Abstract
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) belongs to the nuclear hormone receptor superfamily. Apart from being involved in lipid metabolism, like its other subtypes PPAR α and β, it is implicated to be crucial for successful placentation. While its role in extravillous trophoblast (EVT) differentiation has been studied, the involvement in villous trophoblast (VT) differentiation, fatty-acid metabolism, inflammatory responses, and oxidative pathways during pregnancy deserves more attention. PPAR-γ’s potential role in balancing structural development and functional responsibilities at the maternal-fetal interface suggest a more central role for the receptor. The central role of PPAR-γ in pathways related to placental pathologies suggests a potential role of PPAR-γ in placental function. The molecular regulation of PPAR-γ in this context has been widely disregarded. In this review, we discuss the less explored functions of PPAR-γ in the areas of immunological responses and management of oxidative stress in the placenta. We also shed light on the involvement of PPAR-γ in pathologic pregnancies and briefly discuss the current models in the field. The ability to modulate PPAR-γ’s activity using already available drugs makes it a tempting therapeutic target. Elucidation of the molecular pathways and specific targets regulated by PPAR-γ will provide more information on the role of PPAR-γ in placentation and related disorders in pregnancy. Furthermore it will close the critical gap in our knowledge about the differential regulation of PPAR-γ in the two trophoblast lineages. This will help to evaluate the usefulness and timing of PPAR-γ modulation in at risk pregnancies to improve placental and endothelial function.
Keywords: Differentiation, inflammation, placenta, PPAR-γ, pre-eclampsia, trophoblast
Introduction
Peroxisome proliferator-activated receptors (PPARs), a moniker owed to the early observation that stimulation of these proteins could induce the proliferation of peroxisomes in rodent hepatocytes, are transcription factors belonging to the nuclear hormone receptor superfamily [Issemann and Green 1990]. The spectrum of ligands which effectively target these receptors include endogenously expressed lipid-soluble molecules (e.g., prostacyclin, lysophosphatidic acid), and the thiazolidinedione family of pharmaceutical compounds (e.g., rosiglitazone, Pioglitazone) [Chen et al. 2009; Stapleton et al. 2010]. Like other members of this receptor superfamily, the interaction of PPARs with their corresponding ligands elicits their activation via a change in protein conformation, resulting in dimerization with retinoid X receptor (RXR) and recruitment of co-activators like histone deacetylateses (HDACs), p300/CBP, or members of the steroid receptor co-activator (SRC) family [Lehrke and Lazar 2005; McKenna and O’Malley 2002]. This is followed by either direct binding to a consensus sequence on the DNA or to enable binding to other transcription factors like NF-kappaβ to regulate gene expression [Bright et al. 2007; Yamasaki et al. 2002].
To date, three subtypes of the receptor have been identified - PPAR α, β, and γ. All three subtypes, possess the canonical domain structure common to other nuclear receptor family members. This is structured as the amino-terminal AF-1 trans activation domain, followed by a DNA-binding domain, and a dimerization and ligand-binding domain with a ligand-dependent trans activation function AF-2 located at the carboxy-terminal region [Zoete et al. 2007]. Each subtype is a product of a distinct gene and has an organ specific expression pattern and their functions have been widely studied in different systems. Briefly, PPAR-α is expressed primarily in brown adipose tissue, liver, kidney, heart, and skeletal muscle. It has been implicated in mitochondrialfatty-acid (FA) oxidation, which provides energy for peripheral tissues [Lefebvre et al. 2006]. The involvement in antioxidant pathways is suggested to contribute to the pathogenesis of age-related macular degeneration (AMD) [Del V Cano and Gehlbach 2007; Lefebvre et al. 2006]. PPAR-β is expressed predominantly in the gut, kidney, and heart; and is involved in lipid metabolism, cell survival, wound healing, embryonic implantation, and development of the central nervous system [Berger et al. 2005]. PPAR-γ is mainly expressed in adipose tissue and to a lesser extent in colon, the immune cells – macrophages, and is known to be involved with adipogenesis and macrophage differentiation [Rosen et al. 1999; Tontonoz et al. 1994; Tontonoz et al. 1998].
Interestingly, all three subtypes also show expression in the placenta. In the rodent placenta, they are expressed in the trophoblast cells of the junctional zone as well as the labyrinth zone [Wang et al. 2002]. Expression in lineages of human trophoblast cells has also been reported [Schaiff et al. 2000; Tarrade et al. 2001a; Wang et al. 2002].
