LAY ABSTRACT
Autism spectrum disorders (ASD) are a set of behavioral conditions in children involving language and social interaction disabilities as well as repetitive behaviors and activities. ASDs are certainly inherited, but environmental factors, such nutrition, mother’s health when she is pregnant, and exposure to air pollution during pregnancy or early post-natal life, are also associated with an increased risk. The ways in which genes and environmental factors might interact with each other in influencing risk for autism is poorly understood. In this study we examined one type of genetic change, copy number variation (deletions or duplications of sections of DNA) that is known to have a significant impact on autism, as well as exposure to air pollutants, another established risk factor for developing autism. Specifically we measured the interaction between air pollution and copy number variation on autism risk to determine if their combined action can increase the risk beyond what the two factors might contribute on their own. This study showed that ozone, a major component of what is often referred to as ‘smog’ may increase risk for autism in children who have a high level of copy number changes in their DNA. This is the first study to examine copy number changes in combination with an environmental factor.
Keywords: Autism, copy number variation, air pollution, gene-environment interaction
SCIENTIFIC ABSTRACT
Autism spectrum disorder (ASD) is a complex trait with a high degree of heritability as well as documented susceptibility from environmental factors. In this study the contributions of copy number variation, exposure to air pollutants, and the interaction between the two on autism risk, were evaluated in the population-based case-control Childhood Autism Risks from Genetics and Environment (CHARGE) Study. For the current investigation, we included only those CHARGE children a) who met criteria for autism or typical development and b) for whom our team had conducted both genetic evaluation of copy number burden and determination of environmental air pollution exposures based on mapping addresses from the pregnancy and early childhood. This sample consisted of 158 cases of children with autism and 147 controls with typical development. Multiple logistic regression models were fit with and without environmental variable-copy number burden interactions. We found no correlation between average air pollution exposure from conception to age two years and the child’s CNV burden. We found a significant interaction in which a 1SD increase in duplication burden combined with a 1SD increase in ozone exposure was associated with an elevated autism risk (OR 3.4, P<0.005) much greater than the increased risks associated with either genomic duplication (OR 1.85, 95% CI: 1.25~2.73) or ozone (OR 1.20, 95% CI: 0.93~1.54) alone. Similar results were obtained when CNV and ozone were dichotomized to compare those in the top quartile relative to those having a smaller CNV burden and lower exposure to ozone, and when exposures were assessed separately for pregnancy, the first year of life, and the second year of life. No interactions were observed for other air pollutants, even those that demonstrated main effects; ozone tends to be negatively correlated with the other pollutants examined. These findings appear to be the first indication that global copy number variation may increase susceptibility to certain environmental factors, and underscore the need to consider both genomics and environmental exposures as well as the mechanisms by which each may amplify the risks for autism associated with the other.
INTRODUCTION
Autism is a behavioral disorder that has been the subject of extensive genetic studies (Jeste and Geschwind, 2014). The incidence of ASD in the U.S., currently estimated at 1 in 56 children {Developmental Disabilities Monitoring Network Surveillance Year Principal, 2014 #41}, makes this set of disorders a public health, educational, and economic concern. While autism has been determined to be highly heritable, recent estimates have elevated the proportion of variance attributable to environment. Early family studies of twins estimated the proportion of genetic contribution to autism as high as 90% [see for example, (Bailey et al., 1995, Folstein and Rutter, 1977, Lichtenstein et al., 2010)]. A more recent assessment of twins, while supporting a substantial genetic component, also indicated significant contributions from shared environment (Hallmayer et al., 2011). A large longitudinal study of all births in Sweden between 1982 and 2006 that included more than 14,000 individuals diagnosed with ASD, provided evidence that genetic and environmental contributors to autism are essentially equal, each accounting for about 50% of the variability (Sandin et al., 2014). A parallel study of this same cohort that examined SNP variants in some 3000 subjects, arrived at similar estimates of the heritability proportion (52%), and indicated that common variants comprise the majority of genetic risk for autism (Gaugler et al., 2014). The best estimates at present, therefore, indicate that the etiological architecture of autism includes essentially equal contributions from environment and DNA sequence variation, and both common and rare genomic variants play a role in disease risk and severity. While these studies have established the importance of both genetic and environmental factors in autism, direct measures of their relative contributions and moreover, of the interactions between them are lacking.
Copy number variants are one type of genomic change that contributes to autism susceptibility. The pioneering study by Sebat and colleagues found a ten-fold higher frequency of large and rare de novo variants in children with autism compared to control subjects (Sebat et al., 2007). Subsequent work confirmed these findings (Pinto et al., 2010) and revealed that copy number burden represented in large CNVs correlated with the severity of the phenotypes found in children with neurobehavioral disorders (Girirajan et al., 2011). Genome-wide analysis of both rare and common CNVs demonstrated that autism is associated with increased levels of copy number load, measured as base pairs of change, with a preponderance of duplications (Girirajan et al., 2013). In addition, the level of copy number load negatively correlated with measures of communication and social skills (Girirajan et al., 2013). Further, whole exome sequence analysis has shown that children with autism inherit more CNVs than their unaffected siblings (Krumm et al., 2013). There is therefore good evidence that total genomic copy number burden is a meaningful measure of genomic change that contributes to autism susceptibility and a good sensor for assessing genome-environment interactions.
Phenotypic variation is an expression of several determinants, including genetic variation, environmental exposure, and the interactions between genetic variants and the environment. The magnitude and frequency of gene-environment interactions are largely unknown, and therefore the degree to which environmental impact coupled with genetic variation can explain the so-called “missing heritability” is unresolved. Current genetic studies of complex disorders often assume no appreciable gene-environment interactions, an assumption that has not been validated by experiment (Gaugler et al., 2014). The paucity of gene-environment interaction measures has a simple origin, it requires detailed genetic and environmental data for the same group of individuals, an expensive and time consuming endeavor.
