Abstract
Exercise has profound benefits for brain function in animals and humans. In rodents, voluntary wheel running increases the production of new neurons and upregulates neurotrophin levels in the hippocampus, as well as improving synaptic plasticity, memory function and mood. The underlying cellular mechanisms, however, remain unresolved. Recent research indicates that peripheral organs such as skeletal muscle, liver and adipose tissue secrete factors during physical activity that may influence neuronal function. Here we used an in vitro cell assay and proteomic analysis to investigate the effects of proteins secreted from skeletal muscle cells on adult hippocampal neural progenitor cell (aNPC) differentiation. We also sought to identify the relevant molecules driving these effects. Specifically, we treated rat L6 skeletal muscle cells with the AMP-kinase (AMPK) agonist 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) or vehicle (distilled water). We then collected the conditioned media (CM) and fractionated it using high-performance liquid chromatography (HPLC). Treatment of aNPCs with a specific fraction of the AICAR-CM upregulated expression of doublecortin (DCX) and Tuj1, markers of immature neurons. Proteomic analysis of this fraction identified proteins known to be involved in energy metabolism, cell migration, adhesion and neurogenesis. Culturing differentiating aNPCs in the presence of one of the factors, glycolytic enzyme glucose-6-phosphate isomerase (GPI), or AICAR-CM, increased the proportion of neuronal (Tuj1+) and astrocytic, glial fibrillary acidic protein (GFAP+) cells. Our study provides further evidence that proteins secreted from skeletal muscle cells may serve as a critical communication link to the brain through factors that enhance neural differentiation.
Keywords: Neurogenesis, AICAR, AMPK, Myokines, HPLC, Muscle, Exercise, Doublecortin
1. Introduction
Environmental enrichment results in structural and functional changes in the brain and behavior (Kempermann et al., 1997; van Praag et al., 1999; Nithianantharajah and Hannan, 2006; Eisinger and Zhao, 2017). A critical component of enrichment is physical activity (van Praag, 2008; Kobilo et al., 2011a; Gregoire et al., 2014). In rodents, voluntary wheel running increases neurotransmitters, growth factors, vascularization and adult neurogenesis in the hippocampus (Vivar et al., 2013; Ryan and Kelly, 2016). However, the peripheral factors that may trigger running-induced adult hippocampal neurogenesis remain unclear. Parabiosis studies between young and aged animals show that factors in young blood can regulate vascular remodeling and promote adult neurogenesis in aged animals (Villeda et al., 2011; Katsimpardi et al., 2014). During exercise, peripheral organs such as skeletal muscle, adipose tissue and the liver secrete various factors into circulation that enhance systemic homeostasis (Hansen et al., 2011; Pedersen and Febbraio, 2012). Skeletal muscle releases myokines (Pedersen and Febbraio, 2008; Hawley et al., 2014) that may be linked to neural plasticity (Delezie and Handschin, 2018). This putative association is supported by clinical and basic research. For example, children with Duchenne muscular dystrophy have cognitive deficits (Florek and Karolak, 1977; Hinton et al., 2000). A mouse model of muscular dystrophy, X-linked muscular dystrophy (mdx), exhibits impaired memory function (Vaillend et al., 1995; Anderson et al., 2002) and deficient adult hippocampal neurogenesis (Deng et al., 2009).
An important regulator of muscle physiology is 5′ adenosine monophosphate-activated protein kinase (AMPK, Hardie, 2011). AMPK activation blocks energy-consuming processes and promotes ATP synthesis from fatty acid oxidation, glycosylation and glucose uptake (Vavvas et al., 1997; Kurth-Kraczek et al., 1999). Pharmacological AMPK activation with an agonist, AICAR, decreases fat mass, improves running endurance in sedentary mice (Narkar et al., 2008), and increases muscle mass but not VO2peak (Toedebusch et al., 2016), suggesting it can mimic some aspects of exercise. The effects of this compound extend to brain function in mice, with short-term administration resulting in enhanced adult neurogenesis, brain-derived neurotrophin (BDNF) levels and spatial memory function (Kobilo et al., 2011b; Guerrieri and van Praag, 2015). In mice with a muscle-specific, dominant negative expression of α2-AMPK subunit (AMPKDN) (Zwetsloot et al., 2008), there was no improvement in spatial memory function after AICAR administration, suggesting muscle AMPK activation may be required (Kobilo et al., 2014). Based on these findings, we treated L6 muscle cells with AICAR in culture followed by proteomic analysis of the CM. We identified a novel myokine, Cathepsin B as a mediator of exercise-induced enhancement of hippocampal plasticity and memory function (Moon et al., 2016).
In the present study we aim to identify additional factors that may affect neural function. We utilized a novel and complementary approach to our previous research. We similarly treated L6 myoblasts with AICAR or vehicle and collected the CM. In this study, however, we fractionated the media by HPLC and subsequently used it to treat adult neural progenitor cells (aNPCs). A specific fraction of the AICAR CM increased aNPC expression of DCX and Tuj1, markers of immature neurons. Proteomic analysis of this fraction revealed proteins known to be involved in cell migration, adhesion and neurogenesis. Culturing differentiating aNPCs in AICAR-CM or glucose metabolism regulator GPI, increased the proportion of neuronal and glial cells. Altogether, we observed that secretory proteins from skeletal muscle induced by AICAR treatment influences in vitro neural differentiation.
2. Methods and materials
2.1. Cell culture and preparation of CM
L6 skeletal myoblast cells (ATCC CRL-1458, Virginia, USA) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, NY, USA) supplemented with fetal bovine serum (FBS) to a final concentration of 10%. The cells were passaged every second day and confluency was maintained at less than 80% to prevent spontaneous differentiation. To induce differentiation, cells (4 × 104 cells/mL) were placed into DMEM supplemented with 2% horse serum (HS) for 8 days, with medium refreshed every other day. Differentiation status was routinely monitored under a microscope (Axiovert S100; Zeiss, Germany). On day 9, L6 myotubes were washed three times with DPBS. CM was prepared by adding 12 ml of the unsupplemented DMEM, with or without 100 μM AICAR, to the myotubes and incubating them for 6 h in a 37° C CO2 incubator. The cultured medium was filtered using a 0.22 μm filter and then the filtered medium was concentrated 1:10 by 3,000NMWL centrifugal filter devices (Millipore, MA, USA). Protein concentration was measured using Bradford assay.
