Abstract
Structural, biochemical and biophysical studies of eukaryotic soluble and membrane proteins require their production to milligram quantities. Although large-scale protein expression strategies based on transient or stable transfection of mammalian cells are well established, they are associated with high consumable costs, limited transfection efficiency or long and tedious selection of clonal cell lines. Lentiviral transduction is an efficient method for the delivery of transgenes to mammalian cells and unifies the ease of use and speed of transient transfection with the robust expression of stable cell lines. In this Protocol, we describe the design and step-by-step application of a lentiviral plasmid suite, termed pHR-CMV-TetO2, for the constitutive or inducible large-scale production of soluble and membrane proteins in HEK293 cell lines. Optional features include bicistronic co-expression of fluorescent marker proteins for enrichment of co-transduced cells using cell sorting, and of biotin ligase for in vivo biotinylation. We demonstrate the efficacy of the method for a set of soluble proteins and for the G-protein coupled receptor (GPCR) Smoothened (SMO). We further compare this method with baculovirus transduction of mammalian cells (BacMam), using the type-A γ-aminobutyric acid receptor (GABAAR) β3 homopentamer as a test case. The protocols described here are optimized for simplicity, speed and affordability, lead to a stable polyclonal cell line and milligram-scale amounts of protein in 3-4 weeks, and routinely achieve a ~3-10-fold improvement in protein production yield per cell as compared to transient transduction or transfection.
Keywords: Lentivirus, transduction, protein expression, soluble secreted protein, membrane protein, stable cell line, flow cytometry
Introduction
Technologies for mammalian transient or stable cell line expression have greatly facilitated the production of stabilized and correctly folded recombinant eukaryotic proteins 1. These proteins are often large, have a multi-domain architecture, and contain numerous post-translational modifications (PTMs) such as O- or N-linked glycosylation 2. Accordingly, such proteins are best expressed in native cell types that contain the necessary cellular machinery for protein synthesis, folding and quality control, PTMs and correct subcellular targeting, as well as a near-native membrane and lipid environment.
Previously, we have reported protocols for efficient transient protein expression in mammalian cells using the pHLsec plasmid 3. This plasmid and its derivatives have now been successfully used for more than ten years within the Division of Structural Biology in Oxford, and have been distributed to numerous other labs. During this period, limitations associated with large-scale transient protein expression in HEK293T cells have also become apparent: large-scale plasmid preparation became increasingly costly; the procedure is fairly laborious and generates a significant amount of plastic and biological waste. The volumes of biomass and expression media typically required, and large number of plastic roller bottles often used for a given target, add up to significant financial and environmental burdens. In our hands, large-scale transient transfection efficiencies using polyethylenimine (PEI) are typically limited to ~60-75%, meaning that a significant portion of the total cell mass is wasted.
The generation of stably transfected cell lines overcomes many disadvantages associated with transient transfection 4. It has however one significant drawback; the timeframe to establish a monoclonal cell line through rigorous selection and expansion is approximately 8-10 weeks for every construct 4, which significantly hampers achieving a high sample throughput.
An ideal method would combine the ease of use and speed of transient transfection with the robust expression of stable cell lines. The use of lentiviruses, belonging to the retrovirus genus, presents such a possibility. A recombinant lentivirus, containing genetic elements from human immunodeficiency virus (HIV), is widely used for gene delivery in medical applications 5. Lentiviruses use host cellular machinery to amplify and package their genetic material and the packaged transgene is delivered to the target cell via membrane fusion. Therefore, this method circumvents the need for large-scale DNA preps and laborious transfection procedures while rapidly establishing stable genome integration of the transgene with high efficiency. Although a number of laboratories have designed customized lentiviral vectors for transgene delivery 6–9 and several companies market proprietary lentiviral expression systems, the use of lentiviral transduction for large-scale protein production is not widespread in the structural biology community, perhaps owing to a lack of proof-of-principle examples and detailed protocols.
Here we describe step-by-step procedures for large-scale expression of soluble and membrane proteins using a lentiviral system. We have constructed a plasmid suite around the transfer plasmid pHR-CMV-TetO2 that is specifically designed for large-scale protein expression from HEK293 cell lines and is compatible with subcloning of cDNA inserts from the pHLsec plasmid commonly used for transient transfection 3. Use of this lentiviral method leads to the rapid (~7 days) establishment of polyclonal cell lines that can be selected, expanded and adapted to a variety of protein expression setups (Fig. 1A,B). The typical lead-time for protein production of ~3-4 weeks compares well to transient expression 3. We routinely observe a ~3-10-fold improvement in protein production yield per cell as compared to transient transfection.
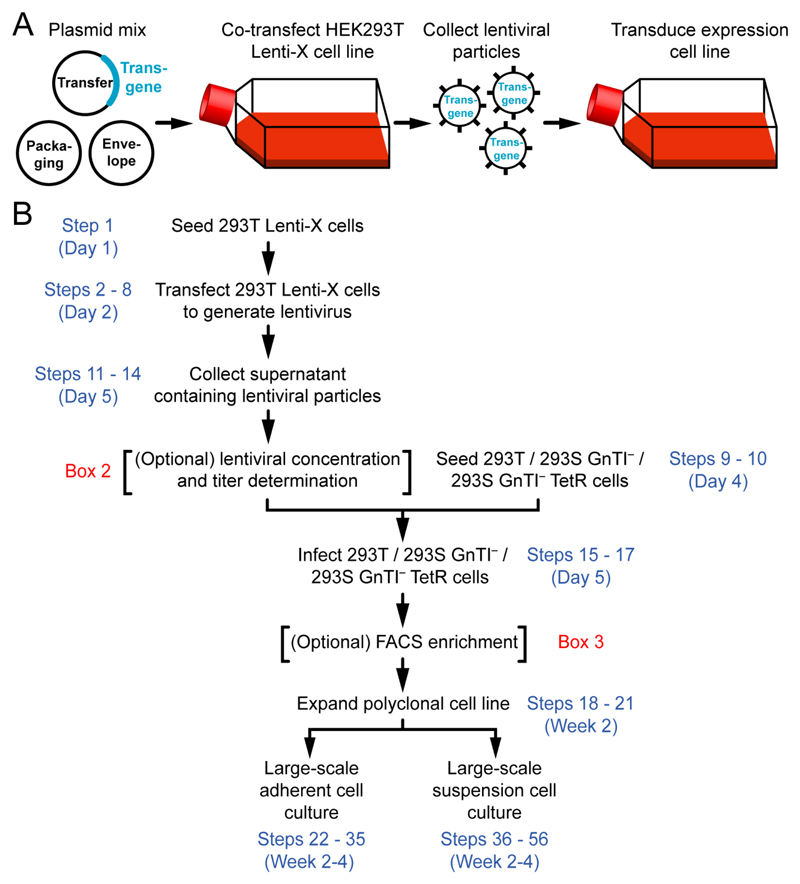
Figure 1. Large-scale expression of soluble or membrane proteins using lentiviral transduction.
(A) Graphical summary of the procedure. (B) Flowchart for the protocol.
Overview of the Procedure
The procedure to transduce expression cells is straightforward; a lentivirus producer cell line (HEK293 Lenti-X) is transiently co-transfected with transfer (encoding the transgene of interest), envelope and packaging plasmids to generate lentiviral particles. The lentivirus-containing supernatant is then, normally without concentration, used to transduce the HEK293 target cell line (Fig. 1A). After genomic integration of the proviral DNA and optional enrichment using cell sorting, these cells are expanded for large-scale protein expression. The procedure can be broken down into four stages (Fig. 1B). In the first stage (Steps 1-8 and Steps 11-14), the lentivirus producer cell line is transfected, and lentiviral particles encoding the transgene(s) are collected. In the second stage (Steps 9-10 and Steps 15-21), expression cells are infected, stably transduced, and expanded. In the third stage (Step 22 Option A i-xiii) adherent expression cells are expanded for large-scale soluble secreted protein expression. Alternatively, in the fourth stage (Step 22 Option B i-xxii), suspension expression cells are expanded for large-scale inducible membrane protein expression.
Features of the pHR-CMV-TetO2 transfer plasmid
We initially developed the lentiviral transfer plasmid pHR-CMV-TetO2 (Table 1) and the protocols described in this paper for high-level expression of the extracellular domain (ECD) of CD45 (protein tyrosine phosphatase receptor type C; PTPRC) for structural studies 10,11. The plasmid contains the minimally necessary cis-acting human immunodeficiency virus (HIV) components: the 5’ and 3’ long terminal repeats (LTRs), the polypurine tract (PPT), the Rev response element (RRE), and the psi (ψ) packaging signal. The genetic elements flanked by the 5’ and 3’ LTRs will be stably integrated into the host cell genome as proviral DNA (Fig. 2A and Supplementary Fig. 1).
Table 1. List of transfer plasmids.
| Plasmid no. | pHR-CMV-TetO2 | Addgene Plasmid # | Purpose |
|---|---|---|---|
| 1 | 3C-Twin-Strep | 113883 | Protein (co-)expression, FACS |
| 2 | 3C-Twin-Strep_IRES-EmGFP | 113884 | |
| 3 | 3C-Twin-Strep_IRES-mRuby2 | 113885 | |
| 4 | 3C-Twin-Strep_IRES-mTurquoise2 | 113886 | |
| 5 | 3C-Avi-His6 | 113887 | Protein (co-)expression, FACS, in vivo biotinylation |
| 6 | 3C-Avi-His6_IRES-EmGFP | 113888 | |
| 7 | 3C-Avi-His6_IRES-mRuby2 | 113889 | |
| 8 | 3C-Avi-His6_IRES-mTurquoise2 | 113890 | |
| 9 | 3C-mVenus-Twin-Strep | 113891 | Membrane protein expression and FSEC-TS screening, FACS |
| 10 | EmGFP | 113892 | Transduction & FACS compensation controls |
| 11 | mVenus | 113893 | |
| 12 | mRuby2 | 113894 | |
| 13 | mTurquoise2 | 113895 | |
| 14 | HA-BirA | 113896 | HA-tagged cytosolic and ER-resident biotin ligase for in vivo biotinylation |
| 15 | HA-BirA-ER | 113897 | |
| 16 | 3C-Avi-His6_IRES-HA-BirA-ER | 113898 | |
| Plasmid no. | pHR-CMV | Addgene Plasmid # | Purpose |
| 17 | TetR-HA-NLS-P2A-BSD-Myc | 113899 | Generation of inducible cell lines |
| Plasmid no. | pHR-SFFV | Addgene Plasmid # | Purpose |
| 18 | 3C-Twin-Strep | 113900 | Alternative for CMV-TetO2 |
| Plasmid no. | pHR-CAG | Addgene Plasmid # | Purpose |
| 19 | 3C-Twin-Strep | 113901 | Alternative for CMV-TetO2 |
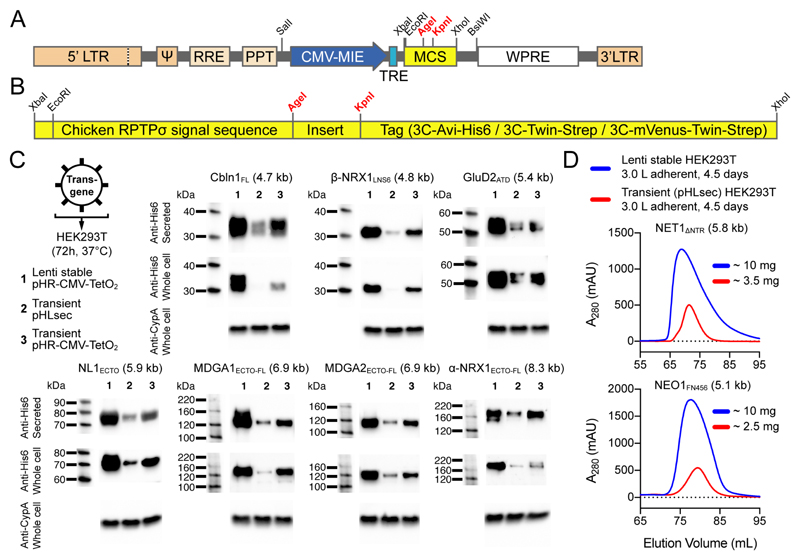
Figure 2. The pHR-CMV-TetO2 transfer plasmid for lentiviral stable expression in HEK293 cells.
(A) Genetic elements between the 5’ and 3’ long terminal repeats (LTRs) are packaged in lentiviral particles and integrated in the expression cell genome as proviral DNA. Total proviral DNA size in the empty pHR-CMV-TetO2 plasmid is 4.2 kb. ψ: psi packaging signal; RRE: Rev response element; PPT: polypurine tract; CMV-MIE: major immediate early cytomegalovirus enhancer and promoter; TRE: tetracycline response element; MCS: multiple cloning site; WPRE: Woodchuck Hepatitis Virus posttranscriptional regulatory element. (B) The pHR-CMV-TetO2 multiple cloning site (MCS). (C) Non-concentrated lentiviral particles were used to infect HEK293T cells and protein was expressed for 72 h. His6-tagged proteins were detected using Western blotting (mouse anti-His6 primary antibody: 1:5,000 dilution, HRP-conjugated goat anti-mouse secondary antibody: 1:5,000 dilution, rabbit anti-Cyclophilin A (CypA) primary antibody: 0.5 μg/mL, HRP-conjugated goat anti-rabbit secondary antibody: 1:5,000 dilution). Proviral DNA sizes are noted in brackets. (D) Comparative size-exclusion chromatograms of secreted NET1ΔNTR and NEO1FN456, showing improved secreted protein yields from lentiviral transduction (blue curves) as compared to transient transfection (red curves) of adherent HEK293T cells.
The transfer plasmid is used in conjunction with a second-generation envelope plasmid (pMD2.G; Addgene Plasmid #12259) and a second-generation packaging plasmid (psPAX2; Addgene Plasmid #12260). pMD2.G encodes the Vesicular Stomatitis Virus G envelope protein (VSV-G) and its use ensures a pseudotyped lentiviral particle with high infectivity and broad host tropism 12; the receptor for VSV-G is the low-density lipoprotein receptor (LDLR) 13. psPAX2 contains the minimally necessary HIV genes required for virus production; gag, pol, rev and tat.
pHR-CMV-TetO2 was derived from the second-generation pHR-SIN-CSGW lentiviral transfer plasmid 14,15. To maximize restriction/ligation subcloning compatibility with our pHLsec plasmid for transient expression 3, we first deleted all internal EcoRI, AgeI, KpnI, XhoI, SalI, XbaI, NotI and BamHI restriction sites from the pHR-SIN-CSGW backbone using PCR. Then, we placed the pHLsec MCS after the pHR-SIN-CSGW Spleen Focus Forming Virus (SFFV) promoter. HEK293 and derived cell lines 16 are widely used for large-scale protein production for structural biology purposes (see Box 1). To optimize the plasmid for protein expression in HEK293 cells, we replaced the SFFV promoter with the major immediate early (MIE) human cytomegalovirus (CMV) enhancer and promoter 17 and two TetO operator sequences, all amplified from the pACMV-tetO plasmid 18, to generate pHR-CMV-TetO2. The CMV promoter 19 is strongly transactivated by the adenoviral E1A protein 20 that is constitutively expressed by immortalized HEK293 cells. The pHR-CMV-TetO2 plasmid retains the Woodchuck Hepatitis Virus (WHP) posttranscriptional regulatory element (WPRE) 21 from the pHR-SIN-CSGW plasmid, leading to improved transcript stability and transgene expression (Fig. 2A and Supplementary Fig. 1).
