Abstract
Objectives
There are limited studies describing the association between ankylosing spondylitis (AS) and osteoporosis. We conducted a nationwide retrospective cohort study to investigate this epidemiologic evidence.
Methods
Data were obtained from the Taiwan National Health Insurance Research Database (NHIRD). Of 10,290 participants, 2,058 patients with AS and 8,232 patients without AS were enrolled from the NHIRD between 2000 to 2013. Cumulative incidences of osteoporosis were compared between 2 groups. Cox regression model was used to estimate the hazard ratio (HR) of developing osteoporosis after controlling for demographic and other co-morbidities, and subgroup analyses were conducted to examine the risk factors for osteoporosis in AS patients.
Results
The incidence rate ratio (IRR) of osteoporosis in AS patients was 2.17 times higher than that non-AS group (95% confidence interval [CI], 1.83–2.57). The adjusted HRs of osteoporosis for AS patients after controlling for demographic characteristics and comorbid medical disorders was 1.99 (95% CI 1.68–2.36). Among AS group, after adjustment for major comorbidities, old age (≥65 years, HR 4.32, 95% CI 3.01–6.18), female sex (HR 2.48, 95% CI 1.87–3.28), dyslipidemia (HR 1.44, 95% CI 1.01–2.06) were risk factors associated with osteoporosis.
Conclusions
This cohort study demonstrated that patients with AS had a higher risk of developing osteoporosis, especially in those aged over 65, female sex and with dyslipidemia in this patient group.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease involving the sacroiliac joints and spine; in some cases, the peripheral joints, entheses, and/or extraarticular structures may also be involved [1]. AS has also been shown to be associated with other extra-articular inflammatory diseases including anterior uveitis, psoriasis, and chronic inflammatory bowel disease [2]. A previous systemic review demonstrated a varied prevalence of AS worldwide; 0.32% in North America, 0.24% in Europe, 0.17% in Asia, 0.10% in Latin America, and 0.07% in Africa [3]. The prevalence of AS in Taiwan is relatively low, between 0.19% and 0.4% [4]. Moreover, in general, AS is more common in males than in females [5].
Osteoporosis is considered to be a musculoskeletal disease identified by a decreased bone mineral density (BMD); the disease has also been shown to correlate with subsequent fragility fractures [6]. Evidence has shown that osteoporosis is relatively common in AS patients; the major pathophysiological mechanisms appear to be systemic inflammation and low BMD resulting from decreased daily physical activities caused by pain, stiffness, and/or ankyloses [7]. In addition, numerous studies have demonstrated that, in AS patients, a low BMD and bone loss are observed within the first 10 years of disease [8–10]. To summarize, studies have indicated that patients with AS are at increased risk of osteoporosis and subsequent osteoporotic fractures due to bone fragility [11].
Several studies have demonstrated that the prevalence of osteoporosis among AS patients varies widely, from 13% to 32% [8, 9, 12]. This wide variance is most likely due to the heterogeneity of the populations [8] or different BMD measurements [13]. Other studies have highlighted the possibility of underdiagnosis of osteoporosis among the AS population [14, 15]. In addition, few studies have explored the incidence of osteoporosis among AS patients, and those that have, are relatively small-scale (504 cases [16] and 17 cases [17]).
Therefore, our study aimed to investigate the association between AS and subsequent osteoporosis; we also sought to explore the potential risk factors and the incidence of new-onset osteoporosis among patients suffering from AS in Taiwan. From these aims, this study sought to strengthen previous small-scale studies and provide additional, clinically relevant, data for this issue. More importantly, we chose to investigate the possibility of underdiagnosis of osteoporosis among AS patients using the nationwide insurance database, which may be more reflective of real-world conditions; we also attempted to provide hypotheses to explain this condition based on both our data and the results of recent epidemiological studies.
Materials and methods
Data sources
The National Health Insurance (NHI) was started as a mandate by the Taiwanese government, with the coverage rate approaching 100% since March 1, 1995. The NHIRD is managed and released for research purposes publicly by the National Health Research Institutes (NHRI), with confidentiality being maintained according to the directives of the Bureau of the National Health Insurance (BNHI). This nationwide study from Taiwan’s NHIRD included all patients with a diagnosis of AS. This dataset included the diagnostic codes according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Database 2000 (LHID 2000) is a sub-dataset of the NHIRD with methodically and randomly screened-out the data in 2000 from the NHIRD; this includes all claim data of one million beneficiaries. In line with the Taiwanese NHRI reports, the distributions of age and sex, or the average insured payroll-related premiums, between the sample groups and all enrollees showed no significant differences [18, 19].
Availability of data and materials section
The NHIRD is addressed in publicity by the NHRI and NHIRD is to be used only for research purposes. All applicants must obey the Computer-Processed Personal Data Protection Law [20] and the corresponding rules of the Bureaus of the NHI and NHRI. Moreover, applicants and their supervisors were asked for signed agreements upon application submission and all applications were reviewed for the approval of the data delivered. The application for the dataset may be mailed to the NHRI at nhird@nhri.org.tw or call at +886-037-246166 ext. 33603 for immediate assistance. Office hours: Monday–Friday, 8:00–17:30 (UTC+8).
The NHIRD, which was open to researchers in Taiwan, was available from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW) (http://www.mohw.gov.tw/cht/DOS/). The data underlying this study was obtained from the NHIRD. Applicants interested in obtaining the data are able to propose a formal application to the Ministry of Health and Welfare of Taiwan. In the last sentence of the paragraph on the webpage, which states "Kindly visit MOHW and NHIA on-site services for NHI Data", the database was delivered to a higher-level government administration, called the "HWDC" for more efficient health-related data linkage, wider application, and better security management.
At present, interested researchers could still obtain the NHI data in Taiwan through a formal application to the Health and Welfare Data Science Center (HWDC), Department of Statistics, Ministry of Health and Welfare (MOHW). HWDC, MOHW website (currently only Chinese): http://dep.mohw.gov.tw/DOS/np-2497-113.html [21].
