Abstract
BACKGROUND:
Psoriasis, the prevalence of which ranges from 2% to 3% of the general population, has been recently recognised as not only a chronic inflammatory skin disorder but also an immunometabolic systemic disease. Dyslipidemia is one of the most important comorbidities of psoriasis. Statins, frequently used as anti-hyperlipidemic agents, may be beneficial in the treatment of several autoimmune diseases, including psoriasis, due to their anti-inflammatory and immunomodulatory characteristics. Hence, we hypothesised that using this medication was not only beneficial for reducing hyperlipidemia but also improving psoriatic conditions.
AIM:
We conducted a study to determine the prevalence of dyslipidemia in psoriatic patients as well as whether the addition of statins (simvastatin prescribed forms) to standard topical antipsoriatic treatment can improve skin lesions in psoriatic patients.
METHODS:
A group of 128 psoriatic patients and 128 healthy controls who were matched with the patients regarding ethnicity, age, and sex were enrolled, and their lipid concentrations were determined. Furthermore, sixty patients were randomly selected from the former group and divided into two treatment subgroups to evaluate the effect of statins on the severity of psoriasis using the PASI score.
RESULTS:
We found that the rate of dyslipidemia in the patient group was significantly higher than in the healthy group (53.9% versus 21.9%, p < 0.001), particularly the triglyceride concentration (1.86 ± 1.17 versus 1.43 ± 0.79 mg/dL, p < 0.001). Also, the PASI score reduction in the simvastatin-treated subgroup was significantly different from that in the placebo-treated one after eight weeks of therapy (8.63 ± 4.78 versus 5.34 ± 3.59, p < 0.01).
CONCLUSION:
This study showed that simvastatin might play a role in controlling hyperlipidemia and in turn decrease the PASI score in psoriatic patients.
Keywords: Simvastatin, Psoriasis, Dyslipidemia
Introduction
Psoriasis is a chronic inflammatory skin disease the prevalence of which ranges from 2% to 3% of the general population, depending on region and ethnic origin [1]. Many recent studies support the hypothesis that psoriasis results from an interaction between an individual’s genetic susceptibility and specific environmental factors. Nearly 20 psoriatic-susceptibility gene loci and genes with a fundamental role in psoriasis pathophysiology have been identified, including PSORS1 (a major susceptibility gene, mapped near the human leucocyte antigen HLACw6) [2]. Currently, the various treatment modalities are divided into local and systemic treatments, including topical steroids, vitamin D3, tar, anthralin, topical retinoids, phototherapy, retinoids, methotrexate, biologic agents and many other treatment modalities. Psoriatic prognosis is unpredictable, and its recurrence almost inevitably appears regardless of treatment [3].
Dyslipidemia is known as one of several comorbidities of psoriasis in addition to cardiovascular abnormalities, hypertension, atherosclerosis, diabetes mellitus type 2, and obesity [4]. Recently, there have been several clinical observations focusing on the risk of developing cardiovascular diseases, such as acute coronary syndromes or arterial hypertension, in psoriatic patients. Additionally, other conditions, including hyperlipidemia, obesity and type 2 diabetes, were found to be more prevalent in the patient group than in the control group [5].
Statins, with the most common drugs being simvastatin and atorvastatin, form one type of compound that can reduce the amount of cholesterol in the blood. They were first discovered in 1971 by Akira Endo and constitute a rapidly growing class of medications that can modulate hypercholesterolemia. The beneficial effect of statins on lipids is directly linked to the inhibition of 3-hydroxy-3-methylglutaryl coenzyme-A reductase, which plays a central role in the synthesis of cholesterol in the liver and underlies the most beneficial effect of statins on lipids. Consequently, statin therapy has been used for the prevention of atherosclerosis and cardiovascular events [5], [6]. Many studies have shown that statins have anti-inflammatory and immunomodulatory properties which would be useful in the treatment of psoriasis and other cutaneous disorders [7], [8], [9].
We conducted a study to determine the prevalence of dyslipidemia in psoriatic patients as well as whether the addition of statins (simvastatin prescribed forms) to standard topical antipsoriatic treatment can improve skin lesions in psoriatic patients.
Patients and methods
Patient population
This study was conducted from January 2011 to December 2014 at the Outpatient Department, Hospital of Dermato-Venereology, Ho Chi Minh City, Vietnam.