A growing body of evidence supports specific environmental contributors to autism susceptibility. In particular, prenatal air pollution exposure has come to the forefront of environmental ASD risk factors as 11 studies from the United Sates, using different study designs and methods, all suggest increased risk with increasing exposure.(Becerra et al., 2013, Kalkbrenner et al., 2010, Kalkbrenner et al., 2015, Raz et al., 2015, Roberts et al., 2013, Talbott et al., 2015a, Talbott et al., 2015b, Volk et al., 2011, Volk et al., 2013, von Ehrenstein et al., 2014, Windham et al., 2006) Criteria air pollutants, including nitrogen dioxide (NO2), particulate matter less than 10 and less than 2.5 microns in diameter (PM10, PM2.5), and ozone, are routinely monitored by the Environmental Protection Agency (EPA) and exposure to both NO2 and PM have been associated with ASD in populations from both Northern and Southern California(Becerra et al., 2013, Kalkbrenner et al., 2015, Volk et al., 2013), North Carolina (Kalkbrenner et al., 2010, Kalkbrenner et al., 2015) West Virginia (Kalkbrenner et al., 2010), Pennsylvania (Talbott et al., 2015b), and in the nation-wide Nurses Health Study(Raz et al., 2015). When studies have attempted to identify critical time periods of exposure, pregnancy and potentially the latter half of pregnancy appear the most important(Weisskopf et al., 2015). Few studies have attempted to include multiple criteria pollutants in the same model, though in the analyses that do, effects of NO2, PM2.5, and ozone persist.(Becerra et al., 2013, Volk et al., 2013) We are aware of only one other paper to date, that has examined genetic susceptibility together with air pollution exposure on ASD risk (Volk et al., 2014).
This study sought to examine the joint effect of genetic susceptibility for ASD, as reflected in copy number variation, and air pollution exposure on risk of ASD in the Childhood Autism Risks from Genetics and Environment (CHARGE) study.
METHODS
Description of sample
CHARGE is a population-based case-control study of preschool children being conducted at the University of California Davis MIND (Medical Investigations of Neurodevelopmental Disorders) Institute (Hertz-Picciotto et al., 2006). Eligibility criteria included: being between the ages of 24 and 60 months at the time of recruitment, living with a biologic parent who speaks either English or Spanish, having been born in California, and residing within the study catchment area of approximately 1.5 hours’ drive to the clinic (covering ~20 counties). Our sampling frame for cases consists of children who receive services through the California Department of Developmental Services (DDS), as well as referrals from health and service providers, from other studies at the UC Davis MIND Institute, and through self-referral. Population-based controls are recruited using California Vital Statistics files of births, from which we randomly sample after frequency-matching on sex, age, and broad geographic region. Regions are large, generally encompassing multiple counties, and correspond to the catchment areas of Regional Centers that coordinate services of the California DDS. This sampling strategy ensures that confounding related to regional characteristics is minimized, and simultaneously that the cases and controls are not over-matched with regard to geographically-related exposures. Additional details on study design are provided elsewhere (Hertz-Picciotto et al., 2006). All autism cases for CHARGE are confirmed on the Autism Diagnostic Observation Schedule (ADOS) and the Autism Diagnostic Interview-Revised (ADI-R). Typically developing controls were children who received a score <15 on the Social Communication Questionnaire and also showed no evidence of other types of developmental delay (composite scores of 70 or greater on Mullen Scales of Early Learning and Vineland Adaptive Behavior Scales). All assessments were conducted at clinics either in the MIND Institute located in Sacramento, CA or the UCLA Neuropsychiatric Institute located in Los Angeles, CA, by trained clinicians with research reliability for the instruments they administered. All components of the data collection were conducted in English or in Spanish by bilingual bicultural staff. For the current investigation, we included only those CHARGE children a) who met criteria for autism or typical development, b) who gave blood and agreed to have their biospecimens shared with researchers outside of the original study team, c) for whom genetic evaluation of copy number burden passed quality control (Girirajan et al., 2013) and d) for whom assignment of air pollution exposures based on residential addresses was successful (Volk et al., 2013). These restrictions resulted in 158 cases of children with autism and 147 controls with typical development eligible for this analysis, which is 79% of those that passed QC for CNV calls in our previous publication (Girirajan et al., 2011). These children were born between 1999 and 2008.
Custom targeted hotspot array
Whole blood was collected for participants’ DNA samples. A custom targeted hotspot Array was used to detect CNVs as described previously (Girirajan et al., 2011). The hotspot arrays comprised 135,000 probes, with higher density probe coverage (median probe spacing 2.6 kbp) for hotspot genomic regions, flanked by segmental duplications, and a lower probe density across the entire genome (median probe spacing 36 kbp). Hybridization, quality control and segmentation analysis were conducted as previously described (Girirajan et al., 2013, Girirajan et al., 2011). Global changes in copy number burden were measured, i.e. bps of duplication or deletion in each individual, or collectively, as total base pairs of altered copy number (i.e. total CNV burden). In the data analysis copy number burden was evaluated as a continuous variable.
Air pollution exposure assignment
Through telephone interviews, we collected demographic characteristics, medical conditions, and environmental exposures, including residential history (Hertz-Picciotto et al., 2006). Residential histories recorded dates and address locations where the mother lived, beginning before conception through the most recent place of residence, as well as any other place of residence where the child lived. These dates and addresses were used to develop air pollution exposure metrics, as commonly implemented in large epidemiologic studies when direct measurements are not feasible (Hertz-Picciotto et al., 2006).
We used the CALINE4 line-source air quality dispersion model in order to acquire model-based estimates of traffic related air pollution (TRP) exposure derived from freeways, non-freeways, and all roads located within 5 km of each child’s home (Bensen, 1992). Information on roadway geometry, link-based traffic volumes, period-specific meteorological conditions (wind speed and direction, mixing heights, and atmospheric stability), and vehicle emission rates were all included in the model (Volk et al., 2013). The CALINE4 model specifically produced estimates of nitrogen oxides (NOx) which were almost perfectly correlated (around 0.99) with estimated concentrations of other traffic-related pollutants, including carbon monoxide and elemental carbon, from this same model. Therefore, our model-based pollutant concentration estimates serve as indicators of the traffic-related air pollutant mixture rather than of any specific pollutant.
In addition, exposure to PM2.5, PM10, ozone, and NO2, used regional data from the US Environmental Protection Agency Air Quality System (www.epa.gov/ttn/airs/airsaqs) supplemented for Southern California by the University of Southern California’s Children’s Health Study data for 1997 through 2009 (Volk et al., 2013). The monthly air quality data from up to four monitoring stations located within 50 km of each residence were used for spatial interpolation of ambient concentrations using inverse distance-squared weighting. If one or more stations were located within 5 km of a residence, only data from those stations were used. For PM2.5, PM10, and NO2, measurements were based on 24 hour average concentrations. For ozone, measurements were based on the average range of ozone measurements from 1000 to 1800 hours (reflecting the high 8-hour daytime exposure).
Based on child date of birth, date of conception, and reported start and end dates of each residence, the average air pollution exposure was assigned for the entire pregnancy and for the child’s first and second year of life. We also created an average exposure for each air pollutant for the period from conception to the child’s second birthday.
Ethnicity estimation
We genotyped 100 SNPs from a custom-designed Illumina based array identified from inherited allele frequencies from four parental populations (Asian, African, European, Amerindian and Indian). To empirically estimate the proportion of ancestry attributable to a particular founding population for each individual we examined these SNPs using the program Structure(Pritchard et al., 2000) to derive 5 continuous variables reflecting each parental population. In our analyses, these variables were included as covariates.