Ethics statement
The NHI dataset consists of de-identified secondary data for research purposes, as such, the Institutional Review Board of Kaohsiung Veterans General Hospital issued a formal written waiver to obviate the need for consent. Therefore, in the present study, written consent was not obtained from the study participants. This study was authorized by the Institutional Review Board (IRB) of the Kaohsiung Veterans General Hospital as No. VGHKS14-CT7-07.
Study population
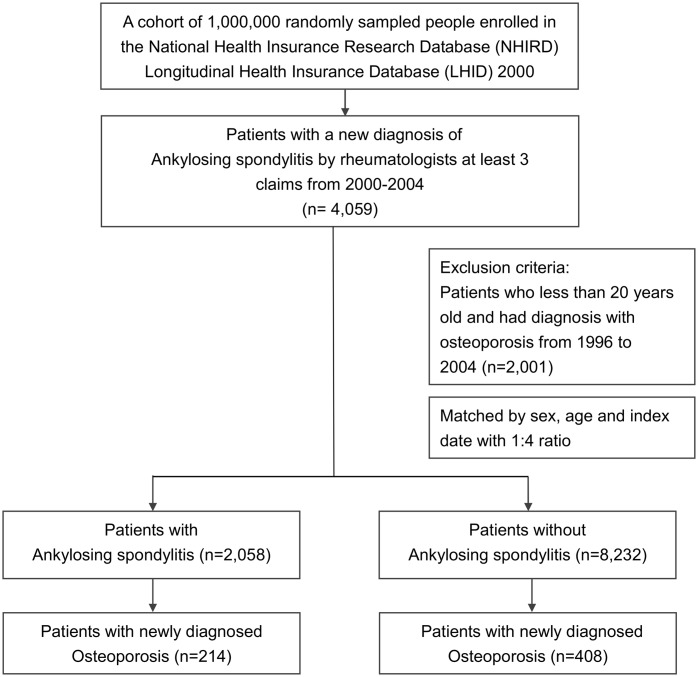
All patients aged 20 years and more, with a new diagnosis of AS (ICD-9-CM code 720.0) between January 1, 2000, and December 31, 2004, were enrolled. Only those who received a diagnosis of AS at least 3 times by a rheumatologist were selected. Since 1984, in Taiwan, the modified version of the New York criteria has been routinely used, in clinical practice and within the medical environment, as criteria for diagnosing patients with suspected AS [22]. We excluded patients who had been diagnosed with AS and osteoporosis (ICD-9-CM code 733.0 or 733.1) prior to the date of enrollment. Finally, we selected 8,232 participants with no history of AS, using the same exclusion criteria and within the same period, and ascertained that they were in accordance with the study cohort at a ratio of 1:4 in terms of age and gender (Fig 1).
Fig 1. A flowchart demonstrating the enrollment of the study cohort.
A total of 2,058 patient with history of Ankylosing spondylitis (AS) and 8,232 patients without history of AS were identified from Taiwan NHIRD. Among patients with and without the diagnosis of AS, 214 and 408 patients developed osteoporosis, respectively.
All the ambulatory medical care and inpatient records for each subject in both groups were tracked from their index visit until December 31, 2013. The date of the first principal diagnosis of osteoporosis during the follow-up period was defined as the primary endpoint. Both cohorts were followed-up until osteoporosis was diagnosed, the death of the patient, withdrawal from the NHI programme, or until the end of 2013, whichever occurred first.
Several risk factors have been established in exploring the association between osteoporosis and other comorbidities [23–28] The variables analyzed in this study were as follows: age; sex; and relevant comorbidities including depressive disorder (ICD-9-CM codes, 296.2, 296.3, 300.4, and 311), hypertension (ICD-9-CM codes, 401–405), diabetes mellitus (DM)(ICD-9-CM code, 250), dyslipidemia (CD-9-CM code, 272), cerebrovascular disease (ICD-9-CM codes, 430–438), chronic obstructive pulmonary disease (COPD)(ICD-9-CM codes, 491, 492, and 496), nephropathy (ICD-9 CM codes, 580–589), autoimmune disease (ICD-9-CM codes, 136.1, 340, 443.1, 446.0–446.2, 446.4–446.7, 555, 556, 694.4, 710.0–710.4, and 714.0), obesity (ICD-9-CM code, 278.00), congestive heart failure (ICD-9-CM code, 428), hepatitis B (ICD-9-CM codes, 070.2 and 070.3), and hepatitis C (ICD-9-CM codes, 070.44, 070.54, and 070.7). All these variables were collected at the index date for the enrollment.
To investigate the urban-rural disparity, we adopted the urbanization stratification of Taiwan townships developed by Taiwan’s National Health Research Institutes. The level of urbanization was determined by the population density, the population ratio of individuals with higher educational levels, the population ratio of elderly individuals over 65 years-old, the population ratio of farmers, and the number of physicians per 100,000 people. The 368 townships in Taiwan were classified into 7 levels of urbanization, excluding the isolated isles in the Kingmen and Lienchiang counties. In the current study, we defined the level 1 and 2 townships as urban, level 3 and 4 townships as suburban, and level 5 to 7 townships, plus the isolated isles, as rural.
The monthly income was included as covariates in the multivariate model in order to calculate the adjusted HR. Insurance premiums, calculated according to the beneficiary’s total income, were used to estimate the monthly income. The monthly income was grouped into no income, low income (monthly income, 0 to 20,000 New Taiwan Dollar [NTD]), median income (monthly income, 20,000 NTD to 40,000 NTD), and high income (monthly income > 40,000 NTD).
Statistical analysis
The incidence of newly diagnosed osteoporosis was calculated in both the AS and control groups, and they were stratified by sex and age (older or younger than 65 years). The variability, in terms of demographic characteristics, was compared between the AS and control groups by using independent t-tests, and chi-square tests when appropriate. The relevant period of person-years until an osteoporosis diagnosis was calculated from the enrollment date to the date of diagnosis of osteoporosis, death, withdrawal from the NHI system, or by the end of the follow-up (December 31, 2013).
