Abstract
Background
Elevated proprotein convertase subtilisin/kexin type 9 (PCSK9) levels have been associated with adverse outcomes in patients hospitalized for sepsis. PCSK9 loss-of-function (LOF) variants area associated with lower low-density lipoprotein cholesterol (LDL-C) levels. Decreased LDL-C is a biomarker of acute and chronic infection and sepsis risk. We examined the association between presence of two genetic PCSK9 LOF variants and risk of infection and sepsis in community-dwelling adults.
Methods
We analyzed data from 10,924 Black participants tested for PCSK9 LOF variants in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. The primary