Abstract
Mesenchymal stem cells (MSCs) have recently generated great interest in the fields of regenerative medicine and immunotherapy due to their unique biologic properties. In this review we attempt to provide an overview of the current clinical status of MSC therapy, primarily focusing on immunomodulatory and regenerative or tissue repair applications of MSCs. In addition, current manufacturing is reviewed with attention to variation in practices (e.g., starting material, approach to culture and product testing). There is considerable variation among the 218 clinical trials assessed here; variations include proposed mechanisms of action, optimal dosing strategy, and route of administration. To ensure the greatest likelihood of success in clinical trials as the field progresses, attention must be given to the optimization of MSC culture.
MESENCHYMAL STEM CELLS
Cellular therapy has evolved quickly over the past decade with valuable experience gained in both preclinical research and clinical trials. Both embryonic and nonembryonic stem cells have been explored as potential therapeutic strategies for a number of diseases. One group of adult stem cells, mesenchymal stem or stromal cells (MSCs), has generated great interest in the fields of regenerative medicine and immunotherapy due to their unique biologic properties. MSCs were first discovered in 1968 by Friedenstein and colleagues1 as adherent fibroblast-like cells in the bone marrow (BM) capable of differentiating into bone. It was subsequently shown that MSCs could be isolated from various tissues such as BM, adipose tissue (AT),2 and umbilical cord blood (UCB).3 These cells can be expanded in vitro, which allows them to rapidly reach the desired cell counts for use in vivo. Using somewhat different strategies, several laboratories have identified, isolated, and cultured MSCs with specific properties.4–6
In an effort to better characterize MSCs, the International Society for Cellular Therapy defined MSCs by the following three criteria:7
MSCs must be adherent to plastic under standard tissue culture conditions;
MSCs must express certain cell surface markers such as CD73, CD90, and CD105, and lack expression of other markers including CD45, CD34, CD14, CD11b, CD79α, or CD19 and HLA-DR surface molecules;
MSCs must have the capacity to differentiate into osteoblasts, adipocytes, and chondroblasts under defined in vitro conditions.
This definition is fairly nonspecific and does little to distinguish MSCs from the classical fibroblasts.8 In this review we attempt to provide an overview of the current clinical status of MSC therapy, primarily focusing on immunomodulatory and regenerative or tissue repair applications of MSCs. In addition, current manufacturing is reviewed with attention to variation in practices (e.g., starting material, approach to culture and product testing).
CLINICAL STATUS
Based on current literature,9 it is thought that MSCs exert their therapeutic effects by several mechanisms including:
The ability to home to sites of inflammation after tissue injury;
The ability to differentiate into various cell types;
The ability to secrete multiple bioactive molecules capable of stimulating recovery of injured cells and inhibiting inflammation;
The lack of immunogenicity and the ability to perform immunomodulatory functions.
These four potential modes of therapeutic efficacy have been demonstrated in various preclinical animal model studies.10 However, this review focuses primarily on clinical applications of MSCs in humans.
The first clinical trial using culture-expanded MSCs was carried out in 1995; in this study, 15 hematooncology patients received injections of autologous (BM-MSCs) cells as part of a safety and feasibility study.11 Since then, the use of MSCs has been further explored. As of October 2012, the clinical trials database (http://www.clinicaltrials.gov) showed 218 clinical trials using MSCs for a wide range of therapeutic applications (Table 1) internationally. Most of these trials are in Phase I (safety studies, n = 42), Phase II (proof of concept for efficacy in human patients, n = 57), or combined Phases I and II studies (n = 105). Only a small number of these trials are in Phase III (comparing a newer treatment to the standard or best known treatment, n = 8) or combined Phases II and III (n = 6). The disease conditions and phase of trials are listed in Table 1 and their sources are summarized in Fig. 1. In general, MSCs appear to be well tolerated; most trials report a lack of any adverse effects except for mild or transient peri-injection effects.10 Encouraging results from these clinical trials have increased research into MSC therapy for a variety of clinical disorders such as acute myocardial infarction, stroke, liver cirrhosis, amyotrophic lateral sclerosis, graft-versus-host disease (GVHD), solid organ transplant rejection, and autoimmune disorders.
TABLE 1.
Current status and enrollment of MSC clinical trials*
| Phases, number of studies [targeted enrollment] |
|||||
|---|---|---|---|---|---|
| Targeted condition | I | I/II | II | II/III | III |
| Bone/cartilage disorders | |||||
| Bone cysts | 1 [6] | 1 [10] | |||
| Bone neoplasms | 1 [50] | ||||
| Cartilage defect | 1 [50] | 2 [38] | 1 [100] | ||
| Degenerative osteoarthritis | 2 [30] | 1 [25] | |||
| Distraction osteogenesis | 1 [6] | ||||
| Fractures | 2 [16] | 1 [24] | 1 [40] | ||
| Ligament injury | 1 [24] | 1 [10] | |||
| Meniscectomy | 2 [110] | ||||
| Osteoarthritis | 5 [42] | 2 [45] | 4 [222] | 1 [104] | |
| Osteodysplasia | 1 [8] | ||||
| Osteogenesis imperfecta | 1 [9] | ||||
| Osteonecrosis | 1 [21] | 2 [39] | 1 [10] | ||
| Osteoporosis | 1 [290] | ||||
| Pseudoarthrosis | 1 [50] | ||||
| Spinal fusion | 1 [62] | ||||
| Hematologic disorders | |||||
| Aplastic anemia | 2 [60] | 1 [30] | |||
| BMT | 1 [125] | 3 [40] | 3 [125] | ||
| GVHD | 2 [59] | 6 [130] | 6 [286] | 1 [100] | 1 [240] |
| Myelodysplastic syndrome | 1 [30] | ||||
| Diabetes | |||||
| Type 1 | 1 [24] | 5 [168] | 1 [60] | 1 [80] | |
| Type 2 | 1 [24] | 3 [170] | |||
| Liver diseases | |||||
| Autoimmune hepatitis | 1 [100] | ||||
| Cirrhosis | 3 [29] | 7 [715] | 5 [266] | ||
| Hypercholesterolemia | 1 [1] | ||||
| Liver failure | 2 [228] | 1 [120] | |||
| Liver transplant | 1 [40] | 1 [60] | |||
| Primary biliary cirrhosis | 1 [100] | ||||
| Cardiovascular diseases | |||||
| Dilated cardiomyopathy | 2 [66] | 2 [80] | |||
| Heart failure | 3 [172] | 4 [160] | |||
| Myocardial infarction | 1 [53] | 2 [45] | 2 [380] | 1 [80] | 2 [165] |
| Myocardial ischemia | 2 [144] | 3 [89] | 1 [60] | ||
| Gastrointestinal diseases | |||||
| Crohn’s disease | 3 [56] | 1 [10] | 4 [696] | ||
| Fistula in ano | 1 [10] | 1 [40] | |||
| Ulcerative colitis | 1 [50] | ||||
| Autoimmune or skin disorders | |||||
| Burns | 1 [20] | ||||
| Epidermolysis bullosa | 1 [75] | ||||
| HIV | 1 [36] | ||||
| SLE | 1 [20] | ||||
| Rheumatoid arthritis | 2 [203] | ||||
| Sjogren’s disease | 1 [20] | ||||
| Systemic sclerosis | 1 [20] | ||||
| Lung diseases | |||||
| Bronchopulmonary dysplasia | 3 [28] | ||||
| COPD | 1 [62] | ||||
| Emphysema | 1 [10] | ||||
| Idiopathic pulmonary fibrosis | 1 [8] | ||||
| Neuromuscular diseases | |||||
| ALS | 1 [25] | 1 [24] | 1 [30] | ||
| Alzheimer’s | 1 [9] | 1 [30] | |||
| Brain injury | 1 [2] | ||||
| Cerebellar ataxia | 1 [8] | 1 [20] | |||
| Disc disease | 3 [55] | ||||
| Hereditary ataxia | 1 [20] | ||||
| ICSOL hemorrhage | 1 [20] | ||||
| Limbus insufficiency | 1 [30] | ||||
| Multiple sclerosis | 1 [24] | 5 [103] | 1 [16] | ||
| Multiple sclerosis and NMO | 1 [20] | ||||
| Multiple system atrophy | 1 [27] | ||||
| Parkinson’s disease | 1 [20] | ||||
| Retinitis pigmentosa | 1 [10] | ||||
| Romberg’s disease | 1 [5] | ||||
| Spinal cord injury | 4 [53] | 2 [100] | 2 [90] | 1 [32] | |
| Stroke | 1 [30] | 3 [203] | 3 [100] | ||
| Muscular dystrophy | 1 [15] | ||||
| Neomyogenesis | 1 [30] | ||||
| Limb ischemia | |||||
| Diabetic foot | 1 [40] | 1 [30] | |||
| Limb ischemia | 9 [245] | 2 [176] | |||
| Renal diseases | |||||
| Kidney injury | |||||
| Kidney transplant | 3 [41] | ||||
| Lupus nephritis | 1 [20] | 1 [25] | |||
| Miscellaneous | |||||
| Endometriosis | 1 [60] | ||||
| Prostate cancer | 1 [31] | ||||
| Total: 218 [9757] | 42 [923] | 105 [3957] | 57 [3285] | 6 [367] | 8 [1225] |
The data were searched on the website of ClinicalTrials.gov (http://www.clinicaltrials.gov) on October 22, 2012. The following key words including “mesenchymal stem cells,” “mesenchymal stromal cells,” “multi-potent stromal cells,” “multi-potent progenitor cells,” “BM stromal cells,” and “connective tissue progenitor” were used.
ALS = amyotrophic lateral sclerosis; COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; ICSOL = intracranial space–occupying lesion; NMO = neuromyelitis optica; SLE = systemic lupus erythematosus.
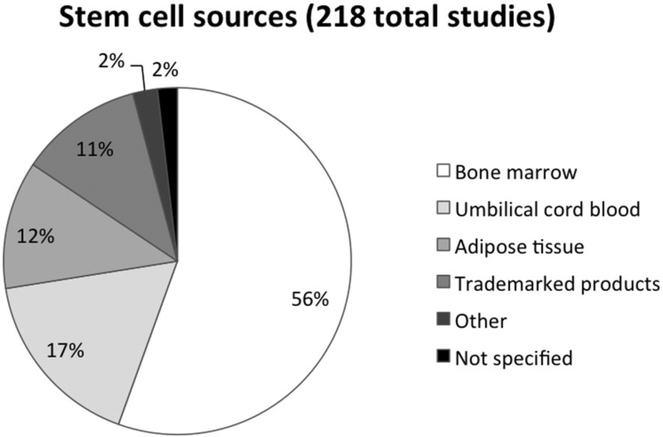
Fig. 1.
Summary of tissue sources for MSCs currently being used in clinical trials. BM is the most common source of MSCs (n = 121), followed by UCB (n = 37) and AT (n = 26).The “Trademarked product” category members are commercial products and do not disclose their cell sources (n = 25). Others include menstrual blood, placenta, and endometrial cells (n = 5).The “not specified” category members did not clearly state the source tissue used to isolate MSCs (n = 4).
