Abstract
Recently studies have aimed at developing transcutaneous spinal direct current stimulation (tsDCS) as a non-invasive technique to modulate spinal function in humans. Independent studies evaluating its after-effects on nociceptive or non-nociceptive somatosensory responses have reported comparable effects suggesting that tsDCS impairs axonal conduction of both the spino-thalamic and the medial lemniscus tracts. The present study aimed to better understand how tsDCS affects, in humans, the spinal transmission of nociceptive and non-nociceptive somatosensory inputs. We compared the after-effects of anodal low-thoracic, anodal cervical and sham tsDCS on the perception and brain responses elicited by laser stimuli selectively activating Aδ-thermonociceptors of the spinothalamic system and vibrotactile stimuli selectively activating low threshold Aβ-mechanoreceptors of the lemniscal system, delivered to the hands and feet. Low-thoracic tsDCS selectively and significantly affected the LEP-N2 wave elicited by nociceptive stimulation of the lower limbs, without affecting the LEP-N2 wave elicited by nociceptive stimulation of the upper limbs, and without affecting the SEP-N2 wave elicited by vibrotactile stimulation of either limb. This selective and segmental effect indicates that the neuromodulatory after-effects of tsDCS cannot be explained by anodal blockade of axonal conduction and, instead, are most probably due to a segmental effect on the synaptic efficacy of the local processing and/or transmission of nociceptive inputs in the dorsal horn.
1. Introduction
In the last years, studies have aimed at developing transcutaneous spinal direct current stimulation (tsDCS) as a non-invasive technique to modulate spinal cord function in humans. Most of these studies have focused on the after-effects of tsDCS on motor-related functions (Cogiamanian et al., 2011; Lim & Shin, 2011; Lamy et al., 2012; Hubli et al., 2013; Bocci et al., 2014; Knikou et al., 2015; Perrotta et al., 2016; Donges et al., 2017; Winkler et al., 2010; Powell et al., 2016). In contrast, although invasive electrical stimulation of the spinal cord (SCS) using epidural electrodes is extensively used as a mean to reduce pain in patients with chronic neuropathic radicular pain (Nizard et al., 2012; Deer et al., 2014), very few experiments have been conducted to assess the possible effects of tsDCS on the spinal transmission and processing of nociceptive inputs. SCS and tsDCS are characterized by very different stimulation parameters such as the use of pulsed versus constant currents, site of stimulation, electrode-target distance, electrode surface size, and intensity of stimulation (de Andrade et al., 2010; Cogiamanian et al., 2012; Deer et al., 2014). Therefore, it is likely that their effects on spinal cord function are very different. Yet, tsDCS might represent a non-invasive and inexpensive alternative to spinal cord stimulation to modulate spinal cord function.
Truini et al. (2011) found that anodal tsDCS applied to the spinal cord at low-thoracic level (anode located over T11, cathode located over the right shoulder) reduces the magnitude of the N2 wave of nociceptive event-related brain potentials elicited by laser stimulation of the foot dorsum. No reduction was observed for the responses elicited by nociceptive stimulation of the face. The finding that tsDCS may modulate the transmission of nociceptive inputs conveyed by the spinothalamic pathway is also supported by the results of Meyer-Friessem et al. (2015) showing that low-thoracic anodal tsDCS reduced the percept elicited by nociceptive mechanical pinprick stimuli delivered to the thigh. Alongside these reports suggesting an effect of tsDCS on the spinal transmission of nociceptive inputs, one study suggested that anodal tsDCS may also modulate the responses to non-nociceptive somatosensory stimuli conveyed by the dorsal column – medial lemniscus pathway (Cogiamanian et al., 2008). Specifically, they reported that low-thoracic anodal tsDCS reduces the magnitude of the cervico-medullary P30 wave elicited by bilateral transcutaneous electrical stimulation of the posterior tibial nerves, without affecting the later cortical P39 wave. They also reported that somatosensory-evoked potentials elicited by stimulation of the median nerve were not affected by low-thoracic anodal tsDCS. Finally, studies have shown an effect of anodal low-thoracic tsDCS on nociceptive (Cogiamanian et al., 2011; Perrotta et al., 2016) and non-nociceptive (Winkler et al., 2010; Lim & Shin, 2011; Lamy et al., 2012; Donges et al., 2017) spinal reflexes. Because the first synaptic relay of non-nociceptive somatosensory inputs conveyed by the dorsal column – medial lemniscus pathway is at the level of the medulla oblonogata, the finding that low-thoracic anodal tsDCS may reduce both supraspinal responses to lower-limb nociceptive inputs conveyed by the spinothalamic tract (Truini et al., 2011; Meyer-Friessem et al., 2015) and supraspinal responses to lower-limb non-nociceptive inputs conveyed by the dorsal columns (Cogiamanian et al., 2008) has led researchers to conclude that the neuromodulatory effects of tsDCS result from a blocking of axonal conduction of action potentials, rather than an inhibitory effect on synaptic efficacy (Cogiamanian et al., 2008; Truini et al., 2011). Such an axonal conduction blockade would be analogous to the “anodal blockade” that can be induced by electrical stimulation of a peripheral nerve (Bhadra & Kilgore, 2004) and would affect indifferently the axons forming both the ascending spino-thalamic tracts and the dorsal column pathways.
Another mechanism, most often put forward to explain the cortical effects of transcranial direct current stimulation (tDCS), is an effect on synaptic transmission (Stagg & Nitsche, 2011). Hyperpolarization or depolarization of post-synaptic neurons induced by a prolonged exposure to negative or positive currents can be expected to reduce or increase synaptic efficacy, respectively. Furthermore, sustained after-effects of tDCS could be explained by the induction of mechanisms related to long-term potentiation and depression (Nitsche & Paulus, 2011). It should be stressed that the neuromodulatory after-effects of anodal tsDCS appear to be opposite to the neuromodulatory after-effects of anodal tDCS. While tsDCS appears to exert inhibitory after-effects (Cogiamanian et al., 2012), anodal tDCS usually leads to excitatory after-effects (Stagg & Nitsche, 2011). This could be explained by a differential effect of tsDCS on different populations of inhibitory or excitatory spinal interneurons and by the fact that cell bodies and axons are differently affected by the tsDCS depending on the orientation of the targeted neurons (Ahmed, 2014, 2016). An inhibitory effect of tsDCS on synaptic transmission would predict an effect of low-thoracic tsDCS on the responses to nociceptive stimuli applied to the lower limb, because the first synaptic relay of these inputs is located in the dorsal horn, i.e. close to the stimulation electrode. This mechanism of action would also predict that low-thoracic tsDCS does not alter the responses to nociceptive stimuli applied to the upper limb or face. However, a synaptic mechanism of action cannot explain an effect of low-thoracic tsDCS on the early-latency brain responses to non-nociceptive somatosensory stimuli as reported in Cogiamanian et al. (2008), because the afferents responsible for these responses ascend directly via the dorsal column to the medulla oblongata.
The aim of the present study was to better understand how tsDCS may affect, in humans, the spinal transmission of nociceptive and non-nociceptive somatosensory inputs projecting to the brain. For this purpose, we compared in 15 healthy participants the after-effects of anodal low-thoracic tsDCS, anodal cervical tsDCS and sham tsDCS on the perception and brain responses elicited by somatosensory stimuli specifically activating heat-sensitive nociceptors of the spinothalamic system (laser heat stimuli) and low-threshold mechanoreceptors of the lemniscal system (vibrotactile stimuli), delivered to the hands and feet.
Depending on the mechanism responsible for the after-effects of tsDCS, we made the following predictions (Fig. 3.1). If the effects of tsDCS are due to an anodal blockade of axonal conduction, low-thoracic tsDCS could be expected to have an inhibitory effect on the responses to both non-nociceptive and nociceptive somatosensory stimuli delivered to the foot, and no effect on the responses to stimuli delivered to the hand. Furthermore, cervical tsDCS could be expected to interfere with the axonal conduction of inputs originating from both the lower limb and the upper limb, resulting in a reduction of the responses to both foot and hand stimulation. In contrast, if the after-effects of tsDCS are due to a change in synaptic efficacy, one would expect low-thoracic tsDCS to only affect the responses elicited by nociceptive stimulation of the foot, and cervical tsDCS to only affect the responses elicited by nociceptive stimulation of the hand dorsum.
Figure 3.1.
