Abstract
The serine-rich repeat (SRR) glycoproteins of Gram-positive bacteria are large, cell wall–anchored adhesins that mediate binding to many host cells and proteins and are associated with bacterial virulence. SRR glycoproteins are exported to the cell surface by the accessory Sec (aSec) system comprising SecA2, SecY2, and 3–5 additional proteins (Asp1 to Asp5) that are required for substrate export. These adhesins typically have a 90-amino acid-long signal peptide containing an elongated N-region and a hydrophobic core. Previous studies of GspB (the SRR adhesin of Streptococcus gordonii) have shown that a glycine-rich motif in its hydrophobic core is essential for selective, aSec-mediated transport. However, the role of this extended N-region in transport is poorly understood. Here, using protein–lipid co-flotation assays and site-directed mutagenesis, we report that the N-region of the GspB signal peptide interacts with anionic lipids through electrostatic forces and that this interaction is necessary for GspB preprotein trafficking to lipid membranes. Moreover, we observed that protein–lipid binding is required for engagement of GspB with SecA2 and for aSec-mediated transport. We further found that SecA2 and Asp1 to Asp3 also localize selectively to liposomes that contain anionic lipids. These findings suggest that the GspB signal peptide electrostatically binds anionic lipids at the cell membrane, where it encounters SecA2. After SecA2 engagement with the signal peptide, Asp1 to Asp3 promote SecA2 engagement with the mature domain, which activates GspB translocation.
Keywords: lipid-protein interaction, protein translocation, liposome, protein targeting, Streptococcus, adhesin, accessory Sec system, anionic lipids, electrostatic interactions, signal peptide, adhesion
Introduction
Serine-rich repeat (SRR)2 glycoproteins of streptococci and staphylococci are virulence factors associated with multiple diseases, including infective endocarditis, meningitis, and pneumonia. These surface glycoproteins function as adhesins that bind tissues and cells through interactions with diverse ligands, including fibrinogen, keratin, and glycans on host cells (1–8). Among the best-characterized of the SRR adhesins is GspB of Streptococcus gordonii, which mediates platelet binding and contributes to the development of endocarditis through protein–sialoglycan interactions (1, 9). The biogenesis of GspB and other SRR proteins requires the coordination of intracellular glycosylation of the adhesins with their transport across the cytoplasmic membrane and to the cell wall, where they undergo sortase-dependent covalent attachment (10, 11). The SRR glycoproteins are translocated by a specialized and dedicated transporter, the accessory Sec (aSec) system. The aSec system includes the ATPase motor protein, SecA2, and the heterotrimeric transmembrane channel comprising SecY2 and accessory Sec proteins 4 and 5 (Asp4 and Asp5) (12–15). Transport via the aSec system also requires three additional cytosolic proteins, Asp1 to Asp3, which have no known homologs outside of aSec systems (13, 14).
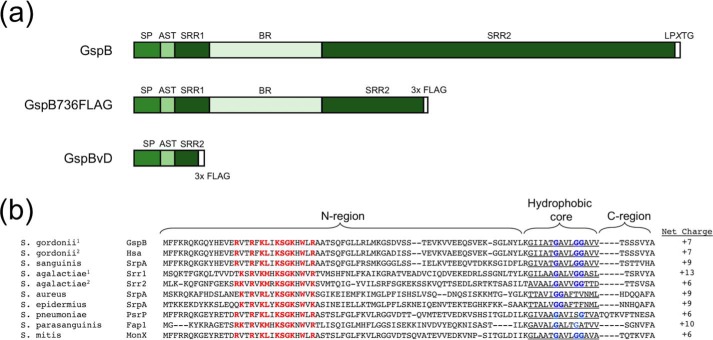
The SRR glycoproteins have a similar domain organization (Fig. 1A). At the N terminus is a 90-amino acid signal peptide, followed by an ∼24-amino acid aSec transport (AST) domain that is required for aSec transport (16–18). At the C terminus is an LPXTG cell wall–anchoring motif. All SRR proteins contain two serine-rich repeat domains (SRR1 and SRR2) that flank a binding region domain, which dictates the ligand binding specificity of the adhesin. The SRR regions undergo extensive O-linked glycosylation by the glycosyltransferase complex GtfAB that is required for protein stability. Depending on the bacterial species, further elongation of the glycan is performed by a variable number of glycosyltransferases (10, 19–21). In addition, Asp2 has recently been shown to be a bifunctional protein that is required for transport and mediates the O-acetylation of GlcNAc moieties on the SRR domains, which is essential for optimal adhesin activity. However, this enzymatic activity is dispensable for aSec transport (22).
Figure 1.
Domain organization and sequence features of SRR proteins. a, diagram of SRR protein domain architecture, truncated variant GspB736FLAG, and GspB variant D (GspBvD) domains. b, alignment of SRR protein signal sequences of representative Streptococcus and Staphylococcus species. S. gordonii1 is strain M99, S. gordonii2 is strain Challis, S. agalactiae1 is strain COH31, and S. agalactiae2 is strain COH1. Shown are species, protein name, signal sequences, and N-region net charge. Red, residues of the conserved polybasic motif. Blue, conserved glycine residues. Highlighted are the predicted N-region, hydrophobic core, and C-regions of the signal peptide sequences.
Like substrates for canonical Sec transport, aSec signal peptides have a tripartite structure, with a basic N-terminal segment (N-region), a hydrophobic core, and a polar C terminus containing the signal peptidase cleavage site (Fig. 1B). SRR preprotein signal peptides have hydrophobic cores of low hydrophobicity that are essential for targeting to the aSec system (23). SRR preproteins also have elongated and highly charged signal peptide N-regions that are required for aSec transport (23). Export also requires the joint activities of Asp1, Asp2, and Asp3, which are cytosolic proteins with no transmembrane segments, with SecA2 and SecY2 at the membrane. Notwithstanding these findings, the mechanisms by which the preprotein substrate is targeted to the membrane and the translocon are not well defined. In particular, neither the ability of the substrate or the Asps to migrate individually or independently to the cell membrane has ever been fully assessed in a native system.
Here, we investigate the importance of streptococcal membrane lipids in promoting membrane localization of the GspB preprotein substrate and the required aSec proteins Asp1 to Asp3. The GspB signal peptide was found to be critical for anionic lipid binding by the preprotein, and consequently lipid binding by the signal peptide proved to be essential for aSec transport of GspB in S. gordonii. Our findings also highlight a potential mechanism of consolidating the cytosolic aSec transport proteins together at the lipid membrane interface. We propose that anionic lipid binding by the aSec proteins and substrate is an important mechanism to drive co-localization of transport components together with the preprotein substrate.
Results
The GspB preprotein preferentially binds anionic lipids
We had previously found that the preprotein GspB, the SRR adhesin of Streptococcus gordonii strain M99, can bind SecA2 in vivo, independently of the other aSec system proteins (18). This suggests that the preprotein itself is sufficient for its own targeting to SecA2 and the aSec translocon. One mechanism by which the preprotein could migrate to the aSec translocon is by localizing at the membrane interface through preprotein–lipid interactions. To determine whether GspB can directly bind lipids, we first assessed preprotein binding to liposomes, using a co-flotation assay. To produce liposomes containing streptococcal lipids, we first examined the lipid composition of the M99 membrane. Studies using two-dimensional thin-layer chromatography (TLC) followed by quantitative phospholipid analysis (Fig. S1A) indicated that the streptococcal membranes contain the phospholipids cardiolipin (CL), phosphatidylglycerol (PG), and phosphatidic acid (PA), as 60, 12, and 17% of the total phospholipids, respectively. The remaining phospholipids were unidentified. Based on orcinol staining of the two-dimensional TLC and published findings of other streptococcal bacteria (24, 25), the remaining lipids are likely to be glycolipids, predominantly a diglycolipid. To confirm this, we conducted one-dimensional TLC followed by orcinol staining (Fig. S1B), which demonstrated that at least four glycolipids, including monoglucosyldiacylglycerol (MGDG), were present.
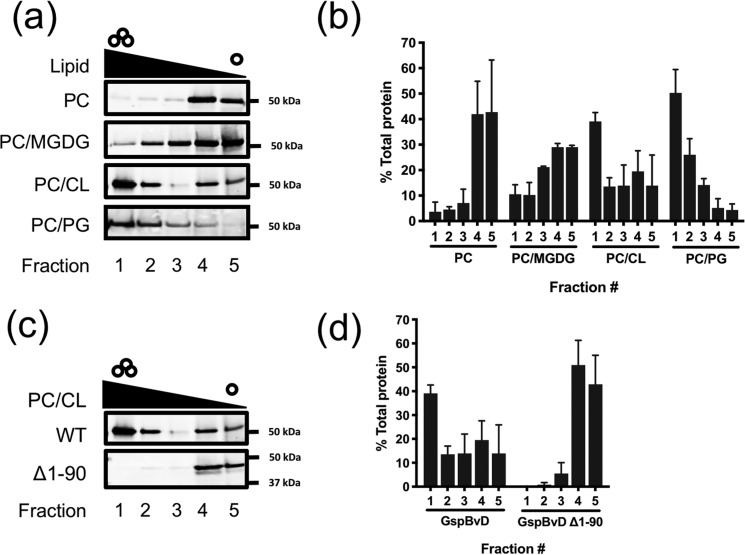
Due to the technical challenges of purifying large amounts of native GspB, we used a truncated form of GspB (GspBvD) to analyze liposome binding. This construct consists of the signal peptide, the first 50 amino acids of the mature region, and 143 amino acids of the second serine-rich repeat region (Fig. 1A). Like the native protein, GspBvD is transported selectively by the aSec system (16). After overexpression in and purification from Escherichia coli, GspBvD was incubated with unilamellar liposomes of differing compositions. Liposome flotation over an Optiprep density gradient was used to separate lipid-bound protein from unbound protein. As S. gordonii membranes do not contain phosphatidylcholine (PC), this lipid was chosen as a negative control for binding studies. Various lipid classes found in S. gordonii were incorporated into PC liposomes, and GspBvD binding was assessed (Fig. 2, A and B). When tested with PC alone, minimal binding of GspBvD was observed. The highest lipid co-flotation by GspBvD was reproducibly seen with liposomes containing CL and PG, with significantly less co-flotation with MGDG-containing liposomes. These results indicate that the GspBvD preprotein is able to bind a lipid interface and that anionic lipids are essential for this interaction.
