Abstract
BACKGROUND:
B-cell activating factor (BAFF) is considered to have a role in the pathogenesis of systemic sclerosis (SSc).
AIM:
We conducted a longitudinal study on early SSc patients to determine the change in BAFF serum level after treatment and its association with organ involvements.
METHODS:
A total of 46 patients (32 diffuse, 14 limited) were recruited, among which 35 patients (24 diffuse, 11 limited) completed 12-month follow-up.
RESULTS:
Higher pretreatment BAFF levels were observed in patients with positive anti-topoisomerase antibody (ATA) (2252.1 ± 899.7 pg/ml versus 1475.5 ± 697.6 pg/ml in ATA-negative patients; p = 0.01) and muscular involvement (2741.9 ± 1039.9 pg/ml versus 1897.2 ± 762.9 pg/ml in patients without muscular involvement; p = 0.005). Lower levels were observed in patients with interstitial lung disease (ILD) (1926.7 ± 757.9 pg/ml versus 2721.6 ± 1131.4 pg/ml in non-ILD patients; p = 0.01). After treatment, BAFF level reduced significantly in diffuse SSc patients (1652.2 ± 892.7 pg/ml versus 2147.6 ± 945.5 pg/ml before treatment; p = 0.03).
CONCLUSION:
Patients with worsening outcome had the highest pretreatment BAFF level and was associated with increased BAFF level after treatment. BAFF can be used to predict and monitor patients’ response to therapy.
Keywords: BAFF, Systemic Sclerosis
Introduction
BAFF (B lymphocyte stimulator, BlyS) plays the most important role in the survival and maturation of B lymphocytes (B cells) [1]. Recent studies have shown that B cells may play an important role in mediating immune responses in systemic sclerosis (SSc). In SSc patients, although the amount of B cells does not increase in skin lesions, activating markers are found to increase. Functional abnormalities of B cells can cause systemic diseases independent with antibody-producing function [2]. The BAFF/BAFFR family are present in almost all the stages of B cell differentiation and maintain the survival of B cells in bone marrow. BAFF also has strong in vitro B cell stimulating function [3]. Therefore, excessive BAFF increase causes self-activation of B cells, playing an important role in the development of autoimmune diseases. Previous studies on SSc Japanese patients with systemic lupus erythematosus (SLE), rheumatoid arthritis, and Sjogren’s syndrome have demonstrated high serum levels of BAFF. Belimumab (a soluble monoclonal anti-BAFF antibody) has been approved by the Food and Drug Administration (FDA) for the treatment of SLE patients [4]. Other studies have also shown that in SSc patients, increased BAFF levels are associated with sclerotic skin lesions, lung’s vital capacity, and musculoskeletal involvements. This suggests that BAFF is a signal of abnormal B cell activation and the development of SSc [5]. A multi-centre clinical randomised controlled trial on rituximab, a monoclonal anti-CD20 antibody that inhibits B cells, has shown improvement on ILD and decreased mRSS [6]. Therefore, BAFF is one of the targets that should be considered in the treatment of SSc.
To evaluate B cells’ activity in early SSc patients, we conducted a study with two aims:
To determine the change in serum BAFF level in SSC patients before and after treatment at the National Hospital of Dermatology and Venerology;
To explore the association of changes in BAFF level with skin and organ involvement in SSC patients.
Methods
We recruited 46 patients diagnosed with SSc based on the 2013 EULAR/ACR classification criteria [7] who were treated at the National Hospital of Dermatology and Venereology (NHDV) from December 2014 to December 2017. Selection criteria included patients who had an onset of ≤ 36 months, were naïve to treatment or had stopped immunosuppressive and antifibrotic therapy for ≥ 2 months, had no concomitant skin diseases such as skin infection or overlap syndrome. The patients were treated with methylprednisolone, methotrexate, vasodilators, and other symptomatic treatments according to the European guidelines on SSc management [8] and were followed up in at least 12 months.