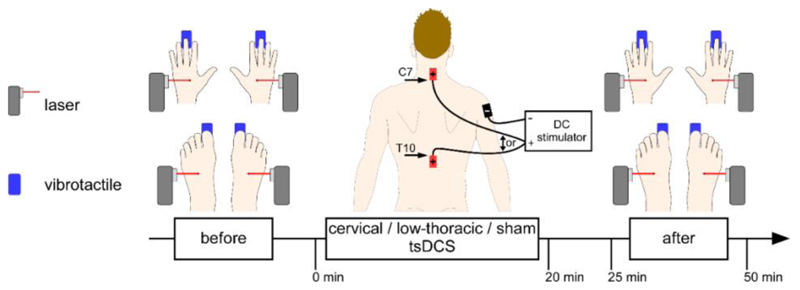
Experimental design. Intensity of perception and ERPs elicited by non-nociceptive and nociceptive stimulation of the hands and feet were recorded before and immediately after anodal cervical tsDCS and sham low-thoracic tsDCS, anodal low-thoracic tsDCS and sham cervical tsDCS, and sham cervical and low-thoracic tsDCS, in three separate experimental sessions. The cervical anode electrode was located above the spinous process of C7. The low-thoracic anode electrode was located below the spinous process of T10. The reference cathode electrode was located on the right shoulder (deltoid muscle). Nociceptive stimuli consisted of short lasting laser pulses delivered on the hand and feet dorsum. Non-nociceptive stimuli were short lasting mechanical vibration delivered on the palmar side of the third fingertip and hallux.
2. Experimental procedures
2.1. Participants
15 healthy volunteers (8 women; 24.1 ± 2.1 years; mean ± SD; range 20-29) took part in the study. They were seated comfortably with their arms and legs resting on a reclining armchair. The ethical committee (Comité Ethique Mont-Godinne Université catholique de Louvain (UCL) Namur, B03920109981) approved the experimental procedures, which were performed in accordance to the Declaration of Helsinki. All the participants were informed about the experimental procedures and gave a written informed consent before participation.
2.2. Procedure
In a blinded fashion, participants received anodal low-thoracic tsDCS, anodal cervical tsDCS or sham tsDCS on three different days separated by at least one week. Immediately before and after tsDCS, event-related brain potentials (ERPs) were recorded while nociceptive and non-nociceptive somatosensory stimuli were delivered to the four limb extremities. Each EEG recording lasted less than 30 minutes, and the recording that followed tsDCS started within 5 minutes after the end of tsDCS (Fig.3.1).
The sensory stimuli were delivered in four blocks, one block per limb. Each block consisted of a sequence of five laser stimuli or five vibrotactile stimuli followed by five vibrotactile stimuli or laser stimuli, repeated three times. Fifteen nociceptive stimuli and fifteen non-nociceptive stimuli were thus delivered to each limb, before and after tsDCS. The modality that was presented first was counterbalanced across participants. Furthermore, the two first blocks of stimulation were delivered to the upper limbs in 8 participants and to the lower limbs in the other 7 participants. After each stimulus, participants were asked to report verbally the intensity of perception using a numerical rating scale (NRS) ranging from 0 (no perception) to 100 (maximal conceivable pain) with 50 representing the limit between non-painful and painful sensations.
2.3. Somatosensory stimulation
The selective activation of Aδ- and C-fiber thermonociceptors was achieved by the use of a temperature-controlled CO2 laser (Laser Stimulation Device (LSD), SIFEC, Ferrières, Belgium) generating transient pulses of radiant heat delivered to the left and right hands and feet dorsum. The same intensity of stimulation (target temperature: 60°C; 10 ms heating ramp; pulse duration: 100 ms; beam diameter: 6 mm) was used for all participants, conditions and sessions. This intensity of stimulation was above the heat activation threshold of quickly-adapting Aδ- and C-fiber thermo-nociceptors (Treede et al., 1995; Treede et al., 1998; Namer et al., 2009; Wooten et al., 2014) and was used for all participants, conditions and sessions.
The selective activation of low threshold Aβ-mechano-receptors was achieved by the use of a 20 mm round-tipped piezo-electric actuator (VTS, Arsalis, UCL, Louvain-la-Neuve, Belgium) generating short lasting mechanical vibrations delivered on the left and right third fingertips and hallux. The same intensity of stimulation (frequency: 300 Hz; displacement 95 μm; duration: 50 ms), above the activation threshold of low-threshold mechano-receptors (Bensmaia, 2008), was used for all participants, conditions and sessions.
2.4. Low-thoracic and cervical tsDCS EEG data acquisition
Real and sham tsDCS were delivered at low-thoracic and cervical levels of the spinal cord using a constant current stimulator (Eldith, NeuroConn GmbH, Germany). In each experimental session, three 7-high × 5-wide cm rectangular sponge electrodes soaked in a 0.9% saline solution were positioned at the same locations. One anode electrode was positioned at cervical level with its lower extremity above the spinous process of C7, covering the entry zone of the cervical dorsal roots C6, C7 and C8. A second anode electrode was positioned at low-thoracic level with its upper extremity below the spinous process of T10, covering the entry zone of the five lumbar dorsal roots L1 to L5. One common cathode return electrode was positioned on the right shoulder (deltoid muscle). The skin sites where the electrodes were placed were prepared by cleaning with ether and alcohol.
Participants were instructed that, in each session, they would undergo concomitant electrical stimulation of the neck and lower back. They were also told that, for technical reasons, stimulation at one of the two sites would start slightly before stimulation of the other site. In the low-thoracic tsDCS condition, sham tsDCS was applied to the cervical location and real tsDCS was applied to the low-thoracic location, as follows. First, the current was ramped up from 0 to 2.5 mA in 20 s at the cervical location, maintained constant for only 15 s, and then ramped down in 20 s (sham stimulation). Immediately after, the current was ramped up from 0 to 2.5 mA in 20 s at the low-thoracic location, maintained constant for 20 minutes, and then ramped down in 20 s (real stimulation). In the cervical tsDCS session, sham tsDCS was applied to the low-thoracic location and real tsDCS was applied to the cervical location. Finally, in the sham tsDCS session, sham tsDCS was applied at both locations, and the order of the stimulation was counterbalanced across participants.
Both real and sham tsDCS elicited a moderate tingling and itching sensation under the electrodes that lasted about one minute after the onset of the stimulation. Because both the sensation elicited by real tsDCS and sham tsDCS faded rapidly, it was possible to blind participants and let them believe that in all three sessions, stimulation was delivered at both low-thoracic and cervical sites.
2.5. EEG data acquisition
The EEG was sampled at 1000 Hz and recorded using an average reference (32-channel ASA-LAB EEG system; Advanced Neuro Technologies, The Netherlands), with 32 actively-shielded Ag-AgCl electrodes mounted in an elastic electrode cap and arranged according to the International 10-20 system (Easycap 32, EASYCAP GmbH, Germany). During each block of stimulation, participants were instructed to keep their gaze fixed on a black cross displayed in front of them and to sit as still as possible. During all EEG recordings, white noise was played through earphones to prevent possible contamination of the EEG signals by responses triggered by the sound of the vibrotactile stimulator. Impedances were kept below 5 kΩ for all leads. The continuous EEG recordings were processed offline using Letswave6 (https://www.letswave.org).
2.6. EEG data analysis
The continuous EEG data were band pass filtered using a 0.3-40 Hz Butterworth zero phase band pass filter and then segmented into 3 s epochs ranging from -500 ms to +2500 ms relative to the stimulus onset. Artefacts related to eye movements and blinks were removed using the FastICA algorithm (Hyvarinen & Oja, 2000). The signals were then baseline corrected by removing the mean of the signal of the reference interval ranging from-500 to 0 ms and re-referenced to M1M2 mastoid electrodes. Epochs containing signals exceeding ±75 µV were rejected. Separate average waveforms were then computed for each participant, condition and stimulus type.
Within the nociceptive ERP waveforms, the LEP-N2 and LEP-P2 waves were identified at electrode Cz referenced to the mastoids M1M2, and the LEP-N1 wave was identified at the contralateral temporal electrode (T7 or T8) referenced to the frontal electrode Fz. The LEP-N2 was defined as the most negative peak occurring 180-280 ms after hand stimulation and 270-480 ms after foot stimulation. The LEP-P2 was defined as the most positive peak following the LEP-N2. Finally, the LEP-N1 was defined as the most negative deflection peaking 140-250 ms after hand stimulation and 160-280 ms after foot stimulation (Bromm & Treede, 1984; Treede et al., 1988; Cruccu et al., 2008; Hu et al., 2010).