Box 1. Growth and maintenance of adherent and suspension HEK293 cells ●TIMING ~30 min.
Adherent HEK293T, HEK293S GnTI– or HEK293S GnTI– TetR cells are normally grown and maintained in standard T75 (75 cm2) or T175 (175 cm2) flasks in a humidified incubator operated at 37°C with 5% CO2. Using flow cytometry, we determined that HEK293 cells reach a density of ~250,000 cell per cm2 flask surface area upon confluency. This number is a useful reference to calculate seeding densities of plates, flasks and bottles. It corresponds to ~2,400,000 cells in a confluent 6-well (9.6 cm2), ~6,250,000 cells in a confluent T25 flask (25 cm2), ~18,750,000 cells in a confluent T75 flask (75 cm2) and ~43,750,000 cells in a confluent T175 flask (175 cm2).
Expand (“split”) confluent T75 or T175 flasks containing adherent cells by removing the complete growth medium, washing the cells with PBS (5 or 10 mL, respectively) and incubating them with Trypsin-EDTA (2 and 5 mL, respectively) for 3 min in a humidified incubator operated at 37°C with 5% CO2. Then, gently dislodge the cells and quench the Trypsin-EDTA solution using 10 or 25 mL complete growth medium, respectively. Pipet up and down a few times using a sterile serological 10 mL pipet to break up any clumped cells. Finally, transfer the required number of cells (typically 1/6th of the total cell slurry) to a new T75 or T175 flask and top up the complete growth medium to the recommended volume (12 or 30 mL, respectively).
Large-scale adherent cultures for soluble secreted protein production are performed in expanded-surface polystyrene roller bottles (2125 cm2) or in HYPERFlasks (1720 cm2). HEK293T and HEK293S GnTI– cells are maintained in DMEM/F-12/10%FBS during expansion, and DMEM/F-12/2%FBS during protein expression, respectively. HEK293S GnTI– TetR cells are grown identically, except for addition of 2 μg/mL blasticidin; this maintains selective pressure on the pcDNA6/TR genetic elements with which the HEK293S GnTI– TetR cells are stably transfected (the cell line was originally selected using 5 μg/mL blasticidin 34, but we observe slowed cell growth at that concentration).
Suspension-adapted HEK293S GnTI– and HEK293S GnTI– TetR cells are normally grown in polycarbonate Erlenmeyer baffled flasks with a filter cap, in a shaking incubator operated at 37°C with 8% CO2 (note that this differs from the 5% CO2 used for adherent cells). Cells are grown in FreeStyle 293/1%FBS medium. Although we add 1% FBS to the FreeStyle 293 medium to facilitate the transfer from adherent to suspension culture (Step 22, Option B, i-ix), it does in principle not require supplementation. The flasks are shaken at 130 rpm and the suspension does not exceed 40% of the flask volume (i.e. for a 2 L flask, a maximum suspension volume of 800 mL is used).
Non-suspension adapted HEK293 cell lines such as HEK293T will require adaptation to suspension growth over a period of several weeks 60. Indeed, full suspension adaptation of the original HEK293 cell line to yield the HEK293S cell line took ~7 months 16. FreeStyle 293-F cells, derived from the fast-growing suspension adapted HEK293-F cell line, are an alternative.
We prefer performing lentiviral transduction in adherent culture, because suspension adapted HEK293S GnTI– and HEK293S GnTI– TetR expression cells adapt readily from an adherent monolayer to suspension culture (Step 22, Option B, i-ix), and because the method relies on direct transfer of lentivirus-containing medium from the adherent HEK293T Lenti-X producer cells to the expression cells (Steps 11-17).
We routinely do not use Penicillin Streptomycin (“Pen Strep”) to prevent bacterial growth, relying instead on sterile technique and well-maintained tissue culture infrastructure.
Cell lines should be regularly checked to ensure that they are authentic and not infected with mycoplasma.
The American Type Culture Collection (ATCC) animal cell culture guide (https://www.atcc.org/~/media/PDFs/Culture%20Guides/AnimCellCulture_Guide.ashx) contains a wealth of tips and techniques for culturing continuous cell lines.
The multiple cloning site (MCS) of the pHR-CMV-TetO2 plasmid is fully compatible with that of pHLsec 3, and is organized as follows; EcoRI – chicken RPTPσ signal sequence – AgeI – insert – KpnI – tag + stop codons – XhoI. Thus, by digesting pHLsec-based constructs with EcoRI/AgeI and XhoI, the complete expression cassette can be transferred to the pHR-CMV-TetO2 backbone (Fig. 2B and Supplementary Fig. 2A,B). The chicken RPTPσ signal sequence directs targeting of the nascent protein to the secretory pathway. In case one wants to use the native signal sequence, in case of intracellular proteins, or in case of membrane proteins that do not contain a signal sequence (for example by relying on transmembrane domains for targeting to the secretory pathway), the chicken RPTPσ signal sequence can be removed by digesting the plasmid with EcoRI. We recommend in vivo assembly (IVA) cloning 22, which exploits recA-independent homologous recombination between a PCR amplicon and the linearized pHR-CMV-TetO2 vector (see Supplementary Methods), as a time-efficient alternative to regular restriction/ligation subcloning.
In the empty pHR-CMV-TetO2 plasmid, the purification tag inserted between the KpnI and XhoI restriction sites is either (i) the 3C-Avi-His6 tag (pHR-CMV-TetO2_3C-Avi-His6 plasmid), or (ii) the 3C-Twin-Strep tag (pHR-CMV-TetO2_3C-Twin-Strep plasmid) (Table 1 and Supplementary Fig. 2A,B). The 3C-Avi-His6 tag consists of a sequential Avi-tag and His6-tag preceded by a Human Rhinovirus (HRV) 3C protease site. It allows straightforward purification of proteins using immobilized metal affinity chromatography (IMAC). Use of the Avi-tag for in vivo biotinylation of proteins is described in the section “Other applications of the method”. The 3C-Twin-Strep tag consists of a sequential arrangement of two Strep-tag II® sequences preceded by a HRV 3C protease site, and is used in conjunction with Strep-Tactin XT® resin 23 (IBA GmbH), for one-step protein purification 24. In both cases, the HRV 3C protease site allows removal of the purification tag by digestion with 3C protease, optionally combined with a reverse purification step.
Finally, the pHR-CMV-TetO2 plasmids contain the Simian vacuolating virus 40 (SV40) origin of replication (ori) as well as the SV40 large T-antigen under control of the SV40 promoter (Supplementary Fig. 1); these features allow autonomous episomal replication of the plasmid in mammalian cells to ensure maintenance of high plasmid copy numbers. The pHR-CMV-TetO2 plasmids are hence also suitable for transient transfection, and can be used for initial small-scale screening of expression constructs (Fig. 2C).
Extended features of the pHR-CMV-TetO2 transfer plasmid suite
A limitation of the lentiviral system is that the size of the transgene to be packaged is physically constrained. Viral titers decrease semi-logarithmically with increasing insert size, with an estimated drop of ~1 log for every 2 kb of insert 25. Measurable titers can however still be obtained with proviral DNA sizes in excess of 18 kb 25. We found that lentiviral titers that are obtained with (i) the HEK293 Lenti-X producer cell line, which is clonally selected to support high-level expression of viral proteins to yield higher viral titers, and (ii) proviral DNA sizes not greater than ~8.5 kb, are sufficiently high to lead to >90% transduction efficiency of HEK293 expression cells. For the pHR-CMV-TetO2_3C-Avi-His6 and pHR-CMV-TetO2_3C-Twin-Strep plasmids, this equates to an insert size of ~4.3 kb between the EcoRI and XhoI sites (Supplementary Fig. 1). Practically, this means that neither concentration of lentiviral titer nor selection of the polyclonal stable cell line is necessary, and that the recombinant protein-producing cell population can immediately be expanded, thereby yielding significant time savings.
Foregoing concentration of the lentivirus-containing supernatant is time-efficient, prevents any negative impact on lentiviral particle integrity and also prevents the infectious lentiviral particles from having to be taken out of the flow cabinet. Consequentially, transduction is performed at a variable multiplicity of infection (MOI; the ratio of the number of viral particles to the number of target cells) since insert size correlates strongly with viral titer and viral titer determines the MOI.
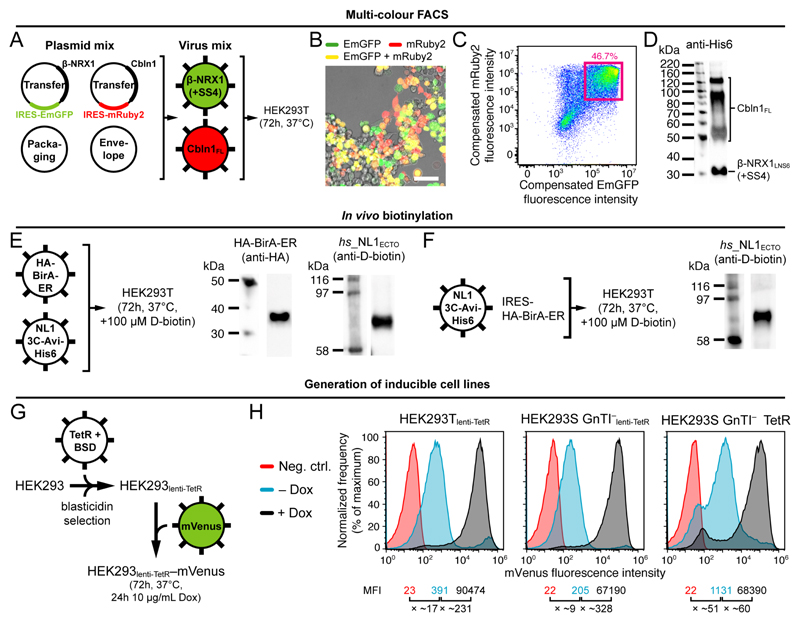
It is desirable to be able to enrich any subset of transduced cells from the total cell pool, especially when working with non-concentrated lentiviral particles encoding proviral DNA sizes greater than ~8.5 kb that yield lower viral titers and corresponding transduction efficiencies. Two strategies are traditionally used; antibiotic selection and enrichment by fluorescence-activated cell sorting (FACS). Antibiotic selection is slower but high selection pressure allows isolation of cells with high copy number transgene integration 7. We opted for FACS because it is faster, and because the presence of fluorescent marker proteins can have multiple downstream applications. The use of FACS to obtain high protein expressing clonal cell lines has long precedence 26–28. We constructed variants of the pHR-CMV-TetO2 plasmid that allow co-expression of fluorescent proteins (FPs) using two strategies;
The 3C-mVenus-Twin-Strep variant (Table 1) encodes mVenus (λEX 515 nm, λEM 528 nm) as a 3C-cleavable C-terminal fusion protein, cloned in frame as a tag between the KpnI and XhoI restriction sites (Supplementary Fig. 3). In this setup, there is a direct 1:1 stoichiometric correspondence between the target protein and the FP.
The IRES-FP variants (Table 1; IRES-EmGFP, IRES-mRuby2 and IRES-mTurquoise2) contain an internal ribosome entry site (IRES) from encephalomyocarditis virus (EMCV) followed by the three different FPs to allow their expression from the same mRNA as the transgene (bicistronic expression) (Fig. 3A and Supplementary Fig. 3). These FPs are the Emerald green fluorescent protein (EmGFP: λEX 487 nm, λEM 509 nm) 29, the red fluorescent protein mRuby2 (λEX 559 nm, λEM 600 nm) 30 and the cyan fluorescent protein mTurquoise2 (λEX 434 nm, λEM 474 nm) 31. The IRES-EmGFP, IRES-mRuby2 and IRES-mTurquoise2 sequences are cloned between the XhoI and BsiWI sites, placing them downstream of the MCS and upstream of the WPRE. A consequence of this setup is that the IRES-FP elements add ~1.3 kb to the proviral DNA size, and so have an impact on lentiviral titer.
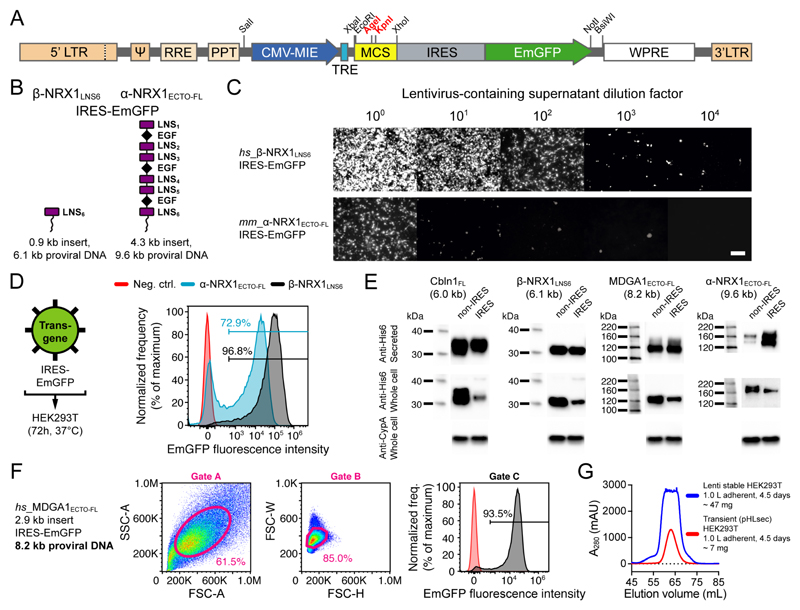
Figure 3. The pHR-CMV-TetO2_IRES-EmGFP transfer plasmid for determination of transduction efficiency and enrichment of transduced cells using FACS.