2.2. Reverse phase HPLC
High-performance liquid chromatography (HPLC; LC20AD, Shimadzu) with a C4 column (2.1 × 150 mm2, 5 mm; Vydac, The Separations Group, Hesperia, CA) was used to analyze the 1% DMSO-DW eluted secretomes. Solvent A was composed of 0.1% TFA (Sigma–Aldrich, St. Louis, MO, USA) in ultrapure water (Fisher Scientific, Pittsburgh, PA, USA), and solvent B was composed of 0.1% TFA in ACN. A linear gradient with a flow rate of 0.3 mL/min was employed, using the following gradient: 1% B (at 7 min), 60% B (at 30 min), 100% B (at 42 min) for 10 min, followed by equilibration with 1% B. The observed UV wavelength was 215 nm. Fractions were concentrated with vacuum centrifuge (Speedvac, Savant, USA) and reconstituted with distilled water (D.W.).
2.3. In-solution digestion and mass spectrometry analysis
Approximately 250 μg of protein from vehicle treated CM and AICAR treated CM mixed secretome were subjected to in solution digestion as described previously (Harsha et al., 2008). The proteins in solution were reduced with 5 mM dithiothreitol followed by alkylation with 10 mM iodoacetamide. Digestion was carried out using trypsin (modified sequencing grade; Promega, Madison, WI) at 37 °C for 16 h. Tandem mass tag (TMT; TMT Mass Tagging kits and reagent, Thermo scientific, Rockford, IL) labeling was carried out as per the manufacturer instructions with minor modifications. Briefly, trypsinized peptides from two conditions were reconstituted in 50 mM TEABC buffer and mixed with the TMT reagent and incubated at RT for 1 h. After the labeling, all samples were pooled and desalted using Sep-Pak C18 cartridges. Peptides were analyzed on an LTQ-Orbitrap Elite mass spectrometer (Thermo Electron, Bremen, Germany) interfaced with Easy-nLC II nanoflow LC system (Thermo Scientific, Odense, Denmark). The pooled TMT labeled peptides were reconstituted in 0.1% formic acid and loaded onto a trap column (75 μm × 2 cm) packed in-house with Magic C18 AQ (Michrom Bioresources, Inc., Auburn, CA, USA). Peptides were resolved on an analytical column (75 μm × 50 cm) at a flow rate of 300 nL/min using a linear gradient of 10–35% solvent B (0.1% formic acid in 95% acetonitrile) over 90 min. The total run time including sample loading and column reconditioning was 120 min. Data dependent acquisition with full scans in 350–1700 m/z range was carried out using an Orbitrap mass analyzer at a mass resolution of 120,000 at 400 m/z. Fifteen most intense precursor ions from a survey scan were selected for MS/MS fragmentation using higher energy collisional dissociation (HCD) fragmentation with 32% normalized collision energy and detected at a mass resolution of 30,000 at 400 m/z. Automatic gain control for full MS was set to 1 × 106 for MS and 5 × 104 ions for MS/MS with a maximum ion injection time of 100 ms. Dynamic exclusion was set to 30 s and singly charged ions were rejected. Internal calibration was carried out using lock mass option (m/z 445.1200025) using ambient air.
2.4. Adult neural progenitor cell culture
Primary NPCs were isolated from the dentate gyrus of 8- to 10-week-old male on C57Bl/6 genetic background as described previously (Li et al., 2016; Guo et al., 2012a,b). Cells independently isolated from three mice served as biological replicates (n = 3). We used only early passage cells (between passages 4 and 10). Differentiation of aNPCs to neurons and astrocytes was carried out as described (Luo et al., 2010; Guo et al., 2011a; Guo et al., 2011b). Briefly, neurospheres with average diameter of 250 μm were disassociated with trypsin and the aNPCs were plated in 24 well plates (Fisher Scientific, 87721) containing poly-L-ornithine and laminin-coated coverslips and cell proliferation medium at the density of 1 × 105 cells/well. At 24 h post plating, differentiation was initiated by mitogen withdrawal. The differentiation media included the following chemical treatments and was partially replaced daily. Specifically, Vehicle CM or AICAR CM (10 μg/ml) or recombinant protein glucose-6-phosphate isomerase (GPI), (0.5 or 1 μg/ml, MBS95581l, Mybiosource) were added to some of the NPCs. Retinoic acid (1 μM, Sigma-Aldrich, R-2625)/forskolin (1 μM, Sigma-Aldrich, F-6886) initiated differentiation was used as a positive control and added to wells that were not used for chemical treatment. At 4 days post-differentiation, cells were fixed using 4% PFA for 30 min. Immunocytochemistry staining was carried out as described (Li et al., 2016). Briefly, cells were pre-blocked using DPBS containing 5% normal goat serum (VECTOR, S-1000) and 0.1% Triton X-100 for 30 min, followed by overnight incubation with primary antibodies: mouse anti-Tuj1 (1:2000, Covance, E10408LF) and rabbit anti-GFAP (1:3000, Dako, Z0334). After washing with DPBS, cells were incubated with secondary antibodies that included goat anti-mouse 568 (1:3000, Invitrogen, A11031) and goat anti-rabbit 568 (1:3000, Invitrogen, A11036) followed by counterstaining with DAPI. After the cells were mounted with VECTASHIELD (VECTOR, H-1000), the numbers of Tuj1, GFAP-positive cells were quantified using an Olympus BX51 microscope using an unbiased stereology method with assistance from StereoInvestigator software. The percentage of differentiated cells was calculated as the number of Tuj1- or GFAP-labeled cells divided by the total number of cells stained with DAPI. Scientists who were blinded to experimental conditions performed quantification.
2.4.1. MTT and RT-PCR assays
aNPC cells were seeded into 96 well plates at an initial density of 103 cells/well and were grown in Neurobasal medium with B27 supplement only for the MTT assay. To evaluate the effect of CM on differentiation marker expression, PCR assays were performed. For PCR assays 105 aNPCs were plated in 6-well plates with the addition of vehicle (distilled water), or fractions of CM (10 μl, approximately 20 μg/μl) to the cells with 0.5% FBS, L-glutamine, Neurobasal medium for 24 h.