Statistical analysis
In order to assess the main effect contributions of CNV burden and air pollution exposure to autism susceptibility, logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for each CNV burden measure and each air pollution exposure adjusted for the other. In initial models, all types of CNV burden (duplication, deletion, or total CNV burden) and air pollutants (TRP, nitrogen dioxide, ozone, PM2.5, or PM10), measured at each of three different time periods spanning pregnancy and the first two years of life were examined as continuous variables, with effect estimates scaled to twice the standard deviation of each exposure distribution. To control confounding, we included as covariates: maternal education, child’s sex and race/ethnicity characterized by the Ancestry Informative Markers (AIMS).
We also examined the correlations between all pairs of CNV burden measures and air pollution exposures using Spearman’s rank correlations. This analysis aimed to determine the presence of broad evidence supporting a molecular interaction whereby prenatal air pollution might play a role in altering copy number burden.
The main analysis was to evaluate the joint effect of CNV and air pollutants on autism. For this question, we added a product term to assess interaction in the logistic regression model, first using both air pollution exposure and CNV burden as continuous variables. To increase interpretability of these results, as air pollution exposure and CNV burden are measured on vastly different scales, we z-score transformed both variables. Estimates were then scaled to reflect a 1SD increase in exposure. Finally, we reparameterized the above model by dichotomizing both CNV burden measures and air pollution exposure at the 75th percentile (the top quartile (‘high’) vs. the bottom 3 quartiles (‘low’)). This model enabled a comparison of the risk for autism among those in the top quartile of both CNV and air pollutant exposures with the risk among those in the bottom three quartiles of both CNV and air pollutant exposures. Moreover, the analyses of dichotomized CNV and air pollution measures were conducted for each of the three time periods (pregnancy, 1st year of life, 2nd year of life) separately. Each of these models also adjusted for confounders of maternal education, child sex, child race/ethnicity and Regional Center.
RESULTS
Interactions between Air Pollution Exposure and CNV Burden Exposure
The correlations between each of the average air pollutant measures across the three time periods and each metric for CNV burden range from −0.088 to +0.081 (Table 1). None were significant, and all of these values are small, indicating that none of our three measures of global CNV has likely been impacted by early life air pollution exposures.
Table 1.
Spearman correlations of CNV burden variable and average air pollution exposure spanning pregnancy through the first two years of life for 305 samplesa
| CNV Burden | Air pollution exposure |
||||
|---|---|---|---|---|---|
| TRP | NO2 | Ozone | PM2.5 | PM10 | |
| Duplication | 0.063 | 0.081 | −0.088 | 0.009 | 0.022 |
| Deletion | −0.007 | 0.016 | 0.042 | 0.079 | 0.023 |
| Total CNV | 0.070 | 0.088 | −0.055 | 0.066 | 0.041 |
All correlation measures were not statistically significant (p-value > .05).
Results for each of the 15 different models representing 3 metrics of CNV and 5 air pollutant measures are presented in Table 2. These analyses confirm the strongest main effects from duplications, total CNV, PM10 and PM2.5, as well as two significant interactions, both involving ozone: with duplications and with total CNV. When examining the average air pollution exposure from pregnancy through the second year of life as a continuous variable we found significant interactions for both CNV duplication burden and total CNV burden in combination with ozone exposure (OR=3.43 and 2.58, respectively) (products of the main effects and interaction, Table 2). These OR’s represent the impact of an increase of 1SD in both ozone exposure and duplications on the risk for autism; the combined exposures result in a noticeably higher level of risk than either duplications (OR=1.85) or ozone (OR=1.20) alone.
Table 2.
Fifteen models predicting risk of autism by main effects and interaction between CNV burden and average air pollution exposure spanning pregnancy through the first two years of life (n=305)a
| CNV Burden | Air Pollutant | CNV Burden | Air Pollutant | Interaction |
|---|---|---|---|---|
| Duplications | TRP | 1.51 (1.08–2.11) | 1.28 (0.98–1.70) | 0.95 (0.73–1.23) |
| NO2 | 1.48 (1.06–2.07) | 1.28 (0.99–1.67) | 0.94 (0.77–1.15) | |
| Ozone | 1.85 (1.25–2.73) | 1.20 (0.93–1.54) | 1.55 (1.09–2.21) | |
| PM10 | 1.46 (1.05–2.03) | 1.52 (1.16–2.01) | 0.93 (0.73–1.19) | |
| PM2.5 | 1.48 (1.06–2.05) | 1.48 (1.13–1.95) | 0.95 (0.72–1.24) | |
| Deletions | TRP | 1.16 (0.89–1.52) | 1.33 (1.00–1.76) | 0.81 (0.59–1.11) |
| NO2 | 1.13 (0.87–1.48) | 1.44 (1.07–1.95) | 0.96 (0.73–1.26) | |
| Ozone | 1.17 (0.90–1.52) | 1.05 (0.81–1.37) | 1.06 (0.83–1.36) | |
| PM10 | 1.15 (0.88–1.51) | 1.71 (1.26–2.32) | 0.98 (0.73–1.33) | |
| PM2.5 | 1.16 (0.89–1.51) | 1.62 (1.19–2.20) | 0.83 (0.61–1.13) | |
| Total | TRP | 1.68 (1.24–2.28) | 1.28 (0.99–1.66) | 0.88 (0.68–1.15) |