Immunomodulatory effects of MSCs
MSCs have unique immunologic characteristics, which promote their survival and growth in allogeneic or xenogeneic environments.12,13 They express very low levels of major histocompatibility complex (MHC) Class I antigens and do not express MHC Class II antigens or costimulatory molecules such as CD40, CD80, and CD86.14 These features protect them from alloreactive natural killer (NK) cell–mediated lysis.15 In addition, human MSCs express HLA-G, a nonclassical MHC Class I antigen, which may prevent the immune response against MSCs (as shown by blocking experiments), although its expression seems to decrease in culture.16 Culture conditions may also affect MSC immunogenicity due to internalization of certain protein molecules of the culture medium.17 However, patients receiving treatment with allogeneic human MSCs did not show antiallogeneic MSC antibody production or T-cell priming.18 The precise mechanisms underlying MSC immunomodulation are still not fully understood, although direct cell-to-cell contact and/or release of soluble immunosuppressive factors may play major roles. MSCs can potentially interact with a wide range of immune cells, including T lymphocytes, B lymphocytes, NK cells, and dendritic cells. MSCs act on both the adaptive and the innate immune systems by suppressing T cells,17 suppressing dendritic cell maturation,19,20 reducing B-cell activation and proliferation,21,22 inhibiting proliferation and cytotoxicity of NK cells,23 and promoting the generation of regulatory T cells via an interleukin (IL)-10 mechanism.24,25 Secretion of prostaglandins and growth factors such as vascular endothelial growth factor, keratinocyte growth factor, and hepatocyte growth factors is also thought to influence immunomodulation and repair of various tissues.26
When influenced by inflammatory cytokines, MSCs are capable of migrating to inflamed tissues and modulating the local inflammatory reactions at two levels via their effects on both innate and adaptive immunity.24,27 One level occurs locally via the secretion of mediators that inhibit the proliferation of immune cells in the vicinity of MSCs. The second induces a systemic response—either an anti-inflammatory Th-2 immune activation or in some instances, the generation of regulatory T cells. In addition, MSCs may recruit and support growth of local autologous stem cells inside the injured tissues, thus promoting cell survival and tissue repair.28
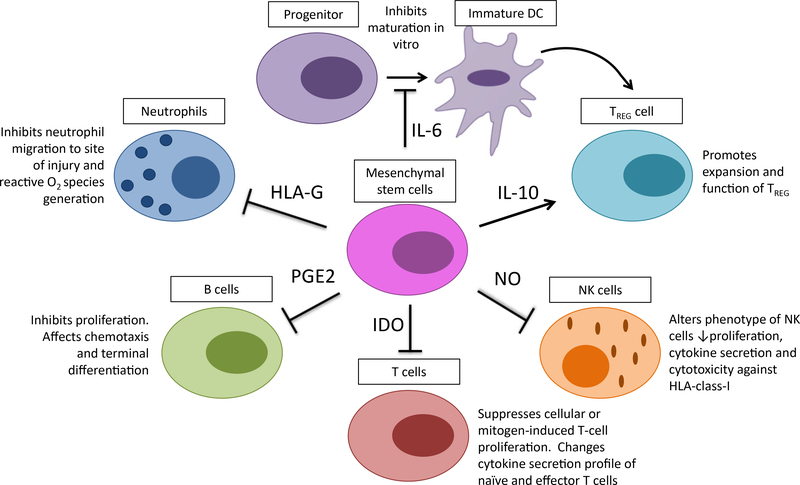
Clinical applications of MSCs are growing rapidly as research progresses and the wide range of MSC-dependent influences on the immune system are further delineated. Figure 2 summarizes the current cell–cell interactions of MSCs with the immune system. Clinical-grade ex vivo expanded MSCs have been used to treat BM and organ transplant rejection and inflammatory and auto- and alloimmune diseases (such as systemic collagen abnormalities and GVHD).
Fig. 2.
Schematic representation of the interactions between MSCs and immune cells. After activation, MSCs secrete soluble mediators—such as nitric oxide (NO), prostaglandin (PGE2), indoleamine 2,3-dioxygenase (IDO), IL-6, IL-10, and human leukocyte antigen (HLA)-G. Production of these mediators regulates the proliferation and function of a variety of immune cells as well as the induction of regulatory T (TREG) cells either directly or indirectly through the generation of immature dendritic cells (DC).
The most significant results on the immunosuppressive effects of MSCs so far have been observed in the treatment of acute GVHD after allogeneic stem cell transplantation. The first case of ex vivo expanded haploidentical MSC infusion in a patient with severe Grade IV GVHD of the gut and liver resulted in a striking improvement of the disease.29 A Phase II study reported that 30 of 55 patients had a complete response and nine patients showed improvement indicating that irrespective of the donor, MSC infusion might be an effective therapy for patients with steroid-resistant acute GVHD.30 Since these studies were performed, several others have produced encouraging responses, both in acute and in chronic GVHD refractory to standard steroid treatment.31–38 Recently, cotransplantation of MSCs39–50 either from the same hematopoietic stem cell (HSC) donor or from a third party has shown rapid engraftment and less severe acute GVHD in most clinical trials. However, a higher incidence of relapse has been reported in a few.43 Cord blood unit cotransplantation or coculture expansion with MSCs has been shown to overcome the limitation posed by low cellularity of cord blood units for unrelated transplants in adults.44,51 The possibility of eliminating this obstacle in transplant would be a major accomplishment and this has opened new avenues of research for studying the properties of MSCs obtained from different sources.
Based on their ability to moderate T-cell proliferation and function, MSCs have also been proposed as a therapeutic option in the treatment of autoimmune diseases,52,53 renal transplantation rejection,54,55 and various immune-mediated neurodegenerative disorders.56–60 The initial Phase I and II clinical trials are summarized in Table 2 and have shown encouraging results to stimulate further research in these areas and the scope of their immunomodulatory and regenerative potential will further expand with better understanding of the underlying mechanism.
TABLE 2.
Summary of the immunomodulatory role of MSCs in clinical trials
| Disease | Patient profile | MSC source | Dose (×106/kg) | Mode of administration | Outcome | Ref [MFR ref] |
|---|---|---|---|---|---|---|
| GVHD (steroid or treatment refractory) | Ac GVHD, n = 10; Chr GVHD, n = 8 | HAP-ID, MRLD (BM-MSC) | 2 (0.3–3.7) | IV 1× | Ac GVHD, CR n = 1, PR n = 6, NR n = 3; Chr GVHD, CR n = 1, PR n = 3, NR n = 4. | 31 [31] |
| Ac GVHD, n = 12; Chr GVHD, n = 6 | Allo (n = 10), MRLD (n = 1), UM (n = 1; BM-MSC) | 1.7–2,3 | IV 2×/week (4 weeks) | Ac GVHD CR n = 7, PR n = 4, NR n = 1; Chr GVHD CR n = 2, PR n = 2, NR n = 3. | 32 [29,30] | |
| Ac GVHD, n = 12 | Universal donor (BM-MSC) | 2–8 | IV 2×/week (4 weeks) | CR n = 7, PR n = 2, mixed n = 3 | 33 [61] | |
| Chr GVHD, n = 12 | MRLD n = 14, HAP-iD n = 2, URLD n = 1 | 0.4–2.1 | IV 1–3× | CR n = 3, PR n = 6, NR n = 3, Response unrelated to donor HLA match. | 35 [35] | |
| Ac GVHD, n = 13 | Allo (BM-MSC) | 0.6–1.1 | 1–5 IV infusions | CR n = 2, PR n = 5 (additional immunosuppression ), NR n = 6. | 36 [36] | |
| Ac GVHD, n = 31 | Allo (BM-MSC) | high = 8, n = 15; low = 2, n = 16 | IV | CR n = 24, PR n = 5, NR n = 2. Response rate did not depend on MSC dose. | 37 [61] | |
| Ac GVHD, n = 55 | MRLD (n = 5), HAP-ID (n = 18), Alio (n = 69; BM-MSC) | 1.4 | IV 1–5× | CR n = 30, PR n = 9, NR n = 13, stable disease n = 3. | 30 [29] | |
| Ac and Chr GVHD, n = 11 Chr GVHD, n = 8 | URLD-UM (BM-MSC) | 0.7–3.7 | 1–5 infusions IV | CR n = 3, PR n = 4, NR n = 4. | 34 [60] | |
| MRLD (n =2), HAP-ID (n = 6), URLD (n = 4; BM-MSC) | 0.7–9 | IV 1–3× | CR n = 5, PR n = 1, not evaluable n = 2 (died). | 38 [38] | ||
| BMT | Leukemia in rem, (MSC, n = 27; Ctrl, n = 28) | BM (n = 4), Allo (n = 23; BM-MSC) | 0.3–0.5 | IV, 24 hr before BMT | Median WBC and PLT engraftment time comparable in two groups. | 50 [50] |
| Hem-one (MSC, n = 13; Ctrl, n = 39) | HAP-ID (n = 15; BM-MSC) | 1–3.9 | IV, 4 hr before UCBT | Significantly ↓ Grade ill and IV compared to historic controls. No difference in engraftment and rejection In two groups. | 39 [39] | |
| Hem-one (n = 20) | Allo (BM-MSC) | 1.4 | 0.5–2 hr before PBSC infusion | Nonrelapse mortality and OS improved in MSC group (p < 0.05) than Ctrl at t = 1 year (historic). | 40 [29] | |
| Hem-onc (n = 12) | MRLD (BM-MSC) | 1.77 | 1 hr after HSC infusion | Rapid engraftment in all, n = 7 alive, n = 5 died (n = 4 relapse 6–18 months, n = 1 liver failure). | 41 [41] | |
| Hem-onc (n = 9) | HAP-ID or URLD (BM-MSC) | 1.04–2.15 | Immediately after UCBT | No difference In cord blood engraftment and incidence of Ac GVHD compared to controls. | 42 [42] | |
| Hem-onc (n = 6) | BM-MSC (HSC donor) | 1 | IV, 50–295 days after HSCT | Rapid recovery, CR n = 2 | 49 [62] | |
| Ac leukemia (n = 15) | HAP-ID (parental donors-BM-MSC) | 5–10 | IV, 4 hr after UCBT | Median neutrophil (19 days) and PLT engraftment (53 days) in all. | 44 [61] | |
| Hem-onc (MSC n = 10; Ctrl n = 15) | HLA-ID sibling donors | 3.4 | IV, 4 hr before HSC | Comparable hematopoietic recovery, Ac GVHD less in MSC group. OS better In Ctrl group at t = 3 years. | 43 [48] | |
| Hem-onc (n = 7) | MRLD n = 5, Allo n = 2 | 0.4–3 | IV | CR n = 3, PR n = 1, NR n = 3, | 45 [45] | |