(A) Schematic representation of the pHR-CMV-TetO2_IRES-EmGFP proviral DNA (5.5 kb) for bicistronic expression of the transgene of interest and free cytosolic EmGFP. (B) Schematic representation of the β-NRX1LNS6 and α-NRX1ECTO-FL constructs. (C) Endpoint dilution assay to estimate functional titer of non-concentrated lentiviral particles encoding β-NRX1LNS6_IRES-EmGFP and α-NRX1ECTO-FL_IRES-EmGFP. Every image was individually enhanced using ImageJ 59 to reveal all cells with fluorescence. Scale bar: 100 μm. (D) Non-concentrated lentiviral particles were used to infect HEK293T cells and protein was expressed for 72 h. Comparison of transduction efficiency of β-NRX1LNS6_IRES-EmGFP and α-NRX1ECTO-FL_IRES-EmGFP. (E) Western blot detection of His6-tagged proteins produced using the pHR-CMV-TetO2_3C-Avi-His6 or pHR-CMV-TetO2_3C-Avi-His6_IRES-EmGFP transfer plasmids (mouse anti-His6 primary antibody: 1:5,000 dilution, HRP-conjugated goat anti-mouse secondary antibody: 1:5,000 dilution, rabbit anti-Cyclophilin A (CypA) primary antibody: 0.5 μg/mL, HRP-conjugated goat anti-rabbit secondary antibody: 1:5,000 dilution). Proviral DNA sizes are noted in brackets. (F) MDGA1ECTO-FL_IRES-EmGFP: forward and side scatter area (A), height (H) and width (W), and fluorescence gates. (G) Comparative size-exclusion chromatograms show secreted MDGA1ECTO-FL yields from 1.0 L (4 roller bottles) of adherent HEK293T cell culture, either transiently transfected (red curve; 6.7 mg total protein) or stably transduced using lentivirus (blue curve; 46.3 mg total protein). Transduced cells were enriched using FACS by applying sorting gates A, B and C from panel F.
To test the efficacy of the IRES-FP elements, we assembled a mRuby2-IRES-EmGFP construct (Supplementary Fig. 4A). Using quantitative fluorescence microscopy, we determined that expression levels of EmGFP are ~30-fold lower when placed after IRES as compared to EmGFP alone, indicating that mRNA translation initiation from an IRES element is less potent than 5’ cap-dependent translation (Supplementary Fig. 4B). Importantly however, we found that the downstream presence of IRES-EmGFP did not adversely affect fluorescence intensity levels in the red channel and that pixel values in the red and green channels correlated with a Pearson’s correlation coefficient (r) of 0.89, indicating that IRES-EmGFP fluorescence can indeed be used as a qualitative and quantitative predictor for the presence and level of the protein of interest encoded by the upstream transgene (Supplementary Fig. 4C). We corroborated these results using flow cytometry; we determined a Pearson’s r of 0.85 between compensated mRuby2 and EmGFP fluorescence in 35,546 single, gated HEK293 cells expressing the mRuby2-IRES-EmGFP construct (Supplementary Fig. 4D).
To verify the inverse relation between proviral DNA size and viral titer, we performed a comparative endpoint dilution assay (see Box 2) for the neuronal proteins β-NRX1LNS6 32 and α-NRX1ECTO-FL 33 cloned into the pHR-CMV-TetO2_3C-Avi-His6_IRES-EmGFP plasmid (6.1 kb and 9.6 kb proviral DNA, respectively; Fig. 3B), and observed a ~100-fold difference in viral titer (~2 logs; Fig. 3C and Supplementary Fig. 5). Using flow cytometry, we determined a transduction efficiency of ~97% and ~73%, respectively (Fig. 3D and Supplementary Fig. 6). To boost transduction efficiency for α-NRX1ECTO-FL, lentiviral particles would need to be concentrated before adding them to the expression cells. Alternatively, or additionally, cells expressing the EmGFP marker protein can be enriched using FACS, and then expanded (see Box 3).
Box 2. Concentration and determination of lentiviral titer. ●TIMING ~4 d.
Concentration and titration of the produced lentivirus allows better control over the MOI, and detailed protocols have been described previously 61. Approaches for titration include (i) quantitative reverse transcription polymerase chain reaction (qRT-PCR) to amplify lentiviral RNA, (ii) detection of the HIV p24 capsid protein, or (iii) qPCR to amplify proviral DNA. These assays are however time consuming, and do not reflect functional viral titers when based on quantification of viral RNA or p24. Instead, we prefer to use a rapid and simple endpoint dilution assay using fluorescence, essentially as described previously for Baculovirus transduction of mammalian cells (BacMam) 44,45. We recommend using EmGFP (pHR-CMV-TetO2_IRES-EmGFP plasmid variant) or mVenus (pHR-CMV-TetO2_3C-mVenus-Twin-Strep plasmid variant) for this purpose since mRuby2 or mTurquoise2 are less bright, which makes it difficult to identify sparsely seeded transduced cells.
Routinely however, we do not concentrate lentiviruses or determine their titer, instead preferring to transfer the lentivirus-containing supernatant (Steps 11-14) directly on top of the expression cells (Step 15). This approach works well for proviral DNA with a size of up to ~8.5 kb, saves time and prevents the virus-containing supernatant from exiting the laminar flow cabinet. IRES-FP fluorescence allows subsequent verification of transduction efficiency using fluorescence microscopy and flow cytometry, as well as FACS-based enrichment of subsets of cells from the total transduced cell pool.
Procedure
CRITICAL The following titration procedure is for one 96-well black plate. It is inspired by published approaches for Baculovirus transduction of mammalian cells (BacMam) 44,45. One 96-well black plate can be used to titer four lentivirus preparations simultaneously (columns 1-3, 4-6, 7-9, and 10-12); the eight dilutions (100 to 107), pipetted in triplicate, fit into rows A to H.
Seed each well of a 96-well black plate with ~4 x 104 HEK293T cells in DMEM/F-12/10%FBS, bringing them to ~50% confluency. It is convenient to do this the day before collection of the lentiviral particle containing supernatant, e.g. simultaneously with Step 9.
Allow the cells to attach and grow overnight at 37°C in a humidified incubator operated with 5% CO2.
Filter 1 mL of lentiviral particle containing supernatant from Step 11 through a 0.45 μm filter unit attached onto a Luer-lock syringe, and into a sterile 1.5 mL tube.
- Make dilution stocks of lentivirus from 100 to 107 in DMEM/F-12/2%FBS in sterile 1.5 mL tubes as follows:
Row Dilution # Dilution Medium Lentiviral stock A 0 100 - 100 μL of lentiviral stock (100) B 1 101 900 μL 100 μL of lentiviral stock (100) C 2 102 900 μL 100 μL of 101 dilution D 3 103 900 μL 100 μL of 102 dilution E 4 104 900 μL 100 μL of 103 dilution F 5 105 900 μL 100 μL of 104 dilution G 6 106 900 μL 100 μL of 105 dilution H 7 107 900 μL 100 μL of 106 dilution Remove the complete medium and replace it with 100 μL of diluted (100 to 107) virus.
Infect the HEK293T cells in triplicate for each virus.
Incubate the flask for 72 h at 37°C in a humidified incubator operated with 5% CO2.
After 72 h, count the number of fluorescent cells in the dilution that contains <10 fluorescent cells.
To calculate lentiviral titer, use the following equation; Transduction Units (TU)/mL = averaged number of fluorescent cells × dilution factor × 10. E.g., when observing an averaged number of 5 fluorescent cells in dilution 5 (row F); TU/mL = 5 x 106. In a confluent T25 flask, which contains ~6,250,000 cells (Box 1), the application of 4 mL of this supernatant leads to a functional MOI of ~3.2 and a theoretical transduction efficiency of ~95%. Examples are shown in Fig. 3C and Supplementary Fig. 5.
Box 3. FACS to enrich subpopulations of transduced cells. ●TIMING ~1 d.
FACS 62–64 presents a fast and convenient method to enrich transduced cell populations. The 3C-mVenus-Twin-Strep and IRES-FP plasmid variants are specifically designed for this purpose. In this box, we provide a number of pointers for optimal experimental results.
When performing cell sorting, we pre-fill our catch tubes with complete medium.
We find that post-sorting cell viability is greatest when using suspension cells.
For adherent cells, we recommend using soybean trypsin inhibitor (SBTI) instead of complete medium to inactivate trypsin and prevent cell clumping. We use trypsin-EDTA solution without phenol red to avoid background fluorescence.
We pass suspension cells and trypsinized adherent cells through a 40 μm cell strainer before sorting, to ensure that cells are mostly single. This prevents clogging of the cell sorter nozzle, which is important when doing prolonged cell sorts.
When performing multi-color FACS, fluorescence compensation is crucial because of the overlap of fluorochrome emission spectra. For this reason, our pHR-CMV-TetO2 suite contains plasmids for the expression of free cytosolic EmGFP, mVenus, mRuby2 and mTurquoise2. Cells expressing these fluorochromes can be used as bright positive control samples. Non-transduced cells serve as negative control sample. Combined, these control samples serve to properly set photomultiplier tube (PMT) detector voltages, and to calculate the fluorescence compensation matrix.
Fluorescence-minus-one (FMO) control samples are cells expressing all but one of the fluorochromes. They ensure that spread of fluorochromes into the channel of interest is properly identified and are ideal for determining accurate gating boundaries.
Viability dyes such as propidium iodide (PI), DRAQ7, 7-AAD or TO-PRO-3 that penetrate dead and dying cells and bind their DNA can be used to design a gating strategy to remove these cells from the transduced cell population, or to monitor cell viability to decide on the appropriate time for harvesting of expression cells or conditioned medium.
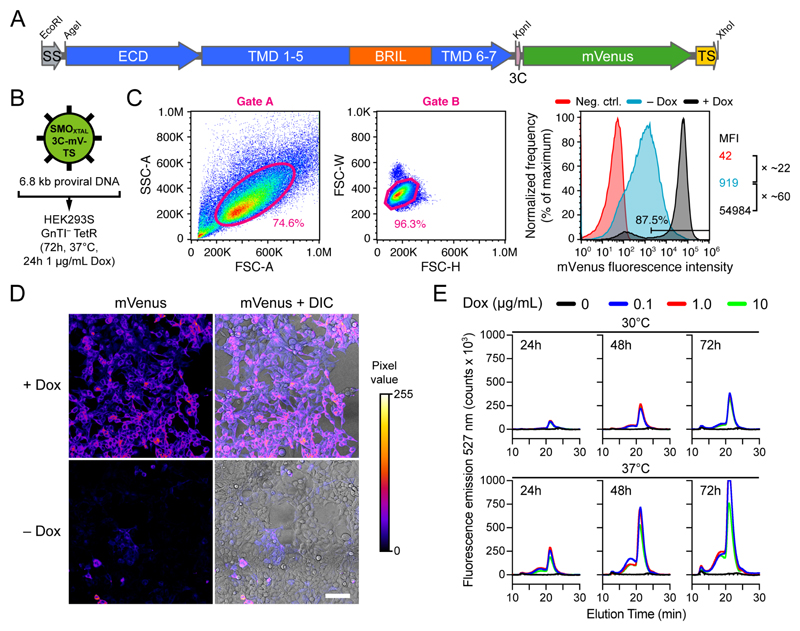
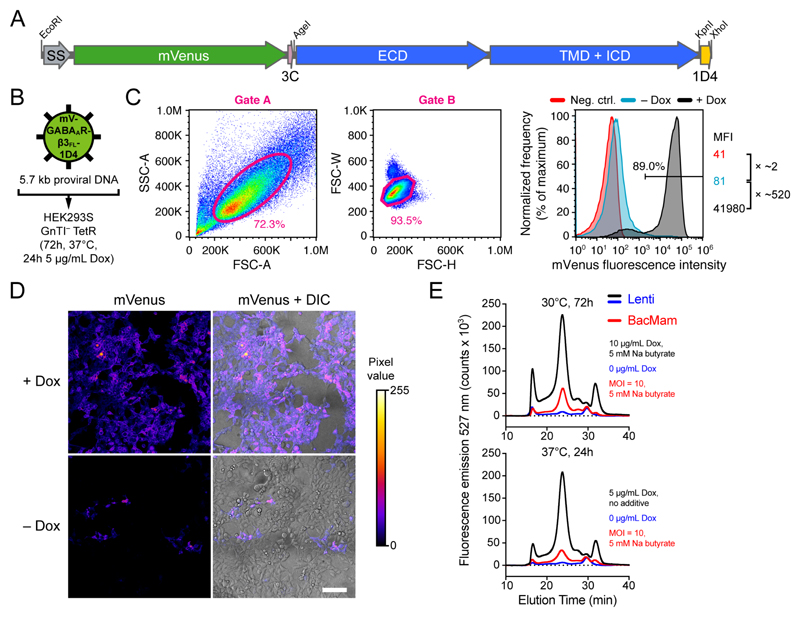
When using inducible cells, FACS can be performed using either the uninduced 65 or the Dox-induced fluorescence. Importantly, the 405 nm laser readily excites Dox, introducing potential bias in fluorescence measurements. Therefore, we induce protein expression 24-48 h before sorting using only 0.1 μg/mL Dox, and spin down the cells (500 g for 5 min) and resuspend them in Dox-free expression medium (without phenol red) or carrier buffer just before cell sorting. FACS compensation and FMO control samples are treated identically. Finally, we return the sorted cells to Dox-free growth media after sorting. Representative –Dox and +Dox fluorescence of HEK293S GnTI– TetR cells expressing the membrane proteins SMOXTAL and GABAAR-β3FL is shown in Fig. 4C and Fig. 5C, respectively.
The pHR-CMV-TetO2 plasmids are optimal for use with HEK293 cell lines
The pHR-CMV-TetO2 plasmid and its variants are optimal for use with the HEK293 cell line and its derivatives 16. We use the HEK293T, the N-acetylglucosaminyltransferase I-negative (GnTI–) HEK293S 34, and the HEK293S GnTI– TetR 34 cell lines for high-level protein production (see Box 1). The latter cell line is identical to HEK293S GnTI– but additionally stably transfected with the pcDNA6/TR vector, leading to constitutive intracellular expression of Tet repressor protein (TetR). TetR binds to the tetracycline response element (TRE) that is just downstream of the CMV promoter in the pHR-CMV-TetO2 plasmids (Fig. 2A, Supplementary Fig. 1 and Supplementary Fig. 3), thereby repressing transcription of the transgene. In the pHR-CMV-TetO2 plasmids, the TRE is composed of 2 repeats of the 19 bp TetO operator sequence (5’-TCCCTATCAGTGATAGAGA-3’). Transgene expression can then be induced (“de-repressed”) by application of tetracycline or its more stable analog doxycycline (Dox); Dox binds TetR, thereby releasing it from the TRE 18,35.