| NO2 | 1.61 (1.19–2.18) | 1.32 (1.01–1.73) | 0.92 (0.75–1.14) | |
| Ozone | 1.76 (1.30–2.39) | 1.08 (0.85–1.38) | 1.36 (1.01–1.81) | |
| PM10 | 1.67 (1.22–2.29) | 1.58 (1.20–2.07) | 0.87 (0.68–1.11) | |
| PM2.5 | 1.70 (1.23–2.33) | 1.50 (1.41–1.97) | 0.84 (0.65–1.08) |
CNV burden and regional pollution effects reflect risk of autism based on a 1 SD of the distribution, specifically per increase of 1,356,513 base pair of duplication burden, 17.7 ppb of TRP, 6.2 ppb of ozone, 5.7 ppb NO2, 6.2 ug/m3 PM10, 3.7 ug/m3 PM2.5
Models adjusted for maximum education level of parents, child’s sex and child’s ethnicity (based on ancestry informative markers in the genome)
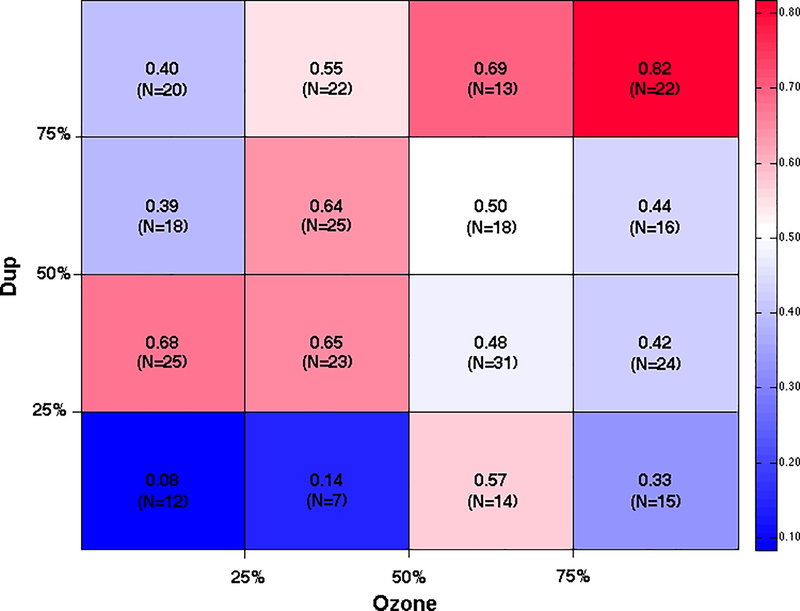
To visualize the risk associated with duplication burden and ozone exposure in combination we divided individuals into 16 subgroups based on quartiles of duplication burden and ozone exposure. Figure 1 shows the proportion of individuals with autism in each of the quartiles of duplication burden on the y-axis and quartiles of ozone exposure on the x-axis. In our case-control study sample with both CNV and air pollution measures, the overall proportion of cases with autism is 0.52. The subgroup from the top quartiles of both duplication burden and ozone exposure showed the highest proportion of children with autism (0.82) while the subgroup from the lowest quartile of duplication burden and ozone exposure showed the lowest proportion of autism cases (0.08). These representations of the data graphically demonstrate how the compound effect of duplication burden and ozone exposure greatly amplifies the odds of autism.
Figure 1. Proportion of individuals with autism by quartile of duplication CNV burden and average ozone exposure.
Each cell represents the intersection of two quartiles, one for duplication CNV burden and the other for average ozone exposure spanning the period from conception through the first two years of life. The proportion provided in each cell is the number of children with autism divided by the total number of individuals in that subgroup. N represents the total number of cases and controls in each cell. Red colors indicate higher proportion of children with autism while blue colors reflect lower proportions (see color bar on the right).
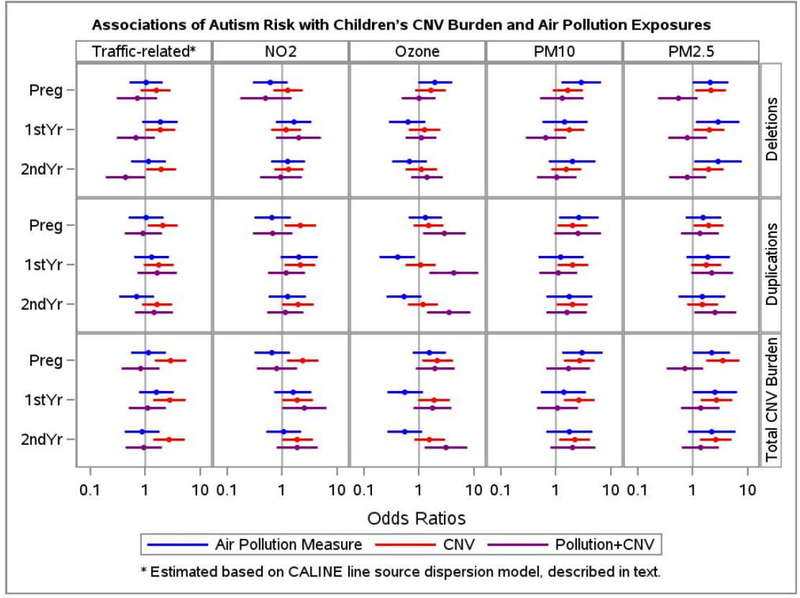
We then investigated the direction, scale and magnitude of the CNV burden-air pollution joint effect on autism but now with variables dichotomized to reflect high vs. low exposure for both air pollution and CNV burden. Moreover, we fit models to separately examine air pollutant exposure in each of the time periods. Results from these analyses for all five air pollutants, all three measures of CNV burden, and three time periods (average pregnancy exposure and the first and second year of the child’s life) are summarized in Figure 2 and presented in detail in Supplemental Table 1. First we see that when air pollution exposures are low, a high total CNV burden (Figure 2, estimates in red) is consistently associated with autism (bottom panel for total CNV burden). These estimates reflect increases in autism risk for the top quartile of total CNV burden with a range from OR=1.5 to OR=3.5, with most models having OR’s near 2.5. Second, we find that when CNV burden is low, especially deletions, high exposures to PM2.5 are associated with autism risk (Figure 2, estimates in blue, for example: comparing high vs. low PM2.5 exposure in postnatal years 1 and 2, respectively, OR=2.8, 95%CI (1.1–7.0), and OR=2.8 (1.1, 7.7)). The associations between autism risk and high exposures to PM10 during pregnancy are consistently elevated among those with low CNV (OR’s range from 2.58 to 2.98), whether measured as deletions, duplications, or their combination.
Figure 2. Estimated effects from genetic copy number variant (CNV) load and from air pollutant exposures, acting separately and in combination, on risk for autism.
Five broad columns represent five different types of air pollutant measurements, and three horizontal panels represent three different metrics for assessing CNV burden. For each cell representing a given air pollutant and CNV metric, three time periods for the air pollutant exposures are examined: pregnancy, 1st postnatal year, and 2nd postnatal year. The colored dots and bars are, respectively, the odds ratios and 95% confidence intervals for associations with autism versus typical development: a) comparing high to low environmental air pollutant exposures (blue) among those with low CNV burden; b) comparing high to low CNV burden (red) among those with low air pollutant exposures; and c) comparing the combined high CNV and high air pollutant exposures to the combined low CNV and low air pollutant exposures (purple). For both CNV burden (genetics) and air pollutant exposure (environment), high is defined as the upper quartile and low as the bottom three quartiles. A number of patterns emerge. These results confirm previous findings that the metric for CNV (red) with the strongest associations is the total CNV burden summing the lengths of both deletions and duplications (bottom panel of results), and reveal additional associations for air pollutants and their interactions with CNV.