Figure 2.
The signal peptide of GspB is required for binding anionic lipids found in S. gordonii but not S. gordonii glycolipids. a, comparison of GspBvD lipid binding to PC, PC/CL, PC/PG, and PC/MGDG liposomes. b, densitometric quantification of GspBvD in gradient fractions from a. c, comparison of binding to PC/CL liposomes by GspBvD with and without SP (GspBvD Δ1–90). d, densitometric quantitation of GspBvD in gradient fractions from c. Representative blots are shown for a and c. Independent experiments were performed in triplicate for b and d, except for MGDG binding, which was performed in duplicate. Bars, mean values ± S.D. (error bars). GspBvD was detected by Western blotting using an anti-FLAG antibody. Representative blots are shown.
To further understand the nature and importance of this preprotein–lipid interaction, we next explored which domains of GspBvD were responsible for membrane binding. Because the GspB signal peptide contains the largest cluster of basic amino acid residues in GspBvD that can interact with anionic lipids, we tested whether the signal peptide of GspBvD was essential for anionic lipid binding. The mature region of GspBvD without the signal peptide was first assessed for lipid binding. In contrast to what was seen with the preprotein and PC/CL liposomes, no co-flotation was observed with the mature region, signifying that the signal peptide is essential for GspB preprotein interactions with anionic lipids (Fig. 2, C and D).
The signal peptide N-region interacts with anionic lipids through electrostatic interactions
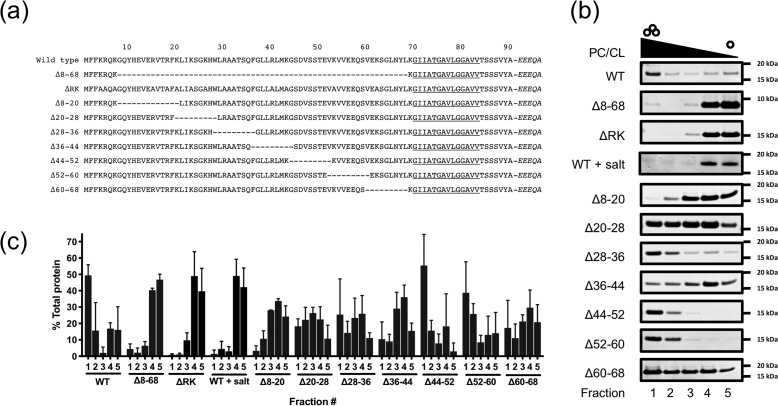
The signal peptide N-region of GspB and all other SRR preproteins contain several basic amino acids, including a conserved polybasic motif (KXYKXGKXW), resulting in an overall net charge of greater than +6 (Fig. 1B). In view of these properties, we next examined the contribution of this region to binding of anionic lipids. We began by generating deletions of segments of the GspB signal peptide, to identify the subregions within the signal peptide responsible for lipid binding (Fig. 3A). Because the lipid-binding sequence is found only within the signal peptide and not in the mature protein, we chose to measure binding of the first 125 amino acids of GspB. This peptide (SP-AST) includes the signal peptide and the AST domain that is essential for export. As was seen with GspBvD, the WT SP-AST peptide preferentially bound to PC/CL liposomes (Fig. 3, B and C), as compared with PC liposomes (Fig. S2). We then deleted residues 8–68 of SP-AST, thereby removing most of the elongated N-region and generating a signal peptide resembling that of SecA/SecY transported preproteins, in terms of overall length and charge. This deletion resulted in almost complete loss of binding to cardiolipin by the signal peptide, confirming that the lipid-binding domain of the signal peptide is localized within the N-region.
Figure 3.
GspB lipid binding is mediated by the N-region. a, diagram of signal peptide N-region mutants studied here. Italics denote residues in the mature region of GspB. b, comparison of lipid binding of GspB signal peptide variants with PC/CL liposomes. Representative Coomassie-stained SDS-PAGE gels are shown. c, densitometric quantification of signal peptides in gradient fractions from b. Independent experiments were performed in triplicate. Plotted are mean values ± S.D. (error bars).
To further define the residues mediating lipid binding, we generated seven SP-AST variants, each containing 9 or 12 amino acid deletions between residues 8 and 68. Upon incubation with CL-containing liposomes, six of the seven constructs showed co-flotation to the top of the gradient, although it was variable across peptides (Fig. 3, B and C). SP-AST Δ8–20 was the only deletion variant with complete loss of co-floatation comparable with levels seen with SP-AST Δ8–68. With this construct, however, we observed that that liposomes did not migrate to the top of the gradient very efficiently, possibly due to permeabilization of the liposomes by the peptide. It was unclear whether other variants, such as Δ20–28, Δ28–36, Δ36–44, and Δ60–68, had subtler but similar effects on liposome integrity and migration, resulting in their more diffuse or inconsistent localization throughout the gradient.
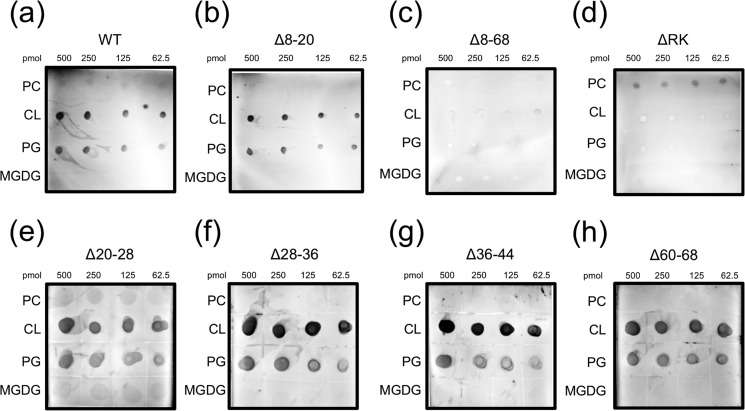
To further investigate these findings, we used a lipid overlay assay to assess protein–lipid interactions. As was seen with the flotation assay, WT SP-AST bound CL and PG, but not MGDG and PC (Fig. 4A). SP-AST Δ8–68 was also screened in this assay, and as was seen with the flotation studies, no lipid binding to anionic lipids was detected (Fig. 4C). SP-AST Δ8–20 displayed strong lipid binding similar to that observed with the WT signal peptide (Fig. 4B), confirming that the low level of lipid binding seen in the flotation assay was likely a technical artifact. All other SP-AST deletion variants screened showed anionic lipid binding using the lipid overlay assay. However, Δ20–28 and Δ60–68 appeared to have reduced binding, indicating lower affinity (Fig. 4, E–H). Taken collectively, cardiolipin binding by the several short deletion variants suggests that multiple areas throughout the signal peptide N-region contribute to membrane binding. However, there was no obvious correlation between charge and strength of binding that could be identified, suggesting that additional properties such as secondary structure may also be important for lipid binding.
Figure 4.
Lipid overlay analysis of signal peptide variants. Lipid binding was assessed for SP-AST variants: WT (a), Δ8–20 (b), Δ8–68 (c), ΔRK (d), Δ20–28 (e), Δ28–36 (f), Δ36–44 (g), and Δ60–68 (h) with PC, CL, PG, and MGDG using a lipid overlay assay and immunoblotting.
To more directly assess the importance of the basic residues in the N-region for lipid binding, all lysine and arginine residues were mutated to alanine, except at residue 69, to yield the SP-AST ΔRK variant. This construct also displayed no binding to CL in the co-flotation assay (Fig. 3, B and C) or the lipid overlay assay (Fig. 4D). When protein–lipid co-flotation assays were performed in high-salt conditions (0.5 m NaCl), lipid binding by WT SP-AST was significantly reduced (Fig. 3, B and C). Collectively, these data indicate that signal peptide interactions with anionic lipids are mediated by charge–charge interactions (Fig. 3).
aSec transport proteins also selectively bind anionic lipids
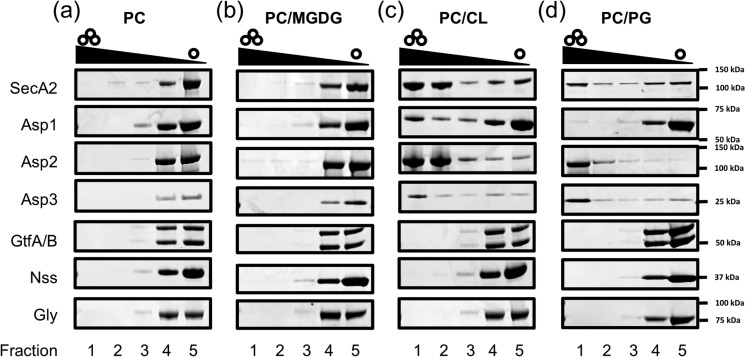
Our finding of specific binding by the GspB preprotein with negatively charged lipids suggests that the signal peptide is able to promote trafficking of GspB to the cell membrane, where it can more readily co-localize with SecA2 and the aSec translocon. This assumes that the preprotein and the aSec system co-localize to the same type of lipids in the cell membrane, but it is unknown whether any of the aSec proteins preferentially associate with anionic lipids. For that reason, we examined lipid binding of each cytosolic aSec protein required for WT GspB biogenesis (Fig. 5 and Fig. S3). Interestingly, a clear demarcation was observed. SecA2, Asp1, Asp2, and Asp3 were all found to bind CL and PG, but not PC or MGDG, thus resembling what was seen with GspBvD. In contrast, the four proteins needed for GspB glycosylation (GtfA, GtfB, Nss, and Gly) showed no binding to any of the four lipids tested. Thus, only proteins required for GspB transport bound anionic lipids, whereas those mediating its post-translation glycosylation did not.