Psoriatic patients, aged more than 18 years old, were recruited into the study. The exclusion criterion included hypersensitivity to simvastatin or calcipotriol/betamethasone dipropionate, renal and liver dysfunction, alcohol abuse, muscular disorders, use of topical or systemic psoriatic medication in the past 1 month, and pregnant and lactating women.
Two hundred fifty-six patients and healthy controls who gave informed consent provided blood samples for the evaluation of their lipid profiles. Also, sixty patients were randomly selected from the patient group and divided into two treatment subgroups. Subgroup 1 was treated with oral simvastatin (40 mg/d divided twice a day) plus topical calcipotriol/betamethasone dipropionate ointment for 8 weeks, and subgroup 2 received only the same topical regimen.
Efficacy assessment
A well-trained dermatologist evaluated the Psoriasis Area and Severity Index (PASI) score of the patients at the baseline, at the 4th week and the 8th week. Also, the lipid profile was assessed simultaneously. Safety was assessed by patients’ reports of adverse events, clinical examination, and liver function tests.
Statistical analysis
Data were expressed as the means ± SDs and the ratio. Statistical analysis was performed using Epi Info™ (Epi Info™ version 3.5.1 for Windows; CDC). The data were analysed using Wilcoxon and Mann-Whitney tests, the Chi-square test, and two-tailed Student’s tests. A P value < 0.05 was considered statistically significant.
Results
Baseline characteristics
The study included 128 patients and 128 healthy controls. The patients (30 patients in each subgroup) were treated for eight weeks. Their characteristics are shown in Table 1.
Table 1.
Baseline patient characteristics
| Characteristics | Group | P value | Subgroup | P value | ||
|---|---|---|---|---|---|---|
| Control (n = 128) | Patient (n = 128) | Subgroup 1 (n = 30) | Subgroup 2 (n = 300) | |||
| Gender: | ||||||
| Men (%) | 64 (50%) | 64 (50%) | 1a | 17 (56.7%) | 17 (56.7%) | 1a |
| Women (%) | 64 (50%) | 64 (50%) | 1a | 13 (43.3%) | 13 (43.3%) | 1a |
| Mean ± SD. age (yr) | 43.3 ± 12.6 | 41.9 ± 14.7 | 0.43b | 36.0 ± 10.0 | 39.1 ± 14.5 | 0.34b |
| Mean ± SD. BMI | 21.9 ± 3.2 | 21.9 ± 3.1 | 0.93b | - | - | - |
| Mean ± SD. Disease duration (yr) | - | 7.7 ± 8.1 | - | 5.8 ± 5.4 | 5.6 ± 5.4 | 0.89b |
| Clinical features | ||||||
| Psoriasis vugaris | 100 (78.1%) | 30 (100%) | 30 (100%) | |||
| Erythrodermic psoriasis | - | 11 (8.6%) | - | 0 | 0 | 1a |
| Pustular psoriasis | 9 (7%) | 0 | 0 | |||
| Arthritis psoriasis | 8 (6.3%) | 0 | 0 | |||
| PASI score: mean ± SD | - | 10.9 ± 7.4 | - | 12.8 ± 5.8 | 11.8 ± 5.1 | 0.51b |
Chi-square test;
Two–tailed Student’s test.
In total, 128 patients (64 male and 64 female) and 128 matched controls (64 male and 64 female) were included. Patients in subgroup 1 (oral simvastatin plus calcipotriol/betamethasone dipropionate ointment) consisted of 17 male and 13 female patients. Subgroup 2 (only calcipotriol/betamethasone dipropionate ointment) consisted of 17 male and 13 female patients. The mean age of the healthy group was 43.3 ± 12.6 years, which was correlated with the patient group (41.9 ± 14.7 years). In subgroup 1, the average age was 36.0 ± 10.0 years, and in subgroup 2, the average age was 39.1 ± 14.5 years. There was no significant difference between sex ratios and mean ages in the two groups.
Figure 1.
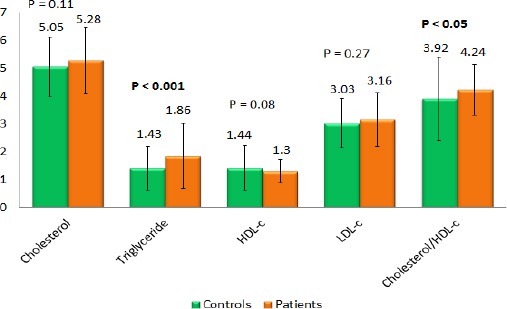
Comparison of lipid concentration profiles between study groups
The mean baseline PASI score in the patient group was 10.9 ± 7.4. Specifically, the mean baseline PASI score in subgroup 1 was 12.8 ± 5.87, and in subgroup 2 it was 11.8 ± 5.1. The difference in the mean baseline PASI scores of the two subgroups was not statistically significant (P = 0.51).