| Hem-onc (n = 14); Ctrl (n = 47; H) | MUD HSC | 1–5 | IV, 4 hr before HSC | Rapid hematopoietic recovery in MSC group (p < 0.05). Carried forward: no graft failure and less severe GVHD. | 46 [46] | |
| Hem-onc (n = 46). | HLA-ID sibling donors | 1 (n = 18), 2.5 (n = 19), 5 (n = 5) | IV, 4 hr before HSC | Rapid hematopoietic recovery in all. Less severe GVHD (Ac = 13, Chr = 22) and relapse n = 12. MSC dose did not influence time to PLT recovery or relapse rate. | 47 [47] | |
| Breast cancer (n = 32) | Auto (BM-MSC) | 1–2.23 | IV, 1–24 hr after HSC | Rapid hematopoietic recovery in all. CR n = 11, PR n = 3, NR n = 10. Progressive disease death n = 4. | 48 [48] | |
| MS and amyotrophic lateral sclerosis | Sec. prog. MS (n = 10) | Auto (BM-MSC) | 1.6 | IV | Improvement In visual acuity (p < 0,003), VER (0.02), and ON thickness (0.06). No improvement In CV, VF, MV, or RNF thickness. | 60 [29] |
| MS (n = 10) | Auto (BM-MSC) | 30–50 | 5 mL Intrathecal, 5 mL | EDSS: improved (n = 5), stabilized (n = 1), worsened (n = 1). No radiologic evidence of improvement by Gad scan. AE (n = 1)ln form of selzure/mild encephalopathy. | 56 [56] | |
| MS (n = 10) | Auto (BM-MSC) | 8.76 | Intrathecal | EDSS: improved (n = 1), stabilized (n = 4), worsened (n = 5). Func, assessment: improved (n = 6), status quo (n = 1), deteriorated (n = 3). At t = 12 months, no signs of Improvement via MRI. | 57 [57] | |
| MSA (MSC n = 16; Ctrl n = 17) | Auto (BM-MSC) | 4 × 107/patlent* | IA (1 × 107 each ICA, 2 × 107 dominant VA) | Smaller Increase in MSC group for total UMSARS and UMSARS Part II compared to Ctrl. | 58 [59] | |
| MSA (MSC, n = 11; Ctrl, n = 18) | Auto (BM-MSC) | 4 × 107 /patient* | IA (1 × 107 each ICA, 2 × 107 dominant VA) | Significant increase In total UMSARS (p < 0.001) for MSC group compared to control. | 59 [59] | |
| Living related renal transplant for end-stage renal disease | (MSC + Std CNI, n = 53; MSC + low CNI, n = 53; CNI n = 53), total = 1 59 | Auto (BM-MSC) | 1–2 | IV 2× (postop at perfusion, 2 weeks later) | Low incidence of acute rejection (7.5% vs, 21.6%), less opportunistic Infection, and better renal function at t = 1 year In MSC group compared to Ctrl, Overall patient and graft survival similar In both groups. | 54 [48] |
| (MSC, n = 2; Ctrl, n = 3) | Auto (BM-MSC) | 1.7 or 2.0 | IV (7 days postop) | Renal biopsy and functions at t = 1 year were normal In MSC patients. | 55 [60] | |
| Systemic iupus/erythematosus | Active SLE (n = 15) | Allo (BM-MSC) | 1 | IV | All (13 patients > 1 year) improved with MSC treatment. Marked decrease In the SLEDAI score (12.2 ± 3.3 to 3.2 ± 2.8) and 24 hours’ proteinuria (2505.0 ± 1323.9 to 858.0 ± 800.7 mg/24 hr, p < 0.05. Decreased anti-dsDNA levels. | 52 [63] |
| Treatment refractory (n = 40) | Allo (BM-MSC) | 1 | IV | All showed stable remission at t = 12–18 months with improvement in serologic markers and renal function. | 53 [53] |
Dose is expressed as total dose rather than per kg body weight.
Ac. = acute; AE = adverse event; Allo = allogeneic; Auto = autologous; Chr. = chronic; CNI = calcineurin inhibitor; cont. = control group; CR = complete response; EDSS = Expanded Disability Status Scale; HAP-ID = haploidentical; hem-onc = hematooncology; IA = intraarterial; ICA = internal carotid artery; Leuks = leukemia patients; MFR ref = MSC manufacturing protocol designed previously and followed in the present study, adverse event—1 (one study Ref.55); MRLD = matched related donor; MS = multiple sclerosis; MUD = matched unrelated donor; NR = no response; OS = overall survival; PR = partial response; Prog. = Progressive; rem. = remission; Sec. = secondary; SLEDAI = SLE disease activity index; UC-MSCs = umbilical cord derived mesenchymal stem cells; UMSAR = unified multiple system atrophy rating scale; URLD-UM = unrelated unmatched donor (universal donor); VA = vertebral artery; VER = visual evoked response
MSCs in tissue repair and regeneration
MSCs have a unique characteristic of selectively homing to the sites of tissue injury and/or inflammation after systemic administration.27 Once located at an inflammation site, MSCs can exert local functional effects in the resident tissue.27,28 Ortiz and coworkers64 showed that murine MSCs home to the lung in response to injury, adopt an epithelium-like phenotype, and reduce inflammation in lung tissue of mice challenged with bleomycin. Cell migration is dependent on a multitude of signals ranging from growth factors to chemokines secreted by injured cells and/or respondent immune cells;65 migration of MSCs may also be regulated by such signals. Studies have demonstrated that MSC migration is influenced by a range of growth factors such as platelet-derived growth factor (PDGF) or insulin-like growth factor-1 (IGF-1) and chemokines such as CCR2, CCR3, CCR4, or CCL5 as assessed by in vitro migration assays.66
Since the 1990s, the differentiation potential of MSCs has attracted much attention. Experimental data have demonstrated that MSCs can differentiate into mesodermal lineages such as bone, cartilage, adipocytes, and connective stromal cells.61 It has also been suggested that MSCs might be capable of differentiating into not only ectodermal lineage cells (e.g., neurons and epithelium), but also endodermal lineage cells (e.g., hepatocytes).67–69 Although these results come from in vitro experiments, they provide exciting indications of how MSCs may differentiate in vivo. Regulated by the subtle microenvironment of local tissue, the differentiation of engrafted MSCs in vivo, of course, may be more complex and much remains unresolved in this regard.70
Based on current knowledge, when induced by a series of signals at the local tissue, engrafted MSCs appear to be capable of differentiating into at least three types of cells in vivo:
Tissue-specific cells necessary for repair of injured tissues. For example, engrafted MSCs can differentiate into cardiomyocytes, smooth muscle cells, and vascular endothelial cells, which are important components of cardiac tissue.71–73
Function-relative cells necessary for optimum growth and proliferation in local tissue. This type of differentiated cell is one component of the specific microenvironment or niche for tissue repair and is used to enhance and promote homing and regeneration (as in BM after a stem cell transplant).74
Regulatory cells, which contribute to tissue repair and regeneration through secretion of cytokines that might possess trophic and immunomodulatory functions.75
The molecular and environmental mechanisms that control MSC differentiation are not fully understood, and no unique phenotype marker has yet been associated with predictable differentiation potential of MSCs. There are currently several hypotheses to explain the differentiation potential for MSCs. For instance, Dennis and colleagues76 suggested that in MSCs, there are storage genes that can express and adjust differentiation into various lineages when exposed to different conditions. Phinney and Prockop28 proposed that MSCs are equipped with motor proteins and a proteolytic arsenal that enables them to interact with and respond to signals from the extracellular matrix and differentiate into unique structures such as muscle, bone, cartilage, or other connective tissues.
Recently, the trophic effects of MSCs have been identified to be of great significance in tissue regeneration. After engraftment, MSCs can contribute to tissue repair by secreting a number of trophic molecules that include soluble extracellular matrix glycoproteins (collagen types I and II, osteopontin), cytokines (transforming growth factor [TGF]-β, IL-10, IL-6), and growth factors (vascular endothelial growth factor, hepatocyte growth factor, keratinocyte growth factor).75 These trophic molecules promote cell–cell connections.77 It has been observed that these trophic molecules can not only reduce inflammation, apoptosis, and fibrosis of damaged tissues, but also stimulate tissue cell regeneration. Although there is evidence that MSCs and certain tissue cells such as cardiomyocytes can interact with one another via small-diameter nanotubes, the underlying mechanism of cell–cell connection and its possible roles during tissue regeneration remains to be further investigated.77,78
Thus, in the acute phase of injury, MSC differentiation does not seem likely. However, MSCs do seem to play a role in regeneration via their trophic function.79 The regenerative role of MSCs in various disease conditions such as myocardial infarction,80–86 ischemic cardiomyopathy,87–90 end-stage liver disease,91–94 peripheral vascular disease with ischemic ulcers,95–98 neurologic stroke,99–101 spinal cord injury,102 cartilage regeneration in degenerative arthritis,103–105 intraosseous bone defects,106 and rare genetic disorders107 are summarized in Table 3.
TABLE 3.