In summary, use of the pHR-CMV-TetO2 plasmids in combination with the HEK293S GnTI– TetR cell line is particularly attractive when producing mammalian proteins for structural biological purposes for a number of reasons; (i) stable expression generally yields higher amounts of protein than transient expression, (ii) the TetO2-TetR system allows inducible expression, which is useful when working with toxic proteins or membrane proteins, (iii) HEK293S cells allow both suspension and adherent growth, (iv) fluorescent marker proteins allow enrichment by FACS, and (v) GnTI– ensures the presence of predominantly homogeneous high mannose-type (Man5GlcNAc2) N-linked glycans that are sensitive to cleavage by endoglycosidase H (EndoH) or F1 (EndoF1) 2.
Other applications of the method
Alternative promoters
The CMV-MIE promoter/enhancer is one of the strongest promoters in HEK293 cells 17. Like other strong viral promoters, it is prone to transcriptional silencing regulated by DNA methylation after prolonged activity 36–38. It is however not prominent in HEK293 cell lines within the timeframe of our procedure and can be reversed by application of the DNA methyltransferase (DNMT) inhibitor 5-Aza-2’-deoxycytidine (5-aza-dC). Since the CMV-MIE promoter/enhancer may not be the best choice for any given cell type 17, we have also included pHR variants containing either the SFFV promoter (pHR-SFFV), or the CAG (CMV enhancer, chicken β-actin promoter, rabbit β-globin splice acceptor) element amplified from pHLsec 3 (pHR-CAG) (Table 1 and Supplementary Fig. 3). All promoters/enhancers are cloned between unique SalI and XbaI restriction sites, allowing their easy replacement with any promoter of choice. Expression cassettes can be transferred between the pHR-CMV-TetO2, pHR-SFFV and pHR-CAG variants (Supplementary Fig. 3).
Co-transduction of different transgenes
Co-infection of cells using separate viruses encoding different transgenes (using FP fusions or using the IRES-FP variants) is useful for the expression of heteromeric receptors or protein complexes. Co-infected cells expressing all subunits can be enriched using multi-color FACS (see Box 3).
In vivo biotinylation
In vivo biotinylation is a convenient way of introducing a biotin label onto a specific 15 residues acceptor sequence (“Avi-tag”; GLNDIFEAQKIEWHE in single-letter amino acid code). We have constructed the pHR-CMV-TetO2_HA-BirA (5.0 kb proviral DNA size) and pHR-CMV-TetO2_HA-BirA-ER (5.2 kb proviral DNA size) transfer plasmids that direct expression of intracellular or ER-resident HA-tagged E. coli biotin ligase (HA-BirA and HA-BirA-ER, respectively) (Table 1). Co-transduction of target cells with lentiviruses encoding HA-BirA or HA-BirA-ER, and lentiviruses encoding the transgene of interest carrying an Avi-tag, leads to site-specific biotinylation of the transgene in the presence of D-biotin. The choice for BirA or BirA-ER is determined by the subcellular location of the Avi-tag during protein trafficking; BirA-ER is used when the Avi-tag is located in the ER-lumen, and BirA is used when the Avi-tag is located in cytoplasm. We routinely use in vivo biotinylation to produce biotinylated protein ligands for biophysical analysis (e.g. surface plasmon resonance (SPR), bio-layer interferometry (BLI) etc.) or for immunohistochemistry. Finally, we constructed the 3C-Avi-His6_IRES-HA-BirA-ER plasmid variant to allow bicistronic expression of Avi-His6-tagged transgene and HA-BirA-ER (Table 1 and Supplementary Fig. 3).
Generation of inducible cell lines
We constructed the pHR-CMV_TetR-HA-NLS-P2A-BSD-Myc transfer plasmid that leads to constitutive expression of HA-tagged TetR and c-Myc-tagged blasticidin deaminase (BSD) from a single transcript (Table 1). The plasmid can be used to make the expression cell line of choice inducible and allows blasticidin selection of the transduced cell pool. It is an alternative to the pcDNA6/TR vector (ThermoFisher Scientific) with which the HEK293S GnTI– TetR cell line was originally constructed 34. The 2A peptide from porcine teschovirus-1 (P2A) directs ribosomal skipping during translation and ensures that TetR and BSD occur as two separate proteins 39. Additionally, we added the SV40 nuclear localization signal (NLS) to TetR to increase its nuclear localization.
Comparison with other methods
The main advantages of our method over transient transfection 3 are that: (i) no large-scale DNA preps (e.g. Mega- or Gigapreps) are necessary since a single mini prep is sufficient, (ii) no tedious large-scale transfection procedures are necessary, (iii) established cell lines can be stored in liquid nitrogen and re-grown when required, (iv) efficient transduction optionally preceded by lentivirus concentration and/or followed by FACS-based enrichment yields close to 100% protein expressing cells, (v) inducible large-scale expression is greatly facilitated, and (vi) protein production yields are larger.
The main advantages of our method over the establishment of a monoclonal stable cell line are simplicity and time-efficiency; a polyclonal stable cell line is generated in ~7 days using our procedure, whereas establishment and selection of stably transfected monoclonal cell lines is laborious and typically takes up to 8-10 weeks 4. Rigorous selection of monoclonal cell lines can lead to isolation of only the most potently expressing cell clones; it is of note that the use of the IRES-FP plasmid variants allows the isolation of single cells or the enrichment of subsets of cells from the total transduced cell pool using FACS (see Box 3). If the user should wish so, the IRES-FP elements can readily be replaced with IRES followed by an antibiotic selection marker gene of choice, such as ble (zeocin resistance), neor (geneticin/G418 resistance), pac (puromycin resistance) or bsd (blasticidin resistance).
piggyBac (PB), a DNA transposon from the cabbage looper moth Trichoplusia ni, is emerging as an effective tool for the generation of inducible, stably transfected mammalian cell lines for large-scale protein production 40,41. Like lentivirus, the piggyBac-based system achieves high and uniform expression levels. Stably transfected cells need to be selected with antibiotic for ~2-3 weeks before scale-up to bulk cell culture.
BacMam 42,43 is the use of baculovirus to transiently deliver genes to mammalian cells. It has been widely used for both screening and large-scale expression of membrane protein constructs 44,45. A key advantage of our method is that lentiviral transduction leads to stable transgene expression, and that successful cell lines can be stored. Additionally, no dedicated infrastructure, media, cell lines or consumables to sustain working with insect cells are necessary. Moreover, the generation of lentivirus is substantially easier and faster than the production of the large quantities of P1 and P2 BacMam virus that are needed to infect large volumes of expression cells 44. Lentivirus thus has a much smaller laboratory footprint than BacMam. Comparative advantages of BacMam are that baculovirus only requires biosafety level 1 (BSL1) practices, and that it is able to carry very large (>15 kb) 42 or multiple inserts. The latter issue can however be circumvented by co-infecting expression cells with multiple lentiviruses and enriching them using multi-color FACS (using the IRES-FP plasmid variants; see Box 3).
Biological Safety Considerations
The lentiviral expression system requires biosafety level 2 (BSL2) or 2+ (BSL2+) practices, depending on the institutional and governmental biosafety guidelines. The two main safety concerns surrounding the use of lentiviral are (i) the potential for generation of replication-competent lentivirus and (ii) the potential for oncogenesis.
The potential for generation of replication-competent lentivirus is determined by the specific design of the plasmids. The 2nd generation lentiviral system described here separates transfer, envelope, and packaging components of the lentivirus onto three separate plasmids. Although 3rd generation lentiviral vectors further improve on the safety of the 2nd generation by splitting the packaging system into two separate plasmids 46, they are more cumbersome to use and result in lower viral titers due to the requirement of one additional plasmid.
The transfer plasmid contains the sequences that will incorporate into the host cell genome, but functional viral particles cannot be produced from it without the genes encoded in the envelope and packaging plasmids. Simultaneous recombination events between the packaging, envelope, and transfer plasmids that produce replication-competent lentiviruses (RCLs) are very unlikely. Replication-competent retroviruses (RCRs) have however been reported 47. The pHR-CMV-TetO2 plasmids are all self-inactivating (SIN 48,49); they have a deletion in the 3’ LTR (ΔU3) of the viral genome that is transferred into the 5’ LTR after one round of reverse transcription. This deletion abolishes transcription of the full-length proviral DNA. SIN vectors also reduce the possibility of recombination to generate RCLs.
The potential for oncogenesis is largely based on the specific insert contained within the lentiviral transfer plasmid and should be considered on a case-by-case basis.
To mitigate risks even further, we do not routinely concentrate the viral supernatant to make lentiviral stock solutions. We directly transfer the lentivirus-containing supernatant onto the expression cells (Steps 11-17 in the Procedure) and use FACS to enrich transduced cell populations. This limits the time that researchers are working with viral particles that are outside of a sealed container and also prevents the viral supernatant from exiting the laminar flow cabinet.
Below is a list of risk reduction measures routinely undertaken within our laboratory;
Only specially trained and signed off laboratory staff are authorized to carry out Class II viral work.
Appropriate personal protective equipment (PPE) is worn at all times; laboratory coat, safety eyewear and disposable long cuff gloves.
Proper decontamination of the working area is performed with 1% (wt/vol) Virkon when working with lentiviral particles, both before work begins and after work is completed.
All manipulations with respect to lentiviral particle generation, packaging, harvesting and infection are carried out in a Class II microbiological safety cabinet (MSC).
A dedicated CO2 cell culture incubator, with affixed warning sign, is used for all lentiviral work.
Care is taken to prevent production of aerosols that may contain viral particles during all liquid handling steps.
In case of accidental spillage, surfaces and items are carefully decontaminated using 1% (wt/vol) Virkon.
All materials exposed to viral supernatant are rinsed with 1% (wt/vol) Virkon before being disposed of into an autoclave bag. These bags are then placed in the biological waste containers for autoclaving as soon as work is complete.
Materials
Reagents
HEK293T Lenti-X cells (Takara/Clontech, cat. no. 632180). !CAUTION The cell lines used in your research should be regularly checked to ensure they are authentic and are not infected with mycoplasma.
HEK293T cells (ATCC, cat. no. CRL-3216).
HEK293S GnTI– cells (ATCC, cat. no. CRL-3022).
HEK293S GnTI– TetR cells 34: obtain by request from the group of Nico Callewaert at the VIB-UGent Center for Medical Biotechnology (nico.callewaert@ugent.vib.be). The cell line is subject to a Material Transfer Agreement (MTA). Alternatively, the cell line can be recreated by (i) stable transfection of HEK293S GnTI– cells with the pcDNA6/TR vector, or (ii) by lentiviral transduction of HEK293S GnTI– cells (Steps 1-17 of the Procedure) using the pHR-CMV_TetR-HA-NLS-P2A-BSD-Myc transfer plasmid (Table 1).
FreeStyle 293-F cells (ThermoFisher Scientific, cat. no. R79007).
Gibco FreeStyle 293 expression medium (ThermoFisher Scientific, cat. no. 12338018).
Gibco fetal bovine serum (FBS; ThermoFisher Scientific, cat. no. 10270).
Gibco MEM non-essential amino acids solution (NEAA; ThermoFisher Scientific, cat. no. 11140-035).
Gibco L-Glutamine (L-Gln; ThermoFisher Scientific, cat. no. 25030-024).
Gibco phosphate buffered saline, calcium- and magnesium-free (PBS; ThermoFisher Scientific, cat. no. 10010015).
Gibco Trypsin-EDTA (0.05% (wt/vol)), phenol red (ThermoFisher Scientific, cat. no. 25300054).
Gibco Trypsin-EDTA (0.05% (wt/vol)), no phenol red (ThermoFisher Scientific, cat. no. 15400054).
Gibco DMEM/F-12; Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (with added L-Gln; ThermoFisher Scientific, cat. no. 11320-074).
Soybean trypsin inhibitor (SBTI; ThermoFisher Scientific, cat. no. 17075029).
Polyethylenimine, 25kDa branched (PEI; Merck, cat. no. 408727).
Polybrene infection reagent (10 mg/mL stock = 1000 ×; Merck, cat. no. TR-1003-G).
Doxycycline hydrochloride (Dox; Merck, cat. no. D3447).
Blasticidin S hydrochloride (ThermoFisher Scientific, cat. no. R21001).
Dimethyl sulfoxide, sterile-filtered, suitable for cell culture (DMSO; Merck cat. no. D2650).
Sodium butyrate (Merck, cat. no. 303410).
Valproic acid (VPA; Merck, cat. no. P4543).
Kifunensine class I α-mannosidase inhibitor (Tocris Bioscience, cat. no. 3207).
(D-)biotin (Merck, cat. no. B4639).
5-Aza-2’-deoxycytidine (5-aza-dC; Merck, cat. no. A3656).
Phosphate buffered saline tablets (PBS; Merck, cat. no. P4417).
RIPA lysis and extraction Buffer (ThermoFisher Scientific, cat. no. 89900).
Benzonase nuclease (Merck, cat. no. 70746).
QIAprep Spin Miniprep Kit (Qiagen, cat. no. 27104).
Lenti-X Concentrator (Takara/Clontech, cat. no. 631231).
Ethanol, absolute (Merck, cat. no. 32221-M).
Rely+On Virkon disinfectant powder (VWR, cat. no. 148-0202).
Microsol 4 decontaminant (Anachem, cat. no. 30312915).
Antibodies
High sensitivity streptavidin-HRP conjugate (Pierce, cat. no. 21130).
Mouse anti-His6 primary antibody (Takara/Clontech, cat. no. 631212).
Mouse anti-HA primary antibody (ThermoFisher Scientific, cat. no. 26183).
Mouse anti-c-Myc primary antibody (ThermoFisher Scientific, cat. no. MA1-980).
HRP-conjugated goat anti-mouse IgG secondary antibody (Merck, cat. no. A0168).
Rabbit anti-Cyclophilin A (CypA) primary antibody (Abcam, cat. no. ab41684).
HRP-conjugated goat anti-rabbit IgG secondary antibody (Abcam, cat. no. ab97051).
Sequencing Primers
pHR-CMV and pHR-CMV-TetO2 forward sequencing primer (5’-CGTCGTCGTGCTCGTTTAGTG-3’).
pHR-SFFV forward sequencing primer (5’-GCTCACAACCCCTCACTCGG-’3).
pHR-CAG forward sequencing primer (5’-GTGCTGGTTATTGTGCTGTCTCATC-’3).
pHR-CMV, pHR-CMV-TetO2, pHR-SFFV and pHR-CAG reverse sequencing primer (5’-CATTAAAGCAGCGTATCCACATAGC-3’).
pHR-CMV-TetO2_IRES-FP reverse sequencing primer (5’-CAAACGCACACCGGCCTTATTCCAAG-3’).
pHR-CMV-TetO2_3C-mVenus-Twin-Strep reverse sequencing primer (5’-GAACAGCTCCTCGCCCTTGCTCAC-3’).
