Abstract
Hyaluronan has a very simple structure. It is a linear glycosaminoglycan composed of disaccharide units of GlcNAc and d-glucuronic acid with alternating β-1,4 and β-1,3 glycosidic bonds that can be repeated 20,000 or more times, a molecular mass >8 million Da, and a length >20 μm. However, it has a very complex biology. It is a major, ubiquitous component of extracellular matrices involved in everything from fertilization, development, inflammations, to cancer. This JBC Review highlights some of these processes that were initiated through publications in the Journal of Biological Chemistry.
Keywords: hyaluronan, glycosaminoglycan, extracellular matrix, inflammation, fertilization
Discovery of hyaluronan (1934–1954)
Karl Meyer and John Palmer published a JBC classic in 1934 titled “The polysaccharide of the vitreous humor” that identified a large glycosaminoglycan that contained a hexuronic acid, an amino sugar, and no sulfoesters. They proposed the name hyaluronic acid from hyaloid (Greek for vitreous) and uronic acid, the macromolecule that is now known as hyaluronan (HA)2 (1). Karl Meyer's continued research with hyaluronan over the next 20 years defined its disaccharide structure ((GlcUAβ(1–3)-(GlcNAcβ(1–4)-) in an article titled “The structure of hyalobiuronic acid from umbilical cord” (2). His research journey is described in the Minireview Prologue (3) for the “Hyaluronan Minireview Series” published in JBC in 2002 (4–7). Based on his discovery and identification of its structure and on his research with other glycosaminoglycans, Karl Meyer is referred to as the father of glycosaminoglycan research.
Hyaluronan in cartilage (1969–1974)
My thesis work at the Rockefeller Institute (now Rockefeller University) with Stanley Sajdera, another graduate student, was published in the JBC in 1969 (8). As Herb Tabor was on the Editorial Board of the Journal at that time, he likely was a reviewer. This study showed that proteoglycans (now named aggrecans) isolated from cartilage in dissociative guanidine hydrochloride solutions reformed large aggregates when dialyzed into associative solvents and that a link protein with two separate binding sites was all that was required. Although I did not know it, this study initiated my extensive research career with hyaluronan (9), which was the missing thread required to form the proteoglycan aggregates and was not known to be present in cartilages at that time. Tim Hardingham and Helen Muir showed that cartilage proteoglycan monomers interacted with hyaluronan in 1972 (10) and that hyaluronan was present in proteoglycan aggregates in 1973 (11). Fortunately, during a sabbatical in Lund, Sweden, working with Dick Heinegard in 1973, we were able to define the correct aggregate structure and role for the link proteins (Fig. 1), published in JBC in 1974 (12).
Figure 1.
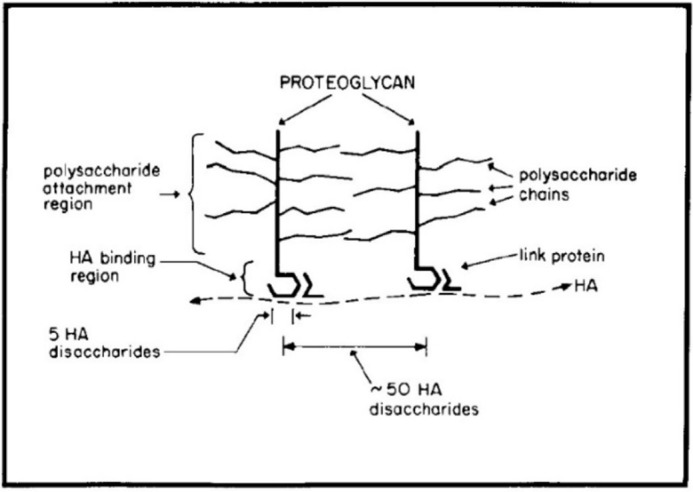
The original model for cartilage proteoglycan aggregates (12).
Hyaluronan matrices in cumulus cell-oophorus expansion (1988–1992)
Antonietta Salustri, a researcher in ovulation and fertilization, joined my lab at the National Institutes of Health for a sabbatical in 1988 to work with Masaki Yanagishita to determine the role of cumulus cells in forming the expanding matrices required for cumulus cell–oocyte complexes (COCs) to ovulate (Fig. 2). Her studies with COC cultures showed that: 1) hyaluronan is the major structural component of the matrix (13); 2) serum is necessary to form it (13); and 3) a factor(s) synthesized by the oocyte is also required (14, 15). The serum factor involved was identified a couple years later by Chen et al. (16) as a member of the inter-α-trypsin inhibitor (IαI) family.
Figure 2.
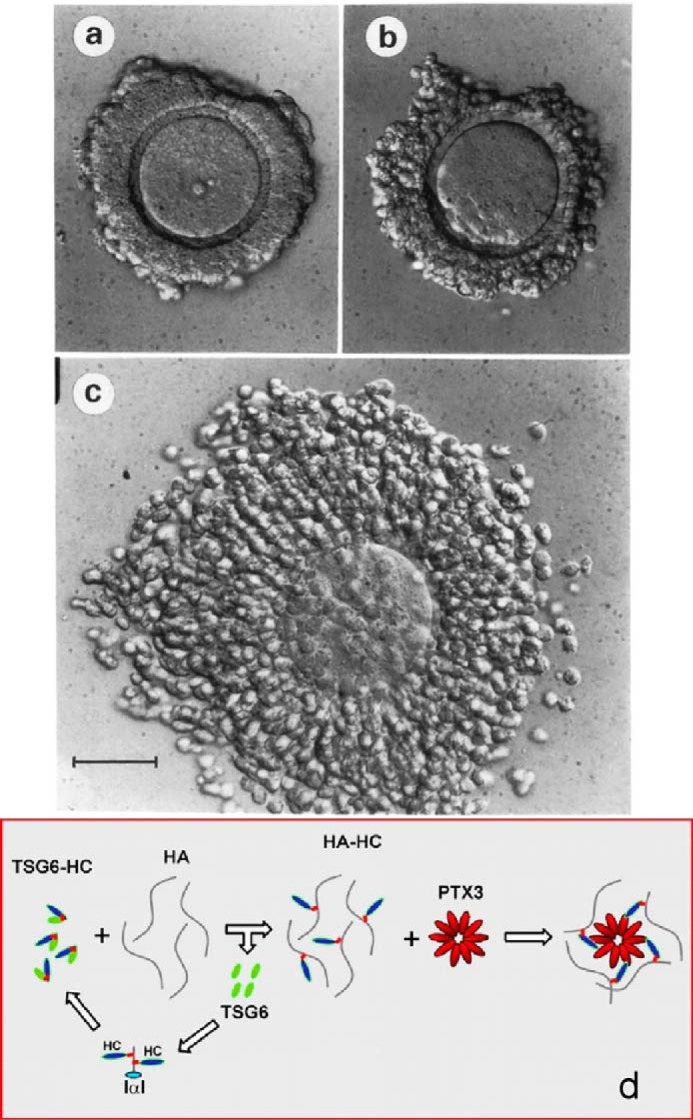
Cumulus cell oocyte complex before (a) and after (c) expansion in culture (13). b was incubated without FBS, which contains IαI that is required for expansion. d is the current model for forming the hyaluronan matrix (27).