Summary of the regenerative role of MSCs in clinical trials
| Disease | Patient profile | MSC Source | Dose (×106) | Administration | Outcome | Ref [MFR ref] |
|---|---|---|---|---|---|---|
| Cardiovascular disorders | ||||||
| Ac myocardial infarction | n = 27 | Auto BM-MSC | Low = 50 (n = 6), high = 700 (n = 21) | Intracoronary | Dose did not affect outcome. Marked improvement in LVEF in patients with low pCO2 and HCO3. | 80 [80] |
| Ac myocardial infarction | n = 53 (MSC n = 39, Ctrl n = 21). | Allo BM-MSC | 0.5, 1.6, 5/kg | IV | GSS and EF significantly better (p = 0.027) in MSC versus Ctrl, with an average AE rate of 5.3 and 7, respectively. | 81 [prochymal] |
| Ac myocardial infarction | AMI with PCI (n = 16) | Auto BM-MSC | 12.2 ± 1.77 (grp-l), 13.2 ± 1.76 (grp-lI) | LAD Group I (n = 8), RCA Group II (n = 8) | Symptomatic improvement at t = 6 months, with MSC infusion. No patient died, was readmitted, or had another MI. No angiographic in-stent restenosis detected in either group. | 82 [82] |
| Ac myocardial infarction | AMI with PCI (MSC n = 35; Ctrl n = 35) | Auto BM-MSC | 8 × 103−1 × 104/mL | Intracoronary | Significant improvement (p < 0.05) in cardiac function in MSC versus Ctrl at t = 3 months and t = 6 months. | 83 [5] |
| Myocardial infarction (old) | MSC n = 8; Ctrl n = 8 | Auto BM-MSC | 5.55 (2.1–9.1) | Injected at CABG or PCI | Significant improvement in NYHA class (p < 0.000), SPECT scan (p < 0.002), and LVEF (<0.005) in MSC versus Ctrl. | 84 [84] |
| Myocardial infarction (old and recent) | MSC n = 11; Ctrl n = 11 | Auto BM-MSC and EPCs | 1–2 | Intracoronary | Intracoronary use of MSCs is feasible, safe, and helps in local regeneration of myocardial tissue early or late following MI. | 85 [85] |
| Refractory angina | n = 31 | Auto BM-MSC | NA | Intramyocardial | All showed significant improvement (p < 0.001) in LVEF and exercise tolerance from baseline level. | 86 [86] |
| Ischemic cardiomyopathy | LV dysfunction with remote Ml (n = 8) | Auto BM-MNC (n = 4), BM-MSC (n = 4) | 200 | TEC injection | MNCs and MSCs help to reverse remodeling of chr, myocardial scar. Significant declines in systolic and diastolic volumes, obscuring → EF Chamber size, MI size, or regional function more likely to improve with treatment. | 87 [87] |
| Dilated cardiomyopathy | n = 40 (PBEP n = 11, BM-MNCs n =29) | Auto-PBEP and BM-MNCs | 5 mL (100 to 1000 cells/mL) | EC n = 9, IC n = 25, IP n = 6 | Significant improvement in EF (25%) at t = 6 months. Repeated stem cell infusion required for sustained improvement. | 88 [88] |
| Ischemic cardiomyopathy | MSC n = 22; Ctrl n = 23 | Auto BM-MSCs | NA | Intracoronary | Significant improvement in exercise tolerance, ↓ reversible defects (p < 0.05) at t = 12 months in MSCs versus baseline and Ctrl. | 89 [89] |
| Dilated cardiomyopathy | MSC n = 12; Ctrl n = 12 | Auto BM-MSCs | NA | Intracoronary | Significant ↓ in plasma BNP levels (p < 0.05) and improvement in 6-minute walk test in MSC versus baseline and Ctrl. | 90 [90] |
| Limb or skin disorders | ||||||
| Type 2 diabetes with limb ischemia | n = 41 (82 limbs): MSC n = 20, MNC n = 21, Ctrl n = 41 | Auto BM-MSCs and MNCs | MSCs, 930 ± 110; MNCs, 960 ± 110 | Intramuscular injection | Marked improvement in rest pain (p< 0.05) and pain free walking in both groups from baseline. MSC Group showed better (p < 0.02) ulcer healing and collateral formation than MNC Group. | 95 [95] |
| Type 2 diabetes with limb ischemia | Limb ischemia (n = 10) | Auto BM-MSCs and MNCs | MSCs and MNCs, 30 | Intramuscular injection | Marked improvement in rest pain (p< 0.05) and pain-free walking from baseline. Better (p < 0.02) ulcer healing and collateral formation. | 96 [96] |
| Cutaneous wound healing | Acute (n = 5); chronic (n = 8) | Auto BM-MSCs | 2/cm2 wound surface area | LA, 4× fibrin polymer spray | All acute (skin cancer surgery) wounds showed complete recovery at 6 weeks. Only three of six chronic wounds showed complete closure, | 98 [98] |
| Nonhealing ulcers (chronic) | BGD (MSC = 9, Ctrl = 9), DM (MSC = 3, Ctrl = 3) | Auto BM-MSCs | 40–50 (>106 cells/cm2 of ulcer) | Intramuscular injection | Marked decrease in ulcer size (p < 0.001) and improvement in pain free walking (p <0.001) in MSC versus Ctrl. | 97 [97] |
| Hepatic disorders | ||||||
| Decompensated liver cirrhosis | Chr-HBV (MSC n = 30, Ctrl n = 15) | UC-MSCs | 0.5 | IV | Significant Improvement liver function and ↓ in ascites vol, for MSC versus Ctrl (p < 0.05). | 91 [108] |
| End-stage liver cell failure | Chr-HCV (MSC n = 20, Ctrl n = 20) | Auto BM-MSCs | 20 hep-lineage (total 200 MNCs) | IS n = 10, IH n = 10 | Improved child score, MELD score, fatigue scale, and ↓ ascites In vol, in MSC versus Ctrl, Route of Infusion not related to outcome. | 92 [92] |
| 4 HBV, 1HCV, 1 alcoholic, 2 crypto LF | Auto BM-MSCs | 30–50 | Peripheral or portal vein | MELD score showed marked improvement (p <0.05) in liver function versus baseline. | 93 [93] | |
| Decompensated liver cirrhosis | n = 4 | Auto BM-MSCs | 31.73 | Peripheral vein | 2/4 patients showed improvement In MELD score >with an overall ↑ QOL in all. | 94 [109] |
| Neurologic disorders | ||||||
| Ischemic stroke | n = 12 | Auto BM-MSCs | 0.6–1.6 | IV, 36–133 days after stroke | Marked Improvement (p< 0.001) In NIHSS change. Mean lesion volume ↓ >20% at 1 week after Infusion. | 99 [110] |
| Ischemic stroke | MSC n = 16, Ctrl n = 36 | Auto BM-MSCs | 50 (2 doses) | IV, 2 weeks apart | MSC group showed clinical Improvement (MRS score p < 0.05) versus Ctrl. Death in 25% of patients In MSC grp and 58.3% In Ctrl at follow-up. | 100 [100] |
| Parkinson’s disease | n =7 (MDD 14.7 ±7.56 years) | Auto BM-MSCs | 1 /kg | Stereotaxic lat, ventrlc, zone | 37 patients with Improved UPDRS. 22.9 and 38% Increase in mean “off” and “on” score versus baseline. Marked ↓ in dose noted in 2. | 111 [111] |
| Spinal cord injury | n = 30 (cervical or thoracic levels) | Auto BM-MSCs | 1 /kg | Lumbar puncture | Auto BM-MSCs are safe and feasible in spinal cord Injury patients. | 102 [102] |
| Ischemic stroke | n = 30 (MSC n = 5, Ctrl | Auto BM-MSCs | 50 (2 doses) | IV, 2 weeks apart | Barthel index in MSC grp better than Ctrl at t = 3, 6, and 12 months In (p = 0.011,0.017, 0.115) and MRS score (p = 0.076, 0.171, 0.286) | 101 [101] |
| Bone and cartilage disorders | ||||||
| Osteoarthritis (knee) | n = 25) n = 4 | Auto BM-MSCs | 8–9 | Intraarticular | Subjective clinical Improvement. No objective evidence of cartilage repair on X-ray. | 103 [103] |
| Osteoarthritis (AVN-F head) | 16 hips; A-Core, n = 8, B-MSC+ Core, n = 8 | Auto BM-MSCs | Not specified | Injected Into femur head | Marked difference in necrosis area of femoral head between group A and B at 12 months (p < 0.05). | 104 [104] |
| Osteoarthritis (knee) | n = 41 | Auto BM-MSCs | 5/mL | Artic, cartilage collagen sheet. | Primarily safety and feasibility study. | 105 [105] |
| Osteoarthritis (knee) | n = 1 | Auto BM-MSCs | 22.4 | Intraarticular | Significant cartilage and menlscal growth on MRI, ↑ In range of motion, ↓ in VAS pain scores. | 112 [112] |
| IOOD | n = 6 (dental implant placement) | Auto BM-MSCs | 400/cc scaffold | Implantation through scaffold | Viable bone substitute leading to bone formation In mice after SC implant. Same construct failed to form bone In IOOD patients. | 106 [106] |
| Miscellaneous | ||||||
| MLD and MPS-IH | MPS-IH (n = 5) MLD (n = 6; post-BMT) | Allo BM-MSCs (ID sib) | 2–10/kg | IV | No clinical Improvement in mental or physical development after MSC infusion, except ↑ in conduction velocity In MLD patients. | 107 [107] |
Ac. = acute; AE = adverse event; Allo = allogeneic; AMI = acute myocardial infarction; Auto = autologous; AWMI = anterior wall myocardial Infarction; BGD = Berger’s disease; CABG = coronary artery bypass graft; CAD = coronary artery disease; Chr, = chronic; Ctrl = control group; DM = diabetes mellitus; EC = eplcardial; grp = group; GSS = global symptom score; 1C = intracoronary; IH = Intrahepatlc; IOOD = intraoral osseous defect; IP = Intrapulmonary; IS = Intrasplenlc; LVEF = left ventricular ejection fraction; MELD = model for end-stage liver disease; MFR ref = MSC manufacturing protocol designed previously and followed in this study; MRS = modified Rankin score; NIHSS = National Institute of Health Stroke Scale; PBEP = peripheral blood endothelial progenitors; PCI = percutaneous coronary implant; QOL = quality of life; TEC = transendocardial; UC-MSC = umbilical cord-derived mesenchymal stem cells, Adverse events—Nil.
Cell dose and frequency
An effective dose without adverse side effects has not yet been optimized and likely differs between diseases, route of administration, frequency of dosing, and other variables. Based on the review of currently applied doses in various clinical trials,29–61,70,80–107,111–113 the clinical dose typically ranges from 0.5 × 106 to 5 × 106 MSCs/kg body weight of the recipient. Testing of high (8 × 106 MSCs/kg) as well as low doses (2 × 106 MSCs/kg) in patients with steroid-refractory acute GVHD37 did not reveal significant differences in response rate or relapse of the primary disease. Similarly, the MSC dose did not affect platelet (PLT) and neutrophil engraftment in post-BMT hematooncology patients.47 However, repeated infusion of MSCs at certain intervals seems to influence the outcome in some studies.30,32,33,36,38,53 Larger randomized trials are needed to determine therapeutic doses and dosing regimens for MSCs in various clinical settings.
MSC manufacturing
With MSCs entering into the clinical arena, the development of production methods in accordance with current Good Manufacturing Practices (GMP) and current Good Tissue Practices is required in the United States. Similar regulations are in place in other countries around the world. Pamphilon and Szczepiorkowski114 and others115 have provided a thorough summary of these regulatory requirements.
Donor, cell sources, and culture processes
MSCs have been derived from several tissue sources (BM, AT, and UCB) listed in Fig. 1 and applied in both autologous and allogeneic settings. With evidence suggesting immune-privileged status, a single allogeneic MSC donor may serve for multiple recipients, raising the demand for well-characterized and even “qualified” donors.116 The screening and testing of donors for MSCs (e.g., health questionnaire, viral testing) is similar to that for other cell- or tissue-based products. The age of the donor seems to be important, with BM from children containing a higher concentration of colony-forming unit fibroblast precursors (CFU-Fs) than that from adults.117 Moreover, increased donor age seems to be directly correlated to detrimental effects in terms of proliferation and multipotency of MSCs.118 The donor should have no abnormalities or risk of abnormalities possibly involving MSCs, which may currently be difficult to assess. No specific regulatory requirement exists for this matter, but the issue should be considered carefully particularly when a single or few universal donors are used for many patients.
Isolation of BM-MSCs
The majority of MSC clinical trials published to date (n = 121) have used BM as the source for the MSCs. BM is removed from the donor’s posterior superior iliac spine or crest using an Illinois needle, or equivalent aspiration needle, in a heparin-containing syringe.119 The sample is subsequently processed by density gradient centrifugation, direct plating, or different enrichment strategies.118 Numerous attempts to enrich MSCs from BM by other methods such as immunomagnetic-based depletion or enrichment strategies have been performed. Selection markers include STRO-1, CD49a, CD105, CD133, CD146, CD271, SSEA-4, antifebrin microbeads, aptamers, and aldehyde dehydrogenase activity.120–123 However, no marker has proven capable of discriminating multipotent, highly proliferating MSCs from other less potent lineage-committed cells. Thus, the most common procedures for obtaining MSCs in clinical-scale numbers utilize density gradient centrifugation for isolation or direct plating to separate mesenchymal and hematopoietic cells by their adhesion to plastic cell culture surfaces.