Dyslipidemia
The rate of dyslipidemia in the patient group was considerably higher than in the healthy control group (53.9% versus 21.9%, p < 0.001). The percentages of hypercholesterolemia in the psoriasis group and control group were 25.0% and 10.9%, respectively, (p < 0.01). When we compared the frequency of hypertriglyceridemia between the two groups, there was a significantly higher frequency in the patient group than in the control group (25.0% in psoriasis versus 8.6% in controls, p < 0.001). The frequency of hypo-HDL-c was significantly different in the patients and the controls (21.9% versus 3.9%, p < 0.001). Each element of the lipid profiles is shown in Table 2.
Table 2.
Comparison of the lipid profiles between the study groups
| Group | P value | ||
|---|---|---|---|
| Patient (n = 128) | Control (n = 128) | ||
| Dyslipidemia | 69 (53.9%) | 28 (21.9%) | < 0.001 |
| Hypercholesterolemia | 32 (25.0%) | 14 (10.9%) | < 0.01 |
| Hypertriglyceridemia | 32 (25.0%) | 11 (8.6%) | < 0.001 |
| Hyper-LDL cholesterolemia | 19 (14.8%) | 12 (9.4%) | 0.18 |
| Hypo-HDL cholesterolemia | 28 (21.9%) | 5 (3.9%) | < 0.001 |
| Cholesterol/HDL-c > 5 | 26 (20.3%) | 8 (6.3%) | < 0.01 |
a Chi-square test.
Analysing the mean concentration of each component in the lipid profiles, our study revealed a difference between the triglyceride levels of the patient group and the control group (1.86 ± 1.17 and 1.43 ± 0.79, respectively, p < 0.001). The ratio of cholesterol/HDL-c of patients was 4.24 ± 0.91. It was significantly higher in the patient group than in control (3.92 ± 1.50) (p < 0.05). However, the means of other components (cholesterol, HDL-c and LDL-c) were not significantly different between the two groups.
Assessment of statins in psoriasis treatment
Lipid profile
In subgroup 1, the mean baseline of cholesterolemia was 5.45 ± 1.21 mm/L, which diminished to 4.20 ± 0.82 and 4.18 ± 0.72 mm/L (p < 0.001) at the 4th week and 8th week, respectively. There was no difference between the triglyceride concentration at pretreatment and after 4 weeks of treatment, which was 2.07 ± 2.00 and 1.32 ± 0.84 mm/L (p = 0.07), respectively; however, after 8 weeks, the triglyceride concentration was 1.26 ± 0.65 mm/L (p < 0.005). While the mean baseline LDL-c level was 3.18 ± 0.7 mm/L, there was a significant difference in the 4th week (2.31 ± 0.80 mm/L, p < 0.001) and 8th week of treatment (2.26 ± 0.7 mm/L, p < 0.001). However, the increasing HDL levels were negligible after 8 weeks of treatment (1.33 ± 0.31, 1.29 ± 0.24 and 1.35 ± 0.24 at pretreatment, 4th week and 8th week, respectively). Meanwhile, in subgroup 2, without treatment, there were no changes in lipid levels over time.
Figure 2.
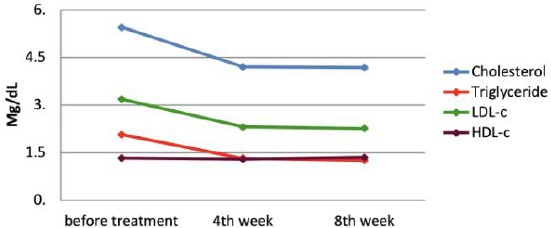
Changes in the lipid profile after 8 weeks of treatment with simvastatin
PASI score
In subgroup 1, in which the treatment was combination of topical therapy and simvastatin, the mean baseline PASI score was 12.8 ± 5.87, which considerably declined to 8.58 ± 5.62 after 4 weeks of treatment (p < 0.01) and ultimately to 4.17 ± 3.81 at the 8th week (p < 0.001) as shown in Table 3. Subgroup 2, treated only with topical therapy, did not have a significant difference in the mean PASI score between pretreatment and the 4th week (11.8 ± 5.13 vs 9.34 ± 5.01, p = 0.21); nevertheless, there was a considerable decrease to 6.52 ± 4.89 after 8 weeks of treatment (p < 0.001) as shown in Table 3.