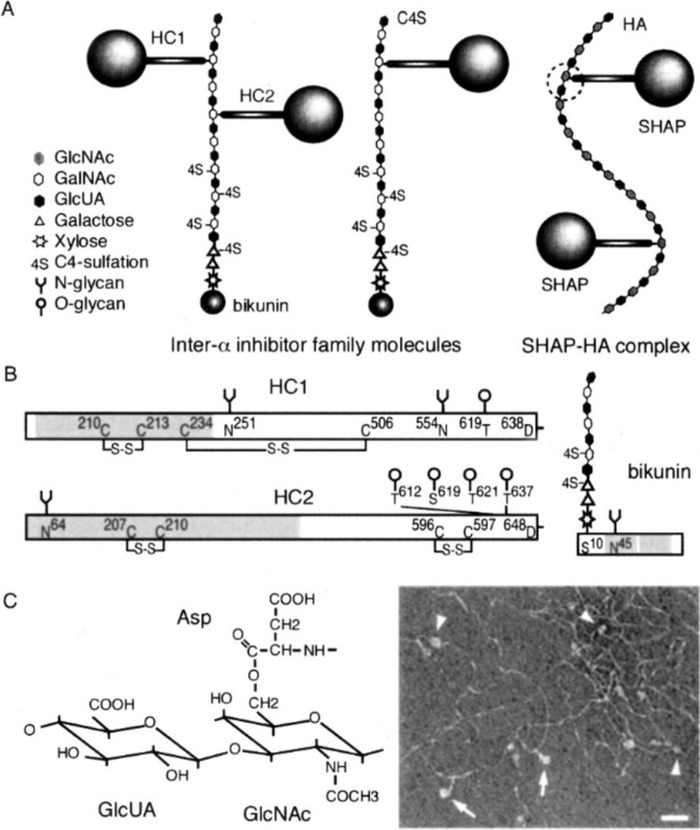
Structure of IαI and pre-α-trypsin inhibitor (PαI) (1989–1991)
During this time frame, Enghild et al. (17) purified two trypsin inhibitors, 225 kDa (IαI) and 125 kDa (PαI), from human plasma. They showed that testicular hyaluronidase digests separated a small trypsin inhibitor protein (HI-30) from both, and they identified three related large “heavy chain” proteins. Their analyses provided strong evidence for the following: 1) a model for IαI consisting of a trypsin inhibitor light chain (designated HI-30) and two heavy chains (HC1 and HC2) that were attached by glycosaminoglycan (GAG) chains; and 2) a model for PαI consisting of HI-30 and one heavy chain (HC3) (see Fig. 9 in Ref. 17). They subsequently showed that the GAG chain was chondroitin-4-sulfate (18).
Identification of serum-derived hyaluronan-associated protein (SHAP) (1990–1995)
Studies by Koji Kimata's lab during this time identified an 85-kDa protein strongly attached to hyaluronan in the cell matrix synthesized by mouse dermal fibroblast cultures (19). It was isolated by hyaluronan lyase digestion of purified hyaluronan isolated from the cultures. They then showed that this protein was not synthesized by the fibroblasts but was derived from the fetal bovine serum (FBS) used in the medium. They also showed that incubation of the FBS alone with high molecular weight HA formed the complex and that the presence of the hyaluronan decasaccharide (HA10), and to a lesser extent the HA8, prevented its formation. The enzyme required to form this complex, TSG-6, described below, must have contaminated the FBS sample.
Subsequent studies by this group identified SHAP as heavy chain 1 (HC1) and HC2 of IαI (20). They then isolated hyaluronan from pathological synovial fluid from human arthritis patients and showed that it contained HCs linked to hyaluronan by ester bonds onto the 6-hydroxyl of N-acetylglucosamines (GlcNAc) (21). This provided evidence that this HC modification of HA is involved in forming pathological HC–HA matrices. This group then developed a transgenic mouse null in the trypsin inhibitor bikunin that is the central protein core on serum IαI, which anchors the chondroitin sulfate chain that covalently binds HC1 and HC2 (22). The homozygous bikunin null mouse exhibited severe female infertility due to the inability to form the expanded cumulus oocyte hyaluronan matrix. Ovulated oocytes had no matrix and were not fertilized. Intraperitoneal injection of IαI fully rescued fertilization.
The 2004 Minireview (23) summarizes early studies that identified IαI and the model for its role in forming HC–HA matrices (Fig. 3). IαI is synthesized by liver hepatocytes and is consistently secreted into circulation. During inflammations, serum IαI is recruited to sites where hyaluronan is being synthesized to form extracellular matrices at the site of inflammation. HCs (SHAPs) are then transferred to form the HC–HA matrices. At the time of this JBC Review, the enzyme responsible for the transfer of the HCs from IαI onto hyaluronan was not known.
Figure 3.
Current model for the structure of IαI and its conversion to HC(SHAP)–HA matrices (23). The schematic structure of IαI family molecules and the SHAP-HA complex are shown in a, and the structures of the HCs and bikunin are shown in b. The electron micrograph shows the globular domains (arrowheads) and stem structures (arrows) of HCs that are bound to the hyaluronan molecules. The ester linkage between hyaluronan and the aspartate in HC is shown in c.