2.5. MTT cell viability assay
Cell viability was quantified by using MTT (3-(4,5-Dimethylthiazo-l2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole; Sigma–Aldrich, St. Louis, MO, USA) reagent. MTT stock solution (5 mg/ml) was prepared in DPBS (GIBCO) and added to the culture medium at a final concentration of 1 mg/ml. After 90 min incubation, the medium was removed, and the chromogen in the cells was dissolved in DMSO containing 0.01 N NaOH. The absorbance at 570 nm was measured using a 96-well microplate spectrophotometer (Thermo scientific, Rockford, IL).
2.6. Semiquantitative RT-PCR analysis
Total RNA was extracted using a total RNA extraction kit (Ribozol, Amresco). First-strand cDNA was synthesized by RT using oligo(dT) primers and SuperScript II reverse transcriptase (Invitrogen). cDNA was amplified for 15–25 cycles using mouse DCX (Forward (F):5′- AAGTG ACCAACAAGGCTAT −3′, Reverse (R):5′- TCATTGTGTTTTCCCGGA-3′), Tuj1 (F):5′-CGCCTTTGGACACCTATTCAG −3′, (R):5′- TTCTCACACTCT TTCCGCAC −3′), NeuN (F):5′- GTAGAGGGACGGAAAATTGAGG −3′, (R): 5′-GGGAAACTGGTCACTGCATAG −3′) and HSP90 (F:5′- GACCAAGGC TGACCTCATAAA −3′, R:5′- GACCAAGGCTGACCTCATAAA - 3′) gene-specific primers. For real-time RT-PCR, total RNA (100 ng) was amplified using the PerfeCTa SYBR Green FastMix (Quanta Biosciences) and ECO PCR system (Illumina).
2.7. Western blot analysis of cultured medium of L6 muscle cells and densitometry
CM (Please see 2.1. Cell culture and preparation of CM) collected from L6 muscle cells was mixed with 1:1 ratio RIPA buffer (Millipore), Protease/Phosphatase Inhibitor Cocktail (Cell Signaling Technology, Danvers, MA) and sample buffer (Bio-rad). For Western blot analysis, containing equal amounts of protein from each fraction were subjected to SDS-PAGE and transferred to a NC membrane (Millipore). The membranes were blocked with 5% nonfat dried milk in PBS containing 0.1% Triton X-100 and then incubated with anti-fibronectin (1:1000, ab2413, Abcam) at 4 °C overnight. Secondary antibody linked to Infrared dye (800 anti-rabbit) were used at a dilution of 1:5000. Specific signals were visualized by Odyssey (Amersham Biosciences). Ponceau staining was used as a loading control. Image J analysis was performed for the densitometry of immunoblot images. The gel analysis tool was used to obtain the absolute intensity for each band (commands used: Analyze > Gel > Select First lane > plot lane > wand tool).
2.8. Bioinformatics analysis
DAVID Bioinformatics Resources 6.7 was used for functional annotation, based on KEGG_Pathway terms. The count threshold was set at 3 and the EASE value was set at 0.1. (Cannistraci et al., 2013).
2.9. Data analysis
Statistical analyses were carried out using Statview and GraphPad software. Comparisons between groups were performed with one-way analysis of variance (ANOVA) followed by Fisher’s PLSD or Tukey’s post-hoc tests. All values were expressed as means ± S.E.M.
3. Results
3.1. Fractionation of conditioned medium from skeletal muscle cell line L6 treated with AICAR
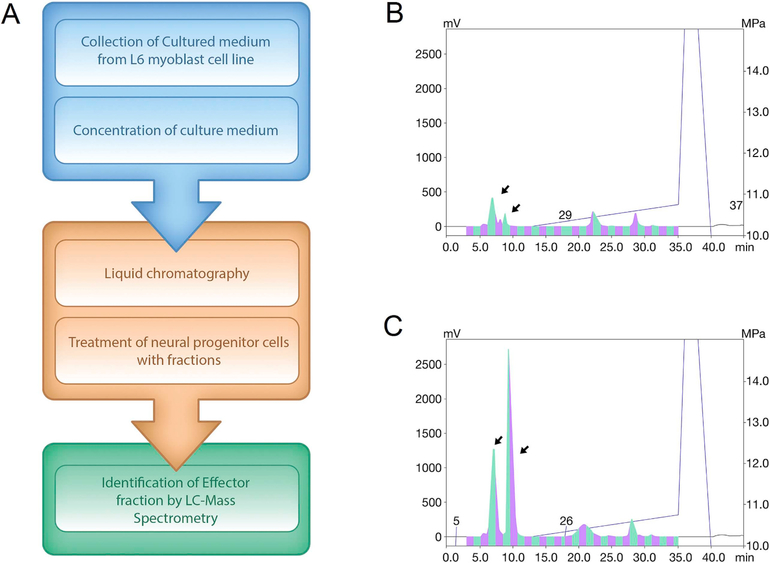
A small-molecule AMPK agonist, AICAR, was used to treat the L6 Rattus skeletal muscle cell line to mimic AMPK modulation of skeletal muscle during the exercise. CM, which contained factors secreted by the skeletal muscle cells, was collected. HPLC equipped with a C4 column was used to fractionate the CM collected from AICAR and vehicle-treated cells and to compare differentially secreted proteins (Fig. 1A). The resulting chromatograms from vehicle-treated and AICAR-treated cells displayed distinct peaks at retention times between 7 and 9 min after injection (Fig. 1B and C). These peaks suggested the presence of uniquely secreted factors in the CM of AICAR-treated muscle cells. Subsequently, the effects of these fractions on neural cell development were evaluated.
Fig. 1.
Schematic model of experiments and HPLC chromatogram results (A) Flow chart shows collection and analysis of CM. (B,C) HPLC chromatograms show that samples from (B) vehicle-treated control and (C) AICAR-treated conditioned medium have distinct retention time of proteins in 215 nm UV wavelength.