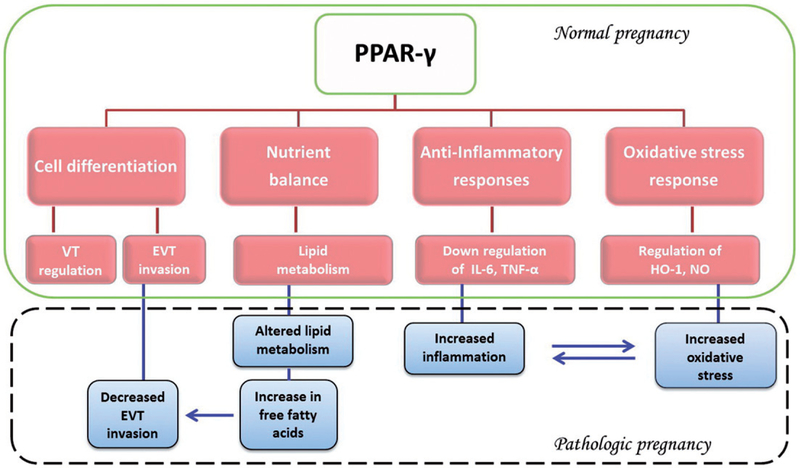
In aspects of placental function, PPAR-γ has emerged as the crucial subtype owing to the embryonic lethality of the knockout mice due to gross placental abnormalities. While, PPAR-γ null mice show increased maternal abortion (20% over wild type), surviving pups develop normally [Lee et al. 1995]. PPAR-β knockout mice also show an embryonic lethality due to defects in placental morphogenesis, but some pups do survive (lethality > 90%) [Barak et al. 2002; Nadra et al. 2006]. In comparison, PPAR-γ knockouts show 100% lethality due to defects in differentiation of labyrinth trophoblast and thinning of the myocardial lining of the ventricles. Interestingly, correcting for the mutation in these embryos (via aggregation with tetraploid embryos which contribute only to the extra-embryonic lineages) rescued the placental as well as the cardiac phenotype suggesting an important role for PPAR-γ in placental function [Barak et al. 1999]. Aspects of PPAR-γ function in placental development have therefore been extensively explored in the human placenta. In this review, we outline the current and potentially new roles of PPAR-γ in placental development and other functions at the maternal-fetal interface (Figure 1).
Figure 1.
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) has been shown to be involved in multiple key metabolic pathways in placentation and pregnancy. These include trophoblast differentiation, inflammatory and oxidative response, and nutrient sensing - specifically fatty acid metabolism. Placental disorders such as preeclampsia often show changes in these pathways which are partially regulated by PPAR-γ. Changes in activity and not necessarily expression may result in altered fatty acid metabolism which in turn may influence villous and extravillous trophoblast differentiation. Similarly, altered activity may cause changes in oxidative stress and inflammation due to regulation and release of inflammatory cytokines such as TNF-α, IL-6, and others which have shown to be associated with conditions like pre-term labor, miscarriage, and pre-eclampsia. The observation that all these factors are at least in part regulated by PPAR-γ supports its potential critical role in placental physiology and disease. VT: villous trophoblast; EVT: extravillous trophoblast; IL-6: inter-leukins-6; TNF-α: tumor necrosis factor alpha; HO-1: heme-oxygenase-1; NO: nitric oxide.
PPAR-γ in Trophoblast Differentiation
Trophoblast differentiation is a critical process towards establishing placental lineages that govern the placental development, maintenance, and function. We distinguish the human trophoblast cells into the decidua invading extravillous trophoblast (EVT) and the placenta residing villous trophoblast (VT) cells which are covered by the syncytium. EVTs invade the maternal decidua to establish pregnancy and secure blood-flow to the implantation site which provides nutrients and oxygen to the fetus. The villous trophoblast forms the main maternal-fetal exchange surface, which is critical for fetal development and has to adapt to environmental changes to secure growth throughout pregnancy. Trophoblast differentiation is a tightly regulated process and is implicated to be abnormally regulated in placental dysfunction disorders [Kliman et al. 1986; Lim et al. 1997; Pijnenborg et al. 1996].
While all three PPAR subtypes are expressed in human trophoblast cells, the expression of α and β subtypes decreases as the cells differentiate, PPAR-γ continues to be expressed strongly in these cells [Georges et al. 2005; Schaiff et al. 2000; Tarrade et al. 2001a; Waite et al. 2000; Wang et al. 2002]. In-vitro studies with first trimester EVTs showed that treatment with PPAR-γ antagonists increased invasion whereas agonists hampered it, implicating the involvement of PPAR-γ in regulating decidua invasion [Fournier et al. 2002; Tarrade et al. 2001b]. A similar study with isolated term villous trophoblast showed induction of differentiation upon treatment with agonists [Schaiff et al. 2000]. Involvement of PPAR-γ in regulating the functions of both EVTs and VT not only suggests its crucial role in trophoblast differentiation but these studies also highlight the differences in response of the trophoblast subtypes to PPAR-γ induction.