Data collection included demographics (sex, age, onset, disease duration) and clinical and laboratory parameters at baseline and 12-month follow-up. Patients were assessed for skin lesions (using the modified Rodnan Skin Score-mRSS), digital tip ulcer, telangiectasia, interstitial lung disease (ILD, using high-resolution computed tomography-HRCT), pulmonary arterial hypertension (PAH, using the cutoff ≥ 35 mmHg of the systolic pulmonary artery pressure-PAPs on transthoracic echocardiography-TTE), urinalysis abnormality (observed on ≥ 2 tests), difficulty swallowing, joint pain, and muscle injury (at least 2 out of 3 findings: muscle pain/weakness, creatinine kinase-CK level higher than normal limit at the time of presentation, and needle electromyography-EMG demonstrating muscle diseases). Severity was classified as mild, moderate, and severe if patients had zero, one, and two or more organ involvements, respectively. Improvement and worsening were determined by changes in constitutional symptoms and at least two systems (healing or new lessons of digital tip ulcer, improved or worsened difficulty swallowing, joint pain, or muscular involvement, a difference of ≥ 2 points in the ILD score, and a difference of ≥ 5 mmHg in PAPs between recruitment and follow-up).
Serum specimens were obtained and stored at baseline and 12 months after treatment, following the standard operating procedures at the NHDV-3 mL of venous blood was drawn into a collecting tube without anticoagulant, left in the room temperature in 30 minutes, and centrifuged at 2000 rpm in 15 minutes. The serum was then extracted to a 1.5 mL Eppendorf tube and stored in a minus 80°C freezer.
Some autoimmune antibodies were tested in Vietnam. Antinuclear antibody (ANA) and anti-centromere antibody (ACA) were tested using indirect immunofluorescence on Hep-2 cells (Fluoro Hepana, MBL, Japan). Anti-topoisomerase antibody (ATA) was tested by enzyme-linked immunosorbent assay (ELISA; Medical & Biological Laboratories, Nakaku, Nagoya, Japan).
Anti-RNA polymerase antibody (RNAP) was tested by ELISA in Japan (Mesacup anti-RNA, Japan). The level of BAFF was measured by ELISA, using Quantikines ELISA Human BAFF/BLyS/TNFSF13B Immunoassay (R&D Systems China Company).
Data analysis was performed using SPSS 20.0 (IBM Corporation). Continuous variables were tested for the difference by the independent t-test (for normally distributed variables) or the Mann-Whitney U test or the Kruskal-Wallis test (for not normally distributed variables). Paired continuous variables were tested for the difference by the paired t-test (for normally distributed variables) or the Wilcoxon Signed Ranks test (for not normally distributed variables). Categorical variables were tested for the difference by the chi-squared test.
The study has been approved by the Hanoi Medical University and the NHDV.
Results
A total of 46 patients were recruited in our study, among which 32 (65.9%) patients had diffuse SSc and 14 (30.4%) limited SSc. The mean age of onset was 50.1 ± 14.1 years, and the mean duration from the onset was 11.5 ± 8.4 months; no difference was observed between diffuse and limited SSc patients. Before treatment, 36 out of 45 patients had positive ATA. The mean level of ATA in diffuse SSc patients was higher than in limited SSc patients (p = 0.008). Only two patients had positive ACA and one positive RNAP. The mean pretreatment mRSS was 15.0 ± 8.9 (18.7 ± 7.9 and 6.6 ± 3.8 in diffuse SSc and limited SSc patients, respectively; p < 0.001). The proportions of other involvements were presented in Table 1.
Table 1.
Patient baseline characteristics
| Total (n = 46) | Diffuse SSc (n = 32) | Limited SSc (n = 14) | p* | |
|---|---|---|---|---|
| Female:male (n) | 32:14 | 2:1 | 11:3 | NS |
| Age of onset (year; mean ± SD) | 50.1 ± 14.1 | 49.0 ± 14.7 | 52.5 ± 12.9 | NS |
| Duration from onset (month; mean ± SD) | 11.5 ± 8.4 | 11.5 ± 7.7 | 11.4 ± 9.9 | NS |
| ATA (mmol/ml; mean ± SD) | 123.1 ± 82.6 | 143.3 ± 75.7 | 73.3 ± 80.4 | 0.008 |
| ATA (n; %) | 36; 80% | 28; 87.5% | 8; 61.5% | NS |
| ACA (n; %) | 2; 4.3% | |||
| RNAP (n; %) | 1; 2.2% | |||
| mRSS (mean ± SD) | 15.0 ± 8.9 | 18.7 ± 7.9 | 6.6 ± 3.8 | 0.0001 |
| Digital tip ulcer (n; %) | 10; 21.7% | 7; 21.9% | 3; 21.4% | NS |
| Telangiectasia (n; %) | 14; 30.4% | 10; 31.2% | 4; 28.6% | NS |
| ILD (n; %) | 36; 78.3% | 25; 78.1% | 11; 78.6% | NS |
| PAH (n; %) | 16; 34.8% | 9; 28.1% | 7; 50.0% | NS |
| Urinalysis abnormality (n; %) | 10; 21.7% | 6; 18.8% | 4; 28.6% | NS |
| Difficulty swallowing (n; %) | 9; 19.6% | 7; 21.9% | 2; 14.3% | NS |
| Joint pain (n; %) | 25; 54.3% | 19; 59.4% | 6; 42.9% | NS |
| Muscular involvement (n; %) | 11; 23.9% | 8; 25.0% | 3; 21.4% | NS |
t-test for continuous variables; chi-squared test for categorical variables. NS: not significant; SD: standard deviation.