These interaction results demonstrate the following: First, similar to the models based on continuous metrics for both CNV and air pollution, the interactions (purple) that emerge as most dramatic and with the strongest significance are for ozone, particularly when combined with duplications, but also with total CNV burden in the second year of life. Thus those in the upper quartile of both CNV and ozone exposure are at exceptionally high risk for ASD. Second, among those with low ozone, CNV (red) appears to have virtually no impact on ASD risk, and only impacts those with high ozone exposure.
Other observations worth noting are: a) The magnitude of genetically induced risk may depend on what exposures are simultaneously accounted for (e.g., for air pollutants during pregnancy, deletions show a stronger impact when adjusted for PM2.5 or the CALINE model of traffic-related pollution (25) than when adjusted for NO2). b) The environmental effects are remarkably consistent across metrics of CNV burden, but show greater variation by time period (e.g., for PM10, associations are stronger for pregnancy than later time periods, but this was not seen for PM2.5 or for NO2)..
Third, results for interactions were similar to the models based on continuous metrics: joint exposures to both high duplication burden and high ozone, as compared with jointly low exposures to both, were associated with substantially elevated risks for autism (Figure 2, estimates in purple). For example, children with high duplication burden who were also exposed during the prenatal period to high ozone levels had nearly a three-fold greater risk to develop autism (OR=2.8 (1.2–6.9)). Even stronger associations were seen for children with high ozone exposures during the first and second years of life, with OR=4.2 (1.5–11.7) and OR=3.4 (1.4–8.6) (Figure 2 / Supplemental Table 1). For the other pollutants, little evidence of interaction was found. Thus we observe a consistent pattern for ozone, as compared to sporadic suggestions of slightly elevated risks for joint exposures involving the other pollutants.
We also conducted a sensitivity analysis by adding maternal age to the models with quartiles of CNV, air pollution and the combination of the two. The impact on the OR’s and their 95% CI’s were negligible (<0.5% change), likely because the models were already adjusted for maternal education, child’s race/ethnicity, and Regional Center; maternal age and education are highly correlated in the CHARGE Study.
DISCUSSION AND CONCLUSIONS
Contributions of Interaction Involving Environmental and Genetic Risk Factors for Autism
There remains an active debate in the research community on the relative contributions of environmental and genetic factors in autism susceptibility, as well as the degree to which interactions might account for the “missing heritability”, namely, our inability to account for even a majority of autism cases with all known genomic variants. Large studies are converging on estimates of heritability in the ranges of 35%−60% (Hallmayer et al., 2011, Rosenberg et al., 2009, Gaugler et al., 2014) (Sandin et al., 2014). As new approaches to environmental exposures, including analyses of biological and environmental samples using non-targeted chemical analyses, are developed and begin to be used in research on ASD etiology, and more comprehensive analyses of interactions are conducted, assessments of the relative contributions from genetics and environment will become feasible. Nevertheless, the question of comparing relative separate contributions from genes and environment may need to be recast when considering a sizable proportion of the risk for autism may be influenced by both types of factors, and moveover, the impacts of environment, broadly defined to include, e.g., nutrition, stress, health of the pregnant mother, the microbiome and so forth, on gene expression mediated by epigenetic modifications are quite likely larger than previously imagined.
Correlations between air pollutant exposure and CNV burden were small. Prenatal exposures could only be expected to influence the de novo mutations, and not those carried by the parents in their somatic cells. However, the proportion of CNVs that are inherited vs. de novo is not clear and we were unable to make estimates, as we did not obtain CNV information from the parents. Hence, we are unable to draw any conclusions regarding the hypothesized pathway in which prenatal air pollution exposures might contribute to altered total CNV burden in the child. If de novo mutations are not a large proportion of the CNV burden, then any impact on de novo mutations from air pollution might have only a small effect on the total CNV burden, and this study would have had very little power to detect that impact. On the other hand, environmentally-induced de novo CNVs in just a few critical genes (for instance, in synaptogenesis pathways), might have large ramifications for ASD risk. Thus further work, in which de novo and inherited CNV are distinguished is warranted,
Our results do support a second type of interaction, namely, one in which ozone exposure may further exacerbate risk for autism in children with susceptibility arising from genetic instability. This finding needs to be viewed in the context of the full set of analyses (three different measures of CNV and five components of air pollution; air pollutant exposures in several time windows, and both continuous and dichotomized variables for both CNV and air pollution). First, we note that many of the pollutants are correlated, as are duplications and total CNV burden, and therefore the results across models are not independent of each other. For this reason, we have elected to report all of the findings, and to examine the findings that did emerge for consistency, since sporadic significance is more likely to be due to chance. Whether looking at ozone as a continuous measure or comparing the top quartile to the bottom three quartiles combined, the pattern of results is similar. Emphasizing only those results supported in several models, the salient observation from this study of gene-by-environment interaction is an ozone association with autism only among individuals who have a high CNV burden. The ozone association with autism in the presence of greater CNV burden stands in contrast to results from other pollutants, which have tended to show consistent associations in the literature with increased risk of ASD when ignoring genetic susceptibility, i.e., in populations as a whole (Becerra et al., 2013, Kalkbrenner et al., 2010, Kalkbrenner et al., 2015, Roberts et al., 2013, Talbott et al., 2015b, Talbott et al., 2015a, Volk et al., 2013, von Ehrenstein et al., 2014). This distinctive pattern is a coherent one in light of a priori scientific evidence establishing a negative correlation of ozone with the other pollutants examined. Notably, ozone overall, or in the absence of high CNV burden, showed essentially no association with autism risk. An association between ozone exposure and autism risk was not identifiable without taking genetic susceptibility into account. This result highlights how both genes and environment risk can contribute to ASD etiology, and highlights the value of incorporating measures of genomic susceptibility into environmental studies.
Mechanisms of ozone-copy number burden interactions
Of the airborne pollutants examined in this study, ozone showed the strongest interactions with CNV burden. What might be the mechanism of this interplay? Ozone is a potent oxidizing agent, known to produce reactive oxygen species (ROS) and cellular damage at exposure levels that can be found in urban environments (Devlin et al., 1991). In model systems, the oxidative and cellular stress produced by ozone has been shown to compromise neural gene expression, function, and behavior (Rivas-Arancibia et al., 2010). There is considerable evidence that autism is associated with elevated levels of oxidative stress both in peripheral blood (Gorrindo et al., 2013) and in the brain (Rossignol and Frye, 2014). Reductions in anti-oxidant molecules that serve to scavenge ROS are also associated with autism (Frye et al., 2013), potentially producing a state sensitive to further oxidative stress. Mitochondrial abnormalities, also found associated with autism, provides another mechanism of affecting oxidative stress in these children (Giulivi et al., 2010). Given the elevated levels of oxidative stress in children with autism it is plausible that ozone exposure potentiates an already compromised metabolic condition, producing a genetic-environment interaction of some magnitude. It is possible that CNVs contributing to autism affect genes altering production of ROS or compromising responses to oxidative stress. For example, a mouse model of Rett Syndrome, shows abnormalities in both oxidative burden and mitochondrial function (Grosser et al., 2012). In this instance our findings suggest that air pollutant exposure may work as an additional risk factor through one or more pathways affected by copy number variants.