Table 3.
Change from baseline PASI score every 4 weeks according to treatment
| Time | Baseline | 4th week | 8th week |
|---|---|---|---|
| Group 1 | 12.8 ± 5.87 | 8.58 ± 5.62 | 4.17 ± 3.81 |
| P < 0.01 | P < 0.001 | ||
| Group 2 | 11.86 ± 5.13 | 9.34 ± 5.01 | 6.52 ± 4.89 |
| P = 0.21 | P < 0.001 |
The PASI score reduction was more significant in subgroup 1 than in subgroup 2 (Mann-Whitney test; p < 0.0001). After 4 weeks of treatment, the rates of patients who achieved PASI75 were 10% and 3.3% in subgroup 1 and subgroup 2, respectively. Patients who achieved PASI75 in subgroup 1 showed a significant increase compared with subgroup 2 after 8 weeks (70% versus 40%, p < 0.05).
Figure 3.
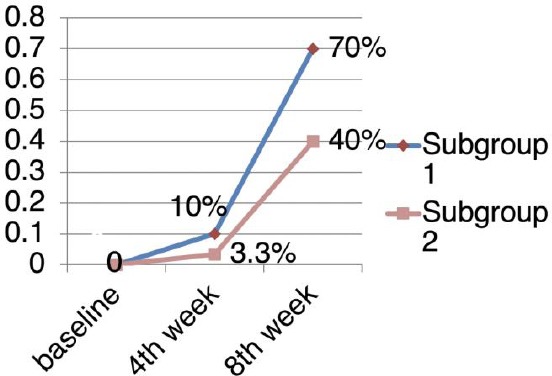
The rates of patient achievement of PASI75
Discussion
Studies on lipid metabolism in psoriasis patients, focusing on skin surface lipids, epidermal phospholipids, serum lipids, LDL-c of the dermis layer, oxidative stress, and the correlation between the inflammatory index of clinical lesions and lipid profiles, have been conducted since the 20th century10. Several studies confirmed that there was dyslipidemia and psoriasis coexist [10], [11].
Our study included 128 patients and 128 controls. There were no differences in age, gender, or BMI between the two study groups (Table 1). Our study used the Adult Treatment Panel III (ATP-III) guidelines from the National Cholesterol Education Program (NCEP) for the management of hypercholesterolemia, which was issued in 2001 and modified in 2004 as follows: cholesterol TP ≥ 6.20 mm/L, triglyceride ≥ 2.26 mm/L, LDL-C ≥ 4.13 mm/L, and HDL-C < 1.03 mm/L. We found that the frequency of dyslipidemia in psoriasis patients was 53.9% for hypercholesterolemia, 25% for hypertriglyceridemia, 21.9% for hypo-HDL-c, 20.3% for cholesterol/HDL-c > 5, and 14.8% for hyper-LDL-c.
The frequency of dyslipidemia in psoriasis patients varies among studies, ranging from 6.4 to 50.9% [12]. In a study on psoriasis, Salihbegovic EM postulated that the frequency of dyslipidemia was 62.8% (the frequencies of hypertriglyceridemia and hypo-HDL-c were 39% and 36%, respectively) [13]. A cross-sectional study of 120 Pakistani psoriasis patients found that the incidence of dyslipidemia was 55.8% [14], which was similar to the incidence found in our study (53.9%). However, the comparison of dyslipidemia frequency among patients with psoriasis was only relative because the standard definition of dyslipidemia was different among authors. Our study compared the rate of dyslipidemia between the two groups. The results showed that the rates of hypercholesterolemia, hypertriglyceridemia, hypo-HDL-c, and cholesterol/HDL-c > 5 in the psoriatic group increased significantly compared with those in the control group (p < 0.05).