Figure 5.
All aSec proteins required for transport bind anionic lipids, but not the SRR protein-specific glycosyltransferases. Shown is lipid binding of aSec transport and glycosylation proteins with PC liposomes (a), PC/MGDG liposomes (b), PC/CL liposomes (c), and PC/PG liposomes (d). Representative Coomassie-stained SDS-polyacrylamide gels are shown.
Relative to SecA2, Asp2, and Asp3, Asp1 showed low binding to CL and PG in these studies. Asp1 is also known to complex with Asp2 and Asp3 (26, 27), perhaps enhancing its lipid localization in vivo. Interestingly, Asp1 also showed high PA binding (data not shown), suggesting that PA may promote membrane localization of Asp1, instead of CL or PG, in S. gordonii. Collectively, these results highlight a likely additional role of anionic lipids in coordinating the individual aSec system proteins at the membrane interface for transport.
Preprotein–lipid binding by the substrate is required for SecA2 cross-linking
Because the aSec transport proteins and GspB preprotein can preferentially localize to negatively charged lipids, we asked whether lipid binding by the preprotein is necessary for an interaction with the transport machinery. To address this issue, we examined the binding of GspBvD-Bpa with SecA2 in E. coli, as measured by in vivo UV cross-linking with p-benzoylphenylalanine (Bpa). This unnatural amino acid is incorporated in the early N-terminal mature region at residue Gln-94 of GspBvD using an amber suppressor tRNA/tRNA synthetase pair (18, 28). After protein induction, cells were treated with UV light, and cross-linking was subsequently detected by Western blot analysis for GspBvD.
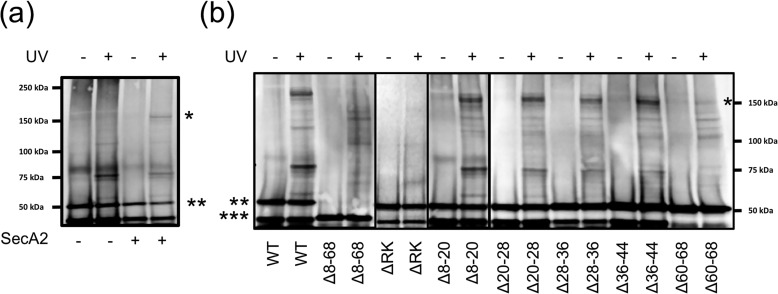
We compared SecA2 cross-linking to GspBvD constructs containing the WT signal peptide or signal peptide variants that showed moderate and significant changes in lipid binding (Fig. 2A). As we have shown previously, the GspBvD preprotein specifically cross-linked to SecA2, even in the absence of the other aSec components (Fig. 6A) (18). In contrast, the GspBvD Δ8–68 preprotein did not cross-link with SecA2 (Fig. 6B), suggesting that membrane binding by the preprotein is needed for subsequent interaction with the ATPase. GspBvD variants with deletions Δ8–20, Δ20–28, Δ28–36, or Δ36–44 cross-linked to SecA2 comparably with the WT protein (Fig. 5B). However, GspBvD Δ60–68 cross-linking to SecA2 was reduced, and GspBvD ΔRK displayed no cross-linking to SecA2 (Fig. 6B). Thus, preprotein variants with signal peptide modifications resulting in no lipid binding showed a total loss of SecA2 cross-linking. This indicates that membrane binding by the preprotein is essential for trafficking to SecA2 and the aSec translocon.
Figure 6.
Lipid binding by the GspB signal peptide is required for SecA2 to bind GspBvD in vivo. a, cross-linking of Bpa-labeled GspBvD in E. coli (GspBvD-Bpa) with or without SecA2 expression and with or without UV treatment. b, cross-linking of GspBvD-Bpa mutants with SecA2 in E. coli with or without UV treatment. GspBvD and cross-linked products were detected by Western blot analysis using an anti-FLAG antibody as described under “Experimental procedures.” *, SecA2-GspBvD-Bpa cross-linked product; **, all GspBvD-Bpa variants except Δ8–68; ***, GspBvD-Bpa Δ8–68. Splice sites are indicated by thick black lines after lanes 4, 6, and 8.
Loss of preprotein membrane binding results in loss of GspB transport in S. gordonii
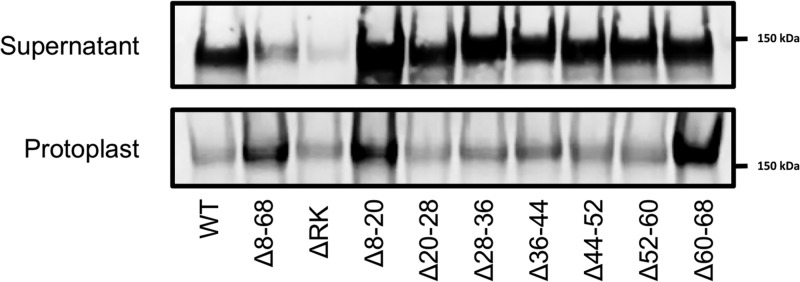
As shown above, all cytosolic aSec proteins required for transport can bind anionic lipids. It is thus possible that one or more of these proteins may facilitate the trafficking of GspB to the membrane, allowing for transport even when a preprotein contains a signal peptide defective in lipid binding. To directly assess the requirement of preprotein lipid binding by the signal peptide for aSec export in S. gordonii, the above signal peptide mutations were incorporated into GspB736FLAG, which is a truncated form of GspB encoding the first 736 amino acids of the WT protein and a C-terminal 3× FLAG tag (Fig. 1A). Export of this substrate was examined by Western blot analysis of streptococcal protoplasts and culture supernatants. As shown in Fig. 7, transport of GspB736FLAG Δ8–68 was significantly reduced relative to transport of WT GspB736FLAG, as indicated by retention of the substrate in the cytosol and loss in the culture supernatants. The transport of the other signal peptide deletion mutants was largely intact, although there was cytosolic accumulation of the Δ8–20 and Δ60–68 variants. GspB736FLAG ΔRK protein levels were reduced relative to other constructs, both secreted and cytosolic protein, suggesting that this mutation results in reduced expression levels or stability. Compared with the WT preprotein, however, export of the substrate was reduced, with increased levels of the glycoprotein in the protoplasts, similar to what was seen with GspB736FLAG Δ8–68. These findings indicate that direct membrane binding by the preprotein is a prerequisite for transport by the aSec system, even in the presence of all other aSec proteins, and that this binding is mediated by charge–charge interactions.
Figure 7.
Membrane binding of the signal peptide is associated with efficient transport of GspB736FLAG in S. gordonii. Shown is export of GspB736FLAG carrying signal peptide variants in S. gordonii M99. Protein was detected by Western blot analysis using an anti-FLAG antibody.
Discussion
Previous studies have shown that substrate export via the aSec pathway requires a specialized signal peptide and that the hydrophobic core of this domain is specifically tailored for aSec transport. Although the N-region of the signal peptide was also known to be important for export, it was unclear which aspects of transport involved this domain. In addition, although the accessory Sec proteins Asp1 to Asp3 were shown to be essential for aSec transport, previous studies had provided conflicting results as to whether they were important for substrate trafficking.
Our current work demonstrates that the signal peptide is necessary and sufficient for the interaction of the GspB preprotein with liposomes, provided the phospholipid content of the liposomes mimics that of streptococcal membranes. In particular, we found that the negatively charged phospholipids, which comprise at least 89% of the total phospholipids in the M99 strain membrane, are essential for signal peptide binding to membrane lipids. Of note, the lipid compositions needed for efficient binding shown here mirror levels of CL in streptococci. Binding of SecA2 and the Asp proteins was robust with liposomes containing 25 mol % CL, a concentration within the range seen in vivo in Streptococcus species.
Furthermore, the preprotein–lipid interaction did not require the aSec transport proteins, including SecA2 or Asp1 to Asp3. These findings indicate that in vivo, the signal peptide mediates preprotein trafficking to the bacterial membrane and that the above aSec transport proteins do not function as chaperones. Instead, they are likely to mediate subsequent events in aSec transport.