Pretreatment BAFF level
There was no difference in the pretreatment BAFF level in diffuse and limited SSc patients (2010.4 ± 907.6 pg/ml versus 2177.2 ± 1009.3 pg/ml; p = 0.58). BAFF level was higher in patients with positive ATA, and this difference was more prominent in diffuse SSc patients. BAFF level was lower in patients with ILD, but the difference was not statistically significant in limited SSc patients. BAFF level was higher in patients with muscular involvement, and this difference was more prominent in limited SSc patients. The number (proportion) of patients with mild, moderate, and severe SSc was 10 (21.7%), 13 (28.3%), and 23 (50%), respectively as shown in Table 2.
Table 2.
BAFF level by SSc types and clinical and laboratory findings
| BAFF (pg/ml) | SSc | Diffuse SSc | Limited SSc | |||
|---|---|---|---|---|---|---|
| Concentration | P* | Concentration | P* | Concentration | p** | |
| ATA | 0.02 | 0.01 | NS | |||
| Present | 2252.1 ± 899.7 | 2202.2 ± 822.7 | 2426.7 ± 1180.0 | |||
| Absent | 1475.5 ± 697.6 | 1104.5 ± 491.7 | 1772.3 ± 737.6 | |||
| Digital tip ulcer | NS | NS | NS | |||
| Present | 2440.3 ± 1033.0 | 2191.2 ± 736.4 | 3021.6 ± 1565.7 | |||
| Absent | 2004.4 ± 853.4 | 2029.7 ± 908.7 | 1946.9 ± 749.5 | |||
| Telangiectasia | NS | NS | NS | |||
| Present | 2142.1 ± 762.8 | 2117.6 ± 822.7 | 2203.4 ± 695.7 | |||
| Absent | 2080.4 ± 966.8 | 2041.1 ± 901.9 | 2166.8 ± 1144.4 | |||
| ILD | 0.01 | 0.03 | NS | |||
| Present | 1926.7 ± 757.9 | 1895.2 ± 783.2 | 1996.9 ± 728.6 | |||
| Absent | 2721.6 ± 1131.4 | 2671.4 ± 930.9 | 2838.7 ± 1769.6 | |||
| PAH | NS | NS | NS | |||
| Present | 2220.2 ± 849.1 | 2431.0 ± 860,6 | 1949.1 ± 813.9 | |||
| Absent | 2034.6 ± 936.0 | 1921.8 ± 842.4 | 2405.4 ± 1192.9 | |||
| Urinalysis abnormality | NS | NS | NS | |||
| Present | 1806.2 ± 661.2 | 1855.8 ± 857.6 | 1731.8 ± 271.2 | |||
| Absent | 2180.6 ± 949.4 | 2113.3 ± 876.7 | 2355.4 ± 1150.4 | |||
| Difficulty swallowing | NS | NS | NS | |||
| Present | 1982.9 ± 1062.1 | 1838.9 ± 1170.0 | 2486.9 ± 396.8 | |||
| Absent | 2127.4 ± 872.5 | 2128.3 ± 778.5 | 2125.6 ± 1081.3 | |||
| Joint pain | NS | NS | NS | |||
| Present | 2158.0 ± 901.7 | 2225.5 ± 953.8 | 1944.3 ± 745.0 | |||
| Absent | 2029.1 ± 918.6 | 1830.4 ± 685.2 | 2351.9 ± 1189.1 | |||
| Muscular involvement | 0.005 | NS | 0.04 | |||
| Present | 2741.9 ± 1039.9 | 2570.3 ± 924.8 | 3199.6 ± 1407.9 | |||
| Absent | 1897.2 ± 762.9 | 1896.6 ± 794.1 | 1898.4 ± 727.1 | |||
| Severity [median (min-max)]*** | NS | NS | NS | |||
| Mild | 1532.0 (649-3012) | 1536.7 (1178-3012) | 1532.0 (650-2499) | |||
| Moderate | 1897.1 (430-3564) | 1764.7 (430-3564) | 2665.1 92563-2767) | |||
| Severe | 2279.1 (1201-4820) | 2279.5 (1201-3735) | 2242.7 (1204-4820) | |||
t-test;
Mann-Whitney U test;
Kruskal-Wallis test. NS: not significant.