Plasmids
Transfer plasmid: pHR-CMV-TetO2/pHR-SFFV/pHR-CAG plasmids. CRITICAL All plasmids are available through the non-profit Addgene plasmid repository and are subject to a Uniform Biological Material Transfer Agreement (UBMTA). Addgene catalog numbers are listed in Table 1.
pMD2.G lentiviral envelope plasmid (Addgene, cat. no. 12259).
psPAX2 lentiviral packaging plasmid (Addgene, cat. no. 12260).
pcDNA6/TR plasmid (ThermoFisher Scientific, cat. no. V102520).
Equipment
Class II microbiological safety cabinet (MSC; Envair, model Bio 2+).
Long cuff nitrile gloves (StarGuard, model PROTECT+).
CO2 incubator (Binder, model C 170).
Shaking incubator (Infors HT, model Multitron Cell).
Roller bottle apparatus (Wheaton, model R2P 2.0Bot, various capacities available).
Roll-in incubator (Wheaton, cat. no. 753680).
Tissue culture plate (6-well plates, flat bottom, TC treated; Greiner Bio-One, cat. no. 657160).
Tissue culture plate (T25, filter cap, 25 cm2; Greiner Bio-One, cat. no. 690175).
Tissue culture plate (T75, filter cap, 75 cm2; Greiner Bio-One, cat. no. 658175).
Tissue culture plate (T175, filter cap, 175 cm2; Greiner Bio-One, cat. no. 660175).
96 well cell culture microplate (black, clear bottom; Greiner Bio-One, cat. no. 655986).
Sterile 30 mL Luer-lock plastic syringes (VWR, cat no. BDAM301229).
40 µm cell strainers (Corning, cat. no. 352340).
Polystyrene roller bottles (2125 cm2; ribbed surface; Greiner Bio-One, cat. no. 681070).
High Yielding Performance Flask cell culture vessel (HYPERFlask; 1720 cm2; Corning, cat. no. 10034).
Syringe filter unit, sterile, 0.45 µm, polyethersulfone (PES; Merck, cat. no. SLHPM33RS).
Polystyrene disposable standard serological pipets (5 mL; Greiner Bio-One, cat. no. 606180).
Polystyrene disposable standard serological pipets (10 mL; Greiner Bio-One, cat. no. 607180).
Polystyrene disposable standard serological pipets (50 mL; Greiner Bio-One, cat. no. 768180).
Bottle-top filter units (Steritop, 500 mL, sterile, 45 mm threaded; Merck, cat no. SCGPT05RE).
Polycarbonate Erlenmeyer vented and baffled flasks (2 L; Corning, cat no. 10092591; see EQUIPMENT SETUP).
Vented caps for plastic 2 L Erlenmeyer cell culture flasks (Merck, cat. no. CLS431339-24EA; see EQUIPMENT SETUP).
Polycarbonate Erlenmeyer vented and baffled flasks (500 mL; Corning, cat no. 10352742; see EQUIPMENT SETUP).
Vented caps for plastic 500 mL Erlenmeyer cell culture flasks (Merck, cat. no. CLS431372-50EA; see EQUIPMENT SETUP).
Haemocytometer (Appleton Woods, cat. no. HC001).
Nunc cell culture cryogenic tubes (1.8 mL; ThermoFisher Scientific, cat. no. 377267).
Controlled rate freezing container (CoolCell LX; BioCision, cat. no. BCS-405G).
Fluorescence-detection size-exclusion chromatography-based thermostability screening (FSEC-TS; see EQUIPMENT SETUP).
Cell sorter (SONY, cat. no. SH800Z).
Reagent Setup
DMEM/F-12/10%FBS: add 50 mL (10% vol/vol) FBS and 5 mL NEAA (1% vol/vol) to 445 mL DMEM/F-12. The medium can be stored at 4°C as long as there are no visual signs of infection or contamination.
DMEM/F-12/2%FBS: add 10 mL (2% vol/vol) FBS and 5 mL NEAA (1% vol/vol) to 485 mL DMEM/F-12. The medium can be stored at 4°C as long as there are no visual signs of infection or contamination.
DMEM/F-12/SFM: add 5 mL NEAA (1% vol/vol) to 495 mL DMEM/F-12. The medium can be stored at 4°C as long as there are no visual signs of infection or contamination.
FreeStyle 293/1%FBS: add 5 mL (1% vol/vol) FBS and 5 mL NEAA (1% vol/vol) to 490 mL FreeStyle 293. The medium can be stored at 4°C as long as there are no visual signs of infection or contamination.
PEI (1 mg/mL stock): the PEI stock solution is highly viscous and cannot be pipetted. First, pour and weigh 1-5 mL straight from the bottle into a 50 mL tube. PEI has a density of 1.030 g/mL at 25°C. Add ultrapure water to prepare a 100 mg/mL stock solution. Rotate the tube overnight to mix. Once the solution is homogeneous, dilute it to 1 mg/mL in ultrapure water and adjust the pH to 7.0 with HCl. Filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. Aliquot and store at −20°C for up to 1 year.
Dox (10 mg/mL stock): dissolve 50 mg in 100% ethanol to a final volume of 5 mL and filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. Aliquot and store at −20°C for up to 8 weeks. CRITICAL Do not expose Dox to direct sunlight.
Blasticidin (2 mg/mL stock): dissolve 20 mg in ultrapure water to a final volume of 10 mL and filter-sterilize using a 0.2 μm syringe filter unit. Aliquot and store at −20°C for up to 8 weeks. !CAUTION Blasticidin is toxic. Always wear personal protective equipment (PPE); laboratory coat, safety eyewear and disposable long cuff gloves. Weigh the powder in a chemical safety cabinet and prepare the solution inside a biological safety cabinet.
Sodium butyrate (500 mM stock): dissolve 1.1 g of sodium butyrate in ultrapure water to a final volume of 20 mL and filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. Aliquot and store at −20°C for up to 8 weeks.
VPA (500 mM stock): dissolve 1.66 g of VPA in ultrapure water to a final volume of 20 mL and filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. Aliquot and store at −20°C for up to 8 weeks.
Kifunensine (500 μM stock): dissolve 11.6 mg of kifunensine in ultrapure water to a final volume of 100 mL and filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. Aliquot and store at −20°C for up to 1 year.
D-biotin (2 mM stock): dissolve 97.7 mg of D-biotin in 1 × PBS to a final volume of 200 mL and filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. Aliquot and store at −20°C for up to 1 year.
5-aza-dC (10 mM stock): dissolve 10 mg of 5-aza-dC in 438 μL DMSO to obtain a 100 mM stock. Dilute 1:10 (vol:vol) in 1 × PBS to obtain the final 10 mM stock. Aliquot and store at −20°C for up to 1 year 50. !CAUTION 5-aza-dC is toxic. Always wear personal protective equipment (PPE); laboratory coat, safety eyewear and disposable long cuff gloves. Prepare the solution inside a biological safety cabinet. 5-aza-dC is supplied in a glass bottle or insert, ready for dissolution.
Soybean trypsin inhibitor (SBTI; 1 mg/mL stock): dissolve 10 mg SBTI in Gibco PBS to a final volume of 10 mL and filter-sterilize using a 0.2 μm syringe filter unit inside a biological safety cabinet. 0.5 mg (= 0.5 mL) of SBTI stock solution will inhibit 0.5 mg trypsin (or 1 mL of a 0.05% (wt/vol) trypsin solution), assuming a specific activity of 10,000 Nα-Benzoyl-L-arginine ethyl ester (BAEE) units/mg SBTI protein. We use 1 mL SBTI per 1 mL of a 0.05% (wt/vol) trypsin solution, yielding a 2:1 wt/wt SBTI:trypsin ratio. Aliquot and store at −20°C for up to 1 year.
Purification of plasmid DNA: purify plasmid DNA using the QIAprep spin miniprep kit (Qiagen) according to the manufacturer’s instructions. Plasmid can be stored indefinitely at –20°C.
Growth and maintenance of adherent and suspension HEK293 cells: culture cells as described in Box 1.
Equipment Setup
FSEC-TS: we essentially perform established procedures for fluorescence-detection size-exclusion chromatography-based thermostability screening 51,52.
The polycarbonate Erlenmeyer flasks for suspension cell culture can be washed, autoclaved and re-used. When the vented caps deteriorate after rounds of autoclaving, the caps can be separately purchased and replaced.
Procedure
HEK293T Lenti-X cell seeding (Day 1) ●TIMING ~30 min
-
1
Add ~9 x 106 HEK293T Lenti-X cells in 12 mL DMEM/F-12/10%FBS to a fresh T75 flask, bringing it to ~50% confluency. Cell culture is described in detail in Box 1. Incubate the flask for 24 h at 37°C in a humidified incubator operated with 5% CO2. One T75 flask will be used per transfection.
? TROUBLESHOOTING
Caution: Cell cultures are a potential biological hazard. Working with HEK293 cells requires biosafety level 2 (BSL2) practices. Perform the work in an approved laminar flow cabinet using sterile techniques and comply with the appropriate institutional biosafety guidelines. This includes wearing protective clothing and eyewear, cleaning of working surfaces, as well as proper disposal of waste before and after performing experiments.
Critical step: Lentivirus can be produced from regular HEK293T cells but the HEK293 Lenti-X cell line used here has been clonally selected to yield viral titers ~30 × higher than HEK293T cells and ~6 × higher than HEK293FT, according to the manufacturer (Takara/Clontech).
Critical step: the health of the HEK293T Lenti-X producer cell line is crucial to obtain maximal lentiviral titer for downstream transduction. Maintain it diligently and split the cells 1/6 twice a week on fixed moments; we suggest Monday morning and Thursday afternoon. We do not use the cells beyond passage 20 (P20; ~10 weeks of culture) to ensure maximum viability and viral yield.
HEK293T Lenti-X cell transfection (Day 2) ●TIMING ~1 h
-
2The next day, prepare the following plasmid DNA transfection mix (30 μg total DNA; 1:1:1 (wt:wt:wt) transfer:packaging:envelope plasmid ratio) in a sterile 1.5 mL tube;
-
○10 μg pHR-CMV-TetO2 transfer plasmid variant containing gene of interest
-
○10 μg psPAX2 packaging plasmid
-
○10 μg pMD2.G envelope plasmid
Top up the transfection mix with DMEM/F-12/SFM to a total volume of 0.25 mL and gently mix the suspension.
? TROUBLESHOOTING
-
○
-
3
Prepare 75 μL PEI (1:2.5 (wt:wt) DNA:PEI ratio) in a sterile 1.5 mL tube. Top up with DMEM/F-12/SFM to a total volume of 0.25 mL and gently mix the suspension.
-
4
When both solutions are ready, add the 0.25 mL PEI mix to the 0.25 mL plasmid DNA transfection mix for a total of 0.5 mL in a 1.5 mL tube.
Critical step: in our experience, both the volume in which the DNA:PEI transfection mix is prepared, as well as the DNA:PEI ratio, have a great effect on the transfection efficiency. We recommend adhering to the values stated in Steps 2-4.
-
5
Vortex the 1.5 mL tube gently for 10 sec, then centrifuge it briefly at low speed to collect the liquid at the bottom of the tube and leave it incubating in the flow cabinet for 15-20 min.
-
6
The T75 flask with HEK293T Lenti-X cells is now >90% confluent (continued from Step 1); take off and discard the complete growth medium; wash with 10 mL PBS and replace with 11.5 mL DMEM/F-12/2%FBS medium.
-
7
Add the 0.5 mL DNA:PEI mix to the 11.5 mL expression medium for a total of 12 mL, and gently tilt flask to cover all cells.
? TROUBLESHOOTING
-
8
Place the T75 flask back at 37°C in a humidified incubator operated with 5% CO2.
Critical Step: The transfection can also be performed in small-scale T25 format or in a T175 flask. The required amounts of plasmid DNA and PEI must be scaled with flask surface area. The T25 format allows the simultaneous screening of multiple constructs and expression conditions before scale-up, while using T175 flasks has the advantage that downstream scale-up is quicker. When performing transfections in T25 format, we use 10 μg total DNA (1:1:1 wt:wt:wt ratio) and a total transfection mix volume of 200 μL (100 + 100 μL) that is added to 4 mL expression medium. When performing transfections in T175 format, we use 70 μg total DNA (1:1:1 wt:wt:wt ratio) and a total transfection mix volume of 1.0 mL (0.5 + 0.5 mL) that is added to 30 mL expression medium. For co-transfection of different transfer plasmids, we usually divide the total amount of transfer plasmid DNA in equal parts (e.g., for a three-plasmid co-transfection in a T75 flask, we use 3.3 μg of each transfer plasmid). Transfer plasmid ratios should however be optimized in situations where there is a large difference in proviral DNA sizes between the transfer plasmids, to ensure approximately equal viral titers for the most efficient co-transduction.
Caution: Lentiviral particles are a potential biological hazard, and require biosafety level 2 (BSL2) or 2+ (BSL2+) practices, depending on the institutional and governmental biosafety guidelines. Perform the work in an approved laminar flow cabinet using sterile techniques, and comply with the appropriate institutional biosafety guidelines. A list of risk reduction measures can be found in the “Biological Safety Considerations” section of the main text.
HEK293T, HEK293S GnTI– or HEK293S GnTI– TetR cell seeding (Day 4) ●TIMING ~30 min
-
9
Two days after transfection, add ~9 x 106 target cells (HEK293T, HEK293S GnTI– or HEK293S GnTI– TetR) in 12 mL DMEM/F-12/10%FBS to a new T75 flask, bringing it to ~50% confluency. Incubate the flask for 24 h at 37°C in a humidified incubator operated with 5% CO2.
-
10
Optional Step: In case of HEK293S GnTI– TetR cells: add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution) to the DMEM/F-12/10%FBS.
Critical step: the health of the HEK293 target cell lines is crucial for efficient transduction and potent protein expression. Maintain them diligently and split the cells 1/6 twice a week on fixed moments; we suggest Monday morning and Thursday afternoon, synchronized with the Lenti-X cells. We do not use the cells beyond passage 20 (P20; ~10 weeks of culture) to ensure maximum viability and protein yield. We do not culture the HEK293S GnTI– TetR cell line beyond passage 10 (P10; ~4-5 weeks of culture) to prevent the loss of TetR expression; TetR is under control of a CMV promoter (pcDNA6/TR vector), which is prone to transcriptional silencing after prolonged culturing. See also the section “Other applications of the method / Alternative promoters”.
Collection of the lentivirus-containing medium (Day 5) ●TIMING ~30 min
-
11
Three days after transfection, collect the 12 mL conditioned medium from the HEK293T Lenti-X T75 flask into a sterile 50 mL tube. This contains the lentiviral particles.
-
12
Add 6 mL fresh DMEM/F-12/10%FBS complete medium to the 50 mL tube, yielding a total of 18 mL.
-
13
Filter the medium through a 0.45 μm filter unit attached onto a Luer-lock syringe, and into a new sterile 50 mL tube.