3.2. Treatment of aNPCs with AICAR CM fraction increases DCX and Tuj1 expression
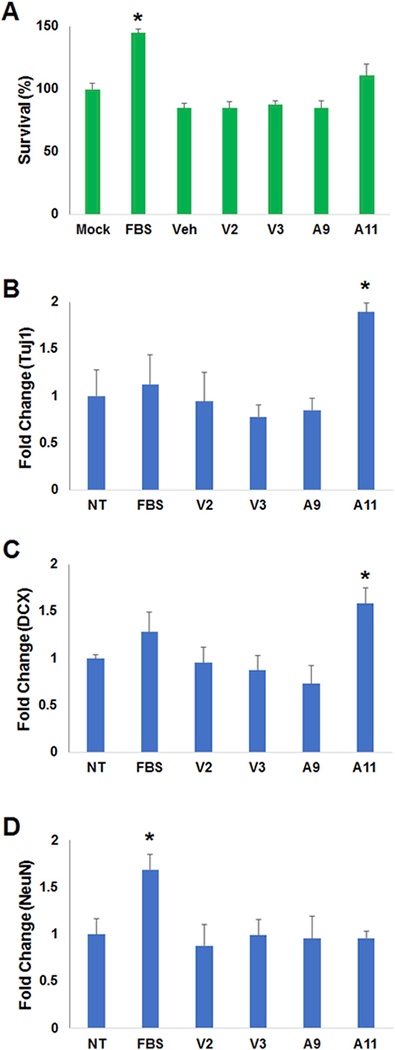
We first investigated whether treatment of aNPCs with fractions from AICAR- or vehicle-treated CM affects cell viability using a MTT assay. Incubating aNPCs for 24 h with various fractions of CM did not significantly impact aNPC viability, whereas treatment with FBS (10%) enhanced cell viability as compared to all the other conditions (F(6,21) = 17.75, p < 0.0001; Fig. 2A). We next assessed the mRNA expression level of well-established neuronal differentiation markers, including Tuj1, doublecortin (DCX) and NeuN. Expression of these markers was evaluated by real-time PCR. We found that fraction A11 of AICAR-treated skeletal muscle cell CM significantly increased the expression of immature neuron markers, Tuj1 (F(5,12) = 3.29, p < 0.05; Fig. 2B) and DCX (F(5,12) = 3.17, p < 0.05; Fig. 2C), as compared to non-treated (NT) cells, and cells exposed to other CM fractions. These findings suggest that a specific factor or factors is present in fraction A11 that may initiate differentiation. This fraction did not increase expression of the mature neuronal marker NeuN. Treatment with 0.5% FBS did enhance NeuN expression (F(5,12) = 3.52, p < 0.05), consistent with previous studies (Guo et al., 2014), (Fig. 2D).
Fig. 2.
Effect of AICAR- or vehicle-treated Conditioned Medium on aNPCs. (A) MTT analysis of the reconstituted fractions from vehicle (V3, V4) or AICAR (A9, A11) conditioned medium (10 μg/μl, respectively) on aNPCs for 24 h. Fetal Bovine Serum (FBS), was used as a positive control. Same volume of distilled water was used as a vehicle (Veh). The FBS (10%) treated samples were significantly different from all other groups (*P < 0.05). (B–D) Real-time PCR analysis of neuronal cell differentiation makers (Tuj1, DCX, NeuN), with administration of reconstituted fractions from vehicle or AICAR treated culture medium (10 μg/μl, respectively) or not treated (NT), on aNPCs for 24 h (B) Tuj1 levels in A11 treated samples were higher than in all other groups. (C) DCX was elevated in the cells treated with fraction A11 as compared to V3, V4, A9 and NT. (D) NeuN levels were enhanced by FBS (0.5%) as compared to all other conditions. Data is expressed as relative target gene expression levels compared with HSP90 expression and presented as the mean of at least three independent experiments, each conducted in triplicate (*P < 0.05).
3.3. Identification of proteins present in CM from AICAR-treated cells
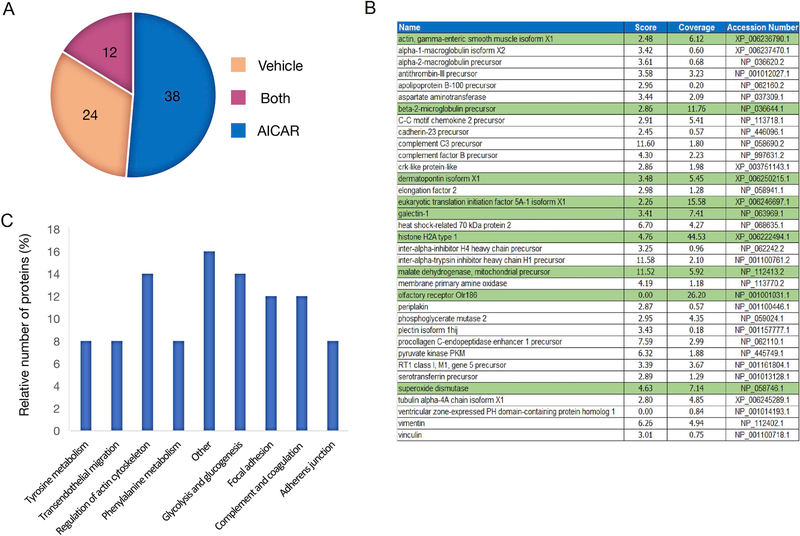
We used LC-MS/MS to identify differentially secreted proteins induced by AICAR treatment of muscle cells. We focused on HPLC “fraction A11” because that fraction enhanced DCX and Tuj1 expression in adult NPCs. To quantify differentially secreted proteins, we generated trypsin-digested peptides from fraction A11, labeled them with stable isotopes, and performed LC/MS. Using this approach, we identified 74 total proteins. Twelve of the proteins were found in both the vehicle and AICAR-treated cells (Supplementary Table 1), resulting in 38 differentially secreted proteins in the AICAR sample (Fig. 3A and B). Nine of these proteins had peptide coverage greater than 5% (highlighted in green).
Fig. 3.
Proteomic analysis of Conditioned Medium. (A) Number of differentially secreted proteins identified by LC/MS in CM fraction A11 from vehicle treated CM. (B) Table of unique proteins identified in AICAR-CM fraction A11. Proteins highlighted in green indicate peptide coverage greater than 5%. (C) Kegg pathway analysis describing the most common pathways represented by proteins secreted from AICAR-treated CM and relative number of proteins in each pathway.
To investigate the functional features of the identified proteins from the AICAR-treated CM, we conducted a functional analysis of all identified proteins using KEGG pathway terms (Fig. 3C). We found that proteins secreted by AICAR-treated cells cluster into eight overlapping categories: glycolysis/gluconeogenesis, phenylalanine metabolism, complement and coagulation cascades, regulation of actin cytoskeleton, tyrosine metabolism, adherens junction, focal adhesion, and leukocyte transendothelial migration. Many of these proteins are cytoskeletal associated (e.g. actin, vimentin and actinin4), or involved in energy metabolism (e.g. pyruvate kinase, phosphoglycerate kinase and L-lactate dehydrogenase A chain-like). One protein secreted by AICAR-treated cells, ventricular zone-expressed PH protein (VEPH), has been reported to be expressed in the embryonic mouse brain (Muto et al., 2004). We note, however, that the peptide coverage of the protein in our AICAR CM was less than 1% (Fig. 3B).