Changes of BAFF level after treatment
In 35 (76.1%) patients (24 diffuse SSc, 11 limited SSc) who completed 12-month follow-up, the mean BAFF level significantly reduced compared to before treatment (1652.2 ± 892.7 pg/ml versus 2147.6 ± 945.5 pg/ml; p = 0.03). However, when stratified by types of SSc, only diffuse SSc patients demonstrated significant reduction in BAFF level (1440.8 ± 637.0 pg/ml versus 2094.7 ± 875.9 pg/ml; p = 0.002) while this reduction in limited SSc patients was not significant [median (min-max) 1641.5 (1333.2-5523.2) versus 2279.1 (648.9-4820.4) pg/ml; p = 0.52] as presented in Figure 1a.
Figure 1.
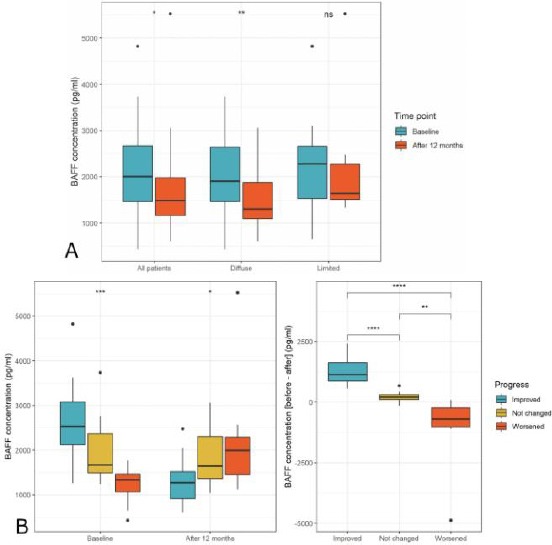
BAFF level before and after treatment; a) BAFF level before and after treatment in all patients and stratified by types of SSc; b) BAFF level before and after treatment among patients with clinically improved, not changed, and worsened outcome. Comparisons between before and after treatment were made by the paired t-test (for all patients and diffuse SSc patients) and the Wilcoxon signed rank test (for limited SSc patients). Comparisons among outcome groups were made by the Kruskal-Wallis test. NS: not significant; *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001
When stratified by outcomes at follow-up (18 improved, 9 not changed, and 8 worsened), there were differences in the BAFF level among outcome groups both at baseline and follow-up, and the differences in the reduction of BAFF level after 12 months were significantly different as presented in Figure 1b. In particular, patients with clinical improvement had the highest baseline BAFF level (median 2531.1, min-max 1257.5-4820.4) and was associated with the greatest reduction in BAFF level (median difference 1137.5, min-max 572.0-2405.5) while patients with clinical worsening had the lowest baseline BAFF level (median 1333.8, min-max 429.5-1764.7) and was associated with an increase in BAFF level (median difference 1993.15, min-max 1123.4-5523.2).
Discussions
In our study, higher BAFF levels were observed in patients with positive anti-topoisomerase antibody (ATA), ILD, and muscular involvement. ATA is more prevalent in diffuse SSc patients, and its presence has been associated with lung involvement [9]. BAFF’s relationship with ATA in SSc has not been established, but a previous study has shown no correlation between ATA and BAFF in systemic lupus erythematosus (SLE) and rheumatoid arthritis [10].
Although decreased lung vital capacity has been observed in patients with elevated BAFF, pulmonary fibrosis, the hallmark of ILD, seems to be associated with high APRIL (a proliferation-inducing ligand) levels but not high BAFF levels [11]. While BAFF is related to the survival and proliferation of B cells, increased levels of BAFF were not shown to correlate with the number of B cells in peripheral blood—in fact, studies have shown that the number of B cells was decreased in SSc [12], [13]. Findings from studies on experimental animals suggested interleukin-6 (IL-6) and IL-10 play a more prominent role in the development and attenuation of lung fibrosis, and BAFF might suppress regulatory B cells (which produces IL-10 and decreases immune response) [14]. Based on a study in 15 years, Yoshizaki and Sato have proposed a model of abnormal B cells’ activity [15]. In this model, augmentation of B-cell antigen receptor (BCR) signalling, along with overproduction of BAFF and TLR stimulation, induces activation of memory B cells and increases apoptosis. This, in turn, decreases the number of peripheral B cells. To maintain the number of inactivated B cells, bone marrow will increase the production of naïve B cells into peripheral blood. Therefore, there is an increase in the number of naïve B cells but a decrease in the number of memory B cells in early SSc patients [16]. B cells are mainly active in peripheral blood but not in organ tissues, and the number of peripheral B cells increases after treatment [17] in this study and another study [18]. BAFF antagonist or genetic ablation of BAFF attenuated lung fibrosis. However, these findings are limited to animal experiments and might not reflect the whole picture of a more complex cytokine network in human.