A Cox proportional hazards regression model was applied to identify possible confounding factors in the univariate analysis and to exclude their effects on the evaluation of the AS-related risk of subsequent osteoporosis in the multivariate analysis. The variables used in the Cox model were as follows: age; sex; and common comorbidities including depressive disorder, hypertension, DM, dyslipidemia, cerebrovascular disease, COPD, nephropathy, autoimmune disease, hepatitis B, and hepatitis C. All variables that displayed a moderately significant statistical relationship with osteoporosis in the univariate analysis (P < 0.1) were included by forward selection in the multivariate analysis (Table 3). Moreover, we applied the Cox proportional hazards regression model to attempt to identify which variables may indicate subsequent osteoporosis among patients with AS (Table 4).
Table 3. Analyses of risk factors for osteoporosis in patients with and without ankylosing spondylitis.
| Predictive variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Ankylosing Spondylitis | 2.17 (1.83–2.55) | < 0.001 | 1.99 (1.68–2.36) | < 0.001 |
| Age (< 65 = 0, ≥ 65 = 1) | 7.17 (6.09–8.43) | < 0.001 | 4.56 (3.70–5.61) | < 0.001 |
| Sex (Male = 0, Female = 1) | 3.27 (2.77–3.85) | < 0.001 | 3.39 (2.87–4.02) | < 0.001 |
| Comorbidities | ||||
| Depressive disorder | 1.11 (0.53–2.34) | 0.781 | ||
| Hypertension | 4.08 (3.48–4.79) | < 0.001 | 1.43 (1.16–1.76) | 0.001 |
| Diabetes mellitus | 3.11 (2.57–3.78) | < 0.001 | 1.20 (0.95–1.50) | 0.124 |
| Dyslipidemia | 3.13 (2.62–3.73) | < 0.001 | 1.43 (1.16–1.77) | 0.001 |
| Cerebrovascular disease | 3.64 (2.96–4.49) | < 0.001 | 1.24 (0.98–1.56) | 0.074 |
| COPD | 3.10 (2.53–3.78) | < 0.001 | 1.53 (1.23–1.90) | < 0.001 |
| Nephropathy | 2.38 (1.90–2.97) | < 0.001 | 1.06 (0.84–1.35) | 0.621 |
| Autoimmune disease | 2.27 (1.72–3.02) | < 0.001 | 1.76 (1.32–2.34) | < 0.001 |
| Hepatitis B | 0.63 (0.33–1.22) | 0.168 | ||
| Hepatitis C | 2.76 (1.31–5.81) | 0.008 | 1.44 (0.67–3.09) | 0.349 |
| Degree of urbanization | ||||
| Urban | Reference | |||
| Suburban | 1.41 (1.19–1.67) | 0.003 | 1.12 (0.94–1.33) | 0.205 |
| Rural | 2.16 (1.66–2.81) | < 0.001 | 1.19 (0.90–1.56) | 0.222 |
| Income group | ||||
| High income | Reference | Reference | ||
| Medium income | 1.12 (0.78–1.62) | 0.544 | 0.96 (0.67–1.39) | 0.836 |
| Low income | 2.42 (1.78–3.29) | < 0.001 | 1.33 (0.97–1.83) | 0.082 |
| No income | 2.64 (1.90–3.67) | 0.378 | 1.10 (0.78–1.56) | 0.585 |
HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease
Table 4. Analyses of risk factors for osteoporosis in patients with ankylosing spondylitis.
| Predictive variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (< 65 = 0, ≥ 65 = 1) | 7.00 (5.27–9.28) | < 0.001 | 4.32 (3.01–6.18) | < 0.001 |
| Sex (Male = 0, Female = 1) | 2.59 (1.97–3.39) | < 0.001 | 2.48 (1.87–3.28) | < 0.001 |
| Comorbidities | ||||
| Depressive disorder | 1.37 (0.61–3.09) | 0.446 | ||
| Hypertension | 2.98 (2.26–3.92) | < 0.001 | 1.10 (0.77–1.57) | 0.595 |
| Diabetes mellitus | 2.73 (1.99–3.76) | < 0.001 | 1.33 (0.91–1.95) | 0.144 |
| Dyslipidemia | 2.67 (2.01–3.57) | < 0.001 | 1.44 (1.01–2.06) | 0.044 |
| Cerebrovascular disease | 2.82 (2.00–3.96) | < 0.001 | 1.15 (0.78–1.70) | 0.478 |
| COPD | 2.12 (1.50–3.00) | < 0.001 | 1.43 (0.98–2.08) | 0.064 |
| Nephropathy | 2.06 (1.47–2.87) | < 0.001 | 1.13 (0.79–1.62) | 0.513 |
| Autoimmune disease | 1.46 (0.95–2.23) | 0.083 | 1.41 (0.91–2.18) | 0.120 |
| Hepatitis B | 0.58 (0.24–1.42) | 0.233 | ||
| Hepatitis C | 2.75 (1.02–7.40) | 0.045 | 1.72 (0.61–4.84) | 0.302 |
| Degree of urbanization | ||||
| Urban | Reference | Reference | ||
| Suburban | 2.03 (1.53–2.68) | < 0.001 | 1.43 (1.06–1.92) | 0.018 |
| Rural | 2.03 (1.25–3.28) | 0.004 | 1.05 (0.63–1.75) | 0.851 |
| Income group | ||||
| High income | Reference | Reference | ||
| Medium income | 0.31 (0.17–0.54) | < 0.001 | 1.09 (0.58–2.06) | 0.780 |
| Low income | 0.40 (0.25–0.65) | < 0.001 | 1.67 (0.97–2.87) | 0.064 |
| No income | 0.94 (0.67–1.30) | 0.697 | 1.74 (0.98–3.11) | 0.060 |
HR, hazard ratio; CI, confidence interval; COPD, chronic obstructive pulmonary disease
The incidence rate ratio (IRR) was calculated from the ratio between the two incidence densities (the rate in the AS group divided by the rate in the control group). To estimate the cumulative incidence of osteoporosis, we performed survival analysis of both cohorts using the Kaplan-Meier method and assessed the significance of the results using the log-rank test.