TNFα-induced protein-6 (TSG-6) is the enzyme that forms HC–HA matrices (2003–2007)
A second infertile null mouse model was described in 2003 (24). In this study a protein often up-regulated in inflammatory processes, TNFα-induced protein-6, now indicated as TSG-6, was deleted, and homozygous females were infertile. As was shown for the bikunin null females, the cumulus–oocyte complexes did not form, nor did any heavy chain–hyaluronan (HC–HA) matrices form in vivo or in vitro. Recombinant TSG-6 catalyzed the covalent transfer of heavy chains from IαI to form HC–HA in a cell-free system. TSG-6 also was able to restore expansion of the null cumulus cell–oocyte complexes in vitro and in vivo to rescue fertility in TSG-6 null females. A subsequent study (25) showed that the TSG-6 mechanism of heavy chain transfer involves two transesterification reactions in which an aspartate ester bond linking a HC to the 6-hydroxyl on an N-acetylgalactosamine (GalNAc) in the chondroitin sulfate in IαI is transferred to TSG-6 to form TSG-6–HC1 or TSG-6–HC2 intermediates. The HC is then transferred to the 6-hydroxyl of a GlcNAc in hyaluronan to form the aspartate HC–HA ester bond (21). A more recent study (2013) showed that the sulfation pattern of the chondroitin sulfate chain is complex with unsulfated, 4S-sulfated, 6S-sulfated, and 2,6S-disulfated regions and that the more fully sulfated regions with the 2,6S-disulfate facilitated the HC transfer mechanism (26). The formation of the cumulus cell–oocyte complex also required an additional molecule, pentraxin, a multimer complex with ∼10 protomers. The Salustri lab (27) showed that pentraxin selectively interacted with HCs in forming the expanding cumulus oocyte HC–HA matrix providing the current model with the TSG-6–HC intermediate for forming its structure (Fig. 2d).
Hyaluronan synthases (1993–1997)
Hyaluronan is a member of the glycosaminoglycan family that includes chondroitin sulfates, heparin/heparan sulfates, and keratan sulfates. Extensive studies showed that the sulfated glycosaminoglycans are synthesized as proteoglycans in the Golgi on protein cores using UDP-sugars that form the backbone disaccharide structures that are extended on the nonreducing ends. Thus, it was thought that hyaluronan would likely be synthesized similarly on a core protein in the Golgi. However, this changed in 1993 when the Weigel lab identified a hyaluronan synthase in Streptococcus pyogenes (28). This study showed that a single gene, named HasA and later as spHAS, was sufficient to synthesize hyaluronan. A subsequent study (29) showed that this enzyme utilizes both substrates, UDP-GlcUA and UDP-GlcNAc, unlike synthesis of all of the proteoglycans that require a different enzyme for each of the two sugars required. Studies to determine the mammalian enzyme converged in 1996–1997 as described in the Minireview (30) and, surprisingly, identified three HASs: HAS1 (mouse (31) and human (32)); HAS2 (mouse (33, 34) and human (35)); and HAS3 (mouse (36)).
It is likely that the HASs evolved from a chitin synthase transforming from synthesis of a large hydrophobic chitin polymer of [GlcNAc(β1,4]n that forms the exoskeleton of crustaceans, arthropods, and insects to synthesis of the large hydrophilic hyaluronan by inserting negatively charged GlcUA in between the GlcNAc residues. The most recent model for hyaluronan synthesis indicates that a short [GlcNAc(β1,4]3,4–UDP chitin oligomer is initially synthesized to induce transfer through the plasma membrane before the negatively charged GlcUA–UDP is added to the reducing end to initiate synthesis and extrusion of the hyaluronan polymer (37, 38).
Monocyte adhesive hyaluronan matrices (1999–2004)
Carol de la Motte, a technician working with Scott Strong, a colorectal surgeon, was studying colon smooth muscle cell cultures. It was known that viral infections of patients with Crohn's disease or ulcerative colitis often had subsequent intestinal inflammations. They showed in 1999 that virus infection of the cultures or treating them with poly(I:C), a viral analogue, induced synthesis of an extensive hyaluronan matrix that mononuclear leukocytes bound to selectively at 4 °C via their cell-surface CD44, a hyaluronan-binding protein (39). Based on a thesis for this discovery, de la Motte received a Ph.D. from Cardiff University and was promoted to Assistant Staff at the Cleveland Clinic. A subsequent study in 2003 showed that this hyaluronan matrix coalesced into cable-like structures and that IαI was necessary for their structure and function (40) at a time prior to the identification of the heavy chain transfer mechanism described above. A subsequent review in early 2004 showed that the bound leukocytes degraded the hyaluronan matrix when the cultures were warmed to 37 °C with capping of their CD44, a mechanism involved in the macrophage responses in inflammations that were shown initially in asthma, colitis, and Crohn's pathologies (see Figs. 7–11 in Ref. 41).
Normal synthesis of hyaluronan induces cell-surface protrusions (2003–2006)
Raija and Markku Tammi's lab showed that activation of rat epidermal keratinocyte cultures with keratinocyte growth factor increased Has2 mRNA and hyaluronan synthesis with concurrent increases in filapodial protrusions (42). A subsequent study transfected several cell lines with green fluorescent protein (GFP)-tagged Has3 (43). Fig. 4A from a following Minireview (44) shows a confocal image of hyaluronan synthesis (red) by live, nondividing human breast adenocarcinoma MCF-7 cells cultured in normal glucose. The Has are transported from the ER in vesicles to the Golgi and from the Golgi to the plasma membrane, embedded (yellow), and then activated to synthesize and extrude the hyaluronan (red) (see supplemental videos in Ref. 43). The HA is on microvillus-like protrusions on the cell surface, which recede back to the cell surface if the hyaluronan is removed by hyaluronidase. The role of these protrusions in modulating the hyaluronan pericellular matrices (coats) is also shown (43).
Figure 4.