Within the non-nociceptive ERP waveforms, the SEP-N2 and SEP-P2 waves were identified at electrode Cz vs. M1M2. The SEP-N2 was defined as the most negative deflection occurring 100-170 ms after hand stimulation and 180-400 ms after foot stimulation. The SEP-P2 was defined as the most positive peak following the SEP-N2 (Miltner et al., 1989; Garcia-Larrea et al., 1995; Kenntner-Mabiala et al., 2008).
2.7. Statistical analyses
To assess whether the responses elicited by stimulation of the left and right limbs were affected differently after tsDCS, a four-way repeated measures ANOVA was performed with the factors ‘side’ (stimulation delivered on left vs. right body side), ‘time’ (before vs. after tsDCS), ‘treatment’ (low-thoracic, cervical and sham tsDCS) and ‘limb’ (responses to stimuli delivered to the lower vs. upper limb). No significant interaction with the factor ‘side’ was observed for any LEP component (LEP-N1, LEP-N2, LEP-P2), SEP component (SEP-N2, SEP-P2), or perceived intensity (all p >.261). Consequently, for further analyses, for each participant, condition and stimulation type, the behavioural and EEG responses elicited by stimulation of the left and right hand, as well as those elicited by stimulation of the left and right foot were averaged such as to obtain a single measure for the upper limb and a single measure for the lower limb. For each measure, a three-way repeated-measures ANOVA was then conducted with the factors ‘time’ (before vs. after tsDCS), ‘treatment’ (low-thoracic, cervical and sham tsDCS) and ‘limb’ (responses to stimuli delivered to the lower vs. upper limb). With this model, a significant two-way interaction between the factors ‘time’ and ‘treatment’ would indicate an effect of real tsDCS (low-thoracic and/or cervical tsDCS) regardless of whether the sensory stimuli are delivered to the lower or upper limbs, and a significant three-way interaction between the factors ‘time’, ‘treatment’ and ‘limb’ would indicate a differential effect of tsDCS on the responses to stimuli delivered to the lower or upper limbs. When necessary a Greenhouse-Geisser correction was performed. Post-hoc analyses of significant interactions were conducted using paired t-tests, Bonferroni-corrected for multiple comparisons. Statistical analyses were conducted using SPSS 25 (SPSS Inc., Chicago, IL, USA). Significance threshold was set at p < .05.
3. Results
None of the participants were able to indicate if the tsDCS protocols were different between the three different sessions. The sensations elicited in all three conditions (real cervical tsDCS + sham low-thoracic tsDCS, real low-thoracic tsDCS + sham cervical tsDCS and sham cervical + sham low-thoracic tsDCS) were similar.
3.1. Effects of low-thoracic and cervical tsDCS on the perception of nociceptive stimuli
Before the tsDCS interventions, the average ratings of the percept elicited by nociceptive stimuli were 46±15/100 and 42±18/100 at the hands and feet, respectively. After the tsDCS intervention, the average ratings 43±18/100 and 40±20/100. On average, the nociceptive stimuli were not perceived as painful.
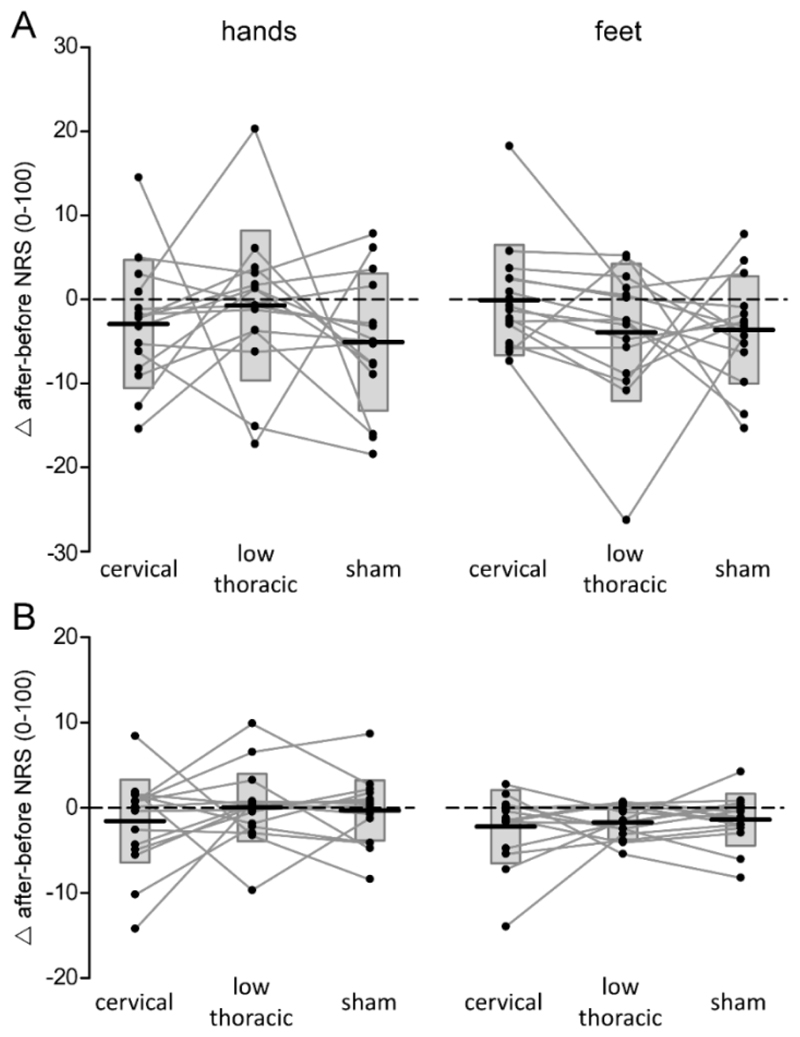
The repeated-measures ANOVA revealed a significant main effect of ‘time’ (before vs. after tsDCS; F1,14 = 6.803; p = .021; η2 = .327). There was no significant two-way ‘time’ × ‘treatment’ interaction (F2,28 = 1.25; p = .291; η2 = .082) and no significant ‘time’ × ‘treatment’ × ‘limb’ interaction (F2,28 = 1.504; p = .240; η2 = .097), indicating that the perception of nociceptive laser stimuli delivered to the hands and feet was not affected by either low-thoracic or cervical tsDCS as compared to sham tsDCS (Fig. 3.2A).
Figure 3.2.
Effects of tsDCS on perceived intensity elicited by somatosensory stimuli. Differences in the intensity of the percept (numerical rating scale [NRS] after minus before tsDCS) elicited by nociceptive laser stimulation (panel A) and non-nociceptive vibrotactile stimulation (panel B) of the hand and foot are for cervical tsDCS, low-thoracic tsDCS and sham tsDCS. Subject-level differences (after minus before tsDCS) are represented by the connected lines and black dots. Box plots represent the group-level average ± SD.
3.2. Effects of low-thoracic and cervical tsDCS on the perception of non-nociceptive stimuli
The average ratings of the percept elicited by non-nociceptive vibrotactile stimuli delivered to the hands and feet were 20±8/100 and 14±7/100 before the tsDCS interventions and 19±8/100 and 12±6/100 after the tsDCS interventions.
The repeated-measures ANOVA revealed a significant main effect of ‘time’ (F1,14 = 5.401; p = .036; η2 = .278), but no significant ‘time’ × ‘treatment’ interaction (F2,28 = .701; p = .46; η2 = .048) and no significant ‘time’ × ‘treatment’ × ‘limb’ interaction (F2,28 = 0.304; p = .666; η2 = .021), indicating that such as for the perception of nociceptive stimuli, the perception of non-nociceptive vibrotactile stimuli delivered to the hands and feet was not affected by low-thoracic or cervical tsDCS as compared to sham tsDCS (Fig. 3.2B).
3.3. Effects of low-thoracic and cervical tsDCS on nociceptive ERPs
In each participant and experimental session, laser stimulation of the hands (Fig. 3.3B) and feet (Fig. 3.4B) dorsum elicited a large biphasic wave maximal at the scalp vertex (electrode Cz vs. M1M2). These laser-evoked potentials (LEPs) consisted of a negative wave (LEP-N2) followed by a positive wave (LEP-P2).
Figure 3.3.