Interestingly, the studies on term villous trophoblast also observed differential behavior of these cells in response to synthetic and naturally occurring ligands of PPAR-γ. Treatment with synthetic ligand troglitazone induced differentiation, whereas the natural ligand PGJ2 hindered it, even inducing apoptosis in cells [Schaiff et al. 2000]. Thus, PPAR-γ seems to have different roles depending upon: 1) trophoblast subpopulations, 2) the gestational age and type, and and 3) the stimulating ligand [Handschuh et al. 2009]. However, one needs to bear in mind that isolated trophoblasts lack their natural environment and tend to differentiate directly in culture conditions which is a critical limitation in some of these studies [Pavan et al. 2004; Schaiff et al. 2005]. While we have substantial evidence for involvement of PPAR-γ in trophoblast differentiation, we still lack the understanding of the molecular regulation. Very few studies focusing on the downstream targets of PPAR-γ exist [Shalom-Barak et al. 2004; Yoon et al. 2000]. Glial cell missing 1 (Gcm-1) has emerged as an interesting candidate in this respect. It regulates differentiation of chorion into labyrinth trophoblast populations and controls syncytiotrophoblast differentiation. Mice lacking Gcm-1 die at E10.5 due to the absence of the placental labyrinth [Anson-Cartwright et al. 2000]. A deficiency of PPAR-γ in mouse trophoblast stem cells was shown to affect labyrinth cell lineages with a concurrent decrease in Gcm-1 [Parast et al. 2009]. Gcm-1 has been shown to also be present in human trophoblast tissue and altered levels of Gcm-1 have also been associated with preeclampsia (PE) placentas [Baczyk et al. 2009; Baczyk et al. 2004; Chen et al. 2004]. Recently, Levystka et al. [2013] showed that Gcm-1 levels could be increased or decreased by PPAR-γ agonists or antagonists in BeWo choriocarcinoma cells suggesting that PPAR-γ via Gcm-1 [Levytska et al. 2013] may play a role in human trophoblast differentiation.
PPAR-γ and Fatty Acid Metabolism in the Placenta
Placental fatty acid (FA) transfer from the mother to the fetus is crucial for adequate development [Munro et al. 1982]. PPAR-γ has been classically known for its role in promoting lipid storage. The observation that the PPAR-γ knockout placentas showed less accumulation of lipid droplets suggested that it may have some similar function in the placenta. Schaiff et al. in 2007 showed that PPAR-γ could alter the FA uptake in the placenta by increasing the expression of FA transport proteins (FTPs) in mice [Schaiff et al. 2007].
A number of FA transporters (FABP, FATP, and CD-36) have also been identified at the microvillous and basal membranes of human placenta. Similar to observations in mice, increase in PPAR-γ activity has been shown to increase fatty acid uptake and accumulation in primary human trophoblast cells by regulating the expression of fatty acid binding proteins (FABP). In turn, oxidized low density lipoproteins (LDLs) were shown to be capable of activating PPAR-γ in primary cytotrophoblast cells and even inhibit trophoblast invasion [Pavan et al. 2004; Schild et al. 2002]. Thus, PPAR-γ appears to be regulating and itself being regulated by lipid metabolites. PPAR-γ might thus act as a nutritional sensor and coordinate FA uptake and trophoblast differentiation in the placenta to ensure growth and function. This might partially help in explaining the pathophysiology of placental insufficiency disorders like PE which are associated with increased lipid peroxidation and defective trophoblast invasion [Hubel 1999; Hubel et al. 1989].
PPAR-γ in Placental Oxidative Stress
Pregnancy is a state of physiological stress including oxidative stress. The initial hypoxia followed by the reoxygenation (ischemic reperfusion) are the major contributors of oxidative stress during early pregnancy [Jauniaux et al. 2000]. In later stages, increased placental mitochondrial activity and production of reactive oxygen species (ROS) further contribute to the oxidative stress [Myatt and Cui 2004]. In moderation, this normally does not cause a problem but excess oxidative stress has been observed in pregnancy complications like intra uterine growth restriction (IUGR), diabetes, and PE [Jauniaux, et al. 2000; Myatt and Cui 2004; Sharma et al. 2006].
Nitric oxide (NO) is a vasodilator at normal levels, but at elevated levels it reacts with ROS to cause lipid peroxidation and nitrosylation of tyrosine residues affecting many signaling pathways. High levels of NO are observed in cases of gestational diabetes mellitus (GDM) with higher levels of oxidative stress [Jawerbaum et al. 2004]. Interestingly, blocking PPAR-γ greatly increases endogenous NO production suggesting that PPAR-γ might be involved in its regulation [Myatt and Cui 2004].