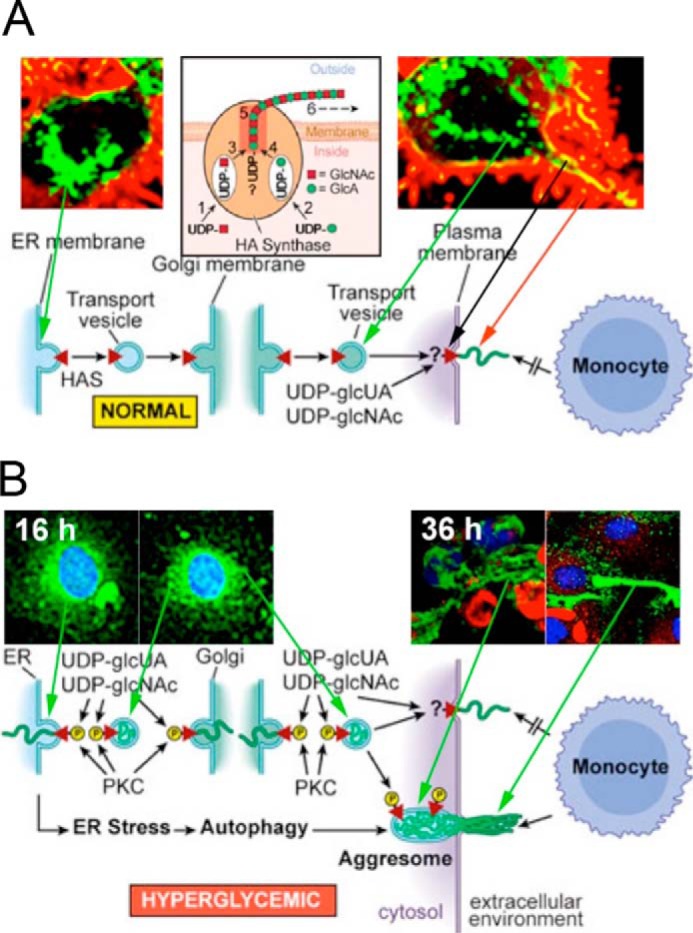
A, normal glucose: Has enzymes (green) travel to the cell surface, activate hyaluronan synthesis (yellow), and extrude hyaluronan (red) along cell-surface protrusions in nondividing cells. This figure (44) was kindly provided by the Tammi laboratory. B, hyperglycemic glucose: Has enzymes in hyperglycemic dividing cells were activated in intracellular membranes. They then synthesized hyaluronan (green) into ER, Golgi, and transport vesicles after entry into S phase as shown at 16 h of division. After division, they extruded the hyaluronan to form an extracellular hyaluronan matrix as shown at 36 h (16 h after division). Cyclin D3 is localized in intracellular regions (red).
Abnormal intracellular hyaluronan synthesis in hyperglycemic dividing cells (2004–2014)
At the time of the Minireview mentioned above (41), there were also several studies showing intracellular localization of hyaluronan, frequently during mitosis (see Fig. 1 in Ref. 44), and it was thought that this might be a normal cellular process. However, a subsequent study published later in 2004 changed this model (45). The problem was the glucose concentrations that were used in many of the previous studies, usually Dulbecco's modified Eagle's medium high glucose (5× normal). This study showed that kidney mesangial cells stimulated to divide from G0/G1 in 3–5× normal glucose, but not in 1–2× normal glucose, activate Has enzymes and synthesize hyaluronan (green) into intracellular compartments (ER, Golgi, and transport vesicles) shortly after entering S phase (Fig. 4B, 16 h). After division, the hyaluronan is extruded into extensive cable-like structures that form a monocyte-adhesive extracellular matrix at the end of division (Fig. 4B, 36 h). Ren et al. (46) showed that the mechanism required cyclin D3, which initiates an autophagic response (Fig. 4B, red). In vivo, kidney glomeruli of streptozotocin-treated type 1 diabetic rats contained abnormal hyaluronan deposits, often with closely associated monocytes/macrophages by 1 week (45).
Subsequent studies showed that low concentrations of heparin (∼0.3 μg/ml) were sufficient 1) to prevent the intracellular hyaluronan synthesis and autophagy and 2) to re-program the cells to finish cell division and then synthesize the extensive extracellular hyaluronan matrix that is monocyte-adhesive (47). Furthermore, the nonreducing trisaccharide of heparin (Hep-Tri) (Kd ∼20 nm) is sufficient (48). These results provide evidence that hyperglycemic dividing cells have a cell-surface receptor that interacts with the Hep-Tri to re-program the cells to block intracellular hyaluronan synthesis. Ongoing experiments provide evidence that dividing cells normally block glucose uptake as they enter cell division, but they cannot do so in high glucose, and that heparin treatment restores a mechanism to block glucose uptake within the 1st h of division.
Hyaluronan in hyperglycemic dividing bone marrow stromal (stem) cells (2014)
3T3-L1 cells have been used extensively to study adipogenesis. However, the mechanism frequently uses high-glucose medium to promote dividing 3T3-L1 precursor cells to adipocytes. This was shown in 1993 to promote synthesis of an excessive amount of hyaluronan by 3T3-L1 cells (49). The importance of this was demonstrated in 2014 when bone marrow stromal (stem) cells were stimulated to divide in normal glucose (1×) compared with high glucose (5×) (50). Normally, these cells divide asymmetrically resulting in one daughter stromal cell and one bone-forming osteoblast. However, in high glucose both daughter cells initiate intracellular synthesis of hyaluronan during division and become pathological adipocytes after division. This mechanism is likely to be a major factor in the current epidemic of diabetic obesity due primarily from the carbohydrate, low-fat Western diet that has been erroneously strongly recommended during the last 40 years as described in the book by Nina Teicholz (51).