There have been many studies with a variety of lipid levels in psoriatic patients. The studies by Sahu S, Taheri Sarvtin, El Asmi MA postulated a statistically significant difference in the concentration of lipids between patients with psoriasis and controls [15], [16], [17]. In our study, the triglyceride level of the patient group was 1.86 ± 1.17 mm/L, and that of the control group was 1.43 ± 0.79 mm/L (p < 0.001). The ratio of cholesterol/HDL-c of patients was 4.24 ± 0.91, which was higher than that of the control group (p < 0.05). Nevertheless, while comparing other elements of the lipid profile, we found no significant differences.
Our study also found that in patients receiving oral simvastatin plus calcipotriol/betamethasone dipropionate steroid ointment, there was a critical decrease in the levels of cholesterol, triglyceride, and LDL-C, while the level of HDL-C remained stable. This finding suggested that simvastatin had a beneficial effect on the modulation of dyslipidemia, as mentioned above. Interestingly, we also found that simvastatin improved the PASI score in psoriatic patients.
Treatment of plaque psoriasis after 8 weeks with calcipotriol/betamethasone dipropionate steroid ointment showed that there was a significant improvement in the PASI score. We also found a faster and more effective decrease in PASI score in patients with additional oral simvastatin than in patients without simvastatin. According to our study, after 4 weeks of treatment, in the subgroup that was treated with simvastatin, the efficacy of adding oral simvastatin was clear. The PASI score of subgroup 1 at the baseline was 12.8 ± 5.87, which significantly diminished after 4 weeks of treatment (8.58 ± 5.62) (p < 0.01). Meanwhile, in the subgroup that did not include simvastatin, the PASI score decreased between pretreatment and the 4th week from 11.86 ± 5.13 to 9.34 ± 5.01, respectively, which were not significantly different. This demonstrated that adding simvastatin (40 mg/d) to calcipotriol/betamethasone dipropionate ointment produced a significantly beneficial effect in psoriatic treatment.
The anti-inflammatory and immune-modulatory effects of statins were identified as a potential experimental treatment for psoriasis in the 1990s. Meanwhile, some studies did not find any effect of statins on psoriasis [18], while others suggested that statins might be useful in the treatment of psoriasis and other dermatologic disorders [9]. We hypothesised that the inhibition of leukocyte function-associated-1 (LFA-1)-mediated adhesion of leukocytes to intercellular adhesion molecule-1 (ICAM-1) or the inhibition of pro-inflammatory mediators produced clinical effects [19], [20].
A variety of mechanisms of cholesterol level modulation produced beneficial effects on psoriasis lesions, including (1) the downregulation of LFA-1, (2) the inhibition of leukocyte-endothelial adhesion, (3) extravasation and natural killer cell activity, (4) the inhibition of pro-inflammatory cytokines such as tumour necrosis factor-alpha and interleukin-1 and -6, (5) lowering the level of C-reactive protein, (6) the promotion of Th1 to Th2 cells and (7) the inhibition of Th1 cytokine receptors on T cells. As mentioned above, the activation of lymphocytes was limited and consequently impaired infiltration into the cutaneous lesion [21]. This result improved plaque psoriasis, which was confirmed by a lower PASI score.
Few studies have reported that statins have been used specifically for the treatment of psoriasis. In 2007, Shirinsky et al. reported the efficacy of simvastatin in treating plaque psoriasis. Seven patients were prescribed simvastatin 40 mg/d and were assessed by the PASI and dermatology quality of life index (DLQI) at the 4th week and 8th week of treatment. After 8 weeks of treatment, they found a statistically significant improvement in the PASI score (47.34%) and DLQI score. Two patients achieved a 50 per cent PASI response, and two patients achieved a 75 per cent PASI response. Although there were many limitations of this study (small sample size, no control group), their results suggest that statins could have clinical benefits in plaque psoriasis treatment [22]. A double-blind study by Naseri M et al. recruited 30 patients with plaque psoriasis and divided them, two groups. One received oral simvastatin (40 mg/day) combined with a topical steroid (50% betamethasone in petrolatum) for 8 weeks, whereas the other group received oral placebo plus the same topical steroid. This study showed that the PASI score decreased significantly in both groups; however, the reduction in the PASI score was greater in patients who received simvastatin [23], which agrees with our results. Also, a study reported by Wolkenstein et al. disclosed that there was a decreased risk of psoriasis associated with the use of oral statins [24], [25], [26], [27].