Donor age, as mentioned earlier, and aspirate quality have been shown to influence MSC numbers.117,118 The frequency of MSCs is approximately 1 per 1 × 106 nucleated cells in adult BM and 1 per 1 × 104 nucleated cells in UCB.120–124 The number of MSCs has been noted to decrease with age, with a 10-fold decrease from birth to teenage and another 10-fold decrease from teenage to elderly.121–125
Isolation of AT-derived MSCs
The discovery of multipotent MSCs within AT has established a second major source of MSCs (n = 26).126 Besides a comparable degree of mesodermal differentiation potential, AT-derived MSCs also appear to have higher frequencies (100–1000× BM) and a high potential for angiogenesis or vasculogenesis compared to that of BM.127 In most cases, lipoaspirates have been used as starting material. Liposuction procedures may yield volumes ranging from milliliters to liters of tissue.128 The most commonly employed procedure, tumescent liposuction, involves the preprocedure infusion of saline solutions containing anesthetics and adrenaline as vasoconstrictors. This approach gives better cell yields than ultrasound-assisted liposuction, which has been shown to compromise recovery as well as expansion capacity of MSCs.129 For obtaining smaller volumes of tissue, machine and syringe aspiration as well as excision can be used instead.130 Further processing steps include removal of cellular debris, oil, excessive blood cells, proteins, and components of the extracellular matrix followed by extensive washing to obtain higher purity of the desired fraction.131 To isolate MSCs from the other tissues, enzymatic treatment is used. Subsequently, centrifugation is performed to remove the adipocyte fraction and pellet the preadipocyte stromal vascular fraction. This fraction is a heterogeneous mixture of cells, including MSCs as well as endothelial, muscle, fibroblastic and mast cells, pericytes, and preadipocytes. After the initial adherence step, all nonadherent cells are discarded by extensive washing, and the remaining adherent cells appear as fibroblastoid cells. These are cultured for approximately 10 days until a 60% to 70% confluent monolayer has developed. Cells can then be split to initiate subsequent culture passage.
To standardize the process, automated devices have been developed to assist in separation and culture. A “bag within a bag” device, composed of an inner mesh and an outer sealed bag, assists to separate the tissue fraction from the contaminating fluid fraction.132 A completely closed system (Celuton system, Cytori Therapeutics, San Diego, CA), which can be used at the patient’s bedside, performs the aspiration, washing, and concentration of the stromal vascular fraction.133 Cells resulting from this process, however, can only be regarded as enriched with MSCs. Only a proportion of approximately 1:1000 cells within the stromal vascular fraction will give rise to CFUs, equivalent to MSCs.5 Admittedly, most studies have used specimens obtained from young and healthy subjects undergoing aesthetic liposuction.To address the effects of age and comorbidity on stem cell frequencies, DiMuzio and Tulenko134 correlated factors such as advanced age (>70 years), obesity, renal failure, and vascular disease and found no significant differences.
Isolation of UCB-derived MSCs
Fresh (i.e., not frozen and thawed) UCB is the third common source for isolating MSCs for clinical use (n = 37). The standard process employed for obtaining UCB is gravity-assisted collection after cannulation of one of the umbilical veins (after delivery of the placenta) under aseptic conditions. This product is then typically processed within 24 hours of collection in a similar manner to BM. Various collection methods result in variable cell yield and viability of MSCs obtained; the success rate in isolating and further expanding MSCs depends on the volume of blood collected, the cell content, and the time between collection and processing,135 which highlights the need for minimal delay between delivery and harvesting. Related cell sources envisioned for clinical applications include neonatal tissues such as the amniotic membrane, the placenta, and Wharton’s jelly of the umbilical cord.136 These sources, like UCB, are of interest due to their relatively unlimited supply of more primitive MSCs with minimal ethical or legal concerns related to tissue sourcing.
MSC expansion
Culture medium
The optimal basal medium for culturing MSCs has not yet been determined. Whereas some investigators favor using α-minimum essential medium,136–139 others favor Dulbecco’s modified Eagle’s medium.61,135,140 The critical ingredient in MSC expansion medium seems to be serum as a source of nutrients, hormones, and growth factors.
Fetal bovine serum
Fetal bovine serum (FBS) has historically been considered essential for obtaining high-quantity and quality MSCs withexvivoexpansion.141 However, concerns for the use of FBS do exist and include risk of transfer of immunogenic xenoproteins as well as transmission of infectious agents, especially transmissible spongiform encephalopathy.142 Accordingly, the European Medicines Agency (EMEA) recommends that “when manufacturers have a choice the use of materials from ‘non transmissible spongiform encephalopathy relevant animal species’ or non-animal origin is preferred.”143,144 If FBS is deemed necessary for culture, extensively tested FBS can be sourced from qualified herds (i.e., animals from countries considered free of risk of variant Creutzfeldt-Jakob disease). Interestingly, FBS-derived proteins have been shown to be internalized by MSCs145 and to be immunogenic, possibly compromising the clinical effectiveness of MSCs.17,146 Accordingly, many in the cell therapy community have already begun implementing non-FBS supplements for large-scale production of MSCs. However, the role of culture ingredients (including FBS) in maintaining MSC immunomodulatory and regenerative properties is still poorly understood, and thus it may be too premature to exclude FBS from MSC culture.
Human supplements
Although acceptable FBS batches are available and are being used for clinical-grade manufacturing of MSCs, the concern outlined above has paved the way for alternative supplements, including human-derived supplements. Of course, a completely chemically defined medium would be optimal for clinical-scale expansion,147 but this has yet to be achieved or implemented. Several working groups have tried to optimize culture media by adding human serum, plasma, or PLT-derived factors. Pooled human PLT lysate (obtained from buffy coat–derived PLT-rich plasma) has growth factors and mitogens released from alpha granules of PLTs during PLT activation either by thrombin or by cell fragmentation during repeated freeze-thaw cycles. Among these potent mediators released from PLTs are epidermal growth factors, basic fibroblast growth factor, PDGFs, TGF-β1, and IGF. These factors enhance proliferation of bone cells and chondrocytes, as well as MSCs, highlighting the role of PLTs in processes such as wound healing and tissue repair.148–151 However, Marx and colleagues152 have observed that regenerative effects of PLT derivatives show extensive variation due to the dependence of growth factor concentration on PLT content, preparation method, white blood cell (WBC) contamination, and mechanisms of PLT growth factor release.153 Recent literature shows a definite advantage of PLT lysate over FBS with regard to MSC proliferation and cloning efficiency and a similar MSC immunophenotype.154 Thus, human PLT lysate may replace FBS in many cell culture systems previously thought to strictly depend on the presence of FBS due to better reproducibility of the lysate preparation protocol without considerable lot-to-lot variation.
Other alternatives to FBS include pooled human serum, blood group AB human serum, and PLT-derived factors, which have been developed by a variety of protocols.155 If an allogeneic source were to be used, large-scale clinical production involving pooled human blood derivatives may require several donors (i.e., to neutralize donor-specific variations and to mimic an off-the-shelf batch). Both blood group AB human serum and thrombin-activated PLT releasate in plasma compared to FBS have been found to be superior in expanding AT-MSCs. Some studies using allogeneic human serum have reported success in isolating and expanding MSCs from BM with preserved differentiation and immune-suppressive properties;156–158 others have observed reduced growth associated with advanced senescence, concluding that autologous serum would be favorable.159,160
Other additives
The growth factor requirements of MSCs have not been defined. However, some growth factors, such as PDGF, epidermal growth factor, TGF-β, and IGF have been tested in culture.161,162 A variety of protocols describe adding fibroblast growth factors to FBS-supplemented medium for expanding MSCs to increase their proliferation rate and maintain multilineage differentiation potential.163 Others indicate that factors like dexamethasone164 or lithium, which both stimulate Wnt signaling, can enhance proliferation of MSCs.165
Oxidative stress can impair MSC qualities. Enhancing the concentration of selenium or selenite has been shown to reduce cell damage induced by reactive oxygen species.166 Likewise, caloric restriction mimicked in vitro by lowering the glucose content has been shown to accelerate MSC proliferation while preventing senescence.167 Contradicting these results, telomerase-immortalized MSCs respond to higher glucose concentrations with enhanced proliferation and osteogenic differentiation.168 Finally, Sotiropoulou and colleagues139 indicate that using astabilized dipeptide form of L-glutamine (GlutaMAX, Life Technologies, Carlsbad, CA) supports better cell growth compared to using L-glutamine.
Cell seeding density
Plating density has emerged as a critical issue for MSC expansion. Due to the adherent nature of MSCs, plating density is an important variable to ensure a good expansion rate and to maintain necessary cellular functions. The initial mononuclear cell (MNC) plating density is extremely variable; published clinical trials30–61,70,80–107,111–113 have involved both high densities (e.g., 1.70 × 105 MNCs/cm2) and lower densities (e.g., 5.0 × 104 MNCs/cm2). On subsequent passages, the plating density should be decreased.48 The choice of cell density remains critical at this stage, and use of a low (e.g., 1.0 × 103-5.0 × 103 MSCs/cm2) or very low (e.g., 1.0 × 101-5.0 × 101 MSCs/cm2) plating density may better maintain a high proliferation rate and multipotentiality of MSCs.169
A consequence of seeding density and length of culture is the proliferative age of MSCs. MSCs have a restricted lifespan and reach a senescent state in which cellular functions become diminished and the risk for acquiring mutations116,170,171 and inflammatory phenotype increases, making them unfit for therapeutic use. Passage numbers are most commonly used to represent proliferative age; however, passage numbers (in contrast to population doublings) do not describe the critical de facto proliferation history when optimal or maximum length of culture is not yet well defined.171 Closed-system bioreactors may have limitations to scale-up due to size or culture capacity.
Devices for expansion
MSCs grow as adherent cells until reaching confluency and are then further expanded by serial passaging. Therefore, the number of cells that can be harvested in an ex vivo expansion culture is determined by the surface area of the culture platform. Typically MSCs are cultivated in conventional monolayer cultures. To achieve a large surface area, multilayered cell factories are used.172,173 This approach is labor-intensive and cost-consuming. Alternatively, it is possible to expand MSCs by using bioreactors.174,175 As closed systems should be preferred in a GMP setting, Rojewski and colleagues176 report a fully automated bioreactor allowing GMP-compliant manufacturing.