The data extraction, computation, linkage, processing, and sampling were performed using the SAS statistical software for Windows, Version 9.3 (SAS Institute, Cary, NC, USA), all other statistical analyses were performed using SPSS 20.0 for Windows (IBM, Armonk, NY, USA). The results of comparisons with a P-value of < 0.05 were considered to indicate a statistically significant relationship.
Results
Participant selection
The demographic and clinical variables between the AS and reference groups are presented in Table 1. According to the inclusion and exclusion criteria of this study, 2,058 patients with AS and 8,232 patients without AS were ultimately included in the outcome analysis. Among both cohorts, the median age at the enrollment date was 38 years (interquartile range [IQR], 28.3–50.1 years) and 63.8% were male. The median follow-up period was 10.87 years (IQR, 9.48–12.42 years) for AS patients and 11.03 years (IQR, 9.65–12.48 years) for non-AS patients. The frequency of comorbidities, including depressive disorder, hypertension, DM, dyslipidemia, cerebrovascular disease, COPD, nephropathy, autoimmune disease, obesity, congestive heart failure, hepatitis B, and hepatitis C, was higher in the AS cohort than the reference cohort. Furthermore, patients in the AS cohort had a significantly higher income than the reference group.
Table 1. Baseline characteristics of patients with and without ankylosing spondylitis.
| Demographic data | Patients with AS n = 2,058 |
Patients without AS n = 8,232 |
P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years)a | 38.01 (28.29–50.11) | 38.01 (28.30–50.11) | |||
| ≥ 65 | 227 | 11.0 | 908 | 11.0 | 0.998 |
| < 65 | 1,831 | 90.0 | 7,324 | 90.0 | |
| Sex | |||||
| Male | 1,314 | 63.8 | 5,256 | 63.8 | 0.999 |
| Female | 744 | 36.2 | 2,976 | 36.2 | |
| Comorbidities | |||||
| Depressive disorder | 44 | 2.1 | 66 | 0.8 | < 0.001 |
| Hypertension | 428 | 20.8 | 1,300 | 15.8 | < 0.001 |
| Diabetes mellitus | 234 | 11.4 | 709 | 8.6 | < 0.001 |
| Dyslipidemia | 333 | 16.2 | 907 | 11.0 | < 0.001 |
| Cerebrovascular disease | 277 | 13.5 | 672 | 8.2 | < 0.001 |
| COPD | 227 | 11.0 | 632 | 7.7 | < 0.001 |
| Nephropathy | 241 | 11.7 | 538 | 6.5 | < 0.001 |
| Autoimmune disease | 76 | 3.7 | 163 | 2.0 | < 0.001 |
| Obesity | 14 | 0.7 | 30 | 0.4 | 0.043 |
| Congestive heart failure | 44 | 2.1 | 102 | 1.2 | 0.003 |
| Hepatitis B | 80 | 3.9 | 158 | 1.9 | < 0.001 |
| Hepatitis C | 17 | 0.8 | 34 | 0.4 | 0.022 |
| Degree of urbanization | 0.541 | ||||
| Urban | 1,253 | 60.9 | 5,096 | 61.9 | |
| Suburban | 665 | 32.3 | 2,623 | 31.9 | |
| Rural | 140 | 6.8 | 513 | 6.2 | |
| Income group | < 0.001 | ||||
| High income | 340 | 16.5 | 1,077 | 13.1 | |
| Medium income | 416 | 20.2 | 1,687 | 20.5 | |
| Low income | 938 | 45.6 | 3,854 | 46.8 | |
| No income | 364 | 17.7 | 1,614 | 19.6 | |
| Follow-up yearsa | 10.87 (9.48–12.42) | 11.03 (9.65–12.48) | < 0.001 | ||
aMedian (interquartile range); COPD indicates chronic obstructive pulmonary disease
The incidence of osteoporosis development
Among the AS group, 214 (10.21 per 1,000 person-years) patients developed osteoporosis during the study period. The incidence rate (IRR) was 2.17 (95% confidence interval [CI], 1.83–2.57, P < 0.001) in the AS group compared to that in the reference group. When stratified by sex and age, the IRR of osteoporosis in the current study remained higher in patients with AS than in the control patients; this was also the case among the male, or younger population (Table 2).
Table 2. Incidence of osteoporosis in patients with and without ankylosing spondylitis (AS).
| Patients with AS | Patients without AS | Rate ratio (95% CI) | P value | |||
|---|---|---|---|---|---|---|
| No. of osteoporosis | Per 1,000 person-years | No. of osteoporosis | Per 1,000 person-years | |||
| Total | 214 | 10.21 | 408 | 4.70 | 2.17 (1.83–2.57) | < 0.001 |
| Age | ||||||
| ≥ 65 | 77 | 53.36 | 164 | 23.34 | 2.29 (1.72–3.02) | < 0.001 |
| < 65 | 137 | 7.02 | 244 | 3.06 | 2.29 (1.85–2.84) | < 0.001 |
| Sex | ||||||
| Male | 88 | 6.51 | 133 | 2.38 | 2.73 (2.06–3.60) | < 0.001 |
| Female | 126 | 16.93 | 275 | 8.89 | 1.91 (1.53–2.36) | < 0.001 |
| Follow-up | ||||||
| 0–1 | 55 | 1747.14 | 59 | 747.21 | 2.34 (1.59–3.43) | < 0.001 |
| 1–5 | 69 | 200.99 | 154 | 128.76 | 1.56 (1.16–2.09) | 0.003 |
| 5–10 | 67 | 15.09 | 143 | 7.90 | 1.91 (1.41–2.57) | < 0.001 |
| ≥ 10 | 23 | 1.43 | 52 | 0.77 | 1.85 (1.08–3.07) | 0.019 |
CI indicates confidence interval
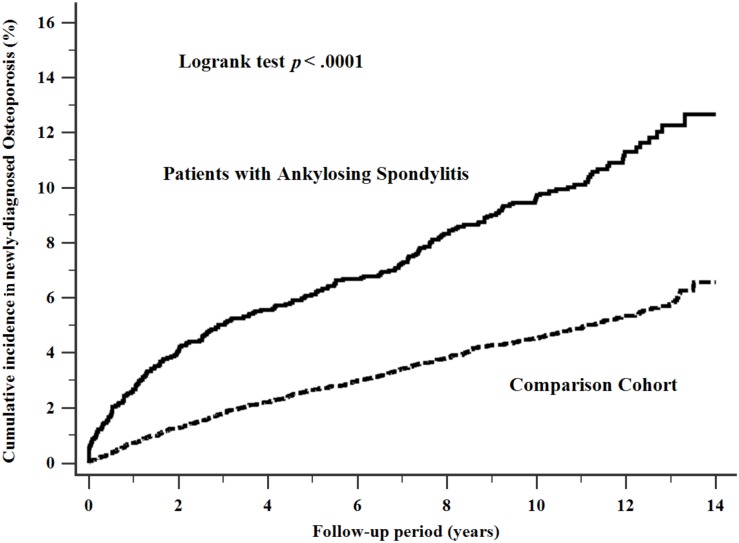
The cumulative incidence of osteoporosis in patients with AS was 10.21 per 1000 person-years, which was higher than the comparison cohort (4.7 per 1000 person-years) during the follow-up period (log-rank test, P < 0.0001, as shown in Fig 2).