Role of CD44 in metabolism of hyaluronan glycocalyces (2014)
The Minireview (52) describes the dynamic synthesis and degradation of hyaluronan glycocalyces on connective tissue cells and how it can be a rheostat to maintain cytosolic UDP-GlcNAc in an acceptable concentration for regulating cytosolic O-GlcNAc transferase activity. Extracellular glucose levels change greatly depending on consumption. If levels decrease below ∼15% of normal during fasting, for example, an AMP-kinase pathway phosphorylates a threonine on active hyaluronan synthases in the plasma membrane and stops their activity. Excessive glucose from feasting, for example, induces synthesis of more hyaluronan glycocalyx, which can then be degraded on the cell-surface catabolic sites. This involves a protease to remove any associated proteoglycan, often versican, a hyaluronidase to fragment the hyaluronan, and CD44 to transport the fragments into an endosome and then recycle to the cell surface (see Fig. 2 of Ref. 52). The inability to prevent increases of cytosolic glucose and the subsequent increased UDP-GlcNAc leads to abnormal O-GlcNAcylation of many cytosolic proteins involved in diabetic pathologies.
Hyaluronan interaction with CD44 variants promotes cancer cell migration and metastasis (2009–2010)
CD44 can be synthesized in up to nine variants. An innovative study by Misra et al. (53) showed that hyaluronan interaction with CD44v6 on murine adenoma tumor cells promoted anti-apoptotic cell-survival pathways. Two transferrin-coated nanoparticles were developed to test their effect on interfering with this pathway in a mouse model of spontaneous colon adenoma tumors: 1) a colon cell-specific promoter-driven Cre recombinase, and 2) a floxed-specific CD44v6shRNA. These two nanoparticles were delivered into the mice every other day, and the mice were sacrificed on day 10. The number of tumors was reduced ∼40%, and the remaining tumors expressed normal amounts of CD44 and excessive amounts of CD44v6, providing evidence for the delivery to the tumor cells and the specificity of the shRNA for the CD44v6. A subsequent study (54) provided evidence that a similar approach may be applicable for prostate cancer in which hyaluronan interaction with CD44v9 has been identified.
The following articles are recent new directions for hyaluronan research.
Hyaluronan glycocalyx on umbilical cord mesenchymal stem cells (2013–2014)
Corneal clouding occurs in mucopolysaccharidosis VII mice (55). Intriguingly, implantation of human umbilical cord mesenchymal stem cells (UMSCs) into the mouse corneas cleared the hazing, and the human cells were not recognized by the mouse immune system. This indicates that exosomes from the human cells had to carry the missing enzyme to the mouse cells so that the lysosomes could clear the accumulating hazing matrices. A following study (56) showed that human UMSCs transplanted into a WT mouse stroma 24 h after an alkali burn suppressed the severe inflammatory response and enabled recovery of corneal transparency within 2 weeks. However, transplanting UMSCs pre-treated with chondroitinase, which cleaves hyaluronan as well as chondroitin sulfate, did not inhibit the inflammatory responses, and the mouse immune system removed the human cells within 2 weeks. Analyses of the USMC glycocalyx showed that it contained HC–hyaluronan, TSG-6, and pentraxin indicating that the composition of the glycocalyx on UMSCs mirrors that formed by the cumulus oocyte complex and that this matrix is capable of protecting the human cells from the immune system of the mouse host. Furthermore, it provides evidence that the exosomes required to clear the mouse mucopolysaccharide VII corneal clouding are likely to have emerged from the hyaluronan-covered microvilli-like protrusions identified in the Tammi and co-workers study (43).
Platelet formation by megakaryocytes requires hyaluronidase 2 (Hyal2) (2016)
The de la Motte lab showed that hyaluronan synthesized by megakaryocytes is involved in platelet formation and biology. They showed that platelets express Hyal2 and that they bind to and cleave hyaluronan produced by TNFα-treated endothelial cells (57). Further studies showed that megakaryocytes synthesize the hyaluronan in intracellular compartments and that its depolymerization by Hyal2 is required for pro-platelet formation (58).
Formation of airway epithelial hyaluronan rafts (2016)
Air–liquid interface cultures of murine airway epithelial cells actively synthesize and release HC3–hyaluronan rafts onto their ciliated apical surfaces (59). To do this, they synthesize the enzyme TSG-6 and a HC3–PαI donor. The rafts were identified in alveolar lavages of lungs from normal mice, but they were not present in alveolar lavages of the TSG-6 null mice. These rafts, then, provide a protective surface in lung airways.
Nuclear HYAL2 has an important role in inhibiting splicing of CD44v7/8 (2017)
Hyaluronidase 2 has been identified as a cell-surface protein with weak enzymatic activity and as a lysosomal enzyme with strong enzymatic activity to fragment hyaluronan. Soma Meran's research (60) has now shown that HYAL2 also has an important nuclear function in regulating alternative splicing events of CD44v7/8 mRNA, thereby modulating these splicing CD44 variants that are involved in the regulation of cellular phenotypes in progressive fibrosis models. Whether its hyaluronidase activity has a role in this novel process remains to be determined.
HC–hyaluronan matrix formation on right side is required for gut rotation (2018)
Induction of left–right asymmetry during development is required for normal gut rotation and function. The Kurpios lab (61) has now shown that the mechanism requires formation of an extensive expanding HC–hyaluronan matrix on the right side with very little hyaluronan matrix on the left side during midgut rotation. TSG-6 null mice fail to initiate midgut rotation, which perturbs vascular development and predisposition to midgut pathology.
Finale
This perspective focuses on many aspects of the ever-increasing roles of hyaluronan in the biology of higher organisms that evolved after a cell learned how to synthesize it, and how the JBC has had a major role in this ongoing research area. It is remarkable that a simple disaccharide structure evolved into so many biological processes–from fertilization to organ development to inflammatory processes to cancer. The exciting progress with this field is due largely to the highly interactive collaborations among the hyaluronan research community. This is reflected by the establishment of the International Society for Hyaluronan Sciences (ISHAS), founded by Endre Balazs in 2004, which now sponsors international meetings every 2 years, with the next one in Cardiff, Wales, in June, 2019. From my focus on hyaluronan research areas that I have been involved in, it is clear that both the high quality of the JBC that was maintained by Herb Tabor and his management of the Associate Editors (which I experienced as an Associate Editor) and the high quality of the manuscript reviews by the Editorial Board have been essential for my career, as well as for continuing the development of the hyaluronan research field. Thank you Herb!