Critical step: Do not use a 0.22 μm filter unit because this may shear the viral particles. Use only cellulose acetate or polyethersulfone (PES) (low protein binding) filters. Avoid the use of nitrocellulose filters; these bind lentiviral envelope proteins and destroy the virus.
-
14
Add 18 μL (from a 10 mg/mL 1000 × stock solution) polybrene to the 18 mL lentivirus-containing medium and mix gently. Polybrene (hexadimethrine bromide) is a cationic polymer that reduces charge repulsion between viral particles and the cell membrane 53. It will promote virus-host cell fusion, leading to higher transduction efficiencies. Optional determination of lentiviral titer is described in Box 2.
? TROUBLESHOOTING
Infection of the target cell line (Day 5) ●TIMING ~30 min
-
15
The T75 flask with either HEK293T, HEK293S GnTI– or HEK293S GnTI– TetR cells (continued from Steps 9-10) is now >90% confluent; remove and discard the complete growth medium; wash with 10 mL PBS and replace with the 18 mL of filtered lentivirus-containing medium.
? TROUBLESHOOTING
-
16
Optional Step: In case of HEK293S GnTI– TetR cells; add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution).
-
17
Place the T75 flask back in the incubator at 37°C in a humidified incubator operated with 5% CO2. The viral particles will now infect the cells and stably integrate their genetic material into the host cell genome.
Expansion of the adherent polyclonal cell line (Day 8) ●TIMING ~3 d
-
18
Three days after infection, the polyclonal stable cell line is established through integration of viral genetic elements into the host cell genome. If the construct is fluorescently tagged, or when using the pHR-CMV-TetO2_IRES-FP plasmid variants, image the cells to verify and quantify cell fluorescence and transduction efficiency. Transduced cells can be enriched via FACS using FP fluorescence (see Box 3). Transgene expression is repressed when using inducible HEK293S GnTI– TetR cells, meaning that either uninduced (“leaky”) or induced fluorescence can be used to evaluate transduction efficiency or for FACS-based enrichment. Cryopreservation of the polyclonal stable cell line is described in Box 4.
Box 4. Long-term storage of cell lines in liquid nitrogen ●TIMING ~1 h.
It is a good idea to cryopreserve the polyclonal stable HEK293 cell line directly after establishment or enrichment, in liquid nitrogen and in the presence of a cryoprotective agent such as dimethyl sulfoxide (DMSO).
Procedure
Gently dislodge and resuspend the adherent cells from the T75 flask in complete growth medium using a sterile serological 10 mL pipet; pipet up and down a few times to break up cell clumps. Alternatively, cells can be trypsinized.
Centrifuge the cells at 500 g for 5 min at room temperature, and discard the supernatant without disturbing the cell pellet.
Resuspend the cells in 9 mL complete growth medium containing 10% (vol/vol) DMSO; this leads to a minimally recommended cell density of ~2.0 x 106 cells/mL.
Dispense aliquots of the cell suspension into cryogenic storage vials. Mix the cells frequently and gently during aliquotting to maintain a homogeneous cell suspension.
Place the cryovials in an isopropanol cooling chamber and store the chamber at –80°C overnight for controlled cooling.
Finally, transfer and store the cryovials in the gas phase above the liquid nitrogen.
In order to restart the cell culture, place a cryovial containing frozen cells in a 37°C water bath for maximally 1 min to rapidly thaw them.
Slowly dilute the thawed cells using pre-warmed complete growth medium.
Plate the thawed cells at high density to optimize their recovery (e.g., use the contents of one cryovial per T75 flask).
CRITICAL The thawing procedure is stressful to cryopreserved cells; use good technique and work quickly to ensure that most of the cells survive the procedure.
Critical Step: A portion of the transduced cells can be split into 6-wells and used for a small-scale induction test, while simultaneously expanding the remainder of the cells (Steps 19-21).
-
19
Wash the T75 flask thoroughly, at least 3 times, with PBS.
-
20
Split the T75 flask into two new T175 flasks (each holding 30 mL DMEM/F-12/10%FBS complete medium) for expansion. Place the T175 flasks back in the incubator at 37°C in a humidified incubator operated with 5% CO2.
-
21
Optional Step: In case of HEK293S GnTI– TetR cells: add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution).
Protein expression in adherent or suspension cells
-
22
To proceed with adherent growth of the HEK293T, HEK293S GnTI– or HEK293S GnTI– TetR polyclonal cell lines in ribbed roller bottles for soluble secreted protein production, follow Option A. To proceed with suspension growth of the HEK293S GnTI– TetR cell line for membrane or intracellular protein production, follow Option B. Option B may also serve as a guideline when producing soluble secreted proteins from suspension adapted cell lines such as FreeStyle 293-F. CRITICAL The adherent cell and suspension cell procedures describe expansion into 1.5 L (six polycarbonate roller bottles) or 1.6 L expression medium (two polycarbonate Erlenmeyer flasks each containing 800 mL expression medium), respectively. These volumes can be up- or downscaled according to the protein target expression levels and desired yields.
Option A: Protein expression in adherent polyclonal cell lines (Week 2-4) ●TIMING ~2-3 wk
-
i
Expansion of the adherent polyclonal cell line (Steps i-ii): After 3.5 days, split each of the two confluent T175 flasks into three new T175 flasks (each holding 30 mL DMEM/F-12/10%FBS complete medium) for expansion. Place the T175 flasks back in the incubator at 37°C in a humidified incubator operated with 5% CO2.
-
ii
Optional Step: In the case of HEK293S GnTI– TetR cells: add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution).
-
iii
Expansion of the adherent polyclonal cell line (Steps iii-v): After 3.5 days, split each of the six T175 flasks into a new ribbed roller bottle (holding 250 mL DMEM/F-12/10%FBS complete medium) for expansion.
-
iv
Optional Step: In the case of HEK293S GnTI– TetR cells: add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution).
-
v
Place the roller bottles in a roller bottle apparatus operated at 37°C and rotate them at 0.80 revolutions per minute (rpm).
-
vi
Protein expression (Steps vi-xi): The ribbed roller bottles are confluent after ~5 days. Take off the complete growth medium and replace with 250 mL DMEM/F-12/2%FBS medium.
-
vii
Optional Step: In the case of HEK293S GnTI– TetR cells: add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution).
-
viii
Optional Step: In the case of HEK293S GnTI– TetR cells: add Dox (from the 10 mg/mL stock solution) to the desired concentration to induce protein expression.
-
ix
Optional Step: Add VPA or sodium butyrate to the desired concentration.
-
x
Optional Step: In the case of HEK293T cells: kifunensine can be added (final concentration of 5 μM from a 500 μM 100 × stock solution) to inhibit class I α-mannosidases to obtain protein carrying endoglycosidase H (endo H) sensitive N-linked glycans 2.
-
xi
Place the roller bottles in a roller bottle apparatus operated at the desired expression temperature (37°C or 30°C) and rotate them at 0.80 rpm.
Critical step: The histone deacetylase inhibitors (iHDACs) VPA and sodium butyrate can enhance stable transgene expression 54. The optimal concentrations of Dox (see Step viii) and of VPA and sodium butyrate (see Step ix), as well as the optimal expression temperature (37°C or 30°C; see Step xi), should be first determined in small-scale culture and optimized for each target protein. The working concentration of Dox can be varied from 0.1 to 10 μg/mL, and that of VPA and sodium butyrate is usually in the 1-10 mM range 54. The goal is to balance these expression parameters such that they lead to maximal production of secreted, correctly folded protein. Our default approach for soluble proteins is to perform expression at 37°C, and to use 1 μg/mL Dox (in the case of HEK293S GnTI– TetR cells) and no VPA or sodium butyrate.
-
xii
Collection of expressed protein (Steps xii-xiii): Collect the conditioned medium. The expression time depends on the cell type:
| Cell type | Collect conditioned medium |
|---|---|
| HEK293T | after 4-5 days |
| HEK293S GnTI– | after 7-10 days |
| HEK293S GnTI– TetR | after 7-10 days |
Critical Step: We advise monitoring cell viability throughout the protein expression and adjust collection times accordingly, i.e. to collect sooner if a significant portion of cells has died. The roller bottles can in principle be re-fed with fresh DMEM/F-12/10%FBS medium if adherent cell death is limited. In that case, consider the addition of 5-aza-dC (final concentration of 10 μM from a 10 mM 1000 × stock solution) to counteract promoter methylation after prolonged activity.
-
xiii
Filter the conditioned medium to sterilize it and to remove remaining cellular debris.
Pause point: Filtered conditioned medium can be temporarily (up to 1 week) stored at 4 °C before proceeding with purification, but we recommend processing the medium quickly to avoid protein degradation.
Option B: Protein expression in polyclonal HEK293S GnTI– TetR cell lines for suspension growth (Week 2-4) ●TIMING ~2-3 wk
-
i
Expansion of the polyclonal HEK293S GnTI– TetR cell line for suspension growth (Steps i-ix): After 3.5 days, pour out the growth media of each of the two confluent T175 flasks and wash them with 10 mL PBS.
-
ii
Trypsinize the cells in each flask by adding 5 mL Trypsin-EDTA for 3 min.
-
iii
Inhibit trypsin by adding 25 mL FreeStyle 293/1%FBS medium to each of the two T175 flasks.
-
iv
Pool the cells and dispense them into one 500 mL polycarbonate Erlenmeyer baffled flask.
-
v
Perform a cell count of this cell suspension; anticipated cell density is ~1.5 x 106 cells/mL.
-
vi
Top up the flask with FreeStyle 293/1%FBS medium to a volume (usually ~200 mL) that corresponds with a final cell density of ~0.5 x 106 cells/mL.
-
vii
Add blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution).
-
viii
Place the flask in the shaking incubator operated at 37°C with 8% CO2 and shaking speed of 130 rpm.
-
ix
Grow the cells until they reach a density of ~2.0 x 106 cells/mL. Monitor cell density by performing a cell count of the cell suspension.
Critical step: For optimal cell growth, seed suspension HEK293S GnTI– TetR cells at a minimum density of ~0.5 x 106 cells/mL. HEK293S GnTI– TetR cells are derived from HEK293S cells, the original suspension adapted HEK293 cell line 16. HEK293S GnTI– TetR cells adapt readily from an adherent monolayer to suspension culture 4, provided a specialized suspension growth medium with added serum (FreeStyle 293/1%FBS) is used and the cells are seeded at sufficiently high density. Anticipate a doubling time of ~24 h for HEK293S GnTI– TetR cells.
-
x
Expansion of the polyclonal HEK293S GnTI– TetR cell line for suspension growth (Steps x-xiii): Transfer the 200 mL of cells to one 2 L polycarbonate Erlenmeyer baffled flask.
-
xi
Top up the flask with FreeStyle 293/1%FBS medium, with added blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution), to a volume of 800 mL. Starting from a cell density of ~2.0 x 106 cells/mL, the final cell density will be ~0.50 x 106 cells/mL.
-
xii
Place the flask in the shaking incubator operated at 37°C with 8% CO2 and shaking speed of 130 rpm.
-
xiii
Grow the cells until they reach a density of ~2.0 x 106 cells/mL. Monitor cell density by performing a cell count of the cell suspension.
-
xiv
Expansion of the polyclonal HEK293S GnTI– TetR cell line for suspension growth (Steps xiv-xviii): Distribute the 800 mL of cells into two 2 L polycarbonate Erlenmeyer baffled flasks.
-
xv
Top up each flask with FreeStyle 293/1%FBS medium, with added blasticidin (final concentration of 2 μg/mL from a 2 mg/mL 1000 × stock solution), to a volume of 800 mL. Starting from a cell density of ~2.0 x 106 cells/mL, the final cell density will be ~1.0 x 106 cells/mL.
-
xvi
Place the flask in the shaking incubator operated at 37°C with 8% CO2 and shaking speed of 130 rpm.
-
xvii
Grow the cells until they reach a density of ~2.0-3.0 x 106 cells/mL. Monitor cell density by performing a cell count of the cell suspension.
-
xviii
Optional Step: Suspension cells can be grown to a density of ~5.0 x 106 cells/mL to further increase total biomass at harvest.
-
xix
Induction of the polyclonal HEK293S GnTI– TetR cell line for protein expression (Steps xix-xxi): Add Dox (from the 10 mg/mL stock solution) to the desired concentration.
-
xx
Optional Step: Add VPA or sodium butyrate to the desired concentration.
-
xxi
Place the flasks in the shaking incubator operated at the desired expression temperature with 8% CO2 and shaking speed of 130 rpm.
? TROUBLESHOOTING
-
xxii
Collection of cell pellets (Step xxii): At the desired time of collection, centrifuge the cells for 10 min at 1,500 g at 4°C and discard the supernatant.
Critical step: The histone deacetylase inhibitors (iHDACs) VPA and sodium butyrate can enhance stable transgene expression 54. The optimal concentrations of Dox (see Step xix) and of VPA and sodium butyrate (see Step xx), as well as the optimal expression temperature (37°C or 30°C 44; see Step xxi) and collection time (see Step xxii), should be first determined in small-scale culture and optimized for each protein. The working concentration of Dox can be varied from 0.1 to 10 μg/mL, and that of VPA and sodium butyrate is usually in the 1-10 mM range 54. The goal is to tune these expression parameters such that they lead to maximal production of correctly folded protein. For membrane proteins, our default initial small-scale FSEC screen varies Dox concentration (at 0.0, 0.1, 1.0 and 10 μg/mL), expression temperature (37°C and 30°C), and collection time (24 h, 48 h and 72 h) giving a total of 24 conditions (0.0 μg/mL Dox samples serve as negative controls), which takes ~24 h to run on an automated UFPLC system. An example for the GPCR Smoothened (SMO) is shown in Fig. 4E.
Figure 4. Lentiviral transduction, inducible expression and FSEC screening of the GPCR Smoothened (SMO) in HEK293S GnTI– TetR cells.
(A) The SMOXTAL-3C-mVenus-Twin-Strep construct (6.8 kb proviral DNA). SS: signal sequence; ECD: extracellular domain; TMD: transmembrane domain; BRIL: thermostabilized apocytochrome b562; 3C: 3C protease cleavage site; TS: Twin-Strep tag. (B) Non-concentrated lentiviral particles encoding SMOXTAL-3C-mVenus-Twin-Strep were used to infect adherent HEK293S GnTI– TetR cells. (C) Flow cytometry analysis of non-transduced (negative control) and transduced (– Dox and + Dox) cells after trypsinization and suspension growth. Dox (1 μg/mL Dox for 24 h) was added when the suspension cell density reached ~1.0 ×106 cells/mL. MFI: mean fluorescence intensity. (D) Fluorescence microscopic imaging of transduced (+ Dox and – Dox) adherent cells. DIC; differential interference contrast. Images were taken on a Leica SP8 SMD X confocal microscope. Fluorescence emission was detected using hybrid detectors (HyD) operated in photon-counting mode, meaning that every incident photon gave rise to one gray value. Scale bar: 100 μm. (E) FSEC screening of detergent-solubilized SMOXTAL-3C-mVenus-Twin-Strep with varied expression temperature, collection time and Dox concentration.