Trafficking to liposomes depended on the N-region of the native signal peptide and was likely to occur via electrostatic interactions with anionic phospholipids. We found that high salt conditions or modification of GspB signal peptides by mutating basic residues or significantly shortening the N-region (i.e. resembling canonical signal peptides) resulted in markedly reduced binding to liposomes. Moreover, these mutations abrogated aSec transport in vivo, indicating that these electrostatic interactions are an important prerequisite for export. Only deletion of the majority of the N-region (residues 8–68) or elimination of most basic residues resulted in significant loss of signal peptide–anionic lipid interactions, highlighting the importance of electrostatic interactions for protein–lipid binding. Of note, the membrane trafficking properties of the N-region are distinct from those described for the hydrophobic core of the signal peptide, where conserved glycine residues within this domain are essential for targeting GspB specifically to the aSec system. It appears, therefore, that different regions of the signal peptide can be functionally mapped to distinct events in aSec transport. The extended length and high saturation of positive charge in the N-region of the GspB signal peptide are conserved properties across the family of SRR glycoproteins, and because the predominant phospholipids of all streptococcal and staphylococcal bacteria that express aSec systems are also CL and/or PG (24, 25, 29–31), binding to anionic lipids may be a general property of SRR glycoprotein signal peptides.
Like the GspB preprotein, SecA2 and Asp1 to Asp3 also preferentially interacted with liposomes containing anionic phospholipids. This suggests that membrane binding may not only result in the co-localization of the substrate with SecA2, but may also promote organization of the accessory proteins at the membrane with the translocon. This clustering of the substrate and aSec proteins is likely to facilitate interactions that are required for transport. Supporting this possibility, previous studies have found SecA2 to be predominantly membrane-associated in vivo (32, 33), suggesting that GspB is most likely to interact with SecA2 at that location. In addition, our cross-linking studies have shown that, although the AST domain of GspB can interact with SecA2, full engagement of this region requires Asp1 to Asp3, and that this more extensive interaction is linked to transport (18). The results presented here are consistent with previously published data demonstrating that Asp1 to Asp3 interact directly with SecA2 and the mature region of the GspB preprotein to facilitate one or more post-targeting steps in translocation (19, 27, 32, 34). Thus, the anionic phospholipids are likely to contribute to aSec export by enhancing membrane localization of the substrate with the export machinery.
Selective transport of SRR glycoproteins by the aSec system is essential for their proper post-translational glycosylation and acetylation, which are required for their optimal function as adhesins (13, 22). Because some components of the aSec system are homologs of the Sec pathway, the aSec substrates have had to evolve mechanisms to establish selective trafficking and targeting to the aSec system. The unique features of the N-region and hydrophobic core of SRR glycoproteins may have evolved to ensure correct trafficking and recognition by their dedicated transport system. We have previously shown that glycine residues in the hydrophobic core are required for targeting the substrate specifically to the aSec system (23). However, these residues result in reduced signal peptide hydrophobicity, which may negatively impact lipid binding. This loss of hydrophobicity may be countered by the high positive charge of the elongated N-region, which drives membrane localization by enhancing electrostatic interactions. Interestingly, signal peptides of SecA transported preproteins of E. coli have also been shown to interact with lipid membranes (35–38). Unlike the GspB signal peptide, this interaction is largely mediated by the hydrophobicity of the hydrophobic core, with minor contribution of the short N-region. Thus, although the signal peptides of SecA and SecA2 substrates may both interact with lipids, the primary mechanism by which they do so is very different.
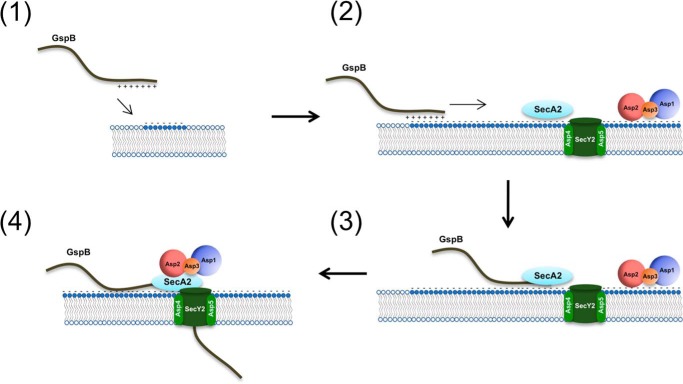
Based on the findings presented here, we propose the following model for aSec transport (Fig. 8). First, the signal peptide of the GspB preprotein binds anionic lipids at the cell membrane through electrostatic interactions (1), where it can laterally diffuse until it encounters SecA2, which is already docked at the membrane, along with Asp1 to Asp3 (2). SecA2 engages with the hydrophobic core of the signal peptide and N terminus of the AST domain (3), after which Asp1 to Asp3 in concert promote SecA2 engagement with the full AST domain of the preprotein, thereby triggering activation of translocation (4). Ongoing experiments will help to further refine the roles of the individual aSec components in this process.
Figure 8.
Model of aSec system transport. GspB traffics (1) and binds directly (2) to the cell membrane via electrostatic interactions with anionic lipids, where it subsequently interacts with SecA2 (3). Asp1 to Asp3 docked at the membrane, in concert, promote engagement of SecA2 with the entire AST domain of GspB to initiate transport (4).
Experimental procedures
Reagents
All lipids were purchased from Avanti Lipids (Alabaster, AL) in chloroform. Lipids include 18:1 PG, 18:1 CL, MGDG, and 18:1 PC. OptiPrep was purchased from Axis-Shield (Oslo, Norway). Bpa was purchased from Bachem (Torrance, CA).
Strains and growth conditions
All plasmids and strains used in this study are listed in Tables 1 and 2, respectively. S. gordonii strains were grown in Todd–Hewitt broth in 5% CO2 at 37 °C. E. coli TOP10 and XL10-Gold strains served as hosts for plasmid cloning. E. coli strains were grown at 37 °C under aeration in Luria–Bertani (LB) broth or Terrific broth, as noted. When appropriate, the following antibiotics were added to the medium at the indicated concentrations for E. coli: 100 μg/ml ampicillin, 30 μg/ml chloramphenicol (or 15 μg/ml chloramphenicol for E. coli–S. gordonii shuttle vectors), 50 μg/ml kanamycin, 400 μg/ml erythromycin, or 50 μg/ml spectinomycin. When appropriate, the following antibiotics were added to the medium at the indicated concentrations for S. gordonii: 100 μg/ml spectinomycin, 5 μg/ml chloramphenicol, or 15 μg/ml erythromycin.
Table 1.
Plasmids used in the present study
| Plasmids | Relevant characteristics | Reference or source |
|---|---|---|
| pVA891 | Streptococcal integration shuttle vector | Ref. 47 |
| pB736FLAG-R | gspB736FLAG and pVA891 rescued from PS919 | Ref. 16 |
| pB736FLAG-R Δ8–20 | pB736FLAG-R with deletion of codons 8–20 | This study |
| pB736FLAG-R Δ20–28 | pB736FLAG-R with deletion of codons 20–28 | This study |
| pB736FLAG-R Δ28–36 | pB736FLAG-R with deletion of codons 28–36 | This study |
| pB736FLAG-R Δ36–44 | pB736FLAG-R with deletion of codons 36–44 | This study |
| pB736FLAG-R Δ44–52 | pB736FLAG-R with deletion of codons 44–52 | This study |
| pB736FLAG-R Δ52–60 | pB736FLAG-R with deletion of codons 52–60 | This study |
| pB736FLAG-R Δ60–68 | pB736FLAG-R with deletion of codons 60–68 | This study |
| pB736FLAG-R Δ8–68 | pB736FLAG-R with deletion of codons 8–68 | Ref. 16 |
| pB736FLAG-R ΔRK | pB736FLAG-R with Ala substitutions of Lys and Arg codons between codons 1–68 | This study |
| pCDFduet-1 | E. coli T7 expression vector | Novagen |
| pCDF.GspBvD.His6 | Vector expressing GspBvD-His6 fusion protein | This study |
| pCDF.GspBvD Δ1–90.His6 | Vector expressing GspBvD-His6 fusion protein with deletion of codons 1–90 | This study |
| pET28c | E. coli T7 expression vector | Novagen |
| pET28c.SP-AST.His6 | Vector expressing GspB SP-AST-His6 fusion protein | This study |
| pET28a.SP-AST.His6 Δ8–20 | pET28c.SP-AST.His6 with deletion of codons 8–20 | This study |
| pET28a.SP-AST.His6 Δ20–28 | pET28c.SP-AST.His6 with deletion of codons 20–28 | This study |
| pET28a.SP-AST.His6 Δ28–36 | pET28c.SP-AST.His6 with deletion of codons 28–36 | This study |
| pET28a.SP-AST.His6 Δ36–44 | pET28c.SP-AST.His6 with deletion of codons 36–44 | This study |
| pET28a.SP-AST.His6 Δ44–52 | pET28c.SP-AST.His6 with deletion of codons 44–52 | This study |
| pET28a.SP-AST.His6 Δ52–60 | pET28c.SP-AST.His6 with deletion of codons 52–60 | This study |
| pET28a.SP-AST.His6 Δ60–68 | pET28c.SP-AST.His6 with deletion of codons 60–68 | This study |
| pET28a.SP-AST.His6 Δ8–68 | pET28c.SP-AST.His6 with deletion of codons 8–68 | This study |
| pET28a.SP-AST.His6 ΔRK | pET28c.SP-AST.His6 with Ala substitutions of Lys and Arg codons between codons 1–68 | This study |
| pMAL-c2x | E. coli T7 expression vector | New England Biolabs |
| pMAL.MBP.His6.SecA2 | Vector expressing MBP-His6-SecA2 fusion protein | This study |
| pET21b | E. coli T7 expression vector | Novagen |
| pET21b.Asp1.GST.His6 | Vector expressing Asp1-GST-His6 fusion protein | Ref. 26 |
| pMal.Asp2-S362A | Vector expressing MBP-Asp2 fusion protein | Ref. 22 |
| pQE-TriSystem His-Strep 2 | E. coli expression vector | Qiagen |
| pET28c.StrepII.Asp3.His8 | Vector expressing StrepII-Asp3-His8 fusion protein | This study |
| pBAD.HisA | E. coli expression vector | Invitrogen |
| pBAD.His6.GtfAB | Vector expressing His6-GtfA and GtfB complex | Ref. 19 |
| pET28a | E. coli T7 expression vector | Novagen |
| pET28a.His6.Nss | Vector expressing His6-Nss fusion protein | This study |
| pET28a.His6.Gly | Vector expressing His6-Gly fusion protein | This study |
| pET28c.SecA2 | Vector expressing His6-SecA2 fusion protein | Ref. 39 |
| pEVOL.pBpF | Vector expressing amber codon tRNA synthetase/tRNA pair | Ref. 48 |
| pCDF.GspBvD-Q94X | Vector expressing GspBvD with TAG amber codon at codon 94 | Ref. 18 |
| pCDF.GspBvD-Q94X Δ8–20 | pCDF.GspBvD-Q94X with deletion of codons 8–20 | This study |
| pCDF.GspBvD-Q94X Δ20–28 | pCDF.GspBvD-Q94X with deletion of codons 20–28 | This study |
| pCDF.GspBvD-Q94X Δ28–36 | pCDF.GspBvD-Q94X with deletion of codons 28–36 | This study |
| pCDF.GspBvD-Q94X Δ36–44 | pCDF.GspBvD-Q94X with deletion of codons 36–44 | This study |
| pCDF.GspBvD-Q94X Δ60–68 | pCDF.GspBvD-Q94X with deletion of codons 60–68 | This study |
| pCDF.GspBvD-Q94X Δ8–68 | pCDF.GspBvD-Q94X with deletion of codons 8–68 | This study |
| pCDF.GspBvD-Q94X ΔRK | pCDF.GspBvD-Q94X with Ala substitutions of Lys and Arg codons between codons 1 and 68 | This study |
Table 2.