This JBC Review is part of a collection honoring Herbert Tabor on the occasion of his 100th birthday. The author declares that he has no conflict of interest with the contents of this article.
- HA
- hyaluronan
- COC
- cumulus cell–oocyte complex
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- GAG
- glycosaminoglycan
- GlcUA
- glucuronic acid
- HAS
- hyaluronan synthase
- HC
- heavy chain
- Hep-Tri
- nonreducing trisaccharide of heparin
- HI-30
- a small trypsin inhibitor protein
- IαI
- inter-α-trypsin inhibitor
- PαI
- pre-α-trypsin inhibitor
- SHAP
- heavy chain 1 (HC1) and HC2 of IαI
- TNFα
- tumor necrosis factor α
- TSG-6
- TNFα-induced protein-6
- UDP
- uridine diphosphate
- and UMSC
- umbilical cord mesenchymal stem cell.
References
- 1. Meyer K., and Palmer J. W. (1934) The polysaccharide of the vitreous humor. J. Biol. Chem. 107, 629–634 [Google Scholar]
- 2. Weissman B., and Meyer K. (1954) The structure of hyalobiuronic acid from umbilical cord. J. Am. Chem. Soc. 76, 1753–1757 10.1021/ja01636a010 [DOI] [Google Scholar]
- 3. McDonald J., and Hascall V. C. (2002) Minireview prologue. J. Biol. Chem. 277, 4575–4579 10.1074/jbc.R100064200 [DOI] [PubMed] [Google Scholar]
- 4. Tammi M. I., Day A. J., and Turley E. A. (2002) Hyaluronan and homeostasis: a balancing act. J. Biol. Chem. 277, 4581–4584 10.1074/jbc.R100037200 [DOI] [PubMed] [Google Scholar]
- 5. Day A. J., and Prestwich G. D. (2002) Hyaluronan-binding proteins: tying up the giant. J. Biol. Chem. 277, 4585–4588 10.1074/jbc.R100036200 [DOI] [PubMed] [Google Scholar]
- 6. Turley E. A., Noble P. W., and Bourguignon L. Y. (2002) Signaling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 10.1074/jbc.R100038200 [DOI] [PubMed] [Google Scholar]
- 7. Toole B. P., Wight T. N., and Tammi M. I. (2002) Hyaluronan–cell interactions in cancer and vascular disease. J. Biol. Chem. 277, 4593–4596 10.1074/jbc.R100039200 [DOI] [PubMed] [Google Scholar]
- 8. Hascall V. C., and Sajdera S. W. (1969) Protein polysaccharide complex from bovine nasal cartilage: the function of glycoprotein in the formation of aggregates. J. Biol. Chem. 244, 2384–2396 [PubMed] [Google Scholar]
- 9. Hascall V. C. (2000) Hyaluronan, a common thread. Glycoconj. J. 17, 607–616 10.1023/A:1011082728155 [DOI] [PubMed] [Google Scholar]
- 10. Hardingham T. E., and Muir H. (1972) The specific interaction of hyaluronic acid with cartilage proteoglycans. Biochim. Biophys. Acta 279, 401–405 10.1016/0304-4165(72)90160-2 [DOI] [PubMed] [Google Scholar]
- 11. Hardingham T. E., and Muir H. (1973) Hyaluronic acid in cartilage. Biochem. Soc. Trans. 1, 282–284 10.1042/bst0010282 [DOI] [Google Scholar]
- 12. Heinegård D., and Hascall V. C. (1974) Aggregation of cartilage proteoglycans III. Characteristics of the proteins isolated from trypsin digests of aggregates. J. Biol. Chem. 249, 4250–4256 [PubMed] [Google Scholar]
- 13. Salustri A., Yanagishita M., and Hascall V. C. (1989) Synthesis and accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell–oocyte complex during FSH-induced mucification. J. Biol. Chem. 264, 13840–13847 [PubMed] [Google Scholar]
- 14. Salustri A., Yanagishita M., and Hascall V. C. (1990) Mouse oocytes regulate hyaluronic acid synthesis and mucification by FSH-stimulated cumulus cells. Dev. Biol. 138, 26–32 10.1016/0012-1606(90)90173-G [DOI] [PubMed] [Google Scholar]
- 15. Salustri A., Ulisse S., Yanagishita M., and Hascall V. C. (1990) Hyaluronic acid synthesis by mural granulosa cells and cumulus cells in vitro is selectively stimulated by a factor produced by oocytes and by transforming growth factor-β1. J. Biol. Chem. 265, 19517–19523 [PubMed] [Google Scholar]
- 16. Chen L., Mao S. J., and Larsen W. J. (1992) Identification of a factor in fetal bovine serum that stabilizes the cumulus extracellular matrix. A role for a member of the inter-α-trypsin inhibitor family. J. Biol. Chem. 267, 12380–12386 [PubMed] [Google Scholar]
- 17. Enghild J. J., Thøgersen I. B., Pizzo S. V., and Salvesen G. (1989) Analysis of inter-α-trypsin inhibitor and a novel trypsin inhibitor, pre-α-trypsin inhibitor, from human plasma. Polypeptide chain stoichiometry and assembly by glycan. J. Biol. Chem. 264, 15975–15981 [PubMed] [Google Scholar]
- 18. Enghild J. J., Salvesen G., Hefta S. A., Thøgersen I. B., Rutherfurd S., and Pizzo S. V. (1991) Chondroitin 4-sulfate covalently cross-links the chains of the human blood protein pre-α-inhibitor. J. Biol. Chem. 266, 747–751 [PubMed] [Google Scholar]
- 19. Yoneda M., Suzuki S., and Kimata K. (1990) Hyaluronic acid associated with the surfaces of cultured fibroblasts is linked to a serum-derived 85-kDa protein. J. Biol. Chem. 265, 5247–5257 [PubMed] [Google Scholar]
- 20. Huang L., Yoneda M., and Kimata K. (1993) A serum-derived hyaluronan-associated protein (SHAP) is the heavy chain of the inter-α-trypsin inhibitor. J. Biol. Chem. 268, 26725–26730 [PubMed] [Google Scholar]
- 21. Zhao M., Yoneda M., Ohashi Y., Kurono S., Iwata H., Ohnuki Y., and Kimata K. (1995) Evidence for the covalent binding of SHAP, heavy chains of inter-α-trypsin inhibitor, to hyaluronan. J. Biol. Chem. 270, 26657–26663 10.1074/jbc.270.44.26657 [DOI] [PubMed] [Google Scholar]