Studies on cytokines in muscular diseases started in 1986 with the identification of IL-2 and interferon (IFN) in polymyositis. Since then, researchers have described multiple cytokines relating to a spectrum of muscular diseases. However, there was no association of muscular involvement with levels of cytokines in SSc [19]. Our study is the first study to show a higher level of BAFF in patients who had muscular involvement.
Although both diffuse and limited SSc patients in our study demonstrated a reduction in the level of BAFF after 12 months of treatment, only diffuse disease patients had a significant reduction. Matsushita et al. found that the reduction of BAFF levels in SSc was more modest than in SLE but still more significant than in dermatomyositis [5]. Patients in both Matsushita’s and our study had an onset within 2–3 years, suggesting BAFF can be elevated at a very early stage of SSc.
The most surprising finding in our study was that higher pretreatment BAFF levels were observed in patients with clinical improvement but not in patients with clinical worsening. Patients with improvement also had more significant reduction of BAFF levels after treatment. In a previous study, increased levels of BAFF have been considered to herald the development or worsening of major events in SSc whereas decreased levels of BAFF was associated with regression of skin involvement [5]. The evidence suggests the level of BAFF before treatment could be used for prognosis and changes in BAFF level can help monitor patients’ treatment progress.
In conclusion, the level of BAFF was significantly higher in early SSc patients and related to ATA production of B lymphocytes. Pretreatment BAFF level was lower in the presence of ILD on HRCT and higher in the presence of muscular involvement. Clinical worsening after 12 months of treatment was associated with higher pretreatment BAFF level and increase in follow-up BAFF level compared to baseline. This suggests that baseline BAFF level as well as it changes after treatment can be used to predict and monitor patients’ response to therapy.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Yoshizaki A. B lymphocytes in systemic sclerosis:Abnormalities and therapeutic targets. The Journal of Dermatology. 2016;43(1):39–45. doi: 10.1111/1346-8138.13184. https://doi.org/10.1111/1346-8138.13184 PMid:26782005. [DOI] [PubMed] [Google Scholar]
- 2.Yoshizaki A, Iwata Y, Komura K, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, Tedder TF. CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. The American journal of pathology. 2008;172(6):1650–63. doi: 10.2353/ajpath.2008.071049. https://doi.org/10.2353/ajpath.2008.071049 PMid:18467694 PMCid:PMC2408424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.François A, Chatelus E, Wachsmann D, Sibilia J, Bahram S, Alsaleh G, Gottenberg JE. B lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis research & therapy. 2013;15(5):R168. doi: 10.1186/ar4352. https://doi.org/10.1186/ar4352 PMid:24289101 PMCid:PMC3978899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, Tanasescu C. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus:a randomised, placebo-controlled, phase 3 trial. The Lancet. 2011;377(9767):721–31. doi: 10.1016/S0140-6736(10)61354-2. https://doi.org/10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis:enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis & Rheumatism:Official Journal of the American College of Rheumatology. 2006;54(1):192–201. doi: 10.1002/art.21526. https://doi.org/10.1002/art.21526 PMid:16385515. [DOI] [PubMed] [Google Scholar]
- 6.Jordan S, Distler JH, Maurer B, Huscher D, van Laar JM, Allanore Y group, ERs. Effects and safety of rituximab in systemic sclerosis:an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74(6):1188–1194. doi: 10.1136/annrheumdis-2013-204522. https://doi.org/10.1136/annrheumdis-2013-204522 PMid:24442885. [DOI] [PubMed] [Google Scholar]
- 7.Hudson M, Fritzler MJ. Diagnostic criteria of systemic sclerosis. Journal of autoimmunity. 2014;48:38–41. doi: 10.1016/j.jaut.2013.11.004. https://doi.org/10.1016/j.jaut.2013.11.004 PMid:24461384. [DOI] [PubMed] [Google Scholar]