Oxygen tension
It has been shown that the most primitive stem cells proliferate and maintain “stemness” under low O2 concentration (e.g., 5%), which is closer to physiologic values.177 Low O2 conditions limit oxidative damage and, thus, may reduce cytogenetic abnormalities.178 Most MSC trials have not involved cells expanded under low O2 culture conditions. However, a trial involving ischemia-tolerant MSCs for treatment of lung injury is in the planning stage.179
Storage and cryopreservation
MSCs for clinical use are most commonly frozen in 10% dimethyl sulfoxide within an electrolyte solution (e.g., PlasmaLyteA) and a protein source (e.g., human serum albumin). The freezing rate is typically 1°C/min through phase change, followed by 2 to 3°C/min until roughly −100°C, at which point the cells are placed in liquid nitrogen or vapor-phase liquid nitrogen. This procedure is based on cryopreservation of HSCs and lymphoid cells and is not optimized for MSCs. MSCs in the frozen state can be transported in liquid nitrogen dry shippers (or equivalent).180
Quality control testing
Quality control testing usually includes viability, immunophenotyping, sterility and mycoplasma testing, and endotoxin level. Viability can be assessed by a variety of assays, including trypan blue, acridine orange-propidium iodide, and 7-aminoactinomycin-D with an accepted specification of at least 70%. Immunophenotyping typically follows the International Society for Cellular Therapy criteria,7 which includes CD73, CD90, and CD105 as positive markers. Samples for sterility and mycoplasma testing are drawn at various time points in manufacturing, such as before culture (i.e., from starting material), during culture, and after culture and before freeze. Automated methods are often used for sterility (bacterial and fungal culture), and several approaches to mycoplasma testing exist (e.g., culture, polymerase chain reaction). Endotoxin content can be evaluated several ways but most often involves limulus amebocyte lysate–based method (chromogenic, turbidometric, etc.). The upper limit for endotoxin is 5 EU/kg/dose for most modes of administration.181
Assays of function and potency
The determination of assays of function or potency may be guided by the presumed mechanism of action. Several general and mechanism-specific examples are highlighted below.
Trilineage differentiation
The standard pathways of MSC differentiation follow osteogenic, chondrogenic, and adipogenic lineages and have been elaborately reported in a large number of publications.61,131,163 This potency assay should be performed if mesenchymal (connective) tissue repair is intended. The lack of evidence of their true biologic role in vivo becomes the limitation of this assay as the hallmark for stem cell characteristics of self-renewal and differentiation has been not accomplished so far.
Immunomodulation
Human MSC surface molecules such as HLA Class I,Thy-1 (CD90), vascular cell adhesion molecule (CD106), intercellular adhesion molecule-1 and −2, activatedWBC adhesion molecule (ALCAM, CD166), lymphocyte functional antigen-3, and various integrins indicate interaction with cognate ligands on T cells.182 In contrast to an expected induction of T-cell response against allogeneic MSCs, T-cell alloreactivity is inhibited by MSCs in mixed lymphocyte cultures or lymphocyte proliferation induced by mitogens, such as phytohemagglutinin or concanavalin A, and are currently accepted in vitro assays to assess inhibitory effect of MSCs on T-cell proliferation.108,182 Some studies have shown that although MSCs in high concentrations (10–40 MSCs per 100 responder lymphocytes) inhibit, low MSC concentrations (≤1 MSC per responder lymphocyte) may stimulate lymphocyte proliferation in mixed lymphocyte cultures.113 These findings stress the importance of determining an optimal MSC dose to achieve intended outcomes.
Regulation of hematopoiesis
The potential benefit of cotransplantation of MSCs with HSCs has been demonstrated.39 This effect can be assayed in vitro in coculture experiments using HSCs and MSCs and thus may be a relevant functional or potency assay for this therapeutic indication.124
Senescence and genomic stability
MSCs have limited lifespan in vitro and enter senescence after multiple passages (25–30 population doublings) in culture. Therefore, for clinical use of MSCs, genomic stability is a major concern during long-term cultures as there is always a risk of cell transformation due to replicative senescence. Cells begin to show telomere shortening, lose a part of their differentiation potential, and exhibit an altered cytokine secretion profile.183,184
All these changes appear to be a continuous process and are hypothesized to start as early as the first passage. However, so far the evidence favoring transformation is low.171 Karyotyping and comparative genomic hybridization have low sensitivity to detect these abnormalities and do not appear to be relevant controls, neither indicating the real risk of MSC transformation or their senescent status. Thus, real relevant controls for transformation and senescence could refer to genes or molecules involved in the senescence and transformation pathways, such as p53, p21, and p16 and may ensure the safety of the product.185
Clonogenicity
The CFU assay is a suitable tool for evaluating the self-renewal capacity of cells. Friedenstein and colleagues186 were the first to describe an assay system to study CFU-F in various hematopoietic cell populations. Analysis of CFU-Fs frequency in BM aspirates requires adequate dilution, minimal manipulation, and low seeding density to get true colony counts in the sample as has been observed previously.123,187
SUMMARY
MSC-based therapies are quickly evolving with more than 200 clinical trials for a variety of therapeutic indications ranging from immunomodulation to tissue repair and regeneration. The field is still in its infancy, however, with lack of consensus on several fronts including: proposed mechanisms of action, optimal dosing strategy, and route of administration. Perhaps most concerning is the broad range of approaches to culture and the poorly understood impact of variables such as source material, medium, supplements, culture technique, and so forth. It may be too premature to suggest standardization of manufacturing, as this may limit the field and overall optimization of manufacturing. However, to ensure the greatest likelihood of success of MSCs in the clinical arena, attention (including from that of granting agencies) should be placed on optimization of culture.
ACKNOWLEDGMENTS
The authors acknowledge the support of Department of Health Research, Indian Council of Medical Research, Ansari Nagar New Delhi, India, for awarding the fellowship to RRS under their fellowship program in the Department of Laboratory Medicine and Pathology, Division of Transfusion Medicine, and Clinical Cell Therapy, Laboratory of Molecular and Cellular Therapeutics, University of Minnesota, Saint Paul, MN.
ABBREVIATIONS:
- AT
adipose tissue
- BM
bone marrow
- CFU-F(s)
colony-forming unit fibroblast precursor(s)
- GMP
Good Manufacturing Practices
- HSC(s)
hematopoietic stem cell(s)
- IGF
insulin-like growth factor
- MSC(s)
mesenchymal stem cell(s)
- PDGF(s)
platelet-derived growth factor(s)
- UCB
umbilical cord blood
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest or funding sources.
REFERENCES
- 1.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968;6:230–47. [PubMed] [Google Scholar]
- 2.Zuk PA, Zuh M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13: 4279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.In’t Anker PS, Scherjon SA, Kleijburg-van Dder Keur C, et al. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004; 22:1338–45. [DOI] [PubMed] [Google Scholar]
- 4.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol 2005;33:1402–16. [DOI] [PubMed] [Google Scholar]
- 5.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006;24: 1294–301. [DOI] [PubMed] [Google Scholar]
- 6.Bochev I, Elmadjian G, Kyurkchiev D, et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immune-globulin production in vitro. Cell Biol Int 2008;32: 384–93. [DOI] [PubMed] [Google Scholar]
- 7.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–17. [DOI] [PubMed] [Google Scholar]
- 8.Haniffa MA, Collin MP, Buckley CD, et al. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica 2009;94:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Qu X, Zhao RC. Clinical applications of mesenchymal stem cells. J Hematol Oncol 2012;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto WR, Wright NA. Mesenchymal stem cells: from experiment to clinic. Fibrogenesis Tissue Repair 2011; 4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells: implications for therapeutic use. Bone Marrow Transplant 1995;16: 557–64. [PubMed] [Google Scholar]
- 12.Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 2000;6:1282–6. [DOI] [PubMed] [Google Scholar]
- 13.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can induce long-term acceptance of solid organ allografts in synergy with low-dose mycophenolate. Transpl Immunol 2008;20:55–60. [DOI] [PubMed] [Google Scholar]
- 14.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003;75:389–97. [DOI] [PubMed] [Google Scholar]
- 15.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells 2006;24:74–85. [DOI] [PubMed] [Google Scholar]
- 16.Nasef A, Mathieu N, Chapel A, et al. Immunosuppressive effects of mesenchymal stem cells: involvement of HLA-G. Transplantation 2007;84:231–7. [DOI] [PubMed] [Google Scholar]
- 17.Sundin M, Ringden O, Sundberg B, et al. No alloantibodies against mesenchymal stromal cells, but presence of anti-fetal calf serum antibodies, after transplantation in allogeneic hematopoietic stem cell recipients. Haematologica 2007;92:1208–15. [DOI] [PubMed] [Google Scholar]
- 18.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838–43. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev 2004;13:263–71. [DOI] [PubMed] [Google Scholar]
- 20.Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 2009;113:46–57. [DOI] [PubMed] [Google Scholar]
- 21.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood 2006; 107:367–72. [DOI] [PubMed] [Google Scholar]
- 22.Asari S, Itakura S, Ferreri K, et al. Mesenchymal stem cells suppress B-cell terminal differentiation. Exp Hematol 2009;37:604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484–90. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–22. [DOI] [PubMed] [Google Scholar]
- 25.English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non redundant roles in human mesenchymal stem cell induction of CD4 + CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol 2009;156:149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthay MA, Thompson BT, Read EJ, et al. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 2010;138:965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devine SM, Cobbs C, Jennings M, et al. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 2003; 101:2999–3001. [DOI] [PubMed] [Google Scholar]
- 28.Phinney DG, Prockop DJ. Concise review: mesenchymal stem / multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells 2007;25:2896–902. [DOI] [PubMed] [Google Scholar]
- 29.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004;363: 1439–41. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371: 1579–86. [DOI] [PubMed] [Google Scholar]
- 31.Pérez-Simon JA, López-Villar O, Andreu EJ, et al. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial. Haematologica 2011;96:1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrmann R, Sturm M, Shaw K, et al. Mesenchymal stromal cell therapy for steroid-refractory acute and chronic graft versus host disease: a phase 1 study. Int J Hematol 2012;95:182–8. [DOI] [PubMed] [Google Scholar]
- 33.Prasad VK, Lucas KG, Kleiner GI, et al. Efficacy and safety of ex vivo cultured adult human mesenchymal stem cells (Prochymal™) in pediatric patients with severe refractory acute graft-versus-host disease in a compassionate use study. Biol Blood Marrow Transplant 2011;17:534–41. [DOI] [PubMed] [Google Scholar]