Heme oxygenase (HO)1 is known for its anti-oxidant properties and cytoprotective effects. In the placenta, Heme oxygenases (HO) are found in vascular endothelium and villous and extravillous trophoblast [Lyall 2000]. HO metabolites carbon monoxide (a potent vasodilator) and bilirubin decrease expression of endothelin 1 and ROS along with anti-angiogenic proteins such as soluble fms-like tyrosine kinase 1 (sFLT-1) [George et al. 2011a; George et al. 2011b]. Recently, PPAR-γ was shown to be involved in regulating HO1 expression. Induction of PPAR-γ in vascular endothelial cells was shown to induce expression of HO1 [Kronke et al. 2007]. This emphasizes the potential role of PPAR-γ in managing oxidative stress and hypertension during pregnancy.
Immune Mediated Effects of PPAR-γ
Pregnancy is a state of dynamic inflammatory phases, wherein the immune status of the mother varies from being pro-inflammatory in the start of pregnancy, to anti-inflammatory in the middle phase to again being pro-inflammatory towards the end [Mor et al. 2011]. There is also a growing body of evidence suggesting that parturition at term is an inflammatory process with pro-inflammatory cytokines like IL-8 and TNF-α playing a role [Christiaens et al. 2008; Elliott 2001; Norman et al. 2007]. Thus, a balance between the pro and anti-inflammatory modulators at the feto-maternal interface is crucial for maintenance of pregnancy.
Indeed, a premature (or untimely) shift towards the pro-inflammatory conditions has been associated with cases of spontaneous abortion and preterm delivery [Challis et al. 2009]. Increased levels of pro-inflammatory cytokines like TNF alpha, IL6, and IL8 have also been reported in PE cases [Rakheja et al. 2002; Sattar et al. 1996].
Interestingly, PPAR-γ has been known for its anti-inflammatory effects [Jiang et al. 1997]. It has also been shown to down regulate expression of pro-inflammatory cytokines like IL-6, IL-8, and TNF-α in human gestational tissue [Lappas et al. 2002]. A drop in the levels of PPAR-γ during labor has also been detected [Dunn-Albanese et al. 2004]. These observations suggest that a drop in PPAR-γ at term might contribute to parturition by regulating the inflammatory cytokines. A premature decline in PPAR-γ levels would thus ensue an inflammatory response which may result in loss of pregnancy.
PPAR-γ Expression Levels and Activity
Studies assessing the protein levels of PPAR-γ associate aberrant levels of PPAR-γ with placental disorders. Decreased levels of PPAR-γ were reported in cases of GDM. Increased levels were observed in IUGR associated PE [Holdsworth-Carson et al. 2010; Rodie et al. 2005].
In comparison, placentas from women with only PE did not show any changes in the levels of PPAR-γ protein [Holdsworth-Carson et al. 2010; Rodie et al. 2005]. These women showed significantly lower levels of the activators (probably a FA derivative) of the receptor in their serum when compared to gestational age matched controls [Waite et al. 2005]. During normal gestation, PPAR-γ activators increase as the pregnancy progresses to term [Waite et al. 2000]. Women with PE showed a drop in these activating factors levels 10–15 weeks before presentation of symptoms [Waite et al. 2005]. Lowered levels of the activators may lead to decreased activation of PPAR-γ which in turn may contribute to the pathology [Waite et al. 2005]. While these studies emphasize the importance of PPAR-γ in placental pathologies, they also suggest that balance between the absolute levels of PPAR-γ protein and the level of activity is crucial.
Levystka et al. [2013] recently showed that PPAR-γ underlies an auto-regulatory mechanism. Using the BeWo choriocarcinoma cell line they showed that inhibition of PPAR-γ activity using an antagonist T0070907 led to an up-regulation of expression whereas activation by an agonist rosiglitazone had the opposite effect. Thus, PPAR-γ increased levels of protein may not always correlate with increased activity. These factors need to be considered when looking at pathologic cases from a PPAR-γ perspective. Studies on pathologic placentas, similar to those in cell lines will add on to our knowledge about molecular regulation of PPAR-γ in such pathologies.
PPAR-γ as a Therapeutic Option in Placental Disorder
Involvement of PPAR-γ with key aspects of pregnancy and the fact that it can be specifically modulated by a vast array of drugs already available makes it an attractive therapeutic option [McCarthy et al. 2011b]. In 2011 McCarthy et al. showed that pregnant rats treated with synthetic PPAR-γ antagonists developed pre-eclamptic phenotypes comparable to what has been described in human suggesting a relevant role in physiology and disease [McCarthy et al. 2011a]. More interestingly the same authors showed in a separate study that activation of the receptor by administration of its synthetic agonist rosiglitazone, to the (reduced uterine perfusion pressure) RUPP rats showed considerable improvement in hyper-tension with no adverse effects on the litters or placental vasculature [McCarthy et al. 2011b]. The RUPP model of rats resembles, in part, the human PE condition, and administration of rosiglitazone significantly reduced hypertension and improved vascular function in an HO1 dependent manner [McCarthy et al. 2011b]. The authors did not observe any adverse effects on placental morphology which is in contrast to some other studies [Nadra et al. 2010; Schaiff et al. 2007]. The group argued that the low dosage used in the study and the time of administration of the drug contributed to the contrasting results. The study demonstrates the ease and potential effectiveness of PPAR-γ modulation using drugs with so far no observed side-effects, albeit in an animal model.