Fig 2. Cumulative incidence of osteoporosis in patients with ankylosing spondylitis.
The cumulative incidence of newly diagnosed osteoporosis in patients with Ankylosing spondylitis was higher than the comparison cohort significantly.
The effects of AS on the risk of developing subsequent osteoporosis
As shown in Table 3, after adjusting for age, sex, the diagnosis of AS and comorbidities, we found that old age, female sex, hypertension, dyslipidemia, COPD and autoimmune disease history were independent risk factors of osteoporosis. The hazard ratio (HR) of the diagnosis of AS, old age, female sex, hypertension, dyslipidemia, COPD and autoimmune disease history was 2.17, 4.56, 3.39, 1.43, 1.43, 1.53 and 1.76, respectively, as compared with those of patients without osteoporosis.
Risk factors of developing osteoporosis in AS patients
Univariate analysis was used to identify the possible risk factors for subsequent osteoporosis in the AS cohort, with the results demonstrated in Table 4. We included these potential confounding variables in a Cox regression model for multivariate analysis. The results of this subgroup analysis showed that old age (≥ 65 years old) (HR = 4.32, 95% CI, 3.01–6.18, P < 0.001), female sex (HR = 2.48, 95% CI, 1.87–3.28, P < 0.001), dyslipidemia (HR = 1.44, 95% CI, 1.01–2.06, P = 0.044) were risk factors in AS patients; these results are consistent with the risk factors within the general population. In addition, living in a suburban area (HR = 1.43, 95% CI, 1.06–1.92, P = 0.018) was an independent risk factor for subsequent osteoporosis development in patients with AS. Furthermore, those with a history of COPD were also shown to have a marginal correlation with a higher risk of developing osteoporosis (HR = 1.43, 95% CI, 0.98–2.08, P = 0.064).
Discussion
Since the NHIRD serves patients from all areas in Taiwan, these patients are sufficiently representative of the nation as a whole. Our study explored the incidence of newly diagnosed osteoporosis in patients with and without AS in Asia. There are two major findings of this study. First, the risk of osteoporosis is significantly higher among patients with AS. Second, we suggest that old age (≥ 65 years old), female sex, and dyslipidemia may be considered as potential risk factors for developing subsequent osteoporosis among patients with AS.
From previous studies, it is well-recognized that AS patients have higher risk of osteoporosis [29]. However, in the present study, the incidence of osteoporosis in AS patients within 1 year of diagnosis was 2.67% (55/2058 = 2.67%); this is much lower than has been previously shown in the Netherlands (9%) [7], China (9.7%) [16], Morocco (25%) [9], and Germany (40.7%) [13]. The difference in follow-up period, as well as differences in geographical region and sex disparity may contribute to this variability.
A current United States cohort study using the registered database and including 46,265 patients with AS, demonstrated that AS patients are significantly more likely to develop osteoporosis than non-AS patients; these results agree with the findings of the present study [30]. However, the incidence rate of osteoporosis among the AS group in the current study was lower than the cohort conducted in the United States (10.21/1,000 person-years vs. 22.6/1,000 person-years); this may be due to the younger population in our study (38 years old vs. 51 years old), the male/female ratio, genetic heterogeneity, and the duration of disease.
We speculated that the number of male AS patients with subsequent osteoporosis is underrecognized and underestimated in our database. There are several potential reasons for this underestimation. First, AS-related syndesmophytes falsely increase the BMD measured by dual-energy x-ray absorptiometry (DXA) [31]. Second, patients with AS usually don’t seek medical treatment unless severe symptoms occur; this, in turn, may result in low DXA or X-ray utilization and thus fewer opportunities for a diagnosis of osteoporosis. One study supports this speculation, reporting that only patients with the most severe vertebral fracture will seek medical advice [32]. Third, clinicians may overlook osteoporosis due to the aim of the initial treatment frequently being directed at the control of symptoms [29]. Fourth, osteoporosis is not usually suspected in a young-male dominant patient group and young men are less likely to be screened according to practice guidelines [33, 34]. Lastly, the unusual results may be due to the fact that many patients and/or physicians are unaware of the increased risk of osteoporosis among the relatively young, and predominantly male, population [8, 29].
AS is recognized as a disease of young men and osteoporosis is usually not suspected in this demographic. Therefore, it would be reasonable to expect most AS patients with subsequent osteoporosis to be male and young. However, our results were not in line with these expectations. We found that old age (≥ 65 years old), female sex, and dyslipidemia are potential risk factors for developing osteoporosis among patients with AS. In the general population, older females, especially postmenopausal females, are well-known to have an increased risk of osteoporosis; this is also the case in AS patients, as has been reported in an earlier study [15].
