [61].Adzemovic MV, Zeitelhofer M, Eriksson U, Olsson T, Nilsson I, Imatinib ameliorates neuroinflammation in a rat model of multiple sclerosis by enhancing blood-brain barrier integrity and by modulating the peripheral immune response, PLoS One 8 (2013) e56586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dinarello CA, Simon A, van der Meer JW, Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases, Nat. Rev. Drug Discov 11 (2012) 633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bernatsky S, Renoux C, Suissa S, Demyelinating events in rheumatoid arthritis after drug exposures, Ann. Rheum. Dis 69 (2010) 1691–1693. [DOI] [PubMed] [Google Scholar]
- [64].Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. , A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases, Nat. Med 21 (2015) 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.