Strains used in the present study
| Strains | Relevant characteristicsa | Reference or source |
|---|---|---|
| S. gordonii | ||
| M99 | Parental stain | |
| PS846 | M99 ΔgspB::pEVP3, Cmr | Ref. 16 |
| PS1740 | M99 ΔgspB | Ref. 12 |
| PS961 | PS846 ΔgspB::pB736FLAG-R, Cmr Ermr | Ref. 16 |
| PS3701 | PS1740 ΔgspB::pB736FLAG-R Δ8–20 Ermr | This study |
| PS3051 | PS1740 ΔgspB::pB736FLAG-R Δ20–28, Ermr | This study |
| PS3702 | PS846 ΔgspB::pB736FLAG-R Δ28–36, Cmr Ermr | This study |
| PS3052 | PS1740 ΔgspB::pB736FLAG-R Δ36–44, Ermr | This study |
| PS3703 | PS846 ΔgspB::pB736FLAG-R Δ44–52, Cmr Ermr | This study |
| PS3704 | PS846 ΔgspB::pB736FLAG-R Δ52–60, Cmr Ermr | This study |
| PS3705 | PS846 ΔgspB::pB736FLAG-R Δ60–68, Cmr Ermr | This study |
| PS1027 | PS846 ΔgspB::pB736FLAG-R Δ8–68, Cmr Ermr | Ref. 16 |
| PS3706 | PS1740 ΔgspB::pB736FLAG-R ΔRK, Ermr | This study |
| E. coli | ||
| TOP10 | Host for cloning | Invitrogen |
| XL10-Gold | Host for cloning | Agilent |
| BL21(DE3) | F− ompT gal dcm lon hsdSB (rB−mB−)λ (DE3 [lacI lacUV5-T7p07 ind1 sam7 nin5]) [malB+]K-12 (λS) | Novagen |
| C43(DE3) | F− ompT gal dcm hsdSB (rB− mB−) (DE3) | Lucigen |
a Cmr, chloramphenicol resistance; Ermr, erythromycin resistance.
DNA manipulations
Plasmid DNA isolation was performed using the QIAprep spin miniprep kit (Qiagen). T4 DNA ligase and restriction enzymes were purchased from New England Biolabs and used according to the manufacturer's recommendations. PCR mutagenesis was performed using QuikChange II XL (Agilent) or QuikChange Lightning (Agilent), per the manufacturer's protocol. Chemically competent E. coli BL21(DE3) or C43(DE3) cells were generated using the Mix & Go E. coli transformation kit (ZymoResearch) and transformed as recommended by the manufacturer. All primers used for cloning or mutagenesis are listed in Table 3. S. gordonii was transformed by competence–induction as described previously (12).
Table 3.
Primers used in the present study
| Primers | Sequence (5′–3′) |
|---|---|
| Gf_His6 insert F | actacaaggatgacgatgacaagcaccaccaccaccaccactaaaattaattaacctaggctgc |
| Gf_His6 insert R | gcagcctaggttaattaattttagtggtggtggtggtggtgcttgtcatcgtcatccttgtagt |
| Gf Δ1–90 F | ttaagtataagaaggagatatacatatggaagaggaacaagcacatgaa |
| Gf Δ1–90 R | ttcatgtgcttgttcctcttccatatgtatatctccttcttatacttaa |
| SP_AST F | accatccatggtttttaaacgtcaaaagggtc |
| SP_AST R | gccggatcctcgagtgtggatgacaaagtagtt |
| Gf Δ8–20 F | catatggtttttaaacgtcaaaagctgattaagtctgggaaacactgg |
| Gf Δ8–20 R | ccagtgtttcccagacttaatcagcttttgacgtttaaaaaccatatg |
| Gf Δ20–28 F | gaacgtgttacgcgttttcttcgtgcggctaca |
| Gf Δ20–28 R | tgtagccgcacgaagaaaacgcgtaacacgttc |
| Gf Δ28–36 F | aactgattaagtctgggaaacacggacttttaagattaatgaaggg |
| Gf Δ28–36 R | cccttcattaatcttaaaagtccgtgtttcccagacttaatcagtt |
| Gf Δ36–44 F | tcgtgcggctacatctcaatctgatgtttcttccac |
| Gf Δ36–44 R | gtggaagaaacatcagattgagatgtagccgcacga |
| Gf Δ44–52 F | tggacttttaagattaatgaagaaggtagtggaggagcagtc |
| Gf Δ44–52 R | gactgctcctccactaccttcttcattaatcttaaaagtcca |
| Gf Δ52–60 F | aggtaattcagtccacttttttcttctgtggaagaaacatcagaac |
| Gf Δ52–60 R | gttctgatgtttcttccacagaagaaaaaagtggactgaattacct |
| Gf Δ60–68 F | gtagtggaggagcagtctaaaggaattatagcgacg |
| Gf Δ60–68 R | cgtcgctataattcctttagactgctcctccactac |
| Gf Δ8–68 F | gatataccatgttttttaaacgtcaaaagaaaggaattatactactaggagc |
| Gf Δ8–68 R | gctcctagtagtataattcctttcttttgacgtttaaaaaacatggtatatc |
| SacI_His6_AvaI F | gactaattcgagctcgcaccaccaccaccaccaccaccacctcgggatcgaggga |
| SacI_His6_AvaI R | tccctcgatcccgaggtggtggtggtggtggtggtgcgagctcgaattagtc |
| BamHI_Asp3 | actcgggatccgaagattcaaaaacataaggaaatttactg |
| Asp3_HindII | taataagcttaccatttgactcctctaaaatttcttc |
| rNss1 | tagaccatggaagttaatattaccaatcta |
| rNss2 | cagactcgagggacaagacttccatcaccg |
| rGly1 | agttggatccgtggataagttagagaataa |
| rGly2 | ttttctcgagcttcattcctgagccagatt |
Generation of recombinant GspB variant D WT and Δ1–90
GspB variant D (GspBvD) WT and Δ1–90 were expressed and purified as C-terminal 3× FLAG–His6 fusion proteins. Generation of pCOLA.GspBvD, which included a 3× FLAG tag, was described previously (18). The sequence for a C-terminal His6 tag was incorporated by PCR mutagenesis using primers Gf_His6 insert F and Gf_His6 insert R to generate pCOLA.GspBvD.His6. The insertion was confirmed by DNA sequencing. Deletion of codons 1–90 was achieved by PCR mutagenesis using primers Gf Δ1–90 F and Gf Δ1–90 R to generate plasmid pCOLA.GspBvDΔ1–90.His6. The deletion was confirmed by DNA sequencing. To generate pCDF.GspBvD.His6 and pCDF.GspBvDΔ1–90.His6, pCDFduet-1, pCOLA.GspBvD.His6, and pCOLA.GspBvDΔ1–90.His6 were digested with NdeI and PacI. The GspBvD inserts were ligated into pCDFduet-1 and confirmed by restriction digest and sequencing. The resulting plasmids (pCDF.GspBvD.His6 and pCDF.GspBvDΔ1–90.His6) were transformed into BL21(DE3) cells. Cells were grown in Terrific broth, and protein expression was induced using 1 mm IPTG at 37 °C for 3 h. Cells were pelleted and lysed in wash buffer (25 mm Tris-HCl (pH 8), 0.15 m KCl) containing 8 m urea and 1× complete protease inhibitor (Roche Applied Science) by sonication on ice. Lysates were clarified by centrifugation at 30,000 × g for 30 min at 4 °C. Clarified lysates were incubated with pre-equilibrated nickel-nitrilotriacetic acid resin at 4 °C. The resin was washed with wash buffer containing 8 m urea and 25 mm imidazole. Protein was eluted with wash buffer containing 8 m urea and 0.3 m imidazole, concentrated using a 10-kDa Amicon centrifugal concentrator (MilliporeSigma), and desalted into wash buffer containing 4 m urea.