Our study expands the limited body of work examining joint environmental and genetic risks for ASD. The joint effects we see between CNV burden and pollution exposure demonstrate an interaction, even when one of the factors has little to no association in the absence of the other. This example demonstrates that exposures with small main effects can also contribute to autism susceptibility via synergism with genetic factors, and that these interactions may be missed when limiting interaction analyses to exposures and genes with significant independent effects. This scenario may be parallel to the numerous examples of genomic regions or loci that showed marginal significance after multiple testing corrections but that have been replicated in larger cohorts or subsequent studies (Hamshere et al., 2013, Athanasiu et al., 2010, Denny et al., 2010, Pendergrass et al., 2013, Hall et al., 2014).
It is striking that the only significant GxE interaction found in the quartile analyses was with ozone, which itself showed no main effect in the CHARGE Study, while pollutants with strong main effects showed no interaction. A likely explanation is that for the other pollutants, CNV burden simply did not amplify susceptibility given an already high effect of air pollution on autism risk. Studies of air pollutants and ASD have not often examined the effect of ozone. To date, ozone has been examined in a report from Taiwan that identified an increased risk for ASD (Jung et al., 2013) and was associated with increased ASD risk when modeled along with PM2.5 in a large case-control study from Los Angeles County (Becerra et al., 2013). Investigation of ozone exposure on the brain has been relatively limited, with data largely coming from the field of cognitive aging, and demonstrating effects which implicate poorer performance over time (Chen and Schwartz, 2009, Gatto et al., 2014). Thus, our finding might represent a chance fluctuation, or it may represent a very novel clue regarding conditions (i.e., vulnerabilities) under which ozone does influence early development and possibly also late cognitive decline.
One limitation to the current study is the small sample size (n = 305), which may have limited our statistical power for subtle interactions. Only two pollutants, ozone and PM10, demonstrated any joint effect and only an ozone interaction was identified over multiple time points examined and with two measures of CNV burden. It should be acknowledged that ozone measures over time are highly correlated, which could suggest lack of specificity in the importance of timing for such exposures. Similarly, total CNV burden also includes duplication burden making it a less-specific measure of genetic susceptibility. As a first analysis of CNV-by-environment interactions, we selected an objectively measured exposure and controlled for only a few potential confounding factors, namely the sociodemographic factors of maternal education, child’s sex and race/ethnicity, and the Regional Center of their residence. Most factors we screened did not exert any confounding effect and after selection of the final model we conducted a sensitivity analysis and determined that maternal age had virtually no impact on any of our results (main effects or interactions); we recognize that identifying covariates that might be associated with the marginal and/or joint distributions of CNV burden and air pollution exposure, as well as with autism status, is uncharted territory. Hence, we cannot exclude a role for confounding by other known, suspected, or unknown risk factors for autism, which could have either masked an interaction with one or more of the other pollutants, or inflated the interaction with ozone. Another limitation is that this analysis was underpowered for more restricted time windows, such as trimesters of pregnancy. Since brain development requires highly orchestrated sequences of processes, it is possible that only certain critical periods are vulnerable to specific air pollutants. Indeed, in our earlier work, we found air pollution in the 3rd trimester and first year of life to be more strongly related to development of ASD than earlier periods.(Volk et al., 2013) Despite these limitations, our findings can help direct future research confirming and extending these relationships.
Our findings emphasize the value and importance of pursuing gene-environment interactions in both candidate and broad discovery modes, which require collecting both genomic and environmental data for large samples in order to discover new interactions or replicate existing interactions. A broad assessment of environmental factors, together with a comprehensive measurement of diverse genetic variants (SNPs, CNVs, etc.) in data resources with sufficient statistical power will likely be necessary to better quantify the joint effects of susceptibility factors for autism. Overall, the findings reported here show that genetic-environment interactions can be substantial and may contribute significantly to risk for complex disorders.
Supplementary Material
Acknowledgements
Support for this work was provided by NIH grant P01 ES011269 to MR, NIEHS R01 ES015359, NIEHS P01 ES011269, NIEHS P30 ES023513, NIH UG3 OD023365, NICHHD U54 HD079125, and EPA STAR #R-829388 & R833292 to IH-P and grants NIEHS R21 ES19002 and ES 007948 to HV. We gratefully acknowledge and thank the hundreds of families who generously participated in the CHARGE Study. The authors declare that there are no conflicts of interest.