- 22. Zhuo L., Yoneda M., Zhao M., Yingsung W., Yoshida N., Kitagawa Y., Kawamura K., Suzuki T., and Kimata K. (2001) Defect in SHAP–hyaluronan complex causes severe female infertility. J. Biol. Chem. 276, 7693–7696 10.1074/jbc.C000899200 [DOI] [PubMed] [Google Scholar]
- 23. Zhuo L., Hascall V. C., and Kimata K. (2004) Inter-α-trypsin inhibitor, a covalent protein–glycosaminoglycan–protein complex. J. Biol. Chem. 279, 38079–38082 10.1074/jbc.R300039200 [DOI] [PubMed] [Google Scholar]
- 24. Fülöp C., Szántó S., Mukhopadhyay D., Bárdos T., Kamath R. V., Rugg M. S., Day A. J., Salustri A., Hascall V. C., Glant T. T., and Mikecz K. (2003) Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-deficient mice. Development 130, 2253–2261 10.1242/dev.00422 [DOI] [PubMed] [Google Scholar]
- 25. Rugg M. S., Willis A. C., Mukhopadhyay D., Hascall V. C., Fries E., Fülöp C., Milner C. M., and Day A. J. (2005) Characterization of complexes formed between TSG-6 and inter-α-inhibitor that act as intermediates in the covalent transfer of heavy chains onto hyaluronan. J. Biol. Chem. 280, 25674–25686 10.1074/jbc.M501332200 [DOI] [PubMed] [Google Scholar]
- 26. Lord M. S., Day A. J., Youssef P., Zhuo L., Watanabe H., Caterson B., and Whitelock J. M. (2013) Sulfation of the bikunin chondroitin sulfate chain determines heavy chain hyaluronan complex formation. J. Biol. Chem. 288, 22930–22941 10.1074/jbc.M112.404186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scarchilli L., Camaioni A., Bottazzi B., Negri V., Doni A., Deban L., Bastone A., Salvatori G., Mantovani A., Siracusa G., and Salustri A. (2007) PTX3 interacts with inter-α-trypsin inhibitor. Implications for hyaluronan organization and cumulus oophorus expansion. J. Biol. Chem. 282, 30161–30170 10.1074/jbc.M703738200 [DOI] [PubMed] [Google Scholar]
- 28. DeAngelis P. L., Papaconstantinou J., and Weigel P. H. (1993) Molecular cloning, identification and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J. Biol. Chem. 268, 19181–19184 [PubMed] [Google Scholar]
- 29. DeAngelis P. L., and Weigel P. H. (1994) Immunochemical confirmation of the primary structure of streptococcal hyaluronan synthase and synthesis of high molecular weight product by the recombinant enzyme. Biochem. J. 33, 9033–9039 10.1021/bi00197a001 [DOI] [PubMed] [Google Scholar]
- 30. Weigel P. H., Hascall V. C., and Tammi M. (1997) Minireview. Hyaluronan synthases. J. Biol. Chem. 272, 13997–14000 10.1074/jbc.272.22.13997 [DOI] [PubMed] [Google Scholar]
- 31. Itano N., and Kimata K. (1996) Expression cloning and molecular characterization of HAS protein, a eukaryotic hyaluronan synthase. J. Biol. Chem. 271, 9875–9878 10.1074/jbc.271.17.9875 [DOI] [PubMed] [Google Scholar]
- 32. Shyjan A. M., Heldin P., Butcher E. C., Yoshino T., and Briskin M. J. (1996) Functional cloning of the cDNA for a human hyaluronan synthase. J. Biol. Chem. 271, 23395–23399 10.1074/jbc.271.38.23395 [DOI] [PubMed] [Google Scholar]
- 33. Spicer A. P., Augustine M. L., and McDonald J. A. (1996) Molecular cloning and characterization of a putative mouse hyaluronan synthase. J. Biol. Chem. 271, 23400–23406 10.1074/jbc.271.38.23400 [DOI] [PubMed] [Google Scholar]
- 34. Fülöp C., Salustri A., and Hascall V. C. (1997) Coding sequence of a hyaluronan synthase homologue expressed during expansion of the mouse cumulus–oocyte complex. Arch. Biochem. Biophys. 337, 261–266 10.1006/abbi.1996.9793 [DOI] [PubMed] [Google Scholar]
- 35. Watanabe K., and Yamaguchi Y. (1996) Molecular identification of a putative human hyaluronan synthase. J. Biol. Chem. 271, 22945–22948 10.1074/jbc.271.38.22945 [DOI] [PubMed] [Google Scholar]
- 36. Spicer A. P., Olson J. S., and McDonald J. A. (1997) Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J. Biol. Chem. 272, 8957–8961 10.1074/jbc.272.14.8957 [DOI] [PubMed] [Google Scholar]
- 37. Yoshida M., Itano N., Yamada Y., and Kimata K. (2000) In vitro synthesis of hyaluronan by a single protein derived from mouse Has1 gene and characterization of amino acid residues essential for the activity. J. Biol. Chem. 275, 497–506 10.1074/jbc.275.1.497 [DOI] [PubMed] [Google Scholar]
- 38. Weigel P. H., Baggenstoss B. A., and Washburn J. L. (2017) Hyaluronan synthase assembles hyaluronan on a [GlcNAc(β1,4]n-GlcNAc(α1-)UDP primer and hyaluronan retains this residual chitin oligomer as a cap at the nonreducing end. Glycobiology 27, 536–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de la Motte C. A., Hascall V. C., Calabro A., Yen-Lieberman B., and Strong S. A. (1999) Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly I:C. J. Biol. Chem. 274, 30747–30755 [DOI] [PubMed] [Google Scholar]
- 40. de la Motte C. A., Hascall V. C., Drazba J., Bandyopadhyay S. K., and Strong S. A. (2003) Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am. J. Pathol. 163, 121–133 10.1016/S0002-9440(10)63636-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hascall V. C., Majors A. K., De La Motte C. A., Evanko S. P., Wang A., Drazba J. A., Strong S. A., and Wight T. N. (2004) Intracellular hyaluronan: a new frontier for inflammation? Biochim. Biophys. Acta 1673, 3–12 10.1016/j.bbagen.2004.02.013 [DOI] [PubMed] [Google Scholar]