3.4. Evaluation of candidate proteins
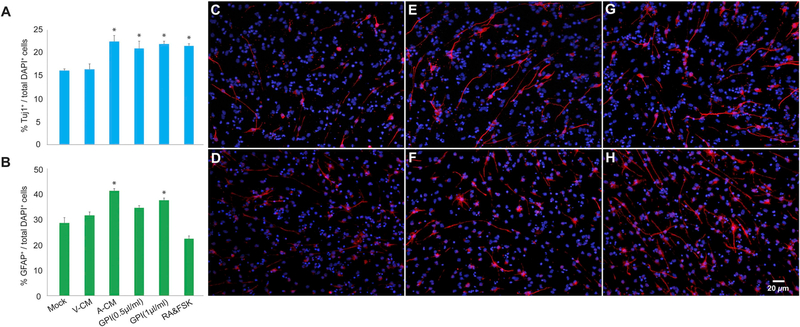
To validate the proteomics data, we evaluated whether candidate factor, fibronectin, was increased in AICAR-CM. Protein levels were significantly elevated as compared to vehicle-CM (t(4) = 3.41, p < 0.05; Supplementary Fig. 1, Supplementary Table 1). In addition, we tested whether another protein, glycolytic enzyme glucose-6-phosphate isomerase (GPI), could affect aNPC differentiation. Specifically, we withdrew differentiation-inhibitory mitogens (FGF2 and EGF) from the aNPC culture media and treated differentiating aNPCs with either vehicle-CM, AICAR-CM, Retonoic acid/Forskolin (RA/FSK) or recombinant GPI. Both concentrations of GPI (0.5 μg/ml and 1 μg/ml), AICAR CM and RA/FSK significantly increased the number of Tuj1-positive neuronal cells as compared to Mock and Vehicle CM treatment conditions (F(5,12) = 10.43, p < 0.0005), (Fig. 4A,C,E,G). GPI (1 μg/ml) and AICAR CM also enhanced gliogenesis as evidenced by an increased number of GFAP-positive astrocytes as compared to Mock and Vehicle CM treatment F(5,12) = 27.83, p < 0.0001), (Fig. 4B,D,F,H).
Fig. 4.
Candidate factor glucose-6-phosphate isomerase (GPI) increases neural differentiation. (A) GPI (0.5 μg/ml and 1 μg/ml), AICAR CM (A-CM) and RA/FSK significantly increased the proportion of aNPCs differentiating into Tuj1-positive neurons as compared to Mock and Vehicle CM (V-CM) treatment conditions (*p < 0.05). (B) GPI (1 μg/ml) and AICAR CM also enhanced gliogenesis as evidenced by an increased number of GFAP-positive astrocytes as compared to Mock and Vehicle CM treatment (*p < 0.05). (C,E,G) Representative photomicrographs of (C) V-CM, (E) A-CM, and (G) GPI treated aNPCs expressing neuronal marker Tuj1 (red), nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI), blue. (D,F,H) Representative photomicrographs of (D) V-CM, (F) A-CM, and (H) GPI treated cells expressing astroglial marker GFAP (red), nuclei were stained with DAPI (blue).
4. Discussion
In the present study we employed an in vitro model to develop a novel approach to understanding the cellular mechanisms underlying the benefits of exercise for memory and mood. Specifically, we aimed to identify novel secreted factors from muscle that may have exercise-like effects on neural cell properties, such as enhancement of survival and differentiation. CM was collected from AICAR- or vehicle-treated muscle cells. Using HPLC fractionation of CM, we observed differentially secreted proteins from AICAR- and vehicle-treated cells. Treatment of aNPCs with various fractions from AICAR- and vehicle-treated cells did not affect viability of aNPCs; however, at least one fraction, Fraction A11, enhanced expression of Tuj1 and DCX, markers of immature neurons. Using liquid chromatography-mass spectrometry, we identified the proteins in the active fraction from AICAR-treated CM. KEGG-pathway analysis revealed that the majority of these proteins are associated with cytoskeletal and energy metabolism pathways, and that one of these factors, glycolytic enzyme GPI, enhanced neuronal and glial differentiation of adult neural progenitor cells.
Human skeletal muscle cells can secrete more than 300 proteins (Hartwig et al., 2014). Many of these myokines affect other tissues such as liver, bone, adipose tissues and immune cells (Pedersen and Febbraio, 2012; Hoffmann and Weigert, 2017). Only a few myokines, such as CTSB, Irisin, Il-6 and vascular endothelial growth factor (VEGF) have so far been associated with brain function (Delezie and Handschin, 2018). CTSB is associated with adult neurogenesis, BDNF levels and memory function (Moon et al., 2016). In muscle cells Fibronectin type III domain containing 5 (FNDC5) is enzymatically cleaved and secreted as myokine irisin, which can elevate hippocampal BDNF gene expression (Wrann, 2015). In humans irisin levels in the blood are elevated by exercise (Jedrychowski et al., 2015). Exercise also increases secretion of VEGF (Hoier et al., 2013) from human muscle. In rodents, blocking circulating VEGF (Fabel et al., 2003) or knockdown of VEGF in skeletal muscle (Rich et al., 2017) inhibits the running-induced increase in adult neurogenesis. Our strategy for identifying additional novel myokines secreted by muscle cells, particularly ones that can affect neural function, has centered on compounds that activate AMPK (Moon et al., 2016; Guerrieri et al., 2017).
Some aspects of exercise (Narkar et al., 2008; see however Toedebusch et al., 2016) are partially mimicked by AICAR treatment. This compound has beneficial effects in mouse models of muscle dystrophy (Al-Rewashdy et al., 2015; Brockhoff et al., 2017), increases adult neurogenesis, BDNF levels and spatial memory function (Kobilo et al., 2011b; Kobilo et al., 2014; Guerrieri and van Praag, 2015) and may alleviate depression-like behavior in mice (Liu et al., 2016). These effects are likely the indirect consequences of peripheral administration, given the limited permeability of the blood brain barrier (Marangos et al., 1990), the lack of spatial memory improvement in AMPKDN mice (Kobilo et al., 2014), and the adverse effects of direct intracranial infusion on synaptic plasticity and spatial memory in rats (Dash et al., 2006). Furthermore, in vitro studies show that direct treatment of neural stem cells results in gliogenesis, but not neurogenesis (Zang et al., 2008; Zhu et al., 2011). In our study, culturing differentiating aNPCs in the presence of AICAR-CM increased the proportion of neuronal and glial cells.