- 34.Arima N, Nakamura F, Fukunaga A, et al. Single intraarterial injection of mesenchymal stromal cells for treatment of steroid-refractory acute graft-versus-host disease: a pilot study. Cytotherapy 2010;12:265–8. [DOI] [PubMed] [Google Scholar]
- 35.Zhang LS, Liu QF, Huang K, et al. [Mesenchymal stem cells for treatment of steroid-resistant chronic graft-versus-host disease]. Zonghua Nei Ke Za Zhi 2009; 48:542–6. [PubMed] [Google Scholar]
- 36.Zhou H, Guo M, Bian C, et al. Efficacy of bone marrow-derived mesenchymal stem cells in the treatment of sclerodermatous chronic graft-versus-host disease: clinical report. Biol Blood Marrow Transplant 2010;16: 403–12. [DOI] [PubMed] [Google Scholar]
- 37.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 2009;15:804–11. [DOI] [PubMed] [Google Scholar]
- 38.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006;81:1390–7. [DOI] [PubMed] [Google Scholar]
- 39.Hou RQ, Wang J, Kong Y, et al. Transfusion of mesenchymal stem cells combined with haploidentical HSCT improves hematopoietic microenvironment. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2010;18:155–60. [PubMed] [Google Scholar]
- 40.Baron F, Lechanteur C, Willems E, et al. Cotransplantation of mesenchymal stem cells might prevent death from graft-versus-host disease (GVHD) without abrogating graft-versus-tumor effects after HLA-mismatched allogeneic transplantation following nonmyeloablative conditioning. Biol Blood Marrow Transplant 2010;16:838–47. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Li JY, Cao K, et al. Cotransplantation of HLA-identical mesenchymal stem cells and hematopoietic stem cells in Chinese patients with hematologic diseases. Int J Lab Hematol 2010;32:256–64. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Bai H, Wang CB, et al. Autologous bone marrow-derived mesenchymal stem cells and peripheral blood stem cells cotransplantation in treatment of hematological malignant diseases. Zhonghua Nei Ke Za Zhi 2009;48: 392–5. [PubMed] [Google Scholar]
- 43.Ning H, Yang F, Jiang M, et al. The correlation between cotransplantation of mesenchymal stem cells and higher recurrence rate in hematologic malignancy patients: outcome of a pilot clinical study. Leukemia 2008;22:593–9. [DOI] [PubMed] [Google Scholar]
- 44.MacMillan ML, Blazar BR, DeFor TE, et al. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I-II clinical trial. Bone Marrow Transplant 2009;43:447–54. [DOI] [PubMed] [Google Scholar]
- 45.Müller I, Kordowich S, Holzwarth C, et al. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol Dis 2008;40:25–32. [DOI] [PubMed] [Google Scholar]
- 46.Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007;110:2764–7. [DOI] [PubMed] [Google Scholar]
- 47.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11:389–98. [DOI] [PubMed] [Google Scholar]
- 48.Koç ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000;18:307–16. [DOI] [PubMed] [Google Scholar]
- 49.Meuleman N, Tondreau T, Ahmad I, et al. Infusion of mesenchymal stromal cells can aid hematopoietic recovery following allogeneic hematopoietic stem cell myeloablative transplant: a pilot study. Stem Cells Dev 2009;18:1247–52. [DOI] [PubMed] [Google Scholar]
- 50.Liu K, Chen Y, Zeng Y, et al. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev 2011;20:1679–85. [DOI] [PubMed] [Google Scholar]
- 51.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012;367:2305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang J, Zhang H, Hua B, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: a pilot clinical study. Ann Rheum Dis 2010;69:1423–9. [DOI] [PubMed] [Google Scholar]
- 53.Sun L, Akiyama K, Zhang H, et al. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells 2009;27:1421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 2012; 307:1169–77. [DOI] [PubMed] [Google Scholar]
- 55.Perico N, Casiraghi F, Introna M, et al. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol 2011;6:412–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamout B, Hourani R, Salti H, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol 2010;227:185–9. [DOI] [PubMed] [Google Scholar]
- 57.Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol 2007;4: 50–7. [DOI] [PubMed] [Google Scholar]
- 58.Lee PH, Lee JE, Kim HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol 2012;72:32–40. [DOI] [PubMed] [Google Scholar]
- 59.Lee PH, Kim JW, Bang OY, et al. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther 2008;83:723–30. [DOI] [PubMed] [Google Scholar]
- 60.Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 2012;11:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 62.Meuleman N, Tondreau T, Delforge A, et al. Human marrow mesenchymal stem cell culture: serum-free medium allows better expansion than classical alpha-MEM medium. Eur J Haematol 2006;76:309–16. [DOI] [PubMed] [Google Scholar]
- 63.Capelli C, Domenghini M, Borleri G, et al. Human platelet lysate allows expansion and clinical grade production of mesenchymal stromal cells from small samples of bone marrow aspirates or marrow filter washouts. Bone Marrow Transplant 2007;40:785–91. [DOI] [PubMed] [Google Scholar]
- 64.Ortiz LA, Gambelli F, McBride C, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 2003;100:8407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spaeth E, Klopp A, Dembinski J, et al. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther 2008;15:730–8. [DOI] [PubMed] [Google Scholar]
- 66.Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant 2010;19:667–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–9. [DOI] [PubMed] [Google Scholar]
- 68.Lee OK, Kuo TK, Chen WM, et al. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004;103:1669–75. [DOI] [PubMed] [Google Scholar]
- 69.Tomita Y, Makino S, Hakuno D, et al. Application of mesenchymal stem cell derived cardiomyocytes as bio-pacemakers: current status and problems to be solved. Med Biol Eng Comput 2007;45:209–20. [DOI] [PubMed] [Google Scholar]
- 70.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gojo S, Gojo N, Takeda Y, et al. In vivo cardiovasculogenesis by direct injection of isolated adult mesenchymal stem cells. Exp Cell Res 2003;288:51–9. [DOI] [PubMed] [Google Scholar]
- 72.Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation 2003;108:863–8. [DOI] [PubMed] [Google Scholar]
- 73.Psaltis PJ, Zannettino AC, Worthley SG, et al. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells 2008;26:2201–10. [DOI] [PubMed] [Google Scholar]
- 74.Petrie Aronin CE, Tuan RS. Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res C Embryo Today 2010;90: 67–74. [DOI] [PubMed] [Google Scholar]
- 75.Ankrum J, Karp JM. Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med 2010;16: 203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dennis JE, Merriam A, Awadallah A, et al. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res 1999;14: 700–9. [DOI] [PubMed] [Google Scholar]
- 77.Plotnikov EY, Khryapenkova TG, Vasileva AK, et al. Cell-to-cell cross-talk between mesenchymal stem cells and cardiomyocytes in co-culture. J Cell Mol Med 2008;12: 1622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cselenyak A, Pankotai E, Horvath EM, et al. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol 2010;11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008;47:1634–43. [DOI] [PubMed] [Google Scholar]
- 80.Miettinen JA, Salonen RJ, Ylitalo K, et al. The effect of bone marrow microenvironment on the functional properties of the therapeutic bone marrow-derived cells in patients with acute myocardial infarction. J Transl Med 2012;10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Z, Zhang F, Ma W, et al. A novel approach to transplanting bone marrow stem cells to repair human myocardial infarction: delivery via a noninfarct-relative artery. Cardiovasc Ther 2010;28:380–5. [DOI] [PubMed] [Google Scholar]
- 83.Chen SL, Fang WW, Qian J, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–8. [PubMed] [Google Scholar]
- 84.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med 2007;10:467–73. [PubMed] [Google Scholar]
- 85.Katritsis DG, Sotiropoulou PA, Karvouni E, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv 2005;65: 321–9. [DOI] [PubMed] [Google Scholar]
- 86.Friis T, Haack-Sørensen M, Mathiasen AB, et al. Mesenchymal stromal cell derived endothelial progenitor treatment in patients with refractory angina. Scand Cardiovasc J 2011;45:161–8. [DOI] [PubMed] [Google Scholar]
- 87.Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res 2011;108:792–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guhathakurta S, Subramanyan UR, Balasundari R, et al. Stem cell experiments and initial clinical trial of cellular cardiomyoplasty. Asian Cardiovasc Thorac Ann 2009;17: 581–6. [DOI] [PubMed] [Google Scholar]
- 89.Chen S, Liu Z, Tian N, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol 2006;18:552–6. [PubMed] [Google Scholar]
- 90.Wang JA, Xie XJ, He H, et al. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:107–10. [PubMed] [Google Scholar]
- 91.Zhang Z, Lin H, Shi M, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol 2012;27(Suppl 2):112–20. [DOI] [PubMed] [Google Scholar]
- 92.Amer ME, El-Sayed SZ, El-Kheir WA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol 2011;23:936–41. [DOI] [PubMed] [Google Scholar]
- 93.Kharaziha P, Hellström PM, Noorinayer B, et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol 2009;21:1199205. [DOI] [PubMed] [Google Scholar]
- 94.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med 2007;10:459–66. [PubMed] [Google Scholar]
- 95.Lu D, Chen B, Liang Z, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011;92:26–36. [DOI] [PubMed] [Google Scholar]
- 96.Lasala GP, Silva JA, Gardner PA, et al. Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology 2010;61:551–6. [DOI] [PubMed] [Google Scholar]
- 97.Dash NR, Dash SN, Routray P, et al. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res 2009;12:359–66. [DOI] [PubMed] [Google Scholar]
- 98.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–312. [DOI] [PubMed] [Google Scholar]
- 99.Honmou O, Houkin K, Matsunaga T, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain 2011;134(Pt 6):1790–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee JS, Hong JM, Moon GJ, et al. ; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–106. [DOI] [PubMed] [Google Scholar]
- 101.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005;57:874–82. [DOI] [PubMed] [Google Scholar]
- 102.Pal R, Venkataramana NK, Bansal A, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy 2009;11:897–911. [DOI] [PubMed] [Google Scholar]
- 103.Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 2011;14:211–15. [DOI] [PubMed] [Google Scholar]
- 104.Chang T, Tang K, Tao X, et al. Treatment of early avascular necrosis of femoral head by core decompression combined with autologous bone marrow mesenchymal stem cells transplantation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2010;24:739–43. [PubMed] [Google Scholar]
- 105.Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med 2011;5:146–50. [DOI] [PubMed] [Google Scholar]
- 106.Meijer GJ, de Bruijn JD, Koole R, et al. Cell based bone tissue engineering in jaw defects. Biomaterials 2008;29: 3053–61. [DOI] [PubMed] [Google Scholar]
- 107.Koç ON, Day J, Nieder M, et al. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH). Bone Marrow Transplant 2002;30:215–22. [DOI] [PubMed] [Google Scholar]
- 108.Bartmann C, Rohde E, Schallmoser K, et al. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion 2007;47:1426–35. [DOI] [PubMed] [Google Scholar]
- 109.Wexler SA, Donaldson C, Denning-Kendall P, et al. Adult bone marrow is a rich source of human mesenchymal “stem” cells but umbilical cord and mobilized adult blood are not. Br J Haematol 2003;121:368–74. [DOI] [PubMed] [Google Scholar]
- 110.Kim S, Honmou O, Kato K, et al. Neural differentiation potential of peripheral blood- and bone-marrow-derived precursor cells. Brain Res 2006;1123:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Venkataramana NK, Kumar SK, Balaraju S, et al. Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Transl Res 2010;155:62–70. [DOI] [PubMed] [Google Scholar]