Grand-average waveforms of the ERPs elicited by nociceptive laser stimulation delivered on the hands. ERPs waveforms obtained after stimulation of the hands are shown before (blue) and after (red) applying cervical, low-thoracic and sham tsDCS. The early latency LEP-N1 component is shown at the contralateral temporal electrode (Tc) vs. Fz (panel A). The LEP-N2 and LEP-P2 components are shown at Cz vs. M1M2 (panel B). The scalp topographies of the different LEP components are shown in the blue (before tsDCS) and red (after tsDCS) frames. Box plots represent the group-level average ± SD of the difference (Δ after - before tsDCS) in magnitude of the three LEP components.
Figure 3.4.
Grand-average waveforms of the ERPs elicited by nociceptive laser stimulation delivered on the feet. ERPs waveforms obtained after stimulation of the feet are shown before (blue) and after (red) applying cervical, low-thoracic and sham tsDCS. The early latency LEP-N1 component is shown at the contralateral temporal electrode (Tc) vs. Fz (panel A). The LEP-N2 and LEP-P2 components are shown at Cz vs. M1M2 (panel B). The scalp topographies of the different LEP components are shown in the blue (before tsDCS) and red (after tsDCS) frames. Box plots represent the group-level average ± SD of the difference (Δ after - before tsDCS) in magnitude of the three LEP components.
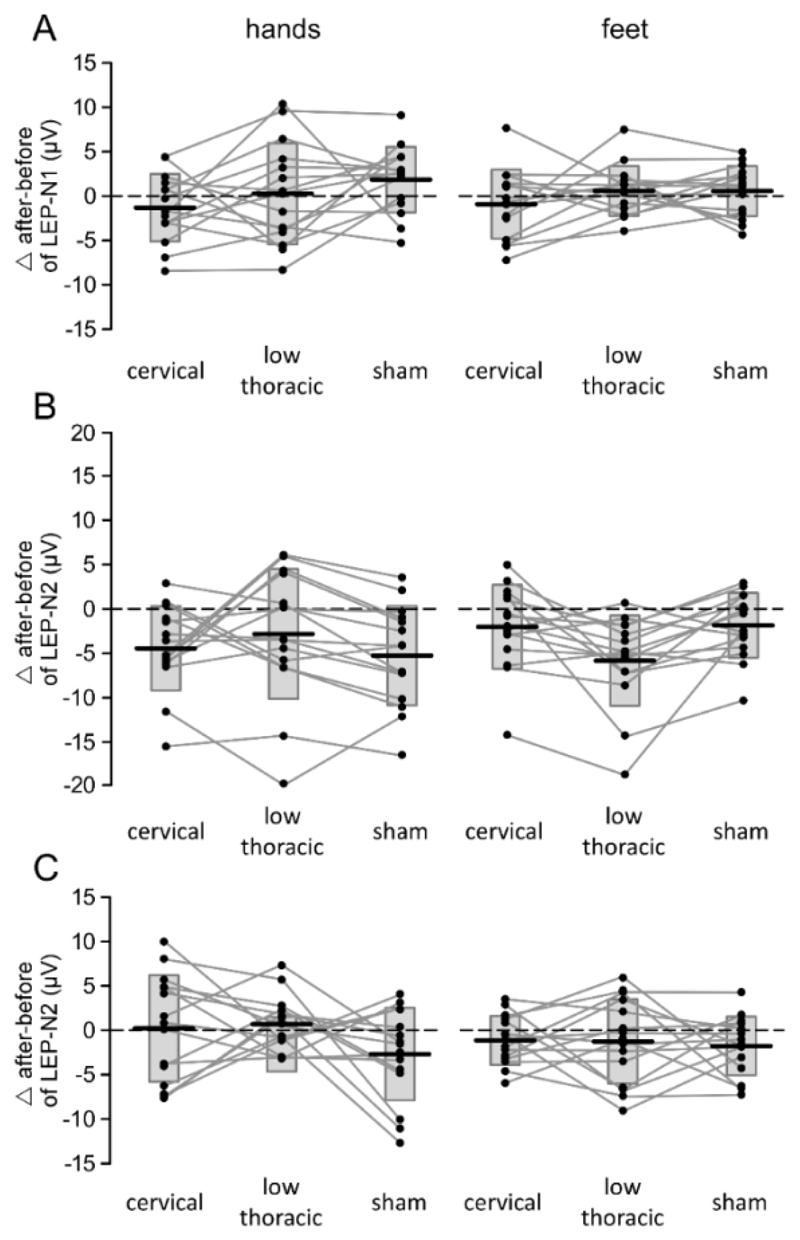
The magnitude of the LEP-N2 elicited by stimulation of the feet was significantly affected after thoracic tsDCS as compared to cervical and sham tsDCS. The repeated-measures ANOVA showed a significant main effect of ‘time’(F1,14 = 15.910; p = .001; η2 = .532), a significant main effect of ‘limb’ (F1,14 = 19.771; p = .001; η2 = .585), but also a significant main effect of ‘treatment’ (F1,14 = 7.075; p = .003; η2 = .335) and, most importantly, a significant three-way ‘time’ × ‘treatment’ x ‘limb’ interaction (F2,28 = 7.938; p = .002; η2 = .362). Post-hoc pairwise comparisons showed that the magnitude of the LEP-N2 wave elicited by laser stimulation of the feet was significantly reduced after low-thoracic tsDCS as compared to cervical tsDCS (t14 = 2.80; p = .041) and as compared to sham tsDCS (t14 = 3.15; p = .021; Fig. 3.5B). The magnitude of the LEP-P2 was not significantly affected by tsDCS. The repeated-measures ANOVA showed a significant main effect of ‘time’ (F1,14 = 4.737; p = .047; η2 = .253) and a significant main effect of ‘limb’ (F1,14 = 8.354; p = .012; η2 = .374), but no effect or interaction with the factor ‘treatment’. The magnitude of the LEP-P2 was similarly reduced after real and sham tsDCS, indicating an unspecific response habituation (Fig 3.5C). On average, its magnitude was greater when stimulating the hands as compared to the feet. Both following stimulation of the hand and following stimulation of the foot, an earlier negative wave (LEP-N1; Figs. 3.3A and 3.4A), clearly distinguishable from the LEP-N2 (Figs. 3.3B and 3.4B), was observed at temporal electrodes (T7/T8) referenced to the frontal electrode Fz. Following stimulation of the hand, the LEP-N1 was maximal over the hemisphere contralateral to the stimulated hand. Following stimulation of the foot, it was symmetrically distributed over the two hemispheres.
Figure 3.5.
Effect of cervical tsDCS, low-thoracic tsDCS and sham tsDCS on the magnitude of LEP-N1 (panel A), LEP-N2 (panel B) and LEP-P2 (panel C) components of the ERPs elicited by nociceptive stimulation of the hands and feet. Subject-level differences are represented by the connecting lines and dots. Box plots represent the group-level average ± SD.
There was no significant effect of tsDCS on the magnitude of the LEP-N1 (Fig 3.5A). The repeated-measures ANOVA showed a significant main effect of ‘limb’ (F1,14 = 11.669; p = .004; η2 = .455; greater responses when stimulating the hands as compared to the feet), but no main effect of ‘time’, and no effect or interaction with the factor ‘treatment’.
The repeated-measures ANOVA conducted on the latencies of the LEP-N2, LEP-P2 and LEP-N1 waves only showed a significant main effect of ‘limb’ (LEP-N2: F1,14 = 140.341; p < .0001; η2 = .909; LEP-P2: F1,14 = 33.142; p <.0001; η2 = .703; LEP-N1: F1,14 = 322.430; p < .0001; η2 = .958) due to the fact that the responses elicited by stimulation of the lower limb had a later latency (Fig. 3.4) than the responses elicited by stimulation of the upper limb (Fig. 3.3).