Generation of recombinant WT SP-AST and variants
The GspB SP-AST WT and variant forms were expressed as His6 fusion proteins. To generate the pET28c.SP-AST.His6 expression plasmid, the previously described plasmid pB736FLAG-R (16) was used to PCR-amplify codons 1–125 using primers SP_AST F and SP_AST R. pET28c and the PCR product were digested with NcoI-HF and XhoI and ligated. sp-ast variants Δ8–68, containing deletion of codons 8–68, and ΔRK, containing mutation of all Lys and Arg codons between codons 1 and 68 to Ala codons, were synthesized using GeneArt Strings DNA Fragments (Thermo Fisher Scientific). DNA fragments were digested and ligated into pET28c as described above. All plasmids were screened by restriction digest and sequencing. Plasmids containing 9 or 12 codon deletions, sp-ast variants Δ8–20, Δ20–28, Δ28–36, Δ36–44, Δ44–52, Δ52–60, and Δ60–68, were generated by PCR mutagenesis using primers in Table 3. Plasmids were screened by DNA sequencing and were transformed into C43(DE3). Proteins were expressed and purified as described above for GspBvD, except Terrific broth was used as the culture medium for protein expression.
Generation of recombinant SecA2
SecA2 was expressed as a fusion protein with N-terminal maltose-binding protein (MBP) and His6 tags. Previously, secA2 was cloned into pMAL-c2x to generate pMAL.SecA2 (39). A His6 tag sequence was incorporated between SecA2 and the Factor Xa cleavage site using primers SacI_His6_AvaI F and SacI_His6_AvaI R. Primers and pMAL.SecA2 were cleaved with Aval and SacI-HF and ligated to generate pMAL.MBP.His6.SecA2. Plasmids were screened by sequencing, and BL21(DE3) cells were subsequently transformed. Cells were grown in Terrific broth, and protein expression was induced using 1 mm IPTG at 16 °C for 18 h. Cells were pelleted and lysed in wash buffer containing 1× complete protease inhibitor tablet by sonication on ice. Lysates were clarified by centrifugation at 30,000 × g for 30 min at 4 °C. Clarified lysates were incubated with amylose resin (New England Biolabs) at 4 °C. Resin was washed with wash buffer, and protein was eluted with wash buffer containing 25 mm maltose. Protein was concentrated using a 100-kDa Amicon centrifugal concentrator. The MBP and histidine tags were removed by incubation with Factor Xa (New England Biolabs) overnight at room temperature. SecA2 was concentrated and partially purified by precipitation using ammonium sulfate (50% final concentration) and then solubilized in wash buffer. Following buffer exchange into 20 mm Tris-HCl (pH 8), 20 mm KCl using a Zeba spin desalting column (Thermo Fisher), SecA2 was further purified by anion-exchange chromatography using a Mini Macro-Prep High Q column (Bio-Rad). SecA2-containing fractions were combined and concentrated using a 100-kDa Amicon centrifugal concentrator followed by buffer exchange into wash buffer.
Generation of recombinant Asp1
Asp1 was expressed as a GST and His6 fusion protein. Previously, asp1 was cloned into pET21b to generate pET21b.Asp1.GST.His6 (26). Plasmids were screened by sequencing, and BL21(DE3) cells were subsequently transformed. Cells were grown in Terrific broth, and protein expression was induced using 1 mm IPTG at 16 °C for 18 h. Cells were collected by centrifugation and lysed in wash buffer containing 1× complete protease inhibitor tablet (Roche Applied Science) by sonication on ice. Lysates were clarified by centrifugation at 30,000 × g for 30 min at 4 °C. Clarified lysates were incubated with GSH Sepharose at 4 °C. The resin was washed with wash buffer, and protein was eluted with wash buffer containing 25 mm GSH. Protein was concentrated using a 100-kDa Amicon centrifugal concentrator. The GST and His6 tags were removed by incubation with thrombin overnight at 4 °C. Asp1 was concentrated and buffer-exchanged into 20 mm Tris-HCl (pH 8), 20 mm KCl. Asp1 was further purified by anion-exchange chromatography as described for SecA2. Asp1 fractions were combined and concentrated using a 50-kDa Amicon centrifugal concentrator and buffer-exchanged into wash buffer.
Generation of recombinant Asp2
Asp2 is a bifunctional protein, essential for both the post-translational acetylation of GspB and its transport via the accessory Sec system. The S362A mutation of Asp2 abolishes its acetyltransferase activity (22) but has no impact on GspB transport. Because the catalytic mutant can be expressed and purified more readily, we used the S362A variant for binding studies. Asp2 was expressed as an MBP fusion protein. The Asp2 expression vector pMAL.Asp2-S362A was generated as described previously (22), and the protein was expressed in BL21(DE3) cells grown in Terrific broth. Protein expression and purification were performed using amylose resin and anion-exchange chromatography as detailed for SecA2 above, except no ammonium sulfate precipitation or Factor Xa cleavage was performed.
Generation of recombinant Asp3
Asp3 was expressed as an N-terminal StrepII and C-terminal His8 fusion protein. Asp3 was cloned into the pQE-TriSystem His-Strep 2 vector (Qiagen) The asp3 gene in plasmid pET28.His6.Asp3 (27) was PCR-amplified using primers BamHI_Asp3 and Asp3_HindII. The vector and PCR product were digested with BamHI-HF and HindIII-HF and ligated to generate pQE2.StrepII.Asp3.His8. Plasmids were screened by restriction digest and sequencing. pQE2.StrepII.Asp3.His8 and pET28c were digested with NcoI-HF and XhoI. The Asp3-containing fragment and pET28c were ligated, and plasmids were screened by restriction digest. Asp3 was expressed in BL21(DE3). Cells were grown in LB broth, and protein expression was induced using 1 mm IPTG at 16 °C for 18 h. Cells were pelleted and lysed in wash buffer containing 1× complete protease inhibitor tablet (Roche Applied Science) by sonication on ice. Lysates were clarified by centrifugation at 30,000 × g for 30 min at 4 °C. Clarified lysates were incubated with pre-equilibrated streptactin resin (Qiagen) at 4 °C. The resin was washed with wash buffer, and protein was eluted with wash buffer containing 5 mm desthiobiotin. Protein was concentrated using a 10-kDa Amicon centrifugal concentrator and buffer-exchanged into wash buffer.
Generation of recombinant GtfAB, Nss, and Gly
The GtfAB complex with a His6 tag was purified as described previously (19). The nss gene was amplified from the M99 chromosome using primers rNss1 and rNss2. The PCR product and pET28a were digested with NcoI and XhoI and ligated to generate pET28a.Nss.His6. Plasmids were screened by restriction digest and sequencing. The gly gene was amplified from the M99 chromosome using primers rGly1 and rGly2. The PCR product and pET28a were digested with BamHI and XhoI and ligated to generate pET28a.His6.Gly. Plasmids were screened by restriction digest and sequencing. Nss and Gly were expressed in BL21(DE3) cells grown in LB broth. Protein expression was induced using 1 mm IPTG at 37 °C for 3 h. Cells were pelleted and lysed in wash buffer containing 1× complete protease inhibitor by sonication on ice. Lysates were clarified by centrifugation at 30,000 × g for 30 min at 4 °C. Clarified lysates were incubated with pre-equilibrated nickel-nitrilotriacetic acid resin at 4 °C. The resin was washed with wash buffer containing 25 mm imidazole. Protein was eluted with wash buffer containing 0.3 m imidazole, concentrated using a 10-kDa Amicon centrifugal concentrator, and desalted into wash buffer.
Liposome preparation
CL is the predominant phospholipid in streptococcal membranes and has been shown to constitute between 18 and 40% of the total membrane lipids (40–45). However, PG dominates in staphylococcal membranes constituting 60% of total lipids (46). Based on orcinol staining of M99 glycolipids, MGDG constitutes 33% of the total glycolipids. In order to closely approximate the molar percentage of these lipids found in vivo, liposome compositions were generated in the indicated molar ratios: PC, 3:1 PC/MGDG, 3:1 PC/CL, and 1:3 PC/PG. Lipids in chloroform were dried using vacuum centrifugation. Lipids were resuspended in 20 mm HEPES (pH 7), 150 mm KCl by incubating at room temperature for 1 h with occasional vortexing to yield a total lipid stock concentration of 50 mm. Unilamellar liposomes were generated by extrusion using the Avanti Mini Extruder (Avanti Lipids) and 0.1-μm polycarbonate membranes. Lipids were stored no longer than 3 days at 4 °C.