- 112.Centeno CJ, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 2008;11:343–53. [PubMed] [Google Scholar]
- 113.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol 2003;57:11–20. [DOI] [PubMed] [Google Scholar]
- 114.Pamphilon DH, Zbigniew M. Szczepiorkowski. Regulation and accreditation in cellular therapy In: Murphy MF, Pamphilon DH, editors. Practical transfusion medicine. 3rd ed. West Sussex, UK: Wiley-Blackwell; 2009. p. 409–20. [Google Scholar]
- 115.Rohde E, Schallmoser K, Bartmann C, et al. GMPcompliant propagation of human multipotent mesenchymal stromal cells In: Gad SC, editor. Pharmaceutical manufacturing handbook: regulations and quality. Hoboken (NJ): John Wiley and Sons; 2008. p. 97–115. [Google Scholar]
- 116.Sensebé L, Bourin P, Tarte K. Good manufacturing practices production of mesenchymal stem/stromal cells. Hum Gene Ther 2011;22:19–26. [DOI] [PubMed] [Google Scholar]
- 117.Baxter MA, Wynn RF, Jowitt SN, et al. Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 2004;22: 675–82. [DOI] [PubMed] [Google Scholar]
- 118.Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev 2008;129:163–73. [DOI] [PubMed] [Google Scholar]
- 119.Bain BJ. Bone marrow aspiration. J Clin Pathol 2001;54: 657–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guo KT, Schaefer R, Paul A, et al. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells 2006;24:2220–31. [DOI] [PubMed] [Google Scholar]
- 121.Gentry T, Foster S, Winstead L, et al. Simultaneous isolation of human BM hematopoietic, endothelial and mesenchymal progenitor cells by flow sorting based on aldehyde dehydrogenase activity: implications for cell therapy. Cytotherapy 2007;9:259–74. [DOI] [PubMed] [Google Scholar]
- 122.Sorrentino A, Ferracin M, Castelli G, et al. Isolation and characterization of CD146(+) multipotent mesenchymal stromal cells. Exp Hematol 2008;36:1035–46. [DOI] [PubMed] [Google Scholar]
- 123.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007; 213:341–7. [DOI] [PubMed] [Google Scholar]
- 124.Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006;91:1017–26. [PubMed] [Google Scholar]
- 125.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol 2009;217:318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells 2007;25: 818–27. [DOI] [PubMed] [Google Scholar]
- 127.Moseley TA, Zhu M, Hedrick MH. Adipose-derived stem and progenitor cells as fillers in plastic and reconstructive surgery. Plast Reconstr Surg 2006;118(suppl 3): 121S–8S. [DOI] [PubMed] [Google Scholar]
- 128.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007;100:1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 2006;8:166–77. [DOI] [PubMed] [Google Scholar]
- 130.Dubois SG, Floyd EZ, Zvonic S, et al. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol Biol 2008;449:69–79. [DOI] [PubMed] [Google Scholar]
- 131.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211–28. [DOI] [PubMed] [Google Scholar]
- 132.Katz AJ, Hedrick MH, Llull R, et al. A novel device for the simple and efficient refinement of liposuctioned tissue. Plast Reconstr Surg 2001;107:595–7. [DOI] [PubMed] [Google Scholar]
- 133.Duckers HJ, Pinkernell K, Milstein AM, et al. The Bedside Celution™ system for isolation of adipose derived regenerative cells. EuroIntervention 2006;2: 395–8. [PubMed] [Google Scholar]
- 134.DiMuzio P, Tulenko T. Tissue engineering applications to vascular bypass graft development: the use of adipose-derived stem cells. J Vasc Surg 2007;45(suppl A):A99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bieback K, Kern S, Klüter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004;22:625–34. [DOI] [PubMed] [Google Scholar]
- 136.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004;22:1330–7. [DOI] [PubMed] [Google Scholar]
- 137.Sekiya I, Larson BL, Smith JR, et al. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 2002;20:530–41. [DOI] [PubMed] [Google Scholar]
- 138.Both SK, van der Muijsenberg AJ, van Blitterswijk CA, et al. A rapid and efficient method for expansion of human mesenchymal stem cells. Tissue Eng 2007;13:3–9. [DOI] [PubMed] [Google Scholar]
- 139.Sotiropoulou PA, Perez SA, Salagianni M, et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006;24:462–71. [DOI] [PubMed] [Google Scholar]
- 140.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 1997;64: 295–312. [PubMed] [Google Scholar]
- 141.Caterson EJ, Nesti LJ, Danielson KG, et al. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol 2002;20:245–56. [DOI] [PubMed] [Google Scholar]
- 142.Asher DM. Bovine sera used in the manufacture of biologicals: current concerns and policies of the U.S. Food and Drug Administration regarding the transmissible spongiform encephalopathies. Dev Biol Stand 1999; 99:41–4. [PubMed] [Google Scholar]
- 143.European Agency for the Evaluation of Medicinal Products, Committee for Proprietary Medicinal Products. Note for guidance on the use of bovine serum in the manufacture of human biological medicinal products. EMEA CPMP/BWP/1793/02; 2003.
- 144.European Agency for the Evaluation of Medicinal Products. Note for guidance on minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products. EMEA/410/01 rev3; 2011.
- 145.Spees JL, Gregory CA, Singh H, et al. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther 2004;9:747–56. [DOI] [PubMed] [Google Scholar]
- 146.Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A 2002;99:8932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mannello F, Tonti GA. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or adhoc formula? All that glitters is not gold! Stem Cells 2007;25:1603–9. [DOI] [PubMed] [Google Scholar]
- 148.Gruber R, Varga F, Fischer MB, et al. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res 2002;13:529–35. [DOI] [PubMed] [Google Scholar]
- 149.Graziani F, Ivanovski S, Cei S, et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 2006;17:212–19. [DOI] [PubMed] [Google Scholar]
- 150.Lucarelli E, Beccheroni A, Donati D, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 2003;24:3095–100. [DOI] [PubMed] [Google Scholar]
- 151.Kilian O, Flesch I, Wenisch S, et al. Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro. Eur J Med Res 2004;9: 337–44. [PubMed] [Google Scholar]
- 152.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1998;85: 638–46. [DOI] [PubMed] [Google Scholar]
- 153.Lucarelli E, Fini M, Beccheroni A, et al. Stromal stem cells and platelet-rich plasma improve bone allograft integration. Clin Orthop Relat Res 2005;435:62–8. [DOI] [PubMed] [Google Scholar]
- 154.Bieback K, Hecker A, Kocaömer A, et al. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells 2009; 27:2331–41. [DOI] [PubMed] [Google Scholar]
- 155.Kocaoemer A, Kern S, Kluter H, et al. Human AB serum and thrombin-activated plateletrich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 2007; 25:1270–8. [DOI] [PubMed] [Google Scholar]
- 156.Yamaguchi M, Hirayama F, Wakamoto S, et al. Bone marrow stromal cells prepared using AB serum and bFGF for hematopoietic stem cells expansion. Transfusion 2002; 42:921–7. [DOI] [PubMed] [Google Scholar]
- 157.Anselme K, Broux O, Noel B, et al. In vitro control of human bone marrow stromal cells for bone tissue engineering. Tissue Eng 2002;8:941–53. [DOI] [PubMed] [Google Scholar]
- 158.Le Blanc K, Samuelsson H, Lonnies L, et al. Generation of immunosuppressive mesenchymal stem cells in allogeneic human serum. Transplantation 2007;84: 1055–9. [DOI] [PubMed] [Google Scholar]
- 159.Shahdadfar A, Fronsdal K, Haug T, et al. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression and transcriptome stability. Stem Cells 2005; 23:1357–66. [DOI] [PubMed] [Google Scholar]
- 160.Gregory CA, Reyes E, Whitney MJ, et al. Enhanced engraftment of mesenchymal stem cells in a cutaneous wound model by culture in allogenic species-specific serum and administration in fibrin constructs. Stem Cells 2006;24:2232–43. [DOI] [PubMed] [Google Scholar]
- 161.Kratchmarova I, Blagoev B, Haack-Sorensen M, et al. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science 2005;308:1472–7. [DOI] [PubMed] [Google Scholar]
- 162.Ng F, Boucher S, Koh S, et al. PDGF, TGF-b and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSC into adipogenic, chondrogenic and osteogenic lineages. Blood 2008;112:295–307. [DOI] [PubMed] [Google Scholar]
- 163.Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 2001;288:413–19. [DOI] [PubMed] [Google Scholar]
- 164.Kogler G, Sensken S, Wernet P. Comparative generation and characterization of pluripotent unrestricted somatic stem cells with mesenchymal stem cells from human cord blood. Exp Hematol 2006;34:1589–95. [DOI] [PubMed] [Google Scholar]
- 165.De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 2004;10:393–401. [DOI] [PubMed] [Google Scholar]
- 166.Ebert R, Ulmer M, Zeck S, et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 2006;24:1226–35. [DOI] [PubMed] [Google Scholar]
- 167.Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res 2006;9:31–5. [DOI] [PubMed] [Google Scholar]
- 168.Li YM, Schilling T, Benisch P, et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Commun 2007;363: 209–15. [DOI] [PubMed] [Google Scholar]
- 169.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A 2001;98:7841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol 2000;28:707–15. [DOI] [PubMed] [Google Scholar]
- 171.Prockop DJ, Brenner M, Fibbe WE, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 2010;12:576–8. [DOI] [PubMed] [Google Scholar]
- 172.Skmoser K, Rohde E, Reinisch A, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods 2008;14:185–96. [DOI] [PubMed] [Google Scholar]
- 173.Sensebé L Clinical grade production of mesenchymal stem cells. Biomed Mater Eng 2008;18:S3–S10. [PubMed] [Google Scholar]
- 174.Chen X, Xu H, Wan C, et al. Bioreactor expansion of human adult bone marrow-derived mesenchymal stemcells. Stem Cells 2006;24:2052–9. [DOI] [PubMed] [Google Scholar]
- 175.Gastens MH, Goltry K, Prohaska W, et al. Good manufacturing practice-compliant expansion of marrow-derived stem and progenitor cells for cell therapy. Cell Transplant 2007;16:685–96. [DOI] [PubMed] [Google Scholar]
- 176.Rojewski M, Dausend J, Schmidtke-Schrezenmeier G, et al. A standardized protocol for GMP compliant expansion of MSC from BM in an animal component free system. Vox Sang 2010;99:43. [Google Scholar]
- 177.Grayson WL, Zhao F, Izadpanah R, et al. Effects of hypoxia on human mesenchymal stem cell expansion and plasticity in 3D constructs. J Cell Physiol 2006;207: 331–9. [DOI] [PubMed] [Google Scholar]
- 178.Berniakovich I, Giorgio M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int J Mol Sci 2013; 14:2119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.McGuigan D Current news releases. Source: December 21; Stemedica Cell Technologies, Inc. Dec 12, 2012. [cited 2012 Dec 27]. Available from: http://www.stemedica.com. [Google Scholar]
- 180.Hanna J, Hubel A. Preservation of stem cells. Organogenesis 2009;5:134–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Munson TE. Guideline for validation of the LAL test as an end-product endotoxin test for human and biological products. Prog Clin Biol Res 1985;189:211–20. [PubMed] [Google Scholar]
- 182.Bifari F, Lisi V, Mimiola E, et al. Immune modulation by mesenchymal stem cells. Transfus Med Hemother 2008; 35:194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim J, Kang JW, Park JH, et al. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res 2009;32:117–26. [DOI] [PubMed] [Google Scholar]
- 184.Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PloS One 2008;3:e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tarte K, Gaillard J, Lataillade JJ, et al. ; Société Française de Greffe de Moelle et Thérapie Cellulaire. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010; 115:1549–53. [DOI] [PubMed] [Google Scholar]
- 186.Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol 1974;2:83–92. [PubMed] [Google Scholar]
- 187.Schallmoser K, Rohde E, Reinisch A, et al. Rapid large-scale expansion of functional mesenchymal stem cells from unmanipulated bone marrow without animal serum. Tissue Eng Part C Methods 2008;14:185–96. [DOI] [PubMed] [Google Scholar]