These parameters of low dosage and exposure times to the drugs seem to hold true in human cases too. A few human case reports assessed the effects of the use of synthetic PPAR-γ agonists (rosiglitazone and pioglitazone) during pregnancy, although the drugs were shown to cross the maternal-fetal barrier no harm to the fetus or induction of PE was noted. [Haddad et al. 2008; Kalyoncu et al. 2004; Yaris et al. 2004]. The dosage in these studies was not as high (~4 mg/day) as recommended for diabetic patients (~8 mg/day) and the time of treatment/exposure to the drug was between 7 – 17 weeks of pregnancy. Additionally, while these drugs were previously thought to increase the risk of cardiovascular diseases in humans, recent reports say otherwise and the drugs have been recently approved by the FDA for widespread use [Bach et al. 2013]. While further studies and risk assessment would be necessary to ensure safety, we need to recognize PPAR-γ as a strong candidate in the development of therapies for managing placental pathologies. The potential reduction in EVT invasion by PPAR-γ agonists could be a problem in efforts to improve placental function in disease [Tarrade et al. 2001b]. Even so, the positive effects on villous trophoblast function could still be assessed in the second and third trimester after most of the spiral artery remodeling and EVT invasion are finished [Milovanov et al. 2013; Robson et al. 2002].
Future Directions
Current studies on the functions of PPAR-γ in the placenta focus mainly on EVTs while studies on villous trophoblast are rare [Pavan et al. 2004; Schaiff et al. 2005]. Villous trophoblast differentiation has been shown to be abnormal in extreme pre-term deliveries. Increased levels of apoptosis has been reported for placental dysfunction disorders such as severe PE and IUGR [Fitzgerald et al. 2011; Ishihara et al. 2001; Longtine et al. 2012]. Additionally the villous trophoblast is the source of many proteins that have been shown to impair systemic endothelial function contributing to hypertension and renal dysfunction in disease [Maynard et al. 2003]. Considering that PPAR-γ might play different roles in EVT and VT, we need better models for addressing the role of PPAR-γ in the overall physiology of the placenta in general and cell types specifically. While the isolated cell culture based models might be appropriate in delineating the molecular players involved in the PPAR-γ pathway, they do not maintain cells in their natural physiological state. In contrast, explant based models preserve the natural micro-structure of tissue, maintain cell proliferation, and prevent terminal differentiation [Miller et al. 2005; Orendi et al. 2011].
In the future we suggest that human studies individually focus on both the extra villous and villous trophoblast as well as in context of each other. Studies concentrating on the role of PPAR-γ in placenta at early stages of gestation (first and second trimester) will add greatly to our knowledge about how both cell lineages develop and contribute to placental disorders. Delineating the PPAR-γ pathway and information about its downstream molecular targets may help in developing better therapeutic intervention strategies for adverse pregnancies before they completely manifest. The currently available PPAR-γ modulating drugs may provide a good starting point for altering placental function in certain pregnancy complications.
Acknowledgment
The authors acknowledge the generous support by March of Dimes.
Abbreviations:
- PPARs
peroxisome proliferator-activated receptors
- FA
fatty acids
- EVT
extravillous trophoblast
- VT
villous trophoblast
- Gcm-1
glial cell missing 1
- PE
pre-eclampsia
- ROS
reactive oxygen species
- IUGR
intra uterine growth restriction
- GDM
gestational diabetes mellitus
- HO
heme oxygenase
- NO
nitric oxide
- RUPP
reduced uterine perfusion pressure
Footnotes
Declaration of interest
March of Dimes Basil O’Connor Scholar Award to SD. The authors report no conflicts of interest.