Our results also suggested that dyslipidemia is a risk factor for developing osteoporosis in AS patients. The link between cardiovascular disease [35], or serum lipids, and low BMD/osteoporosis has been investigated in several studies [36–38]. Osteoporosis and cardiovascular disease share numerous common risk factors, including age and serum oxidized lipid [39]. The underlying mechanisms are thought to originate from a distorted lipid metabolism resulting in higher serum oxidized lipids, which, in turn, suppress osteoblast differentiation [40]. As demonstrated by a murine study by Graham at el al., oxidized lipids will adversely affect bone mechanical strength and impair bone regeneration by inhibiting the differentiation of osteoblasts and enhancing the presence of osteoclasts [41]. Additionally, adipocytokines secreted by adipose cells may enhance osteoclast formation, and oxidized LDL may suppress osteoblast formation [42]. Second, oxidized lipids impair angiogenesis and blood vessel functioning, both of which lead to decreased osteogenesis [43]. The accumulation of oxidized lipids in the atherosclerotic plaque within bone blood vessels will increase the levels of anti-osteoblastogenic inflammatory cytokines and decrease the pro-osteoblastogenic effect [44–46].
Our data demonstrate that the risk of developing osteoporosis is marginally higher in patients with a history of COPD, which is consistent with previous studies [47–49]. The present study also identified a higher risk of osteoporosis in AS patients who live in suburban areas; a lower 25-hydroxy-vitamin D level among the low socio-economic population is one of the possible reasons for this increased risk [50, 51].
The strengths of our study include the large sample size and the fact that we attempted to ensure a high diagnostic accuracy of AS by only obtaining coding from a rheumatologist. Since 1984, modified version of the New York criteria is used routinely in clinical practice in Taiwan as criteria for diagnosing patients with suspected AS. We believe that the diagnosis of AS based on clinical coding by a rheumatologist is reliable in the majority of cases and that the long observation period of the present study offers the opportunity to evaluate the real impact of AS. The national insurance system database is also representative of real conditions in clinical settings. Furthermore, the NHIRD could track each guaranteed case from different medical institutes over a period of time to avoid a dropping-out bias and to minimize the possibility of recall bias.
However, certain limitations in the present study should be taken into consideration. First, personal information related to osteoporosis, such as smoking history, alcohol consumption, lifelong steroid use, low body mass index, lab data including serum inflammation markers, calcium level, vitamin D intake, and serum uric acid [52], may have influenced our results. Although it is widely known that smoking increases the risk of osteoporosis, we attempted to use COPD as a proxy variable for cigarette smoking, which has been accepted in several previous studies [53–55]. Second, details relevant to the disease severity, and those related to the disease activity of AS, are somewhat lacking in the claims data; thus, in the present study, we could not evaluate the impact of the severity and disease activity of AS on osteoporosis. Third, our study did not provide any information about medications administered, which may interfere with the BMD. Finally, we should be aware of some possible investigation bias. For example, patients who were not followed-up adequately and/or perhaps did not receive complete treatment due to a particular disease, could raise the possibility of other subclinical diseases being present within the study population; therefore, the incidence of osteoporosis within the control group may be underestimated.
Conclusions
Although AS is a risk factor for the development of new-onset osteoporosis, there are no existing guidelines to routinely assess patients with AS for osteoporosis, and young men are less likely to be screened. Given that the underestimation of osteoporosis in AS patients can have serious health consequences, a greater emphasis should be placed on this association. This population-based cohort study demonstrated that patients with AS have a higher risk of developing osteoporosis, especially in those aged over 65 years, of female sex, and with dyslipidemia.
Acknowledgments
The authors would like to thank the Research Center of Medical informatics at Kaohsiung Veterans General Hospital for the technical assistance. The study is based on data from the NHIRD provided by the BNHI, Department of Health, Executive Yuan, Taiwan and managed by NHRI, Taiwan. We express our particular gratitude to the government organization, BNHI, and the non-profit foundation, NHRI.
Abbreviations
- AS
Ankylosing spondylitis
- BMD
bone mineral density
- BNHI
Bureau of the National Health Insurance
- COPD
chronic obstructive pulmonary disease
- DM
diabetes mellitus
- HWDC
Health and Welfare Data Science Center
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LHID
Longitudinal Health Insurance Database
- MOHW
Ministry of Health and Welfare
- NHI
National Health Insurance
- NHIRD
National Health Insurance Research Database
- NTD
New Taiwan Dollar
Data Availability
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26): 2563–2574. 10.1056/NEJMra1406182 [DOI] [PubMed] [Google Scholar]
- 2.Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369(9570): 1379–1390. 10.1016/S0140-6736(07)60635-7 [DOI] [PubMed] [Google Scholar]
- 3.Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology. 2014;53(4): 650–657. 10.1093/rheumatology/ket387 [DOI] [PubMed] [Google Scholar]
- 4.Chou CT, Pei L, Chang DM, Lee CF, Schumacher HR, Liang MH. Prevalence of rheumatic diseases in Taiwan: a population study of urban, suburban, rural differences. J Rheumatol 1994;21(2): 302–306 [PubMed] [Google Scholar]