- 42. Karvinen S., Pasonen-Seppänen S., Hyttinen J. M., Pienimäki J., Törrönen K., Jokela T. A., Tammi M. I., and Tammi R. (2003) Keratinocyte growth factor stimulates migration and hyaluronan synthesis in the epidermis by activation of keratinocyte hyaluronan synthases 2 and 3. J. Biol. Chem. 278, 49495–49504 10.1074/jbc.M310445200 [DOI] [PubMed] [Google Scholar]
- 43. Kultti A., Rilla K., Tiihonen R., Spicer A. P., Tammi R. H., and Tammi M. I. (2006) Hyaluronan synthesis induces microvillus-like cell surface protrusions. J. Biol. Chem. 281, 15821–15828 10.1074/jbc.M512840200 [DOI] [PubMed] [Google Scholar]
- 44. Wang A., de la Motte C., Lauer M., and Hascall V. (2011) Hyaluronan matrices in pathobiological processes. FEBS J. 278, 1412–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang A., and Hascall V. C. (2004) Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J. Biol. Chem., 279, 10279–10285 10.1074/jbc.M312045200 [DOI] [PubMed] [Google Scholar]
- 46. Ren J., Hascall V. C., and Wang A. (2009) Cyclin D3 mediates synthesis of a hyaluronan matrix that is adhesive for monocytes in mesangial cells stimulated to divide in hyperglycemic medium. J. Biol. Chem. 284, 16621–16632 10.1074/jbc.M806430200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang A., Ren J., Wang C. P., and Hascall V. C. (2014) Heparin prevents intracellular hyaluronan synthesis and autophagy responses in hyperglycemic dividing mesangial cells and activates synthesis of an extensive extracellular monocyte-adhesive hyaluronan matrix after completing cell division. J. Biol. Chem. 289, 9418–9429 10.1074/jbc.M113.541441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang C. P., Hascall V. C., Zhang F., Linhardt R. J., Abbadi A., and Wang A. (2015) The responses of hyperglycemic dividing mesangial cells to heparin are mediated by the non-reducing terminal trisaccharide. J. Biol. Chem. 290, 29045–29050 10.1074/jbc.M115.677401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Calvo J. C., Gandjbakhche A. H., Nossal R., Hascall V. C., and Yanagishita M. (1993) Rheological effects of the presence of hyaluronic acid in the extracellular media of differentiated 3T3-L1 preadipocyte cultures. Arch. Biochem. Biophys. 302, 468–475 10.1006/abbi.1993.1241 [DOI] [PubMed] [Google Scholar]
- 50. Wang A., Midura R. J., Vasanji A., Wang A. J., and Hascall V. C. (2014) Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes. J. Biol. Chem. 289, 11410–11420 10.1074/jbc.M113.541458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Teicholz N. (2014) The Big Fat Surprise: Why Butter, Meat and Cheese Belong in a Healthy Diet. Simon & Schuster, Inc., New York [Google Scholar]
- 52. Hascall V. C., Wang A., Tammi M., Oikari S., Tammi R., Passi A., Vigetti D., Hanson R. W., and Hart G. W. (2014) The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol. 35, 14–17 10.1016/j.matbio.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Misra S., Hascall V. C., De Giovanni C., Markwald R. R., and Ghatak S. (2009) Delivery of CD44shRNA/nanoparticles within cancer cells: perturbation of hyaluronan/CD44v6 interactions and reduction in adenoma growth in Apc Min/+ mice. J. Biol. Chem. 284, 12432–12446 10.1074/jbc.M806772200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghatak S., Hascall V. C., Markwald R. R., and Misra S. (2010) Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J. Biol. Chem. 285, 19821–19832 10.1074/jbc.M110.104273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coulson-Thomas V. J., Caterson B., and Kao W. W. (2013) Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells 31, 2116–2126 10.1002/stem.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coulson-Thomas V. J., Gesteira T. F., Hascall V., and Kao W. (2014) Umbilical cord mesenchymal stem cells suppress host rejection–The role of the glycocalyx. J. Biol. Chem. 289, 23465–23481 10.1074/jbc.M114.557447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. de la Motte C., Nigro J., Vasanji A., Rho H., Kessler S., Bandyopadhyay S., Danese S., Fiocchi C., and Stern R. (2009) Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 174, 2254–2264 10.2353/ajpath.2009.080831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Petrey A. C., Obery D. R., Kessler S. P., Flamion B., and de la Motte C. A. (2016) Hyaluronan depolymerization by megakaryocyte hyaluronidase-2 is required for thrombopoiesis. Am. J. Pathol. 186, 2390–2403 10.1016/j.ajpath.2016.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Abbadi A., Lauer M., Swaidani S., Wang A., and Hascall V. (2016) Hyaluronan rafts on airway epithelial cells. J. Biol. Chem. 291, 1448–1455 10.1074/jbc.M115.704288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Midgley A. C., Oltean S., Hascall V., Woods E. L., Steadman R., Phillips A. O., and Meran S. (2017) Nuclear hyaluronidase 2 drives alternative splicing of CD44 pre-mRNA to determine profibrotic or antifibrotic cell phenotype. Sci. Signal. 10, eaao1882 10.1126/scisignal.aao1822 [DOI] [PubMed] [Google Scholar]
- 61. Sivakumar A., Mahadevan A., Lauer M. E., Narvaez R. J., Ramesh S., Demler C. M., Souchet N. R., Hascall V. C., Midura R. J., Garantziotis S., Frank D. B., Kimata K., and Kurpios N. A. (2018) Midgut laterality is driven by hyaluronan on the right. Dev. Cell 46, 533–551.e5 10.1016/j.devcel.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]