References
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini RA, and Cross JC (2000) The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet 25:311–14. [DOI] [PubMed] [Google Scholar]
- Bach RG, Brooks MM, Lombardero M, Genuth S, Donner TW, Garber A, et al. (2013) Rosiglitazone and outcomes for patients with diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Circulation 128:785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczyk D, Drewlo S, Proctor L, Dunk C, Lye S, and Kingdom J (2009) Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differ 16:719–27. [DOI] [PubMed] [Google Scholar]
- Baczyk D, Satkunaratnam A, Nait-Oumesmar B, Huppertz B, Cross JC, and Kingdom JC (2004) Complex patterns of GCM1 mRNA and protein in villous and extravillous trophoblast cells of the human placenta. Placenta 25:553–9. [DOI] [PubMed] [Google Scholar]
- Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, et al. (2002) Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA 99:303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. (1999) PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell 4:585–95. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, and Meinke PT (2005) PPARs: Therapeutic targets for metabolic disease. Trends Pharmacol Sci 26: 244–51. [DOI] [PubMed] [Google Scholar]
- Bright JJ, Kanakasabai S, Chearwae W, and Chakraborty S (2008) PPAR Regulation of Inflammatory Signaling in CNS Diseases. PPAR Res 2008:Article ID 658520, 1–12. [DOI] [PMC free article] [PubMed]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, and Petraglia F (2009) Inflammation and pregnancy. Reprod Sci 16:206–15. [DOI] [PubMed] [Google Scholar]
- Chen CP, Chen CY, Yang YC, Su TH, and Chen H (2004) Decreased placental GCM1 (glial cells missing) gene expression in pre-eclampsia. Placenta 25:413–21. [DOI] [PubMed] [Google Scholar]
- Chen HH, Chen TW, and Lin H (2009) Prostacyclin-induced peroxisome proliferator-activated receptor-alpha translocation attenuates NF-kappaB and TNF-alpha activation after renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 297:F1109–18. [DOI] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, and Olson DM (2008) Inflammatory processes in preterm and term parturition. J Reprod Immunol 79:50–7. [DOI] [PubMed] [Google Scholar]
- Del V Cano M, and Gehlbach PL (2007) PPAR-alpha Ligands as Potential Therapeutic Agents for Wet Age-Related Macular Degeneration. PPAR Res 2008:Article ID 821592, 1–5. [DOI] [PMC free article] [PubMed]
- Dunn-Albanese LR, Ackerman WE, Xie Y, Iams JD, and Kniss DA (2004) Reciprocal expression of peroxisome proliferator-activated receptor-gamma and cyclooxygenase-2 in human term parturition. Am J Obstet Gynecol 190:809–16. [DOI] [PubMed] [Google Scholar]
- Elliott CL, Loudon JAZ, Brown N, Slater DM, Bennett PR, and Sullivan MHF (2001) IL-1beta and IL-8 in human fetal membranes: Changes with gestational age, labor, and culture conditions. Am J Reprod Immunol 46:260–7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald B, Levytska K, Kingdom J, Walker M, Baczyk D, and Keating S (2011) Villous trophoblast abnormalities in extremely preterm deliveries with elevated second trimester maternal serum hCG or inhibin-A. Placenta 32:339–45. [DOI] [PubMed] [Google Scholar]
- Fournier T, Pavan L, Tarrade A, Schoonjans K, Auwerx J, Rochette-Egly C, et al. (2002) The role of PPAR-γamma/RXR-alpha heterodimers in the regulation of human trophoblast invasion. Ann N Y Acad Sci 973:26–30. [DOI] [PubMed] [Google Scholar]
- George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, and Granger JP (2011a) Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension 57: 941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EM, Colson D, Dixon J, Palei AC, and Granger JP (2012) Heme Oxygenase-1 Attenuates Hypoxia-Induced sFlt-1 and Oxidative Stress in Placental Villi through Its Metabolic Products CO and Bilirubin. Int J Hypertens 2012:Article ID 486053, 1–6. [DOI] [PMC free article] [PubMed]
- Georges D, Lucie S, Andre´ M, Eric R, and Julie L (2005) Expression of cFABP and PPAR in trophoblast cells: Effect of PPAR ligands on linoleic acid uptake and differentiation. Biochimica et Biophysica Acta (BBA) 1687:181–94. [DOI] [PubMed] [Google Scholar]
- Haddad GF, Jodicke C, Thomas MA, Williams DB, and Aubuchon M (2008) Case series of rosiglitazone used during the first trimester of pregnancy. Reprod Toxicol 26:183–4. [DOI] [PubMed] [Google Scholar]
- Handschuh K, Guibourdenche J, Cocquebert M, Tsatsaris V, Vidaud M, Evain-Brion D et al. (2009) Expression and regulation by PPARgamma of hCG alpha-and beta-subunits: Comparison between villous and invasive extravillous trophoblastic cells. Placenta 30:1016–22. [DOI] [PubMed] [Google Scholar]
- Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M et al. (2010) Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: Gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta 31:222–9. [DOI] [PubMed] [Google Scholar]
- Hubel CA (1999) Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 222:222–35. [DOI] [PubMed] [Google Scholar]
- Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM and McLaughlin MK (1989) Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol 161: 1025–34. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, and Maruo T (2001) Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol 186:158–66. [DOI] [PubMed] [Google Scholar]