In conclusion, the limitations of our study included small sample size, a lack of blinding to treatment, and a lack of assessment of recurrence. Nevertheless, our study found that oral simvastatin could enhance the effect of topical therapy in psoriatic treatment. Considering the safety and benefits of statins, we suggest that statins are worth researching for their dual effects in psoriatic treatment, including decreasing the atherosclerotic disease burden through their lipid-lowering effects and decreasing psoriatic disease activity through their anti-inflammatory, immunomodulatory properties.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Foundation NP. Statistics. 2018. https://www.psoriasis.org/content/statistics .
- 2.Reich K. The concept of psoriasis as a systemic inflammation:implications for disease management. Journal of the European Academy of Dermatology and Venereology. 2012;26:3–11. doi: 10.1111/j.1468-3083.2011.04410.x. https://doi.org/10.1111/j.1468-3083.2011.04410.x PMid:22356630. [DOI] [PubMed] [Google Scholar]
- 3.Van de Kerkhof PCM NF. Psoriasis. 3th ed. Elsevier Saunders; 2012. [Google Scholar]
- 4.Nijsten T, Wakkee M. Complexity of the association between psoriasis and comorbidities. Journal of Investigative Dermatology. 2009;129(7):1601–3. doi: 10.1038/jid.2009.55. https://doi.org/10.1038/jid.2009.55 PMid:19521405. [DOI] [PubMed] [Google Scholar]
- 5.Mosiewicz J, Pietrzak A, Chodorowska G, et al. The rationale for statin use in psoriatic patients. Archives of dermatological research. 2013;305(6):467–472. doi: 10.1007/s00403-013-1374-1. https://doi.org/10.1007/s00403-013-1374-1 PMid:23754638. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM, Becker D, Clark LT, Cooper RS, Denke MA, Howard J, Hunninghake DB, Illingworth DR, Luepker RV, McBride P, McKenney JM. Detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Circulation. 2002;106(25):3143–421. https://doi.org/10.1161/circ.106.25.3143. [PubMed] [Google Scholar]
- 7.Egesi A, Sun G, Khachemoune A, Rashid RM. Statins in skin:research and rediscovery, from psoriasis to sclerosis. Journal of drugs in dermatology:JDD. 2010;9(8):921–7. PMid:20684142. [PubMed] [Google Scholar]
- 8.Jowkar F, Namazi MR. Statins in dermatology. International journal of dermatology. 2010;49(11):1235–43. doi: 10.1111/j.1365-4632.2010.04579.x. https://doi.org/10.1111/j.1365-4632.2010.04579.x PMid:20964647. [DOI] [PubMed] [Google Scholar]
- 9.Namazi MR. Statins:novel additions to the dermatologic arsenal? Experimental Dermatology. 2004;13(6):337–9. doi: 10.1111/j.0906-6705.2004.00208.x. https://doi.org/10.1111/j.0906-6705.2004.00208.x PMid:151↷8. [DOI] [PubMed] [Google Scholar]
- 10.Pietrzak A, Michalak-Stoma A, Chodorowska G, Szepietowski JC. Lipid disturbances in psoriasis:an update. Mediators of inflammation. 2010;2010 doi: 10.1155/2010/535612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clinics in dermatology. 2018;36(1):21–8. doi: 10.1016/j.clindermatol.2017.09.005. https://doi.org/10.1016/j.clindermatol.2017.09.005 PMid:29241748. [DOI] [PubMed] [Google Scholar]
- 12.Daudén E, Casta-Eda S, Suárez C, García-Campayo J, Blasco AJ, Aguilar MD, Ferrándiz C, Puig L, Sánchez-Carazo JL Working Group on Comorbidity in Psoriasis. Clinical practice guideline for an integrated approach to comorbidity in patients with psoriasis. Journal of the European Academy of Dermatology and Venereology. 2013;27(11):1387–404. doi: 10.1111/jdv.12024. https://doi.org/10.1111/jdv.12024 PMid:23134338. [DOI] [PubMed] [Google Scholar]
- 13.Salihbegovic EM, Hadzigrahic N, Suljagic E, Kurtalic N, Sadic S, Zejcirovic A, Mujacic A. Psoriasis and high blood pressure. Medical archives. 2015;69(1):13–15. doi: 10.5455/medarh.2015.69.13-15. https://doi.org/10.5455/medarh.2015.69.13-15 PMid:25870469 PMCid:PMC4384847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamil A AU, Malik LM, Azfar NA, Jahangir M. Frequency of dyslipidemia in patients with psoriasis. Journal of Pakistan Association of Dermatologists. 2018;24(4):307–311. [Google Scholar]