Grant information:
Grant sponsor NIEHS; Grant number: R01 ES015359
Grant sponsor NIEHS; Grant number: P01 ES011269
Grant sponsor NIEHS; Grant number: P30 ES023513
Grant sponsor NIEHS; Grant number: UG3-OD023365
Grant sponsor EPA STAR; Grant number: #R-829388 & R833292
Grant sponsor NICHHD; Grant number: U54 HD079125
Grant sponsor NIEHS; Grant number: ES19002
Grant sponsor NIEHS; Grant number: ES 007948
REFERENCES
- ATHANASIU L, MATTINGSDAL M, KAHLER AK, BROWN A, GUSTAFSSON O, AGARTZ I, GIEGLING I, MUGLIA P, CICHON S, RIETSCHEL M, PIETILAINEN OP, PELTONEN L, BRAMON E, COLLIER D, CLAIR DS, SIGURDSSON E, PETURSSON H, RUJESCU D, MELLE I, STEEN VM, DJUROVIC S & ANDREASSEN OA 2010. Gene variants associated with schizophrenia in a Norwegian genome-wide study are replicated in a large European cohort. J Psychiatr Res, 44, 748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY A, LE COUTEUR A, GOTTESMAN I, BOLTON P, SIMONOFF E, YUZDA E & RUTTER M 1995. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med, 25, 63–77. [DOI] [PubMed] [Google Scholar]
- BECERRA TA, WILHELM M, OLSEN J, COCKBURN M & RITZ B 2013. Ambient air pollution and autism in Los Angeles county, California. Environmental Health Perspectives, 121, 380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSEN P 1992. A review of the development and application of the CA-LINE3 and 4 models. Atmos Environ, 26B, 379–390. [Google Scholar]
- CHEN JC & SCHWARTZ J 2009. Neurobehavioral effects of ambient air pollution on cognitive performance in US adults. Neurotoxicology, 30, 231–9. [DOI] [PubMed] [Google Scholar]
- DENNY JC, RITCHIE MD, BASFORD MA, PULLEY JM, BASTARACHE L, BROWN-GENTRY K, WANG D, MASYS DR, RODEN DM & CRAWFORD DC 2010. PheWAS: demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics, 26, 1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVLIN RB, MCDONNELL WF, MANN R, BECKER S, HOUSE DE, SCHREINEMACHERS D & KOREN HS 1991. Exposure of humans to ambient levels of ozone for 6.6 hours causes cellular and biochemical changes in the lung. Am J Respir Cell Mol Biol, 4, 72–81. [DOI] [PubMed] [Google Scholar]
- FOLSTEIN S & RUTTER M 1977. Infantile autism: a genetic study of 21 twin pairs. J Child Psychol Psychiatry, 18, 297–321. [DOI] [PubMed] [Google Scholar]
- FRYE RE, DELATORRE R, TAYLOR H, SLATTERY J, MELNYK S, CHOWDHURY N & JAMES SJ 2013. Redox metabolism abnormalities in autistic children associated with mitochondrial disease. Transl Psychiatry, 3, e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GATTO NM, HENDERSON VW, HODIS HN, ST JOHN JA, LURMANN F, CHEN JC & MACK WJ 2014. Components of air pollution and cognitive function in middle-aged and older adults in Los Angeles. Neurotoxicology, 40, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUGLER T, KLEI L, SANDERS SJ, BODEA CA, GOLDBERG AP, LEE AB, MAHAJAN M, MANAA D, PAWITAN Y, REICHERT J, RIPKE S, SANDIN S, SKLAR P, SVANTESSON O, REICHENBERG A, HULTMAN CM, DEVLIN B, ROEDER K & BUXBAUM JD 2014. Most genetic risk for autism resides with common variation. Nat Genet, 46, 881–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRIRAJAN S, BRKANAC Z, COE BP, BAKER C, VIVES L, VU TH, SHAFER N, BERNIER R, FERRERO GB, SILENGO M, WARREN ST, MORENO CS, FICHERA M, ROMANO C, RASKIND WH & EICHLER EE 2011. Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes. Plos Genetics, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRIRAJAN S, JOHNSON RL, TASSONE F, BALCIUNIENE J, KATIYAR N, FOX K, BAKER C, SRIKANTH A, YEOH KH, KHOO SJ, NAUTH TB, HANSEN R, RITCHIE M, HERTZ-PICCIOTTO I, EICHLER EE, PESSAH IN & SELLECK SB 2013. Global increases in both common and rare copy number load associated with autism. Hum Mol Genet, 22, 2870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIULIVI C, ZHANG YF, OMANSKA-KLUSEK A, ROSS-INTA C, WONG S, HERTZ-PICCIOTTO I, TASSONE F & PESSAH IN 2010. Mitochondrial dysfunction in autism. JAMA, 304, 2389–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORRINDO P, LANE CJ, LEE EB, MCLAUGHLIN B & LEVITT P 2013. Enrichment of elevated plasma F2t-isoprostane levels in individuals with autism who are stratified by presence of gastrointestinal dysfunction. PLoS One, 8, e68444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSER E, HIRT U, JANC OA, MENZFELD C, FISCHER M, KEMPKES B, VOGELGESANG S, MANZKE TU, OPITZ L, SALINAS-RIESTER G & MULLER M 2012. Oxidative burden and mitochondrial dysfunction in a mouse model of Rett syndrome. Neurobiol Dis, 48, 102–14. [DOI] [PubMed] [Google Scholar]
- HALL MA, VERMA A, BROWN-GENTRY KD, GOODLOE R, BOSTON J, WILSON S, MCCLELLAN B, SUTCLIFFE C, DILKS HH, GILLANI NB, JIN H, MAYO P, ALLEN M, SCHNETZ-BOUTAUD N, CRAWFORD DC, RITCHIE MD & PENDERGRASS SA 2014. Detection of pleiotropy through a Phenome-wide association study (PheWAS) of epidemiologic data as part of the Environmental Architecture for Genes Linked to Environment (EAGLE) study. PLoS Genet, 10, e1004678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALLMAYER J, CLEVELAND S, TORRES A, PHILLIPS J, COHEN B, TORIGOE T, MILLER J, FEDELE A, COLLINS J, SMITH K, LOTSPEICH L, CROEN LA, OZONOFF S, LAJONCHERE C, GRETHER JK & RISCH N 2011. Genetic Heritability and Shared Environmental Factors Among Twin Pairs With Autism. Archives of general psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMSHERE ML, WALTERS JT, SMITH R, RICHARDS AL, GREEN E, GROZEVA D, JONES I, FORTY L, JONES L, GORDON-SMITH K, RILEY B, O’NEILL FA, KENDLER KS, SKLAR P, PURCELL S, KRANZ J, SCHIZOPHRENIA PSYCHIATRIC GENOME-WIDE ASSOCIATION STUDY, C., WELLCOME TRUST CASE CONTROL, C., WELLCOME TRUST CASE CONTROL, C., MORRIS D, GILL M, HOLMANS P, CRADDOCK N, CORVIN A, OWEN MJ & O’DONOVAN MC 2013. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatry, 18, 708–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTZ-PICCIOTTO I, CROEN LA, HANSEN R, JONES CR, VAN DE WATER J & PESSAH IN 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect, 114, 1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JESTE SS & GESCHWIND DH 2014. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol, 10, 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG CR, LIN YT & HWANG BF 2013. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One, 8, e75510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALKBRENNER AE, DANIELS JL, CHEN JC, POOLE C, EMCH M & MORRISSEY J 2010. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology, 21, 631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALKBRENNER AE, WINDHAM GC, SERRE ML, AKITA Y, WANG X, HOFFMAN K, THAYER BP & DANIELS JL 2015. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology, 26, 30–42. [DOI] [PubMed] [Google Scholar]