- Issemann I, and Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347:645–50. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, and Burton GJ (2000) Onset of maternal arterial blood flow and placental oxidative stress. A possible factor in human early pregnancy failure. Am J Pathol 157:2111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawerbaum A, Capobianco E, Pustovrh C, White V, Baier M, Salzberg S, et al. (2004) Influence of peroxisome proliferator-activated receptor gamma activation by its endogenous ligand 15–deoxy Delta12,14 prostaglandin J2 on nitric oxide production in term placental tissues from diabetic women. Mol Hum Reprod 10: 671–6. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, and Seed B (1997) PPAR-γamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391: 82–6. [DOI] [PubMed] [Google Scholar]
- Kalyoncu NI, Yaris F, Ulku C, Kadioglu M, Kesim M, Unsal M, et al. (2004) A case of rosiglitazone exposure in the second trimester of pregnancy. Reprod Toxicol 19:563–4. [DOI] [PubMed] [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, and Strauss JF 3rd., (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118: 1567–82. [DOI] [PubMed] [Google Scholar]
- Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, et al. (2007) Expression of heme oxygenase–1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol 27:1276–82. [DOI] [PubMed] [Google Scholar]
- Lappas M, Permezel M, Georgiou HM, and Rice GE (2002) Regulation of proinflammatory cytokines in human gestational tissues by peroxisome proliferator-activated receptor-gamma: Effect of 15-deoxy-Delta(12,14)-PGJ(2) and troglitazone. J Clin Endocrinol Metab 87:4667–72. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, et al. (1995) Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15:3012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Chinetti G, Fruchart J-CC, and Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116:571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, and Lazar MA (2005) The many faces of PPARgamma. Cell 123:993–9. [DOI] [PubMed] [Google Scholar]
- Levytska K, Drewlo S, Baczyk D, and Kingdom J (2013) PPAR-γ Regulates Trophoblast Differentiation in the BeWo Cell Model. PPAR Res 2014:Article ID 637251, 1–13. [DOI] [PMC free article] [PubMed]
- Lim KH, Zhou Y, Janatpour M, McMaster M, Bass K, Chun SH, et al. (1997) Human cytotrophoblast differentiation/invasion is abnormal in pre–eclampsia. Am J Pathol 151:1809–18. [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Chen B, Odibo AO, Zhong Y, and Nelson DM (2012) Villous trophoblast apoptosis is elevated and restricted to cytotrophoblasts in pregnancies complicated by preeclampsia, IUGR, or preeclampsia with IUGR. Placenta 33:352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall F, Barber A, Myatt L, Bulmer JN, and Robson SC (2000) Hemeoxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. FASEB J 14:208–19. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Min J-YY, Merchan J, Lim K-HH, Li J, Mondal S, et al. (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, et al. (2011a) Evidence implicating peroxisome proliferator-activated receptor-γ in the pathogenesis of preeclampsia. Hypertension 58:882–7. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, and Kenny LC (2011b) Peroxisome proliferator-activated receptor-gamma as a potential therapeutic target in the treatment of preeclampsia. Hypertension 58:280–6. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, and O’Malley BW (2002) Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108: 465–74. [DOI] [PubMed] [Google Scholar]
- Miller RK, Genbacev O, Turner MA, Aplin JD, Caniggia I, and Huppertz B (2005) Human placental explants in culture: Approaches and assessments. Placenta 26:439–48. [DOI] [PubMed] [Google Scholar]
- Milovanov AP, Rasstrigina IM, and Fokina TV (2013) Morphometric evaluation of the frequency distribution and diameter of extravillous trophoblast cells in apparently uncomplicated pregnancy. Arkh Patol 75:18–21. [PubMed] [Google Scholar]
- Mor G, Cardenas I, Abrahams V, and Guller S (2011) Inflammation and pregnancy: The role of the immune system at the implantation site. Ann N Y Acad Sci 1221:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro HN, Pilistine SJ, and Fant ME (1982) The placenta in nutrition. Annu Rev Nutri 3:97–124. [DOI] [PubMed] [Google Scholar]
- Myatt L, and Cui X (2004) Oxidative stress in the placenta. Histochem Cell Biol 122:369–82. [DOI] [PubMed] [Google Scholar]
- Nadra K, Anghel SI, Joye E, Tan NS, Basu-Modak S, Trono D, et al. (2006) Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol Cell Biol 26:3266–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, et al. (2010) PPARgamma in placental angiogenesis. Endocrinology 151:4969–81. [DOI] [PubMed] [Google Scholar]
- Norman JE, Bollapragada S, Yuan M, and Nelson SM (2007) Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 7:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, et al. (2011) Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 32: S49–54. [DOI] [PubMed] [Google Scholar]