Protein–lipid co-flotation assay
Protein was incubated with 4 mm liposomes, as indicated, at room temperature in 20 mm HEPES (pH 7), 150 mm KCl for at least 15 min to equilibrate. Optiprep stock (60%) was added to the protein–lipid mix to make a final 40% Optiprep solution. Optiprep dilutions for the gradient were made in 20 mm HEPES (pH 7). An Optiprep discontinuous gradient were generated by underlaying a 10% solution, 30% solution, and the 40% protein–lipid solution. For high-salt conditions, protein was incubated with lipids in 20 mm HEPES (pH 7), 500 mm NaCl for at least 15 min to equilibrate. Optiprep dilutions for the gradient were made in 20 mm HEPES (pH 7), 500 mm NaCl. Samples were centrifuged for 3 h at 55,000 rpm using a TLA-110 rotor at 4 °C. Five fractions were collected, and proteins were precipitated with TCA (10% final). Samples were centrifuged at 16,000 × g for 5 min. Proteins were resuspended in 1× LDS loading buffer (Life Technologies, Inc.), and 25% of the sample was analyzed by SDS-PAGE using 4–12% BisTris gels (Novex). GspBvD WT and GspBvD Δ1–90 were analyzed by Western blotting using a mouse anti-FLAG antibody (Sigma) and the HiLyte Fluor750 or HiLyte Fluor680 anti-mouse IgG secondary antibody (AnaSpec). For all other proteins (SP-AST variants and aSec proteins), SDS-polyacrylamide gels were stained using SimplyBlue Safestain. All samples were imaged using a LI-COR IR imager (LI-COR Biosciences). Protein quantitation was performed by densitometry of band intensity using ImageJ and normalized as a percentage of the total across all fractions. All experiments were performed in at least triplicate except for binding experiments with PC/MGDG liposomes, which were done in duplicate.
Lipid overlay assay
Lipids in chloroform were spotted onto nitrocellulose membranes at the indicated quantities. Membranes were blocked with 3% BSA in PBS, followed by probing with 20 μg of purified signal peptide variants as indicated. Membranes were washed with PBS, probed with a mouse anti-His6 antibody (GenScript) and with HiLyte Fluor750 anti-mouse IgG antibody. Protein binding was analyzed by imaging using a LI-COR IR imager.
Generation of GspBvD signal peptide variants with Q94X amber codon
The plasmid pCDF.GspBvD-Q94X was used to express GspBvD containing an amber suppression codon at position 94 (18). All deletion variants (Δ8–20, Δ20–28, Δ28–36, Δ36–44, Δ60–68, and Δ8–68) were generated by PCR mutagenesis using the same primers described for sp-ast mutagenesis. The ΔRK variant, which has all Lys and Arg codons between codons 1 and 68 mutated to Ala codons, was synthesized using GeneArt Gene Synthesis (Thermo Fisher Scientific), digested using NdeI and PacI, and ligated into pCDFduet-1. All constructs were confirmed by sequencing.
UV cross-linking of GspBvD in E. coli
pEVOL-pBpF was a gift from Peter Schultz (Addgene plasmid 31190). Plasmids pEVOL.pBpF, pET28c.SecA2, and pCDF.GspBvD-Q94X WT or variants were transformed into C43(DE3) cells. Overnight cultures were grown in LB broth and diluted 20-fold into fresh medium. After 2 h of growth at 37 °C, 0.1 mm Bpa, 0.02% arabinose, and 1 mm IPTG were added to induce expression of the amber suppression tRNA synthetase, GspBvD-Bpa, and SecA2 at 37 °C for 2 h. Cross-linking was initiated by UV irradiation with a 365-nm lamp for 20 min in a 96-well plate on ice. Control samples that were not UV-treated were left in ambient light. Cells were lysed by resuspending in 1× LDS sample buffer. Samples were analyzed by Western blot analysis using an anti-FLAG antibody as described earlier.
Generation of GspB736FLAG signal peptide variants
The plasmid pB736FLAG-R, which contains the gspB736FLAG gene and 1.2-kb upstream sequence in the pVA891 suicide vector, was mutated using PCR and the same primers described above for the SP-AST variants. To generate the pB736FLAG-R ΔRK plasmid, a fragment of the pB736FLAG-R plasmid between restriction sites PciI and HpaI, which includes codons 1–336 of gspb736FLAG, was made using GeneArt Gene Synthesis (Thermo Fisher Scientific) with all Lys and Arg codons between codons 1 and 68 mutated to Ala codons. The gsp736FLAG fragment and pB736FLAG-R were digested using PciI and HpaI and ligated together. The resulting plasmids were screened by restriction digest, and all constructs were confirmed by sequencing. S. gordonii M99 strain PS846 or PS1740 (ΔgspB strains) were transformed with the mutated plasmids as described previously (12).
Analysis of S. gordonii M99 secretion of GspB736FLAG WT and signal peptide variants
Overnight cultures were centrifuged at 16,000 × g to remove cells. Culture supernatants were combined with 4× LDS sample buffer for analysis of secreted protein. To examine protein retained in the cytosol, protoplasts were generated as previously described and lysed by resuspending in 1× LDS buffer (16). All samples were normalized by A600, and GspB736FLAG was detected by Western blot analysis using an anti-FLAG antibody as described earlier.
Author contributions
C. S. and P. M. S. conceptualization; C. S. formal analysis; C. S. and P. M. S. funding acquisition; C. S. and N. N. M. investigation; C. S. visualization; C. S. and P. M. S. writing-original draft; C. S., B. A. B., N. N. M., and P. M. S. writing-review and editing; B. A. B. and N. N. M. methodology; P. M. S. supervision.
Supplementary Material
Acknowledgments
We thank Daisuke Takamatsu for plasmid generation and Peter Schultz for the generous gift of pEVOL-pBpF.
This work was supported by an American Heart Association postdoctoral fellowship (to C. S.); NIAID, National Institutes of Health, Postdoctoral Training Grant 5 T32 AI060537-13 (to C. S.) and Grant R01AI41513 (to P. M. S.); Merck (to N. N. M.); and LA Biomed-Harbor UCLA, Intramural Research Grant 31604-01 (to N. N. M.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S3.
- SRR
- serine-rich repeat
- aSec
- accessory Sec
- Asp
- accessory Sec protein
- AST
- accessory Sec transport
- CL
- cardiolipin
- PG
- phosphatidylglycerol
- PA
- phosphatidic acid
- MGDG
- monoglucosyldiacylglycerol
- PC
- phosphatidylcholine
- Bpa
- p-benzoylphenylalanine
- LB
- Luria–Bertani
- MBP
- maltose-binding protein
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside.
References
- 1. Bensing B. A., López J. A., and Sullam P. M. (2004) The Streptococcus gordonii surface proteins GspB and Hsa mediate binding to sialylated carbohydrate epitopes on the platelet membrane glycoprotein Ibα. Infect. Immun. 72, 6528–6537 10.1128/IAI.72.11.6528-6537.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seo H. S., Mu R., Kim B. J., Doran K. S., and Sullam P. M. (2012) Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PLoS Pathog. 8, e1002947 10.1371/journal.ppat.1002947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Samen U., Eikmanns B. J., Reinscheid D. J., and Borges F. (2007) The surface protein Srr-1 of Streptococcus agalactiae binds human keratin 4 and promotes adherence to epithelial HEp-2 cells. Infect. Immun. 75, 5405–5414 10.1128/IAI.00717-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shivshankar P., Sanchez C., Rose L. F., and Orihuela C. J. (2009) The Streptococcus pneumoniae adhesin PsrP binds to Keratin 10 on lung cells. Mol. Microbiol. 73, 663–679 10.1111/j.1365-2958.2009.06796.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bensing B. A., Khedri Z., Deng L., Yu H., Prakobphol A., Fisher S. J., Chen X., Iverson T. M., Varki A., and Sullam P. M. (2016) Novel aspects of sialoglycan recognition by the Siglec-like domains of streptococcal SRR glycoproteins. Glycobiology 26, 1222–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deng L., Bensing B. A., Thamadilok S., Yu H., Lau K., Chen X., Ruhl S., Sullam P. M., and Varki A. (2014) Oral streptococci utilize a Siglec-like domain of serine-rich repeat adhesins to preferentially target platelet sialoglycans in human blood. PLoS Pathog. 10, e1004540 10.1371/journal.ppat.1004540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seo H. S., Minasov G., Seepersaud R., Doran K. S., Dubrovska I., Shuvalova L., Anderson W. F., Iverson T. M., and Sullam P. M. (2013) Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J. Biol. Chem. 288, 35982–35996 10.1074/jbc.M113.513358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pyburn T. M., Bensing B. A., Xiong Y. Q., Melancon B. J., Tomasiak T. M., Ward N. J., Yankovskaya V., Oliver K. M., Cecchini G., Sulikowski G. A., Tyska M. J., Sullam P. M., and Iverson T. M. (2011) A structural model for binding of the serine-rich repeat adhesin GspB to host carbohydrate receptors. PLoS Pathog. 7, e1002112 10.1371/journal.ppat.1002112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xiong Y. Q., Bensing B. A., Bayer A. S., Chambers H. F., and Sullam P. M. (2008) Role of the serine-rich surface glycoprotein GspB of Streptococcus gordonii in the pathogenesis of infective endocarditis. Microb. Pathog. 45, 297–301 10.1016/j.micpath.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takamatsu D., Bensing B. A., and Sullam P. M. (2004) Four proteins encoded in the gspB-secY2A2 operon of Streptococcus gordonii mediate the intracellular glycosylation of the platelet-binding protein GspB. J. Bacteriol. 186, 7100–7111 10.1128/JB.186.21.7100-7111.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seepersaud R., Bensing B. A., Yen Y. T., and Sullam P. M. (2012) The accessory Sec protein Asp2 modulates GlcNAc deposition onto the serine-rich repeat glycoprotein GspB. J. Bacteriol. 194, 5564–5575 10.1128/JB.01000-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bensing B. A., and Sullam P. M. (2002) An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44, 1081–1094 10.1046/j.1365-2958.2002.02949.x [DOI] [PubMed] [Google Scholar]