- KRUMM N, O’ROAK BJ, KARAKOC E, MOHAJERI K, NELSON B, VIVES L, JACQUEMONT S, MUNSON J, BERNIER R & EICHLER EE 2013. Transmission disequilibrium of small CNVs in simplex autism. Am J Hum Genet, 93, 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LICHTENSTEIN P, CARLSTROM E, RASTAM M, GILLBERG C & ANCKARSATER H 2010. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry, 167, 1357–63. [DOI] [PubMed] [Google Scholar]
- PENDERGRASS SA, BROWN-GENTRY K, DUDEK S, FRASE A, TORSTENSON ES, GOODLOE R, AMBITE JL, AVERY CL, BUYSKE S, BUZKOVA P, DEELMAN E, FESINMEYER MD, HAIMAN CA, HEISS G, HINDORFF LA, HSU CN, JACKSON RD, KOOPERBERG C, LE MARCHAND L, LIN Y, MATISE TC, MONROE KR, MORELAND L, PARK SL, REINER A, WALLACE R, WILKENS LR, CRAWFORD DC & RITCHIE MD 2013. Phenome-wide association study (PheWAS) for detection of pleiotropy within the Population Architecture using Genomics and Epidemiology (PAGE) Network. PLoS Genet, 9, e1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINTO D, PAGNAMENTA AT, KLEI L, ANNEY R, MERICO D, REGAN R, CONROY J, MAGALHAES TR, CORREIA C, ABRAHAMS BS, ALMEIDA J, BACCHELLI E, BADER GD, BAILEY AJ, BAIRD G, BATTAGLIA A, BERNEY T, BOLSHAKOVA N, BOLTE S, BOLTON PF, BOURGERON T, BRENNAN S, BRIAN J, BRYSON SE, CARSON AR, CASALLO G, CASEY J, CHUNG BH, COCHRANE L, CORSELLO C, CRAWFORD EL, CROSSETT A, CYTRYNBAUM C, DAWSON G, DE JONGE M, DELORME R, DRMIC I, DUKETIS E, DUQUE F, ESTES A, FARRAR P, FERNANDEZ BA, FOLSTEIN SE, FOMBONNE E, FREITAG CM, GILBERT J, GILLBERG C, GLESSNER JT, GOLDBERG J, GREEN A, GREEN J, GUTER SJ, HAKONARSON H, HERON EA, HILL M, HOLT R, HOWE JL, HUGHES G, HUS V, IGLIOZZI R, KIM C, KLAUCK SM, KOLEVZON A, KORVATSKA O, KUSTANOVICH V, LAJONCHERE CM, LAMB JA, LASKAWIEC M, LEBOYER M, LE COUTEUR A, LEVENTHAL BL, LIONEL AC, LIU XQ, LORD C, LOTSPEICH L, LUND SC, MAESTRINI E, MAHONEY W, MANTOULAN C, MARSHALL CR, MCCONACHIE H, MCDOUGLE CJ, MCGRATH J, MCMAHON WM, MERIKANGAS A, MIGITA O, MINSHEW NJ, MIRZA GK, MUNSON J, NELSON SF, NOAKES C, NOOR A, NYGREN G, OLIVEIRA G, PAPANIKOLAOU K, PARR JR, PARRINI B, PATON T, PICKLES A, PILORGE M, et al. 2010. Functional impact of global rare copy number variation in autism spectrum disorders. Nature, 466, 368–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRITCHARD JK, STEPHENS M & DONNELLY P 2000. Inference of population structure using multilocus genotype data. Genetics, 155, 945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZ R, ROBERTS AL, LYALL K, HART JE, JUST AC, LADEN F & WEISSKOPF MG 2015. Autism spectrum disorder and particulate matter air pollution before, during, and after pregnancy: a nested case-control analysis within the Nurses’ Health Study II Cohort. Environ Health Perspect, 123, 264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVAS-ARANCIBIA S, GUEVARA-GUZMAN R, LOPEZ-VIDAL Y, RODRIGUEZ-MARTINEZ E, ZANARDO-GOMES M, ANGOA-PEREZ M & RAISMAN-VOZARI R 2010. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol Sci, 113, 187–97. [DOI] [PubMed] [Google Scholar]
- ROBERTS AL, LYALL K, HART JE, LADEN F, JUST AC, BOBB JF, KOENEN KC, ASCHERIO A & WEISSKOPF MG 2013. Perinatal Air Pollutant Exposures and Autism Spectrum Disorder in the Children of Nurses’ Health Study II Participants. Environ Health Perspect, 121, 978–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG RE, LAW JK, YENOKYAN G, MCGREADY J, KAUFMANN WE & LAW PA 2009. Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med, 163, 907–14. [DOI] [PubMed] [Google Scholar]
- ROSSIGNOL DA & FRYE RE 2014. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol, 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDIN S, LICHTENSTEIN P, KUJA-HALKOLA R, LARSSON H, HULTMAN CM & REICHENBERG A 2014. The familial risk of autism. JAMA, 311, 1770–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEBAT J, LAKSHMI B, MALHOTRA D, TROGE J, LESE-MARTIN C, WALSH T, YAMROM B, YOON S, KRASNITZ A, KENDALL J, LEOTTA A, PAI D, ZHANG R, LEE YH, HICKS J, SPENCE SJ, LEE AT, PUURA K, LEHTIMAKI T, LEDBETTER D, GREGERSEN PK, BREGMAN J, SUTCLIFFE JS, JOBANPUTRA V, CHUNG W, WARBURTON D, KING MC, SKUSE D, GESCHWIND DH, GILLIAM TC, YE K & WIGLER M 2007. Strong association of de novo copy number mutations with autism. Science, 316, 445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALBOTT EO, ARENA VC, RAGER JR, CLOUGHERTY JE, MICHANOWICZ DR, SHARMA RK & STACY SL 2015a. Fine particulate matter and the risk of autism spectrum disorder. Environ Res, 140, 414–20. [DOI] [PubMed] [Google Scholar]
- TALBOTT EO, MARSHALL LP, RAGER JR, ARENA VC, SHARMA RK & STACY SL 2015b. Air toxics and the risk of autism spectrum disorder: the results of a population based case-control study in southwestern Pennsylvania. Environ Health, 14, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLK HE, HERTZ-PICCIOTTO I, DELWICHE L, LURMANN F & MCCONNELL R 2011. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect, 119, 873–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLK HE, KERIN T, LURMANN F, HERTZ-PICCIOTTO I, MCCONNELL R & CAMPBELL DB 2014. Autism spectrum disorder: interaction of air pollution with the MET receptor tyrosine kinase gene. Epidemiology, 25, 44–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLK HE, LURMANN F, PENFOLD B, HERTZ-PICCIOTTO I & MCCONNELL R 2013. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry, 70, 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EHRENSTEIN OS, ARALIS H, COCKBURN M & RITZ B 2014. In Utero Exposure to Toxic Air Pollutants and Risk of Childhood Autism. Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSKOPF MG, KIOUMOURTZOGLOU MA & ROBERTS AL 2015. Air Pollution and Autism Spectrum Disorders: Causal or Confounded? Curr Environ Health Rep, 2, 430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDHAM GC, ZHANG L, GUNIER R, CROEN LA & GRETHER JK 2006. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the san francisco bay area. Environ Health Perspect, 114, 1438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.