Pause point: The cell pellets can be snap-frozen in liquid nitrogen (LN2) and stored at –80 °C indefinitely for future use.
Timing
The entire protocol, starting from the transfection of Lenti-X cells to the liter-scale collection of conditioned medium containing secreted protein or suspension cells containing membrane or intracellular proteins, takes ~3-4 weeks. The hands-on timing for each stage of the PROCEDURE is summarized below.
Step 1, HEK293T Lenti-X cell seeding: ~30 min
Steps 2-8, HEK293T Lenti-X cell transfection: ~1 h
Steps 9-10, HEK293T, HEK293S GnTI– or HEK293S GnTI– TetR cell seeding: ~30 min
Steps 11-14, collection of the lentivirus-containing medium: ~30 min
Steps 15-17, infection of the target cell line: ~30 min
Steps 18-21, expansion of the adherent polyclonal cell line: ~3 d
Step 22 Option A, protein expression in adherent polyclonal cell lines: ~2-3 wk
Steps i-v, expansion of the adherent polyclonal cell line: ~8 d
Steps vi-xi, protein expression: ~5-10 d
Steps xii-xiii, collection of expressed protein: ~1 h
Step 22 Option B, protein expression in polyclonal HEK293S GnTI– TetR cell lines for suspension growth: ~2-3 wk
Steps i-xviii, expansion of the polyclonal HEK293S GnTI– TetR cell line for suspension growth: ~12 d
Steps xix-xxi, induction of the polyclonal HEK293S GnTI– TetR cell line and protein expression: ~1-3 d
Step xxii, collection of cell pellets: ~1 h
Box 1, growth and maintenance of adherent and suspension HEK 293 cells: ~30 min
Box 2, endpoint dilution assay to determine functional lentiviral titer: ~4 d
Box 3, FACS to enrich subpopulations of transduced cells: ~1 d
Box 4, long-term storage of cell lines in liquid nitrogen: ~1 h
? TROUBLESHOOTING
Troubleshooting guidance can be found in Table 2.
Table 2. Troubleshooting Table.
| Step | Problem | Possible reason | Possible solution |
|---|---|---|---|
| 1 | Mammalian cell contamination | Improper sterile technique or problem with tissue culture infrastructure. | Adhere strictly to proper tissue culture technique. Ensure that all surfaces are uncluttered and cleaned with 70% (vol/vol) ethanol or 1% (wt/vol) Virkon before and after use. The use of Penicillin Streptomycin (“Pen Strep”) is not encouraged, as it can lead to reduced protein yields. |
| 1 | Low lentiviral titer | Old or badly maintained HEK293T Lenti-X cells | Start fresh HEK293T Lenti-X cell culture from a low-passage liquid nitrogen stock. |
| 2 | Low transduction efficiency | Low quality lentivirus | Ascertain the use of the correct packaging and envelope plasmids. |
| 7 | Low transduction efficiency | Unsuccessful HEK293T Lenti-X cell transfection | Transfect HEK293T Lenti-X cells at appropriate confluency, use fresh PEI or other transfection reagent, verify plasmid DNA integrity and purity. Use fluorescence to verify HEK293T Lenti-X cell transfection efficiency if construct is fluorescently tagged. |
| 14 | Low transduction efficiency | No polybrene added to viral supernatant | Ascertain the use of polybrene. |
| 15 | Low transduction efficiency | Non-compatible cell line | Cell line needs to express the LDL receptor on its surface. |
| 22 Option B xxi | Reduced HEK293S GnTI– TetR cell viability after induction of expression | Protein toxicity, mainly an issue for membrane and intracellular proteins | Screen for optimal harvesting time (e.g. limit expression time to 24 h) and expression parameters (Temperature, and Dox, VPA, sodium butyrate concentration). Increase concentration of serum in the expression medium. |
Anticipated Results
The described protocols are used within our laboratories at the Division of Structural Biology (STRUBI) and the MRC-LMB to express a large number of soluble and membrane proteins varying in size, topology and oligomeric state, as well as protein complexes. The time required to identify promising constructs varies considerably on a case-by-case basis. For soluble proteins, we identify constructs suitable for scale-up by evaluating their transient or stable expression and secretion in small-scale format using Western blotting. For membrane proteins, we follow established procedures for FSEC-TS 51,52. We emphasize the importance of exploring expression time, expression temperature and Dox concentration as variables 44. Given the scalability of the method, polyclonal cell lines can be readily established and expanded in small-scale adherent cultures or low-volume suspension cultures, allowing the simultaneous screening of these variables before scale-up.
Examples illustrating the Protocol
To illustrate the broad applicability of the method, we cloned a set of cDNA inserts into the various pHR-CMV-TetO2 plasmids. These inserts code for neuronal proteins of varying sizes, folds and expression levels and have been previously successfully transiently expressed in HEK293 cells using the pHLsec plasmid. Soluble secreted proteins include 3C-Avi-His6-tagged Cbln1FL 32, β-NRX1LNS6 32, GluD2ATD 32, NL1ECTO 33, MDGA1ECTO-FL and MDGA2ECTO-FL 33, and α-NRX1ECTO-FL 33, listed in order of increasing proviral DNA and protein size.
We compared secreted and whole cell protein levels from HEK293T cells transduced with non-concentrated lentiviral particles (pHR-CMV-TetO2_3C-Avi-His6 plasmid) in T25 flask format, with levels from HEK293T cells transiently transfected with equimolar amounts of either the pHLsec or the pHR-CMV-TetO2_3C-Avi-His6 plasmids, using anti-His6 western blotting. For all targets, we noted markedly increased levels of protein production and secretion from the lentivirus-transduced cells, regardless of proviral DNA size and absolute expression levels. Interestingly, we also observed higher transient expression levels using pHR-CMV-TetO2 as compared to pHLsec, indicating that pHR-CMV-TetO2 is very well suited to initial transient small-scale screening of expression constructs and that subcloning in pHLsec is unnecessary for this purpose (Fig. 2C). For the large-scale (12 roller bottles) production of axon guidance molecules Netrin-1 (NET1ΔNTR) and Neogenin-1 (NEO1FN456) 55,56, we achieved a 3-fold and 4-fold improvement in secreted protein yield after purification, respectively, when using lentiviral stable expression as compared to transient expression using pHLsec (Fig. 2D).
We compared secreted and whole cell protein levels from HEK293T cells transduced with non-concentrated lentiviral particles produced using the pHR-CMV-TetO2_3C-Avi-His6 or pHR-CMV-TetO2_3C-Avi-His6_IRES-EmGFP plasmid variants, using anti-His6 western blotting (Fig. 3E). Interestingly, although markedly lower signal was observed from the cell pellets of the IRES-EmGFP samples, the levels of secreted protein were not adversely affected. For the α-NRX1ECTO-FL construct, a marked increase in secreted protein levels was even observed. In summary, we prefer to use the IRES-FP plasmid variants over the non-IRES-FP plasmid variants since they lead to at least as much secreted protein, while also enabling routine verification of transfection and transduction efficiency using fluorescence microscopy or flow cytometry, as well as enrichment of the cell line using FACS. As an example, we established a polyclonal stable cell line for 3C-Avi-His6-tagged MDGA1ECTO-FL 33 (8.2 kb proviral DNA). Using flow cytometry, we measured a transduction efficiency of ~94% (Fig. 3F). We applied this sort gate and expanded the sorted cells into four roller bottles (1L expression medium). From this volume, we obtained ~47 mg of purified secreted MDGA1ECTO-FL, as compared to ~7 mg from large-scale transient transfection using pHLsec (Fig. 3G).
We further illustrate the usefulness of the method for membrane proteins using the GPCR Smoothened (SMO) 57 and the GABAA receptor (GABAAR) β3 homopentamer 58. We established polyclonal stable HEK293S GnTI– TetR cell lines for mVenus-fused SMOXTAL (6.8 kb proviral DNA; Fig. 4A,B) and mVenus-fused full-length GABAAR-β3 (GABAAR-β3FL; 5.7 kb proviral DNA; Fig. 5A,B), using non-concentrated lentiviral particles. 72 h post-transduction, we initiated suspension culture. After 48 h, we induced gene expression for 24 h using Dox. For both SMOXTAL (Fig. 4C,D) and GABAAR-β3FL (Fig. 5C,D), we determined using flow cytometry that ~90% of gated suspension HEK293S GnTI– TetR cells robustly expressed protein. Interestingly, the analysis also revealed that repression of GABAAR-β3FL expression was more efficient (less “leaky”) than that of SMOXTAL, leading to a larger fold change in median fluorescence intensity (MFI) upon Dox application (Fig. 4C and 5C).
Figure 5. Lentiviral transduction and inducible expression of the GABAA receptor β3 homopentamer (GABAAR-β3FL) in HEK293S GnTI– TetR cells, and comparison with BacMam.
(A) The mVenus-GABAAR-β3FL-1D4 construct (5.7 kb proviral DNA). SS: signal sequence; 3C: 3C protease cleavage site; ECD: extracellular domain; TMD: transmembrane domain; ICD: intracellular domain; 1D4: 1D4 tag. (B) Non-concentrated lentiviral particles encoding mVenus-GABAAR-β3FL-1D4 were used to infect adherent HEK293S GnTI– TetR cells. (C) Flow cytometry analysis of non-transduced (negative control) and transduced (– Dox and + Dox) cells after trypsinization and suspension growth. Dox (5 μg/mL Dox for 24h) was added when the suspension cell density reached ~1.0 ×106 cells/mL. MFI: mean fluorescence intensity. (D) Fluorescence microscopic imaging of transduced (+ Dox and – Dox) adherent cells. DIC; differential interference contrast. Images were taken on a Leica SP8 SMD X confocal microscope. Fluorescence emission was detected using hybrid detectors (HyD) operated in photon-counting mode, meaning that every incident photon gave rise to one gray value. Scale bar: 100 μm. (E) Comparative lentivirus vs. BacMam FSEC screening. mVenus-GABAAR-β3FL-1D4 yield using lentiviral transduction is ~5-fold greater than using BacMam when comparing the best respective conditions (Supplementary Fig. 7A,B). MOI: multiplicity of infection.
To determine the optimal expression parameters for SMOXTAL, we expanded the polyclonal stable HEK293S GnTI– TetR cell line into 6-well format and performed a FSEC screen, varying Dox concentration (0.0, 0.1, 1.0 and 10 μg/mL), expression temperature (30°C and 37°C 44), and collection time (24 h, 48 h and 72 h). From the analysis, we determined that SMOXTAL expression is best when induced using 0.1 or 1.0 μg/mL Dox and collected after 72 h at 37°C (Fig. 4E). We routinely perform this FSEC analysis for our membrane protein samples; it takes less than 24 h to screen a total of 24 conditions using an automated UFPLC system. The 0.0 μg/mL Dox samples serve as negative controls.
We compared the BacMam procedure 44,45 with our lentiviral procedure for the expression of GABAAR-β3FL, using FSEC. Common screening parameters were expression temperature (30°C and 37°C), collection time (24 h, 48 h and 72 h), and additives (5 mM VPA or sodium butyrate). For BacMam and lentiviral transduction, we additionally screened MOI ratios (0, 1, 4 and 10) or Dox concentration (0, 5 and 10 μg/mL), respectively (Supplementary Fig. 7A,B). From this matrix of conditions, we determined that in the case of lentiviral transduction, inducible expression at 37°C for 24 h without additives or at 30°C for 72 h with 5 mM sodium butyrate is optimal, whereas in the case of BacMam expression at 30°C for 72 h using an MOI ratio of 10 as well as 5 mM sodium butyrate is optimal. However, protein yield using lentiviral transduction was at least 5-fold greater than using BacMam when comparing the best respective conditions (Fig. 5E and Supplementary Fig. 7A,B).
For co-infection of cells using lentiviruses encoding distinct transgenes, two strategies can be followed; (i) separately produced lentiviruses can be mixed in the desired stoichiometric ratio after titer determination, or (ii) multiple pHR-CMV-TetO2 plasmids can be co-transfected into the HEK293T Lenti-X producer cell line. In both cases, co-transduced cells can then be enriched using multi-color FACS. We applied the second strategy to the secreted Cbln1FL–β-NRX1LNS6 complex 32 (Fig. 6A); from the total transduced cells pool (Fig. 6B), the subset of cells expressing both transgenes was isolated using FACS and using IRES-EmGFP and IRES-mRuby2 fluorescence (Fig. 6C,D). The approach can be extended to multi-subunit complexes using more fluorochromes; our plasmid suite currently supports up to three colors (IRES-EmGFP, IRES-mRuby2 and IRES-mTurquoise2; Supplementary Fig. 8A,B), but this can be readily modified or extended since the IRES-FP elements can be excised and replaced using the unique XhoI-BsiWI restriction sites (Supplementary Fig. 3).
Figure 6. Tools for multi-color FACS, in vivo biotinylation, and the generation of inducible cell lines.
(A-D) Multi-color FACS. (A) Non-concentrated lentiviral particles encoding β-NRX1LNS6_IRES-EmGFP (6.1 kb proviral DNA) and Cbln1FL_IRES-mRuby2 (6.0 kb proviral DNA) were used to co-infect HEK293T cells. (B) Fluorescent imaging indicated a mixture of non-, single- and double-transduced cells. The image was taken on a Leica SP8 SMD X confocal microscope. Scale bar: 50 μm. (C) Two-dimensional compensated fluorescence plot. (D) Western blot detection of His6-tagged β-NRX1LNS6(+SS4) and Cbln1FL secreted from co-infected cells after cell sorting (mouse anti-His6 primary antibody: 1:5,000 dilution, HRP-conjugated goat anti-mouse secondary antibody: 1:5,000 dilution). (E-F) In vivo biotinylation. (E) Non-concentrated lentiviral particles encoding either HA-BirA-ER (5.1 kb proviral DNA) or 3C-Avi-His6-tagged NL1ECTO (5.9 kb proviral DNA) were used to co-infect HEK293T cells. (F) Non-concentrated lentiviral particles encoding 3C-Avi-His6-tagged NL1ECTO and IRES_HA-BirA-ER (7.6 kb proviral DNA) were used to infect HEK293T cells. A D-biotin concentration of 100 μM was maintained in the DMEM/F-12/2%FBS medium. Anti-D-biotin western blot detection of in vivo biotinylated NL1ECTO (high sensitivity streptavidin-HRP conjugate; 1:4000 dilution). Anti-HA western blot detection of HA-tagged TetR (mouse anti-HA primary antibody: 1:5,000 dilution, HRP-conjugated goat anti-mouse secondary antibody: 1:5,000 dilution). (G-H) Generation of inducible cell lines. (G) Non-concentrated lentiviral particles encoding TetR-HA-NLS-P2A-BSD-Myc (5.1 kb proviral DNA) and mVenus (4.7 kb proviral DNA) were used to infect HEK293T and HEK293S GnTI– cells. (H) Flow cytometry analysis of the resulting HEK293Tlenti-TetR and HEK293S GnTI–lenti-TetR inducible cell lines, and comparison with the HEK293S GnTI– TetR cell line 34. MFI: mean fluorescence intensity.