3.4. Effects of low-thoracic and cervical tsDCS on non-nociceptive ERPs
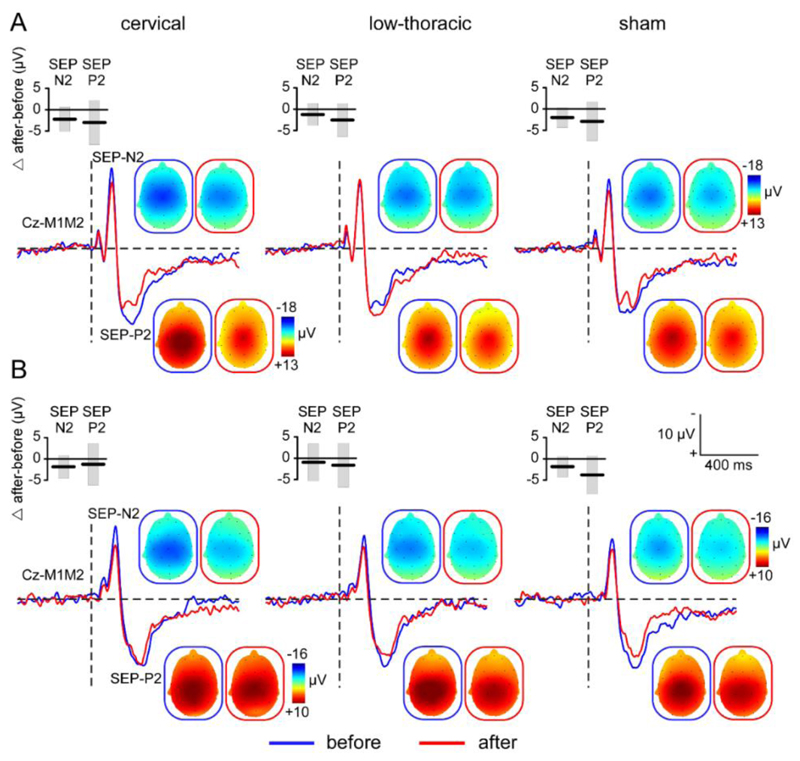
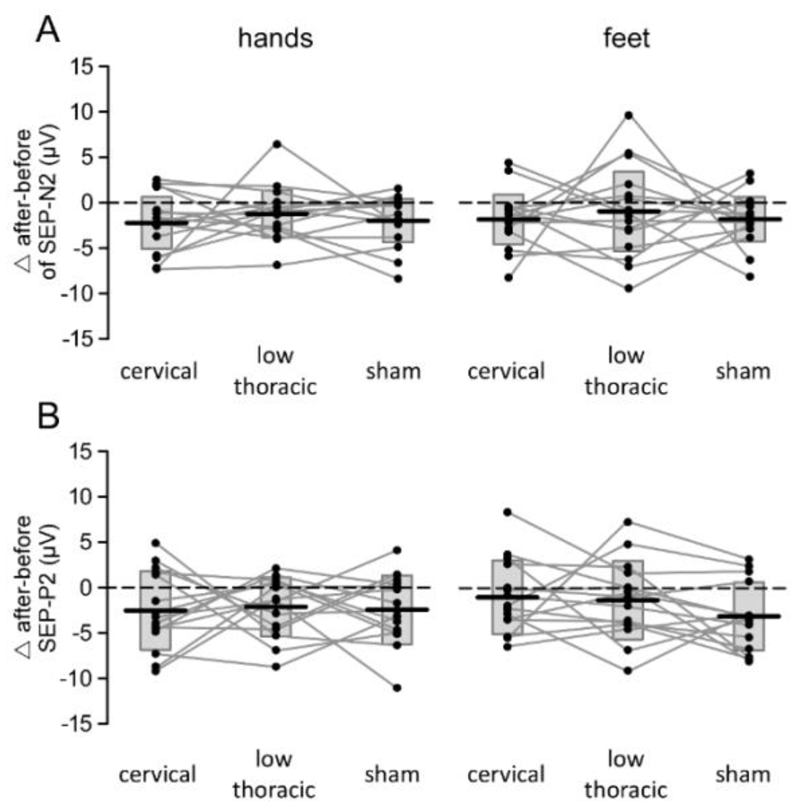
In each participant and experimental session, vibrotactile stimulation of the hands (Fig. 3.6A) and feet (Fig. 3.6B) elicited a large biphasic wave maximal at the scalp vertex (Cz vs. M1M2). These somatosensory-evoked potentials (SEPs) consisted of a negative wave (SEP-N2) followed by a positive wave (SEP-P2). Neither the magnitude of the SEP-N2 (Fig. 3.7A) nor the magnitude of the SEP-P2 were significantly affected by tsDCS (Fig. 3.7B). The repeated-measures ANOVA only showed a significant main effect of ‘time’ (SEP-N2: F1,14 = 15.236; p = .002; η2 = .521; SEP-P2: F1,14 = 16.459; p = .001; η2 = .540) compatible with response habituation, and a significant main effect of ‘limb’ (SEP-N2: F1,14 = 10.0; p = .007; η2 = .417; SEP-P2: F1,14 = 47.001; p < .0001; η2 = .770) due to the fact that the responses elicited by stimulation of the hands (Fig. 3.6A) were, on average, greater than the responses elicited by stimulation of the feet (Fig. 3.6B). There was no effect or interaction with the factor ‘treatment’.
Figure 3.6.
Grand-average waveforms of the ERPs elicited by non-nociceptive vibrotactile stimulation. ERPs waveforms obtained after stimulation of the hands (panel A) and feet (panel B) are shown before (blue) and after (red) applying cervical, low-thoracic and sham tsDCS. The SEP-N2 and SEP-P2 components are shown at Cz vs. M1M2 with their respective scalp topographies before (blue frame) and after (red frame) tsDCS. Box plots represent the group-level average ± SD of the difference (Δ after - before tsDCS) in magnitude of the two SEP components.
Figure 3.7.
Effect of cervical tsDCS, low-thoracic tsDCS and sham tsDCS on the magnitude of SEP-N2 (panel A) and SEP-P2 (panel B) components of non-nociceptive ERPs elicited by non-nociceptive stimulation of the hands and feet. Subject-level differences (Δ after - before tsDCS) are represented by connecting lines and dots. Box plots represent the group-level average ± SD.
The repeated-measures ANOVA conducted on the latencies of the SEP-N2 and SEP-P2 waves also showed no significant effect of tsDCS. There was only a main effect of ‘limb’ on the latency of the SEP-N2 (F1,14 = 126.293; p < .0001; η2 = .900; later responses when stimulating the foot) and a main effect of ‘time’ on the latency of the SEP-P2 (F1,14 = 6.584; p = .022; η2 = .320).
4. Discussion
The present work is the first controlled double-blind study comparing, within subjects, the after-effects of low-thoracic and cervical anodal tsDCS on the behavioural and EEG responses to nociceptive and non-nociceptive somatosensory stimulation of the upper and lower limbs. The results show that low-thoracic tsDCS significantly affects the LEP-N2 wave elicited by nociceptive laser stimulation of the lower limbs, without affecting the LEP-N2 wave elicited by nociceptive stimulation of the upper limbs, and without affecting the SEP-N2 wave elicited by non-nociceptive vibrotactile stimulation of either limbs. This selective and segmental effect of tsDCS on the responses to nociceptive stimulation indicates that the neuromodulatory after-effects of low-thoracic tsDCS are not due to an anodal blockade of axonal conduction within the spinal cord. Instead, it suggests that the after-effects of low-thoracic tsDCS are related to a synaptic modulation of the local processing and/or transmission of nociceptive input at the level of the dorsal horn.
4.1. Selective effect of low-thoracic tsDCS on the responses to nociceptive stimulation of the lower limbs
The finding that low-thoracic anodal tsDCS induces a significant decrease of the magnitude of the LEP-N2 wave elicited by nociceptive stimulation of the foot as compared to cervical and sham tsDCS is in line with the results of Truini et al. (2011). In contrast, the finding that low-thoracic anodal tsDCS does not modulate the responses to non-nociceptive vibrotactile stimulation is in disagreement with the study of Cogiamanian et al. (2008) reporting an effect on an early-latency subcortical component of somatosensory-evoked potentials elicited by transcutaneous electrical stimulation of the left and right posterior tibial nerves. However, the statistical comparison of the effects of anodal vs. cathodal vs. sham tsDCS on the responses elicited by tibial nerve stimulation was based on results obtained in only five participants. Furthermore, the authors did not observe any effect of tsDCS on later cortical components of the elicited responses.