Enhancement of neurogenesis and cognitive function by exercise is a complex process that likely involves multiple interacting pathways (Vivar et al., 2013). While studies of single molecules linked to skeletal muscle have been conducted (Wrann et al., 2013; Agudelo et al., 2014; Moon et al., 2016), none has examined a combination of factors on neuronal cell function. In this study, we used the differentially expressed fractions from AICAR-treated conditioned medium to begin to investigate whether pools of proteins, associated with specific fractions, may influence cell survival or neurogenesis, two outcomes closely associated with in vivo running-induced neurogenesis (Vivar et al., 2013). We performed a MTT viability assay and real-time PCR assays to assess the expression of neurogenic markers. We observed that protein fractions from AICAR-treated conditioned media did not affect cell viability as assessed by the MTT assay. However, markers of immature neurons, differentiation and migration, such as Tuj1 and DCX, increased significantly in aNPCs when exposed to fraction A11 of AICAR-treated conditioned media. Our proteomic and bioinformatic analysis identified 38 proteins in fraction A11. Two subgroups of secretory proteins were adhesion junction and focal adhesion proteins, which are both involved in cell-to-cell adhesion dynamics or migration of cells (De Pascalisa and Etienne-Mannevillea, 2017; Mosher and Schaffer, 2017). Thus, our bioinformatics analysis is consistent with the results of the neuronal differentiation markers.
The fraction included factors such as complement C3 precursors (Shinjyo et al., 2009) and fibronectin (Reh and Radke, 1988; Buzanska et al., 2009; Szymczak et al., 2010) that have been reported to play a role in neurogenesis. Immunoblotting showed that fibronectin levels were significantly upregulated in AICAR-CM. In addition, a protein that regulates glucose metabolism, glucose-6-phosphate isomerase (GPI) was present in the fraction. GPI is a multifunctional protein, that is not only a glycolytic enzyme in the cytosol but also serves as a secreted neuroleukin and autocrine motility factor (Jeffrey et al., 2000) that promotes cell proliferation and migration (Zong et al., 2015). A study showed that GPI is associated with gp78/autocrine motility factor receptor signaling and regulates calcium release from the endoplasmic reticulum, which is important for protein unfolding during neurodegenerative processes (Fu et al., 2011; Hetz and Saxena, 2017). Furthermore, mutations in GPI have been identified in patients with neuromuscular dysfunction and mental retardation (Schroter et al., 1985). Our findings suggest a novel function for this protein, enhancing adult neural stem cell differentiation into neurons and glia. Another protein secreted by AICAR-treated cells, ventricular zone-expressed PH protein (VEPH), is expressed in the embryonic mouse brain (Muto et al., 2004). VEPH is a poorly-characterized protein containing a pleckstrin homology domain. In addition to being expressed in the mouse embryonic brain, expression was also observed in the eye and kidney (Muto et al., 2004). The amino terminal region of VEPH is highly conserved, suggesting a functional significance. However, VEPH knockout mice do not have any observable abnormalities in the brain, retina or other tissues. VEPH warrants further study, as a potential role in behavior, and in indices of functional and structural brain plasticity have not yet been assessed.
Although we observed a direct effect on neural differentiation mediated by secretory proteins from skeletal muscle, many questions remain unresolved. First, the in vitro system used in this study does not completely reflect the multiple and complex benefits of exercise. Second, the pattern of proteins secreted from the skeletal muscle by AICAR treatment cannot fully represent the whole secretome from various types of skeletal muscles during exercise. Furthermore, recent research suggests that other tissues also play an important role. For instance, adipose tissue-derived cytokines, called adipokines, are regulated by exercise (Golbidi and Laher, 2014). Adiponectin can enhance adult hippocampal neurogenesis in mice and in cell culture models (Zhang et al., 2011, 2016) and has been suggested to cross the blood-brain barrier to elevate adult neurogenesis in vivo (Yau et al., 2014). Running-induced neurogenesis and anti-depression-like behavior was not detected in adiponectin knockout mice (Yau et al., 2014). In addition, ketone bodies secreted from liver (hepatokines), such as exercise-induced beta-hydroxybutyrate (BHA) can increase BDNF expression through HDAC2/HDAC3 inhibition and Histone H3 acetylation in the hippocampus (Sleiman et al., 2016). Applying pharmacological compounds or molecules that are considered exercise-mimetics (Guerrieri et al., 2017) or enviromimetics (McOmish and Hannan, 2007) to cell cultures of different organs (e.g. muscle, adipose tissue, liver) and analyzing the proteins in the conditioned medium may be an effective approach for revealing novel cellular mechanisms underlying the effects of physical activity on the brain.
Together, we observed that secretory proteins from skeletal muscle induced by AICAR treatment can influence in vitro expression of neuronal differentiation markers. We suggest that secreted proteins from skeletal muscle may play a role in neurogenesis as mediated by enhanced brain function during exercise. Further studies to validate these effects and elucidate the potential mechanism in adult neuronal differentiation during exercise will be of particular interest.
Supplementary Material
H I G H L I G H T S.
Conditioned media (CM) from AICAR treated muscle cells affects neural function.
AICAR-CM enhances adult neural progenitor cell neuronal and glial differentiation.
AICAR-CM fraction A11 contains proteins important for cell migration and neurogenesis.
Glucose regulator glucose-6-phosphate isomerase increases neural differentiation.
Acknowledgments
This work was supported by grants to H.Y.M. from the National Research Foundation, South Korea (NRF, grant number: 700-20170032) and Korea Mouse Phenotype Center (R1710921) and Research Resettlement Fund for new faculty of Seoul National University, South Korea, in part by the Intramural Research Program of the NIH, National Institute on Aging, United States (NIA) and by NIH/NIMH, United States (R01MH116582) to XZ. We are grateful to Dr. Seungwoo Yoo and Linda R. Kitabayashi for assistance with figure preparation.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2018.10.041.