To illustrate in vivo biotinylation, we either (i) co-infected HEK293T cells with non-concentrated lentiviral particles encoding HA-tagged ER-resident BirA (HA-BirA-ER) and lentiviral particles encoding 3C-Avi-His6-tagged NL1ECTO (Fig. 6E), or (ii) performed bicistronic expression of NL1ECTO and HA-BirA-ER using the 3C-Avi-His6_IRES-HA-BirA-ER plasmid (Fig. 6F). D-biotin was maintained in the expression medium at 100 μM. Using western blotting, we confirmed successful biotinylation of secreted NL1ECTO (Fig. 6E,F).
Finally, we generated inducible HEK293T and HEK293S GnTI– cells by infecting them with non-concentrated lentiviral particles encoding TetR-HA-NLS-P2A-BSD-Myc. After two passages that included selection with 10 μg/mL blasticidin to kill non-transduced cells, we infected the resulting HEK293Tlenti-TetR and HEK293S GnTI–lenti-TetR cell lines with non-concentrated lentiviral particles encoding cytosolic mVenus (Fig. 6G). Using flow cytometry, we confirmed inducible mVenus expression in both polyclonal cell lines (Fig. 6H). Interestingly, HEK293S GnTI–lenti-TetR cells showed less uninduced (“leaky”) mVenus expression than HEK293S GnTI– TetR cells, leading to a larger fold change in median fluorescence intensity (MFI) upon Dox application (Fig. 6H).
Supplementary Material
Supplementary Methods
Small-scale (6-well) transient transfection procedure for soluble secreted proteins.
Large-scale (roller bottle) transient transfection procedure for soluble secreted proteins.
Compatibility of pHR-CMV-TetO2 with in vivo assembly (IVA) cloning.
FSEC screening of the GPCR Smoothened (SMO).
GABAAR-β3FL: comparison of BacMam and lentiviral transduction.
Editorial Summary.
This Protocol describes a suite of lentiviral transfer plasmids that can be used for high-yield, time- and cost-efficient, and constitutive or inducible production of soluble and membrane proteins in mammalian cell lines.
Acknowledgements
We thank Graham Davies and Catherine Green (University of Oxford) for assistance with flow cytometry, Sergi Padilla-Parra and Luis AJ Alvarez (University of Oxford) for assistance with microscopic imaging, Duncan Laverty (MRC-LMB) for the modified pEZT-BM vector, Jake Watson (MRC-LMB) for advice on in vivo assembly (IVA) cloning, and Susanne Ressl (Indiana University Bloomington) for comments on the manuscript. pMD2.G (Addgene plasmid # 12259) and psPAX2 (Addgene plasmid # 12260) were a gift from Didier Trono. This work was supported by the Marie-Curie (FP7-328531) long-term postdoctoral fellowship (to J.E.); the UK Medical Research Council grants MR/L009609/1 and MC_UP_1201/15 (to A.R.A.); the UK Biotechnology and Biological Sciences Research Council grant BB/M024709/1 (to A.R.A.); the Cancer Research UK grants C20724/A14414 (to C.S.) and C375/A10976 (to E.Y.J.); the European Research Council (ERC) grant 647278 (to C.S.); the Wellcome Trust studentships 105247/Z/14/Z (to S.S.) and 203726/Z/16/Z (to R.E.W.). The Wellcome Centre for Human Genetics is funded by Wellcome Trust Core Award 203852/Z/16/2.
Footnotes
Author contributions
J.E. and E.B. designed and constructed the pHR-CMV-TetO2 plasmid suite. J.E., E.B., B.B. and V.T.C. established and optimized the protocol. J.E., B.B. and E.B. performed the cloning, western blotting, flow cytometry and microscopic imaging. S.S. performed the GABAA-β3FL comparative expression tests. R.E.W. performed the SMOXTAL expression tests. S.C.G. performed the NET1/NEO1 large-scale expressions. E.F.X.B. performed the generation of the inducible HEK293lenti-TetR cell lines. D.I.S., E.Y.J., C.S. and A.R.A. provided funding and oversaw the research. J.E. and A.R.A. conceptualized the research. J.E. wrote the paper. All authors provided feedback on the final manuscript version.
Competing financial interests
The authors declare no competing financial interests.
Please indicate up to four primary research articles where the protocol has been used and/or developed.
1. Chang, V. T. et al. Initiation of T cell signaling by CD45 segregation at 'close contacts'. Nat Immunol 17, 574-582 (2016).
References
- 1.Aricescu AR, Owens RJ. Expression of recombinant glycoproteins in mammalian cells: towards an integrative approach to structural biology. Curr Opin Struc Biol. 2013;23:345–356. doi: 10.1016/j.sbi.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang VT, et al. Glycoprotein structural genomics: Solving the glycosylation problem. Structure. 2007;15:267–273. doi: 10.1016/j.str.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta crystallographica. Section D, Biological crystallography. 2006;62:1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary S, et al. Overexpressing human membrane proteins in stably transfected and clonal human embryonic kidney 293S cells. Nat Protoc. 2012;7:453–466. doi: 10.1038/nprot.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naldini L, Trono D, Verma IM. Lentiviral vectors, two decades later. Science. 2016;353:1101–1102. doi: 10.1126/science.aah6192. [DOI] [PubMed] [Google Scholar]
- 6.Benabdellah K, et al. Development of an all-in-one lentiviral vector system based on the original TetR for the easy generation of Tet-ON cell lines. PloS one. 2011;6:e23734. doi: 10.1371/journal.pone.0023734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Groote P, et al. Generation of a new Gateway-compatible inducible lentiviral vector platform allowing easy derivation of co-transduced cells. Biotechniques. 2016;60:252–259. doi: 10.2144/000114417. [DOI] [PubMed] [Google Scholar]
- 8.Campeau E, et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PloS one. 2009;4:e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandaranayake AD, et al. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic acids research. 2011;39:e143. doi: 10.1093/nar/gkr706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang VT, et al. Initiation of T cell signaling by CD45 segregation at 'close contacts'. Nat Immunol. 2016;17:574–582. doi: 10.1038/ni.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang VT, Spooner RA, Crispin M, Davis SJ. Glycan Remodeling with Processing Inhibitors and Lectin-Resistant Eukaryotic Cells. Methods Mol Biol. 2015;1321:307–322. doi: 10.1007/978-1-4939-2760-9_21. [DOI] [PubMed] [Google Scholar]
- 12.Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkelshtein D, et al. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci U S A. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demaison C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- 15.Zufferey R, et al. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nature biotechnology. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 16.Lin YC, et al. Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations. Nature communications. 2014;5 doi: 10.1038/ncomms5767. 4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin JY, et al. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PloS one. 2010;5:e10611. doi: 10.1371/journal.pone.0010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc Natl Acad Sci U S A. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foecking MK, Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45:101–105. doi: 10.1016/0378-1119(86)90137-x. [DOI] [PubMed] [Google Scholar]
- 20.Gorman CM, Gies D, McCray G, Huang M. The human cytomegalovirus major immediate early promoter can be trans-activated by adenovirus early proteins. Virology. 1989;171:377–385. doi: 10.1016/0042-6822(89)90605-3. [DOI] [PubMed] [Google Scholar]
- 21.Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Nafria J, Watson JF, Greger IH. IVA cloning: A single-tube universal cloning system exploiting bacterial In Vivo Assembly. Sci Rep. 2016;6 doi: 10.1038/srep27459. 27459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt TG, et al. Development of the Twin-Strep-tag and its application for purification of recombinant proteins from cell culture supernatants. Protein Expr Purif. 2013;92:54–61. doi: 10.1016/j.pep.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt TG, Skerra A. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat Protoc. 2007;2:1528–1535. doi: 10.1038/nprot.2007.209. [DOI] [PubMed] [Google Scholar]
- 25.Kumar M, Keller B, Makalou N, Sutton RE. Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther. 2001;12:1893–1905. doi: 10.1089/104303401753153947. [DOI] [PubMed] [Google Scholar]
- 26.Knox R, et al. A streamlined implementation of the glutamine synthetase-based protein expression system. BMC Biotechnol. 2013;13:74. doi: 10.1186/1472-6750-13-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancia F, et al. Optimization of protein production in mammalian cells with a coexpressed fluorescent marker. Structure. 2004;12:1355–1360. doi: 10.1016/j.str.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Oberbek A, Matasci M, Hacker DL, Wurm FM. Generation of stable, high-producing CHO cell lines by lentiviral vector-mediated gene transfer in serum-free suspension culture. Biotechnol Bioeng. 2011;108:600–610. doi: 10.1002/bit.22968. [DOI] [PubMed] [Google Scholar]
- 29.Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- 30.Lam AJ, et al. Improving FRET dynamic range with bright green and red fluorescent proteins. Nat Methods. 2012;9:1005–1012. doi: 10.1038/nmeth.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goedhart J, et al. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93% Nature communications. 2012;3:751. doi: 10.1038/ncomms1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elegheert J, et al. Structural basis for integration of GluD receptors within synaptic organizer complexes. Science. 2016;353:295–299. doi: 10.1126/science.aae0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elegheert J, et al. Structural Mechanism for Modulation of Synaptic Neuroligin-Neurexin Signaling by MDGA Proteins. Neuron. 2017;95:896–913 e810. doi: 10.1016/j.neuron.2017.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao F, et al. Tetracycline repressor, tetR, rather than the tetR-mammalian cell transcription factor fusion derivatives, regulates inducible gene expression in mammalian cells. Hum Gene Ther. 1998;9:1939–1950. doi: 10.1089/hum.1998.9.13-1939. [DOI] [PubMed] [Google Scholar]
- 36.Brooks AR, et al. Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J Gene Med. 2004;6:395–404. doi: 10.1002/jgm.516. [DOI] [PubMed] [Google Scholar]
- 37.Hsu CC, et al. Targeted methylation of CMV and E1A viral promoters. Biochemical and biophysical research communications. 2010;402:228–234. doi: 10.1016/j.bbrc.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 38.He J, Yang Q, Chang LJ. Dynamic DNA methylation and histone modifications contribute to lentiviral transgene silencing in murine embryonic carcinoma cells. J Virol. 2005;79:13497–13508. doi: 10.1128/JVI.79.21.13497-13508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JH, et al. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PloS one. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, et al. Simple piggyBac transposon-based mammalian cell expression system for inducible protein production. Proc Natl Acad Sci U S A. 2013;110:5004–5009. doi: 10.1073/pnas.1218620110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matasci M, Baldi L, Hacker DL, Wurm FM. The PiggyBac transposon enhances the frequency of CHO stable cell line generation and yields recombinant lines with superior productivity and stability. Biotechnol Bioeng. 2011;108:2141–2150. doi: 10.1002/bit.23167. [DOI] [PubMed] [Google Scholar]
- 42.Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci U S A. 1996;93:2348–2352. doi: 10.1073/pnas.93.6.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dukkipati A, et al. BacMam system for high-level expression of recombinant soluble and membrane glycoproteins for structural studies. Protein Expr Purif. 2008;62:160–170. doi: 10.1016/j.pep.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goehring A, et al. Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat Protoc. 2014;9:2574–2585. doi: 10.1038/nprot.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morales-Perez CL, Noviello CM, Hibbs RE. Manipulation of Subunit Stoichiometry in Heteromeric Membrane Proteins. Structure. 2016;24:797–805. doi: 10.1016/j.str.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otto E, et al. Characterization of a replication-competent retrovirus resulting from recombination of packaging and vector sequences. Hum Gene Ther. 1994;5:567–575. doi: 10.1089/hum.1994.5.5-567. [DOI] [PubMed] [Google Scholar]
- 48.Miyoshi H, et al. Development of a self-inactivating lentivirus vector. J Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zufferey R, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schermelleh L, Spada F, Leonhardt H. Visualization and measurement of DNA methyltransferase activity in living cells. Curr Protoc Cell Biol. 2008;Chapter 22 doi: 10.1002/0471143030.cb2212s39. Unit 22 12. [DOI] [PubMed] [Google Scholar]
- 51.Hattori M, Hibbs RE, Gouaux E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure. 2012;20:1293–1299. doi: 10.1016/j.str.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14:673–681. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Davis HE, Rosinski M, Morgan JR, Yarmush ML. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophysical journal. 2004;86:1234–1242. doi: 10.1016/S0006-3495(04)74197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Backliwal G, et al. Valproic acid: a viable alternative to sodium butyrate for enhancing protein expression in mammalian cell cultures. Biotechnol Bioeng. 2008;101:182–189. doi: 10.1002/bit.21882. [DOI] [PubMed] [Google Scholar]
- 55.Bell CH, et al. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science. 2013;341:77–80. doi: 10.1126/science.1232322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Healey EG, et al. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nature structural & molecular biology. 2015;22:458–465. doi: 10.1038/nsmb.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byrne EFX, et al. Structural basis of Smoothened regulation by its extracellular domains. Nature. 2016;535:517–522. doi: 10.1038/nature18934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014;512:270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rueden CT, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017;18:529. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meissner P, et al. Transient gene expression: recombinant protein production with suspension-adapted HEK293-EBNA cells. Biotechnol Bioeng. 2001;75:197–203. doi: 10.1002/bit.1179. [DOI] [PubMed] [Google Scholar]
- 61.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 62.Telford WG, et al. Flow cytometry of fluorescent proteins. Methods. 2012;57:318–330. doi: 10.1016/j.ymeth.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Robinson JP, Roederer M. HISTORY OF SCIENCE. Flow cytometry strikes gold. Science. 2015;350:739–740. doi: 10.1126/science.aad6770. [DOI] [PubMed] [Google Scholar]
- 64.Davies D. Cell separations by flow cytometry. Methods Mol Biol. 2012;878:185–199. doi: 10.1007/978-1-61779-854-2_12. [DOI] [PubMed] [Google Scholar]
- 65.Andrell J, et al. Generation of Tetracycline-Inducible Mammalian Cell Lines by Flow Cytometry for Improved Overproduction of Membrane Proteins. Methods Mol Biol. 2016;1432:63–78. doi: 10.1007/978-1-4939-3637-3_5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.