In the present study, lower-limb nociceptive laser stimuli were applied to the foot dorsum, which is primarily innervated by the superficial fibular nerve, and lower-limb non-nociceptive vibrotactile stimuli were applied to the hallux which is primarily innervated by the medial plantar nerve. These sensory afferents are conveyed to the spinal cord through the dorsal roots L4, L5 and S1 (Lee et al., 2008; Canbay et al., 2014). The anode electrode delivering low-thoracic tsDCS (7 cm length) was positioned just below the spinous process of T10, overlying T11-T12 vertebrae, considered to correspond to the entry zone of the lumbar dorsal roots L2-L5 (Goshgarian, 2010). Therefore, the anode electrode was located close to the spinal cord levels where nociceptive primary afferent fibres originating from the foot dorsum synapse with spinothalamic neurons in the dorsal horn (D'Mello & Dickenson, 2008). In contrast, tactile inputs originating from the hallux ascend directly the dorsal column-medial lemniscus pathway and synapse at the level of the medulla oblongata in the gracile nucleus. Therefore, the selective effect of low-thoracic tsDCS on the responses to nociceptive inputs originating from the foot dorsum can be explained by a segmental-specific effect of low-thoracic tsDCS on the synaptic efficacy of spinal neurons involved in the local processing and/or transmission of nociceptive inputs within the dorsal horn. It should be stressed that the reduction of the output of the spinal cord leading to the observed decrease of the brain response to nociceptive inputs could be related to the synaptic modulation of different spinal interneurons involved in the spinal processing of nociceptive inputs. Indeed, although our results support that tsDCS affects the synaptic efficacy of nociceptive transmission within the spinal dorsal horn, it remains unknown whether this after-effect of low-thoracic tsDCS is related to a direct modulation of the first synaptic relay of the ascending nociceptive input, or whether it results from an indirect effect on other neurons modulating spinal transmission neurons and/or peripheral axon terminals, including the descending systems.
The differential effect of low-thoracic anodal tsDCS on the transmission of nociceptive vs. non-nociceptive inputs originating from the lower limbs contradicts the hypothesis that the after-effects of tsDCS result from an axonal blockade. Indeed, such a mechanism would be expected to affect conduction both within ascending fibers of the spinothalamic tracts and ascending fibers of the dorsal columns, resulting in a similar effect on the responses to nociceptive and non-nociceptive stimuli. Taking into account the high variability in the location of lumbar spinal cord segments relative to the low thoracic spinous processes, the low-thoracic anode electrode could, in some participants, have been located higher than the dorsal root entry zones of the lumbar dorsal roots L4 and L5. If this were the case, a conduction blockade would still be expected to affect the transmission of ascending inputs originating from lower segments. Furthermore, simulation studies have suggested that the threshold to induce an anodal conduction blockade decreases with increasing axonal diameter, and increases as a function of the distance separating the electrode from the axon (Tai et al., 2009). Considering that the dorsal columns are more posterior than the spinothalamic tracts, the electrode distance to the dorsal column should be slightly shorter than the electrode distance to the spinothalamic tract. Furthermore, the ascending fibers of the spinothalamic tract which convey the LEP-N2 response to the nociceptive laser stimulus are small-diameter sparsely-myelinated Aδ-fibers, whereas the ascending fibers of the dorsal column which convey the SEP-N2 response to the non-nociceptive vibrotactile stimulus are predominantly large-diameter thickly-myelinated Aβ-fibers. Hence, one would expect that the threshold to elicit anodal blockade through low-thoracic tsDCS would be lower for the dorsal column pathway as compared to the spinothalamic tract.
Studies have reported that low-thoracic tsDCS increases the threshold of the lower-limb nociceptive withdrawal reflex (RIII) (Cogiamanian et al., 2011; Perrotta et al., 2016). This could be explained both by an inhibitory effect on synaptic transmission in the spinal cord (Cogiamanian et al., 2011; Perrotta et al., 2016), or an inhibitory effect on axonal conduction within the reflex circuit (Cogiamanian et al., 2011). Studies on the non-nociceptive H-reflex showed opposite results, where tsDCS tended to increase H-reflex excitability (Winkler et al., 2010; Lamy et al., 2012). This discrepancy does not rule out the possibility that tsDCS induces local changes in synaptic efficacy. Indeed, the opposite effects of tsDCS on the RIII and H-reflex might be due to the different orientation of the spinal neurons involved in these different reflexes and their modulation, resulting in net effects that can be either excitatory or inhibitory (Winkler et al., 2010; Lamy et al., 2012; Rahman et al., 2013).
It must also be stressed that the after-effects of tsDCS have been reported to last 15 to 60 minutes (Cogiamanian et al., 2008; Winkler et al., 2010; Cogiamanian et al., 2011; Lamy et al., 2012; Meyer-Friessem et al., 2015; Perrotta et al., 2016). In the present study, the measurements after tsDCS took approximately 30 minutes to be completed. Because no change was observed in the effect of tsDCS at the beginning vs. the end of the measurement session after tsDCS, we may assume that the after-effects of tsDCS remained relatively stable during the entire test period. The duration of these after-effects are similar to the duration of the after-effects of transcranial direct current stimulation, and are compatible with changes in synaptic efficacy resulting from the induction of long term potentiation- or depression-like mechanisms (Nitsche et al., 2008; Stagg & Nitsche, 2011). Such durations strongly contradict the notion that the after-effects of tsDCS are due to anodal blockade of axonal conduction. Indeed, when anodal stimulation succeeds in producing an axonal conduction blockade, conduction is restored almost immediately after the stimulation is interrupted (Bhadra & Kilgore, 2004).
Finally, considering the intensity of the current passed through the anodal electrode, and the distance between the cutaneous electrode and the spinal cord, it seems unlikely that low-thoracic anodal tsDCS is able to induce an anodal block. Indeed, in nerve conduction studies, Dreyer et al. (1993) failed to induce an anodal block with surface electrodes on the skin targeting the median or superficial radial nerve with intensities above 5 mA. Frahm et al. (2016) showed in a modelling study that no anodal block occurred during peripheral nerve stimulation for pain relief using an implanted subcutaneous electrode, even at high intensities of stimulation. In humans, indirect evidence of the occurrence of a nerve conduction block on sacral nerve roots have been reported during invasive electrical stimulation applied at intensities exceeding 2 mA (Rijkhoff et al., 1997). In the present setup the actual distance between the electrodes located on the skin and the axons of the spinal cord is approximately 4 to 7 cm (Brinkley & Masters, 1967), and the current density at the level of the spinal cord is thought to be considerably reduced due the impedance of the underlying tissues (Parazzini et al., 2014; Fernandes et al., 2016; Miranda et al., 2016).
4.2. Lack of effect of low-thoracic tsDCS on the early LEP-N1 wave elicited by nociceptive stimulation of the lower limbs
Low-thoracic tsDCS induced a significant reduction of the LEP-N2 wave elicited by nociceptive stimulation of the lower limbs, but had no significant effect on the magnitude of the earlier LEP-N1 wave. This lack of effect could be due to the relatively low signal-to-noise ratio of the LEP-N1 elicited by stimulation of the foot, and is in contradiction with the results of Truini et al. (2011) who reported a significant decrease of both components after low-thoracic tsDCS. The cortical generators of the early-latency LEP-N1 remains a matter of debate. Source analysis studies have suggested a contribution of the contralateral primary somatosensory cortex (S1) and bilateral secondary somatosensory cortices (S2) (Tarkka & Treede, 1993; Xu et al., 1995; Spiegel et al., 1996; Valeriani et al., 1996; Valeriani et al., 2000; Garcia-Larrea et al., 2003; Valentini et al., 2012). Considering that in S1, the representation of the foot is more medial as compared to the hand, the contribution of S1 activity to the LEP-N1 might be strongly dependent on the location of the nociceptive stimulus. This could explain why, in the present study, although a LEP-N1 was clearly identified when stimulating the foot dorsum, its signal-to-noise ratio was lower as compare to the LEP-N1 elicited by stimulation of the hand. This could also explain why the scalp topography of LEP-N1 elicited by stimulation of the hand was clearly lateralized over the contralateral hemisphere, whereas the scalp topography of the LEP-N1 elicited by stimulation of the foot was symmetrically distributed over the two hemispheres.
4.3. Lack of effect of low-thoracic tsDCS on pain perception
Low-thoracic tsDCS appeared to have no immediate effect on the intensity of the percept elicited by transient nociceptive laser stimuli delivered to the lower limb. This observation is in line with previous studies (Cogiamanian et al., 2011; Truini et al., 2011; Meyer-Friessem et al., 2015; Perrotta et al., 2016; Schweizer et al., 2017). Dissociations between perception and brain activity elicited by nociceptive stimuli have been frequently observed (Garcia-Larrea et al., 1997; Iannetti et al., 2008; Mouraux & Iannetti, 2009; Legrain et al., 2012b) suggesting that the neuronal processes responsible for perception could be different than those responsible for the stimulus-evoked brain activity sampled with EEG. One can wonder whether the delay at which the effect of tsDCS was assessed played a role. Indeed, the only three studies that have reported a reduction of pain perception after low-thoracic tsDCS found that the effect took at least 30 minutes to build up (Meyer-Friessem et al., 2015; Perrotta et al., 2016). Meyer-Friessem et al. (2015) observed that the pain perception triggered by high intensity mechanical pinprick stimulation of the thigh was significantly decreased from 30 to 60 minutes after low-thoracic tsDCS. A similar delay was reported by Perrotta et al. (2016). It should be also considered that assessing the changes in intensity of perception elicited by stimuli delivered at a constant intensity does not fully assess the possible changes in the perceptual experience. Future studies should also examine whether tsDCS affects spatial or intensity discrimination abilities, or the quality of the elicited sensations to investigate more extensively the potential effects of tsDCS on perception.