Footnotes
Conflicts of interest
The authors have no conflict of interest to report.
References
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, et al. , 2014. Skeletal muscle PGC-1a1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45. [DOI] [PubMed] [Google Scholar]
- Al-Rewashdy H, Ljubicic V, Lin W, Renaud JM, Jasmin BJ, 2015. Utrophin A is essential in mediating the functional adaptations of mdx mouse muscle following chronic AMPK activation. Hum. Mol. Genet 24 (5), 1243–1255. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Head SI, Rae C, Morley JW, 2002. Brain function in Duchenne muscular dystrophy. Brain 125, 4–13. [DOI] [PubMed] [Google Scholar]
- Brockhoff M, Rion N, Chojnowska K, Wiktorowicz T, Eickhorst C, Erne B, Frank S, Angelini C, Furling D, Rüegg MA, Sinnreich M, Castets P, 2017. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J. Clin. Invest 127 (2), 549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzanska L, Ruiz A, Zychowicz M, Rauscher H, Ceriotti L, Rossi F, Colpo P, Domańska-Janik K, Coecke S, 2009. Patterned growth and differentiation of human cord blood-derived neural stem cells on bio-functionalized surfaces. Acta Neurobiol. Exp. 69 (1), 24–36. [DOI] [PubMed] [Google Scholar]
- Cannistraci CV, Ogorevc J, Zorc M, Ravasi T, Dovc P, Kunej T, 2013. Pivotal role of the muscle-contraction pathway in cryptorchidism and evidence for genomic connections with cardiomyopathy pathways in RASopathies. BMC Med. Genom 6, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN, 2006. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J. Neurosci 26 (31), 8048–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pascalisa C, Etienne-Mannevillea S, 2017. Single and collective cell migration: the mechanics of adhesions. Mol. Biol. Cell 28 (14), 1833–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delezie J, Handschin C, 2018. Endocrine crosstalk between skeletal muscle and the brain. Front. Neurol 9, 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B, Glanzman D, Tidball JG, 2009. Nitric oxide generated by muscle corrects defects in hippocampal neurogenesis and neural differentiation caused by muscular dystrophy. J. Physiol 587 (Pt 8), 1769–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger BE, Zhao X, 2017. Identifying molecular mediators of environmentally enhanced neurogenesis. Cell Tissue Res. 371 (1), 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD, 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci 18, 2803–2812. [DOI] [PubMed] [Google Scholar]
- Florek M, Karolak S, 1977. Intelligence level of patients with the Duchenne type of progressive muscular dystrophy (pmd-d). Eur. J. Pediatr 126, 275–282. [DOI] [PubMed] [Google Scholar]
- Fu M, Li L, Albrecht T, Johnson JD, Kojic LD, Nabi IR, 2011. Autocrine motility factor/phosphoglucose isomerase regulates ER stress and cell death through control of ER calcium release. Cell Death Differ. 18 (6), 1057–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golbidi S, Laher I, 2014. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res 2014, 726861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire CA, Bonenfant D, Le Nguyen A, Aumont A, Fernandes KJL, 2014. Untangling the influences of voluntary running, environmental complexity, social housing and stress on adult hippocampal neurogenesis. PLoS One 9 (1), e86237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri D, van Praag H, 2015. Exercise-mimetic AICAR transiently benefits brain function. Oncotarget 4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri D, Moon HY, van Praag H, 2017. The latest on exercise mimetics. Brain Pl (2), 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Patzlaff NE, Jobe EM, et al. , 2012a. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat. Protoc. 7 (11), 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Allan AM, Zong R, et al. , 2011a. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med 17 (5), 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zhang L, Christopher DM, et al. , 2011b. RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron 70 (5), 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Patzlaff NE, Jobe EM, Zhao X, 2012b. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat. Protoc 7 (11), 2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G, 2014. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14 (2), 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, Pedersen BK, Plomgaard P, 2011. Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152, 164–171. [DOI] [PubMed] [Google Scholar]
- Hardie DG, 2011. AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsha HC, Molina H, Pandey A, 2008. Quantitative proteomics using stable isotope labeling with amino acids in cell culture. Nat. Protoc 3 (3), 505–516. [DOI] [PubMed] [Google Scholar]
- Hartwig S, Raschke S, Knebel B, Scheler M, Irmler M, Passlack W, et al. , 2014. Secretome profiling of primary human skeletal muscle cells. Biochim. Biophys. Acta 1844 (5), 1011–1017. [DOI] [PubMed] [Google Scholar]
- Hawley JA, Hargreaves M, Joyner MJ, Zierath JR, 2014. Integrative biology of exercise. Cell 159, 738–749. [DOI] [PubMed] [Google Scholar]
- Hetz C, Saxena S, 2017. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol 13 (8), 477–491. [DOI] [PubMed] [Google Scholar]
- Hinton VJ, De Vivo DC, Nereo NE, Goldstein E, Stern Y, 2000. Poor verbal working memory across intellectual level in boys with Duchenne dystrophy. Neurology 54, 2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Weigert C, 2017. Skeletal Muscle as an Endocrine Organ: the Role of Myokines in Exercise Adaptations. Cold Spring Harbor Laboratory Press, pp. pp237–258 Perspectives in Medicine, The Biology of Exercise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoier B, Prats C, Qvortrup K, Pilegaard H, Bangsbo J, Hellsten Y, 2013. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. Faseb. J 27 (9), 3496–3504. [DOI] [PubMed] [Google Scholar]
- Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM, 2015. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metabol. 22, 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery CJ, Bahnson BJ, Chien W, Ringe D, Petsko GA, 2000. Crystal structure of rabbit phosphoglucose isomerase, a glycolytic enzyme that moonlights as neuroleukin, autocrine motility factor, and differentiation mediator. Biochemistry 39 (5), 955–964. [DOI] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, 2014. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH, 1997. More hippocampal neurons in adult mice living in an enriched environment. Nature 386 (6624), 493–495. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H, 2011a. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn. Mem 18, 605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H, 2011b. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn. Mem. 18, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Guerrieri D, Zhang Y, Collica SC, Becker KG, van Praag H, 2014. AMPK agonist AICAR improves cognition and motor coordination in young and aged mice. Learn. Mem. 21, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Kraczek EJ, Hirshman MF, Goodyear LJ, Winder WW, 1999. 5’ AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48 (8), 1667–1671. [DOI] [PubMed] [Google Scholar]