- 5.Reveille JD, Hirsch R, Dillon CF, Carroll MD, Weisman MH. The prevalence of HLA-B27 in the US: data from the US National Health and Nutrition Examination Survey, 2009. Arthritis Rheum 2012;64(5): 1407–1411. 10.1002/art.33503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J. The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol 2015;765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Weijden MA, van Denderen JC, Lems WF, Heymans MW, Dijkmans BA, van der Horst-Bruinsma IE. Low bone mineral density is related to male sex and decreased functional capacity in early spondylarthropathies. Clin Rheumatol. 2011;30(4): 497–503. 10.1007/s10067-010-1538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Weijden MA, Claushuis TA, Nazari T, Lems WF, Dijkmans BA, van der Horst-Bruinsma IE. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: a systematic review. Clin Rheumatol. 2012;31(11): 1529–1535. 10.1007/s10067-012-2018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghozlani I, Ghazi M, Nouijai A, Mounach A, Rezqi A, Achemlal L, et al. Prevalence and risk factors of osteoporosis and vertebral fractures in patients with ankylosing spondylitis. Bone. 2009;44(5): 772–776. 10.1016/j.bone.2008.12.028 [DOI] [PubMed] [Google Scholar]
- 10.Briot K, Roux C. Inflammation, bone loss and fracture risk in spondyloarthritis. RMD Open. 2015;1(1): e000052 10.1136/rmdopen-2015-000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pray C, Feroz NI, Nigil Haroon N. Bone mineral density and fracture risk in ankylosing spondylitis: a meta-analysis. Calcif Tissue Int 2017;101(2): 182–92. 10.1007/s00223-017-0274-3 [DOI] [PubMed] [Google Scholar]
- 12.Ulu MA, Batmaz I, Dilek B, Cevik R. Prevalence of osteoporosis and vertebral fractures and related factors in patients with ankylosing spondylitis. Chin Med J 2014;127(15): 2740–2747 [PubMed] [Google Scholar]
- 13.Karberg K, Zochling J, Sieper J, Felsenberg D, Braun J. Bone loss is detected more frequently in patients with ankylosing spondylitis with syndesmophytes. J Rheumatol 2005;32(7): 1290–1298 [PubMed] [Google Scholar]
- 14.Klingberg E, Geijer M, Gothlin J, Mellstrom D, Lorentzon M, Hilme E, et al. Vertebral fractures in ankylosing spondylitis are associated with lower bone mineral density in both central and peripheral skeleton. J Rheumatol. 2012;39(10): 1987–1995. 10.3899/jrheum.120316 [DOI] [PubMed] [Google Scholar]
- 15.Klingberg E, Lorentzon M, Mellstrom D, Geijer M, Gothlin J, Hilme E, et al. Osteoporosis in ankylosing spondylitis—prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14(3): R108 10.1186/ar3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang DM, Zeng QY, Chen SB, Gong Y, Hou ZD, Xiao ZY. Prevalence and risk factors of osteoporosis in patients with ankylosing spondylitis: a 5-year follow-up study of 504 cases. Clin Exp Rheumatol. 2015;33(4): 465–470 [PubMed] [Google Scholar]
- 17.Emohare O, Cagan A, Polly DW Jr., Gertner E. Opportunistic computed tomography screening shows a high incidence of osteoporosis in ankylosing spondylitis patients with acute vertebral fractures. J Clin Densitom. 2015;18(1): 17–21. [DOI] [PubMed] [Google Scholar]
- 18.Cheng TM. Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood). 2003;22(3): 61–76. [DOI] [PubMed] [Google Scholar]
- 19.Insurance BoNH. National Health Insurance Research Database, Taiwan: 2000 [Google Scholar]
- 20.Lawrence Ong KC. Data Protection 2018 Taiwan [updated 12/06/2018; cited 2018. https://iclg.com/practice-areas/data-protection-laws-and-regulations/taiwan.
- 21.National Health Insurance Administration MoHaW, Taiwan, R.O.C. National Health Insurance Annual Report 2014–2015 Taiwan: Center for Biomedical Resources of NHRI; 2014. https://nhird.nhri.org.tw/en/.
- 22.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4): 361–368 [DOI] [PubMed] [Google Scholar]
- 23.Wong SK, Chin KY, Suhaimi FH, Ahmad F, Ima-Nirwana S. The relationship between metabolic syndrome and osteoporosis: a review. Nutrients. 2016;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou YC, Lu CL, Lu KC. Mineral bone disorders in chronic kidney disease. Nephrology. 2018;23 Suppl 488–494. [DOI] [PubMed] [Google Scholar]
- 25.Inoue D, Watanabe R, Okazaki R. COPD and osteoporosis: links, risks, and treatment challenges. Int J Chron Obstruct Pulmon Dis. 2016; 11637–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briot K, Geusens P, Em Bultink I, Lems WF, Roux C. Inflammatory diseases and bone fragility. Osteoporos Int. 2017;28(12): 3301–3314. 10.1007/s00198-017-4189-7 [DOI] [PubMed] [Google Scholar]
- 27.Mezuk B, Eaton WW, Golden SH. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos Int. 2008;19(1): 1–12. 10.1007/s00198-007-0449-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gennari L, Bilezikian JP. Osteoporosis in men. Endocrinol Metab Clin North Am. 2007;36(2): 399–419. 10.1016/j.ecl.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 29.Davey-Ranasinghe N, Deodhar A. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2013;25(4): 509–516. 10.1097/BOR.0b013e3283620777 [DOI] [PubMed] [Google Scholar]
- 30.Walsh JA, Song X, Kim G, Park Y. Evaluation of the comorbidity burden in patients with ankylosing spondylitis using a large US administrative claims data set. Clin Rheumatol. 2018;37(7): 1869–1878. 10.1007/s10067-018-4086-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vosse D, de Vlam K. Osteoporosis in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2009;27(4 Suppl 55): S62–S67 [PubMed] [Google Scholar]
- 32.Geusens P, Vosse D, van der Linden S. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2007;19(4): 335–339. 10.1097/BOR.0b013e328133f5b3 [DOI] [PubMed] [Google Scholar]
- 33.Force USPST. Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 2011;154(5):356–364. 10.7326/0003-4819-154-5-201103010-00307 [DOI] [PubMed] [Google Scholar]
- 34.Liu H, Paige NM, Goldzweig CL, Wong E, Zhou A, Suttorp MJ, et al. Screening for osteoporosis in men: a systematic review for an American College of Physicians guideline. Ann Intern Med. 2008;148(9): 685–701 [DOI] [PubMed] [Google Scholar]
- 35.Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. 2008;5(1): 19–34 [PMC free article] [PubMed] [Google Scholar]