- Parast MM, Yu H, Ciric A, Salata MW, Davis V, and Milstone DS (2009) PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One 4:e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan L, Tsatsaris V, Hermouet A, Therond P, Evain-Brion D, and Fournier T (2004) Oxidized low-density lipoproteins inhibit trophoblastic cell invasion. J Clin Endocrinol Metab 89:1969–72. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Luyten C, Vercruysse L, and Van Assche FA (1996) Attachment and differentiation in vitro of trophoblast from normal and preeclamptic human placentas. Am J Obstet Gynecol 175:30–6. [DOI] [PubMed] [Google Scholar]
- Rakheja D, Bennett MJ, Foster BM, and Domiati-Saad R and Rogers BB (2002) Evidence for fatty acid oxidation in human placenta, and the relationship of fatty acid oxidation enzyme activities with gestational age. Placenta 23:447–50. [DOI] [PubMed] [Google Scholar]
- Robson SC, Simpson H, Ball E, Lyall F, and Bulmer JN (2002) Punch biopsy of the human placental bed. Am J Obstet Gynecol 187: 1349–55. [DOI] [PubMed] [Google Scholar]
- Rodie VA, Young A, Jordan F, Sattar N, Greer IA, and Freeman DJ (2005) Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig 12:320–9. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–17. [DOI] [PubMed] [Google Scholar]
- Sattar N, Gaw A, Packard CJ, and Greer IA (1996) Potential pathogenic roles of aberrant lipoprotein and fatty acid metabolism in pre-eclampsia. Br J Obstet Gynaecol 103:614–20. [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, and Sadovsky Y (2005) Peroxisome proliferator-activated receptor-gamma and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab 90: 4267–75. [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM, and Sadovsky Y (2000) Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocrinol Metab 85:3874–81. [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Knapp FF Jr.,, Barak Y, Biron-Shental T, Nelson DM, and Sadovsky Y (2007) Ligand-activated peroxisome proliferator activated receptor gamma alters placental morphology and placental fatty acid uptake in mice. Endocrinology 148:3625–34. [DOI] [PubMed] [Google Scholar]
- Schild RL, Schaiff WT, Carlson MG, Cronbach EJ, Nelson DM, and Sadovsky Y (2002) The activity of PPAR gamma in primary human trophoblasts is enhanced by oxidized lipids. J Clin Endocrinol Metab 87:1105–10. [DOI] [PubMed] [Google Scholar]
- Shalom-Barak T, Nicholas JM, Wang Y, Zhang X, Ong ES, Young TH, et al. (2004) Peroxisome proliferator-activated receptor gamma controls Muc1 transcription in trophoblasts. Mol Cell Bio 24: 10661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma JB, Sharma A, Bahadur A, Vimala N, Satyam A, and Mittal S (2006) Oxidative stress markers and antioxidant levels in normal pregnancy and pre-eclampsia. Int J Gynaecol Obstet 94:23–7. [DOI] [PubMed] [Google Scholar]
- Stapleton CM, Mashek DG, Wang S, Nagle CA, Cline GW, Thuillier P, et al. (2011) Lysophosphatidic acid activates peroxisome proliferator activated receptor-gamma in CHO cells that over – express glycerol 3-phosphate acyltransferase-1. PLoS One 6(4):e18932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrade A, Schoonjans K and Guibourdenche J (2001a) PPARγ/RXRα heterodimers are involved in human CGb synthesis and human trophoblast differentiation [DOI] [PubMed]
- Tarrade A, Schoonjans K, Pavan L, Auwerx J, Rochette-Egly C, Evain-Brion D, et al. (2001b) PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Meta 86: 5017–24. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, and Spiegelman BM (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79:1147–56. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, and Evans RM (1998) PPAR promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93:241–52. [DOI] [PubMed] [Google Scholar]
- Waite LL, Louie RE, and Taylor RN (2005) Circulating activators of peroxisome proliferator-activated receptors are reduced in pre-eclamptic pregnancy. J Clin Endocrinol Metab 90:620–6. [DOI] [PubMed] [Google Scholar]
- Waite LL, Person EC, Zhou Y, Lim KH, Scanlan TS, and Taylor RN (2000) Placental peroxisome proliferator-activated receptor-gamma is up-regulated by pregnancy serum. J Clin Endocrinol Metab 85:3808–14. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fujii H, and Knipp GT (2002) Expression of PPAR and RXR isoforms in the developing rat and human term placentas. Placenta 23:661–71. [DOI] [PubMed] [Google Scholar]
- Yamasaki S, Nakashima T, Kawakami A, Miyashita T, Ida H, Migita K, et al. (2002) Functional changes in rheumatoid fibroblast-like synovial cells through activation of peroxisome proliferator-activated receptor gamma-mediated signalling pathway. Clin Exp Immunol 129:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaris F, Yaris E, Kadioglu M, Ulku C, Kesim M, and Kalyoncu NI (2004) Normal pregnancy outcome following inadvertent exposure to rosiglitazone, gliclazide, and atorvastatin in a diabetic and hypertensive woman. Reprod Toxicol 18:619–21. [DOI] [PubMed] [Google Scholar]
- Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, et al. (2000) Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 20: 5343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoete V, Grosdidier A, and Michielin O (2007) Peroxisome proliferator-activated receptor structures: Ligand specificity, molecular switch and interactions with regulators. Biochimica et biophysica acta 1771:915–25. [DOI] [PubMed] [Google Scholar]