- Li Y, Stockton ME, Bhuiyan I, et al. , 2016. MDM2 inhibition rescues neurogenic and cognitive deficits in a mouse model of fragile X syndrome. Sci. Transl. Med. 8, 336 336ra61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang Y, Li H, Ji L, 2016. The role of nitric oxide in the antidepressant actions of 5-Aminoimidazole-4-Carboxamide-1-β-D-Ribofuranoside in insulin-resistant mice. Psychosom. Med 78 (1), 102–112. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shan G, Guo W, et al. , 2010. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 6 (4), e1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangos PJ, Loftus T, Wiesner J, Lowe T, Rossi E, Browne CE, et al. , 1990. Adenosinergic modulation of homocysteine-induced seizures in mice. Epilepsia 31 (3), 239–246. [DOI] [PubMed] [Google Scholar]
- McOmish CE, Hannan AJ, 2007. Enviromimetics: exploring gene environment interactions to identify therapeutic targets for brain disorders. Expert Opin. Ther. Targets 11 (7), 899–913. [DOI] [PubMed] [Google Scholar]
- Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H, 2016. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metabol. 24, 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher KI, Schaffer DV, 2017. Influence of hippocampal niche signals on neural stem cell functions during aging. Cell Tissue Res. 371 (1), 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto E, Tabata Y, Taneda T, Aoki Y, Muto A, Arai K, Watanabe S, 2004. Identification and characterization of Veph, a novel gene encoding a PH domain-containing protein expressed in the developing central nervous system of vertebrates. Biochimie 86, 523–531. [DOI] [PubMed] [Google Scholar]
- Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, et al. , 2008. AMPK and PPARdelta agonists are exercise mimetics. Cell 134, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ, 2006. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci 7 (9), 697–709. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA, 2008. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA, 2012. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol 8, 457–465. [DOI] [PubMed] [Google Scholar]
- Reh TA, Radke K, 1988. A role for the extracellular matrix in retinal neurogenesis in vitro. Dev. Biol 129 (2), 283–293. [DOI] [PubMed] [Google Scholar]
- Rich B, Scadeng M, Yamaguchi M, Wagner PD, Breen EC, 2017. Skeletal myofiber vascular endothelial growth factor is required for the exercise training-induced increase in dentate gyrus neuronal precursor cells. J. Physiol 595 (17), 5931–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Kelly ÁM, 2016. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Ageing Res. Rev 27, 77–92. [DOI] [PubMed] [Google Scholar]
- Schröter W, Eber SW, Bardosi A, Gahr M, Gabriel M, Sitzmann FC, 1985. Generalised glucosephosphate isomerase (GPI) deficiency causing haemolytic anaemia, neuromuscular symptoms and impairment of granulocytic function: a new syndrome due to a new stable GPI variant with diminished specific activity (GPI Homburg). Eur. J. Pediatr 144 (4), 301–305. [DOI] [PubMed] [Google Scholar]
- Shinjyo N, Stahlberg A, Dragunow M, Pekny M, Pekna M, 2009. Complement-derived anaphylatoxin C3a regulates in vitro differentiation and migration of neural progenitor cells. Stem Cell. 27 (11), 2824–2832. [DOI] [PubMed] [Google Scholar]
- Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Abou Haidar E, Stringer T, Ulja D, Karuppagounder SS, Holson EB, et al. , 2016. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 5, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak P, Wojcik-Stanaszek L, Sypecka J, Sokolowska A, Zalewska T, 2010. Effect of matrix metalloproteinases inhibition on the proliferation and differentiation of HUCB-NSCs cultured in the presence of adhesive substrates. Acta Neurobiol. Exp. 70 (4), 325–336. [DOI] [PubMed] [Google Scholar]
- Toedebusch RG, Ruegsegger GN, Braselton JF, Heese AJ, Hofheins JC, Childs TE, Thyfault JP, Booth FW, 2016. AMPK agonist AICAR delays the initial decline in lifetime-apex V̇o2 peak, while voluntary wheel running fails to delay its initial decline in female rats. Physiol. Genom. 48 (2), 101–115. [DOI] [PubMed] [Google Scholar]
- Vaillend C, Rendon A, Misslin R, Ungerer A, 1995. Influence of dystrophin-gene mutation on mdx mouse behavior. I. Retention deficits at long delays in spontaneous alternation and bar-pressing tasks. Behav. Genet 25, 569–579. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci 2, 266–270. [DOI] [PubMed] [Google Scholar]
- van Praag H, 2008. Neurogenesis and exercise: past and future directions. NeuroMol. Med 10, 128–140. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, et al. , 1997. Contraction-induced changes in acetyl-CoA carboxylase and 5’-AMP-activated kinase in skeletal muscle. J. Biol. Chem 272 (20), 13255–13261. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. , 2011. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivar C, Potter MC, van Praag H, 2013. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr. Top Behav. Neurosci 15, 189–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM, 2013. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metabol. 18, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, 2015. FNDC5/Irisin – their role in the nervous system and as a mediator for beneficial effects of exercise on the brain. Br. Plast. 1, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau SY, Li A, Hoo RLC, Ching YP, Christie BR, Lee TMC, Xu A, So K-F, 2014. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. U. S. A 111 15810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Yu LF, Pang T, Fang LP, Feng X, Wen TQ, Nan FJ, Feng LY, Li J, 2008. AICAR induces astroglial differentiation of neural stem cells via activating the JAK/STAT3 pathway independently of AMP-activated protein kinase. J. Biol. Chem 283 (10), 6201–6208. [DOI] [PubMed] [Google Scholar]
- Zhang D, Guo M, Zhang W, Lu X-Y, 2011. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade. J. Biol. Chem 286, 44913–44920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wang X, Lu X, 2016. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology 157, 2853–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Kremer P, Tadmori I, Ren Y, Sun D, He X, Young W, 2011. Lithium suppresses astrogliogenesis by neural stem and progenitor cells by inhibiting STAT3 pathway independently of glycogen synthase kinase 3 beta. PLoS One 6 (9), e23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong M, Lu T, Fan S, Zhang H, Gong R, Sun L, Fu Z, Fan L, 2015. Glucose-6-phosphate isomerase promotes the proliferation and inhibits the apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis Res. Ther 17 (1), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP, 2008. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J. Physiol 586 (24), 6021–6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.