- 8.Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, Clements P, Denton C, Farge D, Fligelstone K, Földvari I. EULAR recommendations for the treatment of systemic sclerosis:a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Annals of the rheumatic diseases. 2009;68(5):620–8. doi: 10.1136/ard.2008.096677. https://doi.org/10.1136/ard.2008.096677 PMid:19147617. [DOI] [PubMed] [Google Scholar]
- 9.Walker UA, Tyndall A, Czirjak L, Denton C, Farge-Bancel D, Kowal-Bielecka O, Müller-Ladner U, Bocelli-Tyndall C, Matucci-Cerinic M. Clinical risk assessment of organ manifestations in systemic sclerosis:a report from the EULAR Scleroderma Trials And Research group database. Annals of the rheumatic diseases. 2007;66(6):754–63. doi: 10.1136/ard.2006.062901. https://doi.org/10.1136/ard.2006.062901 PMid:17234652 PMCid:PMC1954657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15(9):570–6. doi: 10.1177/0961203306071871. https://doi.org/10.1177/0961203306071871 PMid:17080911. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita T, Fujimoto M, Hasegawa M, Tanaka C, Kumada S, Ogawa F, Takehara K, Sato S. Elevated serum APRIL levels in patients with systemic sclerosis:distinct profiles of systemic sclerosis categorized by APRIL and BAFF. The Journal of rheumatology. 2007;34(10):2056–62. PMid:17896803. [PubMed] [Google Scholar]
- 12.Forestier A, Guerrier T, Jouvray M, Giovannelli J, Lefèvre G, Sobanski V, Hauspie C, Hachulla E, Hatron PY, Zéphir H, Vermersch P. Altered B lymphocyte homeostasis and functions in systemic sclerosis. Autoimmunity reviews. 2018;17(3):244–55. doi: 10.1016/j.autrev.2017.10.015. https://doi.org/10.1016/j.autrev.2017.10.015 PMid:29343447. [DOI] [PubMed] [Google Scholar]
- 13.López-Cacho JM, Gallardo S, Posada M, Aguerri M, Calzada D, Mayayo T, Cárdaba B. Association of Immunological Cell Profiles with Specific Clinical Phenotypes of Scleroderma Disease. BioMed Research International. 2014:1–8. doi: 10.1155/2014/148293. https://doi.org/10.1155/2014/148293 PMid:24818126 PMCid:PMC4004116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita T, Kobayashi T, Mizumaki K, Kano M, Sawada T, Tennichi M, Okamura A, Hamaguchi Y, Iwakura Y, Hasegawa M, Fujimoto M. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Science advances. 2018;4(7):eaas9944. doi: 10.1126/sciadv.aas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshizaki A, Sato S. Abnormal B lymphocyte activation and function in systemic sclerosis. Annals of dermatology. 2015;27(1):1–9. doi: 10.5021/ad.2015.27.1.1. https://doi.org/10.5021/ad.2015.27.1.1 PMid:25673924 PMCid:PMC4323585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mavropoulos A, Simopoulou T, Varna A, Liaskos C, Katsiari CG, Bogdanos DP, Sakkas LI. Breg cells are numerically decreased and functionally impaired in patients with systemic sclerosis. Arthritis & Rheumatology. 2016;68(2):494–504. doi: 10.1002/art.39437. https://doi.org/10.1002/art.39437 PMid:26414243. [DOI] [PubMed] [Google Scholar]
- 17.Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis:expanded naive B cells and diminished but activated memory B cells. Arthritis & Rheumatism:Official Journal of the American College of Rheumatology. 2004;50(6):1918–27. doi: 10.1002/art.20274. https://doi.org/10.1002/art.20274 PMid:15188368. [DOI] [PubMed] [Google Scholar]
- 18.François A, Gombault A, Villeret B, Alsaleh G, Fanny M, Gasse P, Adam SM, Crestani B, Sibilia J, Schneider P, Bahram S. B cell activating factor is central to bleomycin-and IL-17-mediated experimental pulmonary fibrosis. Journal of autoimmunity. 2015;56:1–1. doi: 10.1016/j.jaut.2014.08.003. https://doi.org/10.1016/j.jaut.2014.08.003 PMid:25441030. [DOI] [PubMed] [Google Scholar]
- 19.Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor α, and interferon-γlevels in sera from patients with scleroderma. Arthritis & Rheumatism:Official Journal of the American College of Rheumatology. 1992;35(1):67–72. doi: 10.1002/art.1780350111. https://doi.org/10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]