- 13. Takamatsu D., Bensing B. A., and Sullam P. M. (2004) Genes in the accessory sec locus of Streptococcus gordonii have three functionally distinct effects on the expression of the platelet-binding protein GspB. Mol. Microbiol. 52, 189–203 10.1111/j.1365-2958.2004.03978.x [DOI] [PubMed] [Google Scholar]
- 14. Takamatsu D., Bensing B. A., and Sullam P. M. (2005) Two additional components of the accessory sec system mediating export of the Streptococcus gordonii platelet-binding protein GspB. J. Bacteriol. 187, 3878–3883 10.1128/JB.187.11.3878-3883.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bandara M., Corey R. A., Martin R., Skehel J. M., Blocker A. J., Jenkinson H. F., and Collinson I. (2016) Composition and activity of the non-canonical Gram-positive SecY2 complex. J. Biol. Chem. 291, 21474–21484 10.1074/jbc.M116.729806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bensing B. A., Takamatsu D., and Sullam P. M. (2005) Determinants of the streptococcal surface glycoprotein GspB that facilitate export by the accessory Sec system. Mol. Microbiol. 58, 1468–1481 10.1111/j.1365-2958.2005.04919.x [DOI] [PubMed] [Google Scholar]
- 17. Bensing B. A., and Sullam P. M. (2010) Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide. J. Bacteriol. 192, 4223–4232 10.1128/JB.00373-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bensing B. A., Yen Y. T., Seepersaud R., and Sullam P. M. (2012) A Specific interaction between SecA2 and a region of the preprotein adjacent to the signal peptide occurs during transport via the accessory Sec system. J. Biol. Chem. 287, 24438–24447 10.1074/jbc.M112.378059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y., Seepersaud R., Bensing B. A., Sullam P. M., and Rapoport T. A. (2016) Mechanism of a cytosolic O-glycosyltransferase essential for the synthesis of a bacterial adhesion protein. Proc. Natl. Acad. Sci. U.S.A. 113, E1190–E1199 10.1073/pnas.1600494113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu F., Zhang H., Yang T., Haslam S. M., Dell A., and Wu H. (2016) Engineering and dissecting the glycosylation pathway of a streptococcal serine-rich repeat adhesin. J. Biol. Chem. 291, 27354–27363 10.1074/jbc.M116.752998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y. L., Jin H., Yang H. B., Zhao R. L., Wang S., Chen Y., and Zhou C. Z. (2017) Defining the enzymatic pathway for polymorphic O-glycosylation of the pneumococcal serine-rich repeat protein PsrP. J. Biol. Chem. 292, 6213–6224 10.1074/jbc.M116.770446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seepersaud R., Sychantha D., Bensing B. A., Clarke A. J., and Sullam P. M. (2017) O-Acetylation of the serine-rich repeat glycoprotein GspB is coordinated with accessory Sec transport. PLoS Pathog. 13, e1006558 10.1371/journal.ppat.1006558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bensing B. A., Siboo I. R., and Sullam P. M. (2007) Glycine residues in the hydrophobic core of the GspB signal sequence route export toward the accessory Sec pathway. J. Bacteriol. 189, 3846–3854 10.1128/JB.00027-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brundish D. E., Shaw N., and Baddiley J. (1966) Bacterial glycolipids. Glycosyl diglycerides in Gram-positive bacteria. Biochem. J. 99, 546–549 10.1042/bj0990546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mishra N. N., Tran T. T., Seepersaud R., Garcia-de-la-Maria C., Faull K., Yoon A., Proctor R., Miro J. M., Rybak M. J., Bayer A. S., Arias C. A., and Sullam P. M. (2017) Perturbations of phosphatidate cytidylyltransferase (CdsA) mediate daptomycin resistance in Streptococcus mitis/oralis by a novel mechanism. Antimicrob. Agents Chemother. 61, e02435–16 10.1128/AAC.02435-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen Y., Bensing B. A., Seepersaud R., Mi W., Liao M., Jeffrey P. D., Shajahan A., Sonon R. N., Azadi P., Sullam P. M., and Rapoport T. A. (2018) Unraveling the sequence of cytosolic reactions in the export of GspB adhesin from Streptococcus gordonii. J. Biol. Chem. 293, 5360–5373 10.1074/jbc.RA117.000963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seepersaud R., Bensing B. A., Yen Y. T., and Sullam P. M. (2010) Asp3 mediates multiple protein-protein interactions within the accessory Sec system of Streptococcus gordonii. Mol. Microbiol. 78, 490–505 10.1111/j.1365-2958.2010.07346.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Young T. S., and Schultz P. G. (2010) Beyond the canonical 20 amino acids: expanding the genetic lexicon. J. Biol. Chem. 285, 11039–11044 10.1074/jbc.R109.091306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adams H. M., Joyce L. R., Guan Z., Akins R. L., and Palmer K. L. (2017) Streptococcus mitis and S. oralis lack a requirement for CdsA, the enzyme required for synthesis of major membrane phospholipids in bacteria. Antimicrob. Agents Chemother. 61, e02552–02516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meiers M., Volz C., Eisel J., Maurer P., Henrich B., and Hakenbeck R. (2014) Altered lipid composition in Streptococcus pneumoniae cpoA mutants. BMC Microbiol. 14, 12 10.1186/1471-2180-14-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaw N. (1970) Bacterial glycolipids. Bacteriol. Rev. 34, 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yen Y. T., Cameron T. A., Bensing B. A., Seepersaud R., Zambryski P. C., and Sullam P. M. (2013) Differential localization of the streptococcal accessory sec components and implications for substrate export. J. Bacteriol. 195, 682–695 10.1128/JB.01742-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen Q., Wu H., Kumar R., Peng Z., and Fives-Taylor P. M. (2006) SecA2 is distinct from SecA in immunogenic specificity, subcellular distribution and requirement for membrane anchoring in Streptococcus parasanguis. FEMS Microbiol. Lett. 264, 174–181 10.1111/j.1574-6968.2006.00455.x [DOI] [PubMed] [Google Scholar]
- 34. Yen Y. T., Seepersaud R., Bensing B. A., and Sullam P. M. (2011) Asp2 and Asp3 interact directly with GspB, the export substrate of the Streptococcus gordonii accessory Sec System. J. Bacteriol. 193, 3165–3174 10.1128/JB.00057-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahn T., Oh D.-B., Kim H., and Park C. (2002) The phase property of membrane phospholipids is affected by the functionality of signal peptides from the Escherichia coli ribose-binding protein. J. Biol. Chem. 277, 26157–26162 10.1074/jbc.M203445200 [DOI] [PubMed] [Google Scholar]
- 36. Hoyt D. W., and Gierasch L. M. (1991) Hydrophobic content and lipid interactions of wild-type and mutant OmpA signal peptides correlate with their in vivo function. Biochemistry 30, 10155–10163 10.1021/bi00106a012 [DOI] [PubMed] [Google Scholar]
- 37. Keller R. C. A., Killian J. A., and de Kruijff B. (1992) Anionic phospholipids are essential for α-helix formation of the signal peptide of prePhoE upon interaction with phospholipid vesicles. Biochemistry 31, 1672–1677 10.1021/bi00121a014 [DOI] [PubMed] [Google Scholar]
- 38. McKnight C. J., Briggs M. S., and Gierasch L. M. (1989) Functional and nonfunctional LamB signal sequences can be distinguished by their biophysical properties. J. Biol. Chem. 264, 17293–17297 [PubMed] [Google Scholar]
- 39. Bensing B. A., and Sullam P. M. (2009) Characterization of Streptococcus gordonii SecA2 as a paralogue of SecA. J. Bacteriol. 191, 3482–3491 10.1128/JB.00365-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brundish D. E., Shaw N., and Baddiley J. (1965) The isomerization and transesterification of phosphodiester groups in phospholipids. Biochem. J. 97, 37C–38C 10.1042/bj0970037C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. dos Santos Mota J. M., den Kamp J. A., Verheij H. M., and van Deenen L. L. M. (1970) Phospholipids of Streptococcus faecalis. J. Bacteriol. 104, 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fischer W. (1970) A new phosphoglycolipid from Streptococcus lactis. Biochem. Biophys. Res. Commun. 41, 731–736 10.1016/0006-291X(70)90074-4 [DOI] [PubMed] [Google Scholar]
- 43. Umemoto Y., and Sato Y. (1978) Fatty acid composition of various lipid fractions of Streptococcus lactis grown at low temperature. Agric. Biol. Chem. 42, 213–219 10.1080/00021369.1978.10862960 [DOI] [Google Scholar]
- 44. Brundish D. E., Shaw N., and Baddiley J. (1965) The glycolipids from the non-capsulated strain of Pneumococcus I-192R, A.T.C.C. 12213. Biochem. J. 97, 158–165 10.1042/bj0970158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fischer W. (1977) The polar lipids of group B Streptococci: II. Composition and positional distribution of fatty acids. Biochim. Biophys. Acta 487, 89–104 10.1016/0005-2760(77)90046-7 [DOI] [PubMed] [Google Scholar]
- 46. Komaratat P., and Kates M. (1975) The lipid composition of a halotolerant species of staphylococcus epidermidis. Biochim. Biophys. Acta 398, 464–484 10.1016/0005-2760(75)90197-6 [DOI] [PubMed] [Google Scholar]
- 47. Macrina F. L., Evans R. P., Tobian J. A., Hartley D. L., Clewell D. B., and Jones K. R. (1983) Novel shuttle plasmid vehicles for Escherichia-Streptococcus transgeneric cloning. Gene 25, 145–150 10.1016/0378-1119(83)90176-2 [DOI] [PubMed] [Google Scholar]
- 48. Chin J. W., Martin A. B., King D. S., Wang L., and Schultz P. G. (2002) Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl. Acad. Sci. 99, 11020–11024 10.1073/pnas.172226299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.