- 15.Sahu S, Devi E, Poddar A, Ray S. Lipid profile:a conduit in the progression from psoriasis to cardiovascular disease. International Journal of Advances in Medicine. 2017;4(1):173–5. https://doi.org/10.18203/2349-3933.ijam20170105. [Google Scholar]
- 16.HajHeydari Z. Serum lipids and lipoproteins in patients with psoriasis. Archives of Iranian medicine. 2014;17(5):343–346. PMid:24784863. [PubMed] [Google Scholar]
- 17.El Asmi MA, Zidi W, Mebazaa A, et al. Serum lipid level in Tunisian patients with psoriasis. Clinical laboratory. 2014;60(6):1043–1047. doi: 10.7754/clin.lab.2013.130535. PMid:25016711. [DOI] [PubMed] [Google Scholar]
- 18.Aronson PJ, Friedman DB. Pharmacologic doses of lovastatin do not predictably affect the course of psoriasis. Archives of dermatology. 1992;128(1):124. https://doi.org/10.1001/archderm.1992.01680110138028. [PubMed] [Google Scholar]
- 19.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease:from protein prenylation to immunomodulation. Nature Reviews Immunology. 2006;6(5):358–370. doi: 10.1038/nri1839. https://doi.org/10.1038/nri1839 PMid:16639429 PMCid:PMC3842637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nature medicine. 2001;7(6):687. doi: 10.1038/89058. https://doi.org/10.1038/89058 PMid:11385505. [DOI] [PubMed] [Google Scholar]
- 21.Ghazizadeh R, Tosa M, Ghazizadeh M. Clinical improvement in psoriasis with treatment of associated hyperlipidemia. The American journal of the medical sciences. 2011;341(5):394–8. doi: 10.1097/MAJ.0b013e3181ff8eeb. https://doi.org/10.1097/MAJ.0b013e3181ff8eeb PMid:21233693. [DOI] [PubMed] [Google Scholar]
- 22.Shirinsky IV, Shirinsky VS. Efficacy of simvastatin in plaque psoriasis:A pilot study. Journal of the American Academy of Dermatology. 2007;57(3):529–31. doi: 10.1016/j.jaad.2007.05.040. https://doi.org/10.1016/j.jaad.2007.05.040 PMid:17707157. [DOI] [PubMed] [Google Scholar]
- 23.Naseri M, Hadipour A, Sepaskhah M, Namazi MR. The remarkable beneficial effect of adding oral simvastatin to topical betamethasone for treatment of psoriasis:a double-blind, randomized, placebo-controlled study. Nigerian journal of medicine:journal of the National Association of Resident Doctors of Nigeria. 2010;19(1):58–61. doi: 10.4314/njm.v19i1.54216. https://doi.org/10.4314/njm.v19i1.54216 PMid:20232758. [DOI] [PubMed] [Google Scholar]
- 24.Wolkenstein P, Revuz J, Roujeau JC, Bonnelye G, Grob JJ, Bastuji-Garin S. Psoriasis in France and associated risk factors:results of a case-control study based on a large community survey. Dermatology. 2009;218(2):103–9. doi: 10.1159/000182258. https://doi.org/10.1159/000182258 PMid:19060463. [DOI] [PubMed] [Google Scholar]
- 25.Wollina U, França K, Lotti T, Tirant M. Adjuvant treatment of chronic plaque psoriasis in adults by a herbal combination:Open German trial and review of the literature. Dermatologic Therapy. 2018:e12624. doi: 10.1111/dth.12624. https://doi.org/10.1111/dth.12624 PMid:30175556. [DOI] [PubMed] [Google Scholar]
- 26.Damevska K, França K, Lotti T, Nikolovska S, Pollozhani N. Complementary and integrative therapies for psoriasis:Looking forward. Dermatologic Therapy. 2018;31:e12627. doi: 10.1111/dth.12627. https://doi.org/10.1111/dth.12627 PMid:30133906. [DOI] [PubMed] [Google Scholar]
- 27.El-Gammal A, Nardo VD, Daaboul F, et al. Is There a Place for Local Natural Treatment of Psoriasis? Open Access Maced J Med Sci. 2018;6(5):839–842. doi: 10.3889/oamjms.2018.106. https://doi.org/10.3889/oamjms.2018.106. [DOI] [PMC free article] [PubMed] [Google Scholar]