- 36.Muka T, Trajanoska K, Kiefte-de Jong JC, Oei L, Uitterlinden AG, Hofman A, et al. The association between metabolic syndrome, bone mineral density, hip bone geometry and fracture risk: the Rotterdam study. PloS one. 2015;10(6): e0129116 10.1371/journal.pone.0129116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen YY, Wang WW, Yang L, Chen WW, Zhang HX. Association between lipid profiles and osteoporosis in postmenopausal women: a meta-analysis. Eur Rev Med Pharmacol Sci. 2018;22(1): 1–9. 10.26355/eurrev_201801_14093 [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto T, Sato M, Dehle FC, Brnabic AJ, Weston A, Burge R. Lifestyle-related metabolic disorders, osteoporosis, and fracture risk in Asia: a systematic review. Value Health Reg Issues. 2016;949–956. [DOI] [PubMed] [Google Scholar]
- 39.Lello S, Capozzi A, Scambia G. Osteoporosis and cardiovascular disease: an update. Gynecol Endocrinol. 2015;31(8): 590–594. 10.3109/09513590.2015.1041908 [DOI] [PubMed] [Google Scholar]
- 40.Pirih F, Lu J, Ye F, Bezouglaia O, Atti E, Ascenzi MG, et al. Adverse effects of hyperlipidemia on bone regeneration and strength. J Bone Miner Res. 2012;27(2): 309–318. 10.1002/jbmr.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Graham LS, Tintut Y, Parhami F, Kitchen CM, Ivanov Y, Tetradis S, et al. Bone density and hyperlipidemia: the T-lymphocyte connection. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2010;25(11): 2460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koshiyama H, Ogawa Y, Tanaka K, Tanaka I. The unified hypothesis of interactions among the bone, adipose and vascular systems: ‘osteo-lipo-vascular interactions’. Medical hypotheses. 2006;66(5): 960–3. 10.1016/j.mehy.2005.11.024 [DOI] [PubMed] [Google Scholar]
- 43.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood 2014;123(5):625–31. 10.1182/blood-2013-09-512749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Filipowska J, Tomaszewski KA, Niedzwiedzki L, Walocha JA, Niedzwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20(3): 291–302. 10.1007/s10456-017-9541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcified Tissue Int. 2013;93(4): 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alagiakrishnan K, Juby A, Hanley D, Tymchak W, Sclater A. Role of vascular factors in osteoporosis. J Gerontol A Biol Sci. 2003;58(4): 362–366 [DOI] [PubMed] [Google Scholar]
- 47.Miller J, Edwards LD, Agusti A, Bakke P, Calverley PM, Celli B, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med. 2013;107(9): 1376–1384. 10.1016/j.rmed.2013.05.001 [DOI] [PubMed] [Google Scholar]
- 48.Chen SJ, Liao WC, Huang KH, Lin CL, Tsai WC, Kung PT, et al. Chronic obstructive pulmonary disease and allied conditions is a strong independent risk factor for osteoporosis and pathologic fractures: a population-based cohort study. QJM. 2015;108(8): 633–640. 10.1093/qjmed/hcv012 [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Olmos L, Alberquilla A, Ayala V, Garcia-Sagredo P, Morales L, Carmona M, et al. Comorbidity in patients with chronic obstructive pulmonary disease in family practice: a cross sectional study. BMC Fam Pract. 2013;1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Navarro Mdel C, Saavedra P, Jodar E, Gomez de Tejada MJ, Mirallave A, Sosa M. Osteoporosis and metabolic syndrome according to socio-economic status, contribution of PTH, vitamin D and body weight: The Canarian Osteoporosis Poverty Study (COPS). Clin Endocrinol. 2013;78(5): 681–686. [DOI] [PubMed] [Google Scholar]
- 51.Lim SK, Kung AW, Sompongse S, Soontrapa S, Tsai KS. Vitamin D inadequacy in postmenopausal women in Eastern Asia. Current medical research and opinion. 2008;24(1): 99–106. 10.1185/030079908X253429 [DOI] [PubMed] [Google Scholar]
- 52.Kang KY, Hong YS, Park SH, Ju JH. Low levels of serum uric Acid increase the risk of low bone mineral density in young male patients with ankylosing spondylitis. JRheumatol. 2015;42(6): 968–974. [DOI] [PubMed] [Google Scholar]
- 53.Raaschou P, Simard JF, Asker Hagelberg C, Askling J. Rheumatoid arthritis, anti-tumour necrosis factor treatment, and risk of squamous cell and basal cell skin cancer: cohort study based on nationwide prospectively recorded data from Sweden. BMJ (Clin Res ed). 2016;352i262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang KH, Hsu YC, Hsu CC, Lin CL, Hsu CY, Lee CY, et al. Prolong exposure of NSAID in patients with RA will decrease the risk of dementia: a nationwide population-based cohort Study. Medicine. 2016;95(10): e3056 10.1097/MD.0000000000003056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang KH, Chang MY, Muo CH, Wu TN, Chen CY, Kao CH. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PloS one. 2014;9(8): e103078 10.1371/journal.pone.0103078 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study is from the National Health Insurance Research Database (NHIRD), which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
The NHIRD is addressed in publicity by the NHRI and NHIRD is to be used only for research purposes. All applicants must obey the Computer-Processed Personal Data Protection Law [20] and the corresponding rules of the Bureaus of the NHI and NHRI. Moreover, applicants and their supervisors were asked for signed agreements upon application submission and all applications were reviewed for the approval of the data delivered. The application for the dataset may be mailed to the NHRI at nhird@nhri.org.tw or call at +886-037-246166 ext. 33603 for immediate assistance. Office hours: Monday–Friday, 8:00–17:30 (UTC+8).
The NHIRD, which was open to researchers in Taiwan, was available from the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW) (http://www.mohw.gov.tw/cht/DOS/). The data underlying this study was obtained from the NHIRD. Applicants interested in obtaining the data are able to propose a formal application to the Ministry of Health and Welfare of Taiwan. In the last sentence of the paragraph on the webpage, which states "Kindly visit MOHW and NHIA on-site services for NHI Data", the database was delivered to a higher-level government administration, called the "HWDC" for more efficient health-related data linkage, wider application, and better security management.
At present, interested researchers could still obtain the NHI data in Taiwan through a formal application to the Health and Welfare Data Science Center (HWDC), Department of Statistics, Ministry of Health and Welfare (MOHW). HWDC, MOHW website (currently only Chinese): http://dep.mohw.gov.tw/DOS/np-2497-113.html [21].