4.4. Lack of effect of cervical tsDCS on the responses to nociceptive and non-nociceptive stimulation of the lower and upper limbs
One could object that the lack of effect of cervical tsDCS on the responses to nociceptive stimulation of the upper limb goes against the hypothesis that the after-effects of tsDCS are due to a segmental effect on the synaptic transmission of nociceptive inputs in the dorsal horn.
Despite interindividual anatomical and topographical differences of the spinal cord and spinal segments responsible for cutaneous innervation, it is very unlikely that the lack of effect of cervical tsDCS was due to the electrode not being placed over the spinal cord levels receiving input from the hand dorsum. Indeed, because of its large size, the cervical electrode covered at least spinal cord levels C6, C7 and C8. Therefore, even if interindividual differences exist in the relative contribution of C6, C7 and C8 dorsal roots to hand dorsum innervation (Lee et al., 2008), it is unlikely that this could explain why cervical tsDCS did not modulate the responses elicited by nociceptive stimulation of the hand dorsum.
It seems more likely that the differential effects of cervical and low-thoracic tsDCS was due to the fact that the distance between the skin and the spinal cord varies along the spinal segments and, on average, is greater at cervical level than at low-thoracic level (Brinkley & Masters, 1967; Hirabayashi et al., 1988). Studies have suggested that the magnitude of the current density reaching the spinal cord could be more than 30 times weaker than the current density within spinal muscles (Parazzini et al., 2014). Another explanation could be related to the fact that the location of the return electrode influences the distribution of the electric field generated by tsDCS. In a recent modelling study, Fernandes et al. (2016) reported that cervical tsDCS with active and return electrodes respectively located over C7 and at the shoulder generates an electric field four time smaller than cervical tsDCS targeting C3 with a return electrode located over T3. Further studies are needed to determine whether significant after-effects of cervical tsDCS can be observed using optimized electrode configurations and/or greater intensities of stimulation.
5. Conclusion
In conclusion, we demonstrate that low-thoracic tsDCS exerts a selective effect on the brain responses to nociceptive stimulation of the lower limbs, without concomitantly affecting the responses to non-nociceptive stimulation of the lower limbs, and without affecting the responses to nociceptive and non-nociceptive stimulation of the upper limbs. These findings provide evidence that the after-effects of tsDCS are due to a (direct or indirect) local modulation of synaptic efficacy at the level of the dorsal horn.
Given the ambiguous effects of tsDCS on pain perception, future studies are needed to evaluate the clinical relevance of these effects. For example, further research could be conducted to assess the after-effects of tsDCS on sustained pain and/or examine the long-lasting changes that might be induced by repeated sessions of tsDCS. Furthermore, future work could examine whether stronger effects might be observed using electrode configurations optimized to maximize current density at the level of the spinal cord.
References
- Ahmed Z. Trans-spinal direct current stimulation modifies spinal cord excitability through synaptic and axonal mechanisms. Physiol Rep. 2014;2 doi: 10.14814/phy2.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z. Modulation of gamma and alpha spinal motor neurons activity by trans-spinal direct current stimulation: effects on reflexive actions and locomotor activity. Physiol Rep. 2016;4 doi: 10.14814/phy2.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensmaia SJ. Tactile intensity and population codes. Behav Brain Res. 2008;190:165–173. doi: 10.1016/j.bbr.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve. IEEE Trans Neural Syst Rehabil Eng. 2004;12:313–324. doi: 10.1109/TNSRE.2004.834205. [DOI] [PubMed] [Google Scholar]
- Bocci T, Vannini B, Torzini A, Mazzatenta A, Vergari M, Cogiamanian F, Priori A, Sartucci F. Cathodal transcutaneous spinal direct current stimulation (tsDCS) improves motor unit recruitment in healthy subjects. Neurosci Lett. 2014;578:75–79. doi: 10.1016/j.neulet.2014.06.037. [DOI] [PubMed] [Google Scholar]
- Brinkley D, Masters HE. The depth of the spinal cord below the skin. Br J Radiol. 1967;40:66–68. doi: 10.1259/0007-1285-40-469-66. [DOI] [PubMed] [Google Scholar]
- Bromm B, Treede RD. Nerve fibre discharges, cerebral potentials and sensations induced by CO2 laser stimulation. Hum Neurobiol. 1984;3:33–40. [PubMed] [Google Scholar]
- Canbay S, Gurer B, Bozkurt M, Comert A, Izci Y, Baskaya MK. Anatomical relationship and positions of the lumbar and sacral segments of the spinal cord according to the vertebral bodies and the spinal roots. Clin Anat. 2014;27:227–233. doi: 10.1002/ca.22253. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Pulecchi F, Marceglia S, Priori A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2008;119:2636–2640. doi: 10.1016/j.clinph.2008.07.249. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Vergari M, Schiaffi E, Marceglia S, Ardolino G, Barbieri S, Priori A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain. 2011;152:370–375. doi: 10.1016/j.pain.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Ardolino G, Vergari M, Ferrucci R, Ciocca M, Scelzo E, Barbieri S, Priori A. Transcutaneous spinal direct current stimulation. Front Psychiatry. 2012;3:63. doi: 10.3389/fpsyt.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, Rossini PM, Treede RD, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–1719. doi: 10.1016/j.clinph.2008.03.016. [DOI] [PubMed] [Google Scholar]
- D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- de Andrade DC, Bendib B, Hattou M, Keravel Y, Nguyen JP, Lefaucheur JP. Neurophysiological assessment of spinal cord stimulation in failed back surgery syndrome. Pain. 2010;150:485–491. doi: 10.1016/j.pain.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:515–550. doi: 10.1111/ner.12208. discussion 550. [DOI] [PubMed] [Google Scholar]
- Donges SC, D'Amico JM, Butler JE, Taylor JL. The effects of cervical transcutaneous spinal direct current stimulation on motor pathways supplying the upper limb in humans. PLoS One. 2017;12:e0172333. doi: 10.1371/journal.pone.0172333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer SJ, Dumitru D, King JC. Anodal block V anodal stimulation. Fact or fiction. Am J Phys Med Rehabil. 1993;72:10–18. doi: 10.1097/00002060-199302000-00004. [DOI] [PubMed] [Google Scholar]
- Fernandes SR, Salvador R, Wenger C, de Carvalho MA, Miranda PC. Influence of electrode configuration on the electric field distribution during transcutaneous spinal direct current stimulation of the cervical spine. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:3121–3124. doi: 10.1109/EMBC.2016.7591390. [DOI] [PubMed] [Google Scholar]
- Frahm KS, Hennings K, Vera-Portocarrero L, Wacnik PW, Morch CD. Nerve Fiber Activation During Peripheral Nerve Field Stimulation: Importance of Electrode Orientation and Estimation of Area of Paresthesia. Neuromodulation. 2016;19:311–318. doi: 10.1111/ner.12371. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Lukaszewicz AC, Mauguiere F. Somatosensory responses during selective spatial attention: The N120-to-N140 transition. Psychophysiology. 1995;32:526–537. doi: 10.1111/j.1469-8986.1995.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Peyron R, Laurent B, Mauguiere F. Association and dissociation between laser-evoked potentials and pain perception. Neuroreport. 1997;8:3785–3789. doi: 10.1097/00001756-199712010-00026. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Frot M, Valeriani M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol Clin. 2003;33:279–292. doi: 10.1016/j.neucli.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Development, Anatomy, and Function of the spinal Cord. In: Lin VW CD, Cutter NC, Frost FS, Hammond MC, Lindblom, LB PI, Waters R, Woolsey RM, editors. Spinal Cord Medicine: Principles and Practice. New-York: Demos Medical; 2010. pp. 3–21. [Google Scholar]
- Hirabayashi Y, Matsuda I, Inoue S, Shimizu R. The distance from the skin to the epidural space. J Anesth. 1988;2:198–201. doi: 10.1007/s0054080020198. [DOI] [PubMed] [Google Scholar]
- Hu L, Mouraux A, Hu Y, Iannetti GD. A novel approach for enhancing the signal-to-noise ratio and detecting automatically event-related potentials (ERPs) in single trials. Neuroimage. 2010;50:99–111. doi: 10.1016/j.neuroimage.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Hubli M, Dietz V, Schrafl-Altermatt M, Bolliger M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin Neurophysiol. 2013;124:1187–1195. doi: 10.1016/j.clinph.2012.11.021. [DOI] [PubMed] [Google Scholar]
- Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Netw. 2000;13:411–430. doi: 10.1016/s0893-6080(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Hughes NP, Lee MC, Mouraux A. Determinants of laser-evoked EEG responses: pain perception or stimulus saliency? J Neurophysiol. 2008;100:815–828. doi: 10.1152/jn.00097.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenntner-Mabiala R, Andreatta M, Wieser MJ, Muhlberger A, Pauli P. Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biol Psychol. 2008;78:114–122. doi: 10.1016/j.biopsycho.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Knikou M, Dixon L, Santora D, Ibrahim MM. Transspinal constant-current long-lasting stimulation: a new method to induce cortical and corticospinal plasticity. J Neurophysiol. 2015;114:1486–1499. doi: 10.1152/jn.00449.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy JC, Ho C, Badel A, Arrigo RT, Boakye M. Modulation of soleus H reflex by spinal DC stimulation in humans. J Neurophysiol. 2012;108:906–914. doi: 10.1152/jn.10898.2011. [DOI] [PubMed] [Google Scholar]
- Lee MW, McPhee RW, Stringer MD. An evidence-based approach to human dermatomes. Clin Anat. 2008;21:363–373. doi: 10.1002/ca.20636. [DOI] [PubMed] [Google Scholar]
- Legrain V, Mancini F, Sambo CF, Torta DM, Ronga I, Valentini E. Cognitive aspects of nociception and pain: bridging neurophysiology with cognitive psychology. Neurophysiol Clin. 2012;42:325–336. doi: 10.1016/j.neucli.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Lim CY, Shin HI. Noninvasive DC stimulation on neck changes MEP. Neuroreport. 2011;22:819–823. doi: 10.1097/WNR.0b013e32834b939d. [DOI] [PubMed] [Google Scholar]
- Meyer-Frießem CH, Haag LM, Schmidt-Wilcke T, Magerl W, Pogatzki-Zahn EM, Tegenthoff M, Zahn PK. Transcutaneous spinal DC stimulation reduces pain sensitivity in humans. Neurosci Lett. 2015;589:153–158. doi: 10.1016/j.neulet.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Miltner W, Johnson R, Jr, Braun C, Larbig W. Somatosensory event-related potentials to painful and non-painful stimuli: effects of attention. Pain. 1989;38:303–312. doi: 10.1016/0304-3959(89)90217-0. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Salvador R, Wenger C, Fernandes SR. Computational models of non-invasive brain and spinal cord stimulation. Conf Proc IEEE Eng Med Biol Soc. 2016;2016:6457–6460. doi: 10.1109/EMBC.2016.7592207. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- Namer B, Barta B, Orstavik K, Schmidt R, Carr R, Schmelz M, Handwerker HO. Microneurographic assessment of C-fibre function in aged healthy subjects. J Physiol. 2009;587:419–428. doi: 10.1113/jphysiol.2008.162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, Paulus W, Hummel F, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Transcranial direct current stimulation--update 2011. Restor Neurol Neurosci. 2011;29:463–492. doi: 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- Nizard J, Raoul S, Nguyen JP, Lefaucheur JP. Invasive stimulation therapies for the treatment of refractory pain. Discov Med. 2012;14:237–246. [PubMed] [Google Scholar]
- Parazzini M, Fiocchi S, Liorni I, Rossi E, Cogiamanian F, Vergari M, Priori A, Ravazzani P. Modeling the current density generated by transcutaneous spinal direct current stimulation (tsDCS) Clin Neurophysiol. 2014;125:2260–2270. doi: 10.1016/j.clinph.2014.02.027. [DOI] [PubMed] [Google Scholar]
- Perrotta A, Bolla M, Anastasio MG, Serrao M, Sandrini G, Pierelli F. Modulation of temporal summation threshold of the nociceptive withdrawal reflex by transcutaneous spinal direct current stimulation in humans. Clin Neurophysiol. 2016;127:755–761. doi: 10.1016/j.clinph.2015.01.031. [DOI] [PubMed] [Google Scholar]
- Powell ES, Carrico C, Raithatha R, Salyers E, Ward A, Sawaki L. Transvertebral direct current stimulation paired with locomotor training in chronic spinal cord injury: A case study. NeuroRehabilitation. 2016;38:27–35. doi: 10.3233/NRE-151292. [DOI] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol. 2013;591:2563–2578. doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkhoff NJ, Hendrikx LB, van Kerrebroeck PE, Debruyne FM, Wijkstra H. Selective detrusor activation by electrical stimulation of the human sacral nerve roots. Artif Organs. 1997;21:223–226. doi: 10.1111/j.1525-1594.1997.tb04654.x. [DOI] [PubMed] [Google Scholar]
- Schweizer LM, Zahn PK, Pogatzki-Zahn EM, Magerl W, Tegenthoff M, Meyer-Frießem CH. Influence of transcutaneous spinal stimulation on human LTP-like pain amplification. A randomized, double-blind study in volunteers. Clinical Neurophysiology. 2017 doi: 10.1016/j.clinph.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Spiegel J, Hansen C, Treede RD. Laser-evoked potentials after painful hand and foot stimulation in humans: evidence for generation of the middle-latency component in the secondary somatosensory cortex. Neurosci Lett. 1996;216:179–182. doi: 10.1016/0304-3940(96)13025-1. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Tai C, Roppolo JR, de Groat WC. Analysis of nerve conduction block induced by direct current. J Comput Neurosci. 2009;27:201–210. doi: 10.1007/s10827-009-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkka IM, Treede RD. Equivalent electrical source analysis of pain-related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol. 1993;10:513–519. doi: 10.1097/00004691-199310000-00009. [DOI] [PubMed] [Google Scholar]
- Treede RD, Kief S, Holzer T, Bromm B. Late somatosensory evoked cerebral potentials in response to cutaneous heat stimuli. Electroencephalogr Clin Neurophysiol. 1988;70:429–441. doi: 10.1016/0013-4694(88)90020-x. [DOI] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483(Pt 3):747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol. 1998;80:1082–1093. doi: 10.1152/jn.1998.80.3.1082. [DOI] [PubMed] [Google Scholar]
- Truini A, Vergari M, Biasiotta A, La Cesa S, Gabriele M, Di Stefano G, Cambieri C, Cruccu G, et al. Transcutaneous spinal direct current stimulation inhibits nociceptive spinal pathway conduction and increases pain tolerance in humans. Eur J Pain. 2011;15:1023–1027. doi: 10.1016/j.ejpain.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Valentini E, Hu L, Chakrabarti B, Hu Y, Aglioti SM, Iannetti GD. The primary somatosensory cortex largely contributes to the early part of the cortical response elicited by nociceptive stimuli. Neuroimage. 2012;59:1571–1581. doi: 10.1016/j.neuroimage.2011.08.069. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Rambaud L, Mauguiere F. Scalp topography and dipolar source modelling of potentials evoked by CO2 laser stimulation of the hand. Electroencephalogr Clin Neurophysiol. 1996;100:343–353. doi: 10.1016/0168-5597(96)95625-7. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Barba C, Le Pera D, Tonali P, Mauguiere F. Sources of cortical responses to painful CO(2) laser skin stimulation of the hand and foot in the human brain. Clin Neurophysiol. 2000;111:1103–1112. doi: 10.1016/s1388-2457(00)00273-x. [DOI] [PubMed] [Google Scholar]
- Winkler T, Hering P, Straube A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin Neurophysiol. 2010;121:957–961. doi: 10.1016/j.clinph.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Wooten M, Weng HJ, Hartke TV, Borzan J, Klein AH, Turnquist B, Dong X, Meyer RA, et al. Three functionally distinct classes of C-fibre nociceptors in primates. Nat Commun. 2014;5:4122. doi: 10.1038/ncomms5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Kanda M, Shindo K, Fujiwara N, Nagamine T, Ikeda A, Honda M, Tachibana N, et al. Pain-related somatosensory evoked potentials following CO2 laser stimulation of foot in man. Electroencephalogr Clin Neurophysiol. 1995;96:12–23. doi: 10.1016/0013-4694(94)00223-8. [DOI] [PubMed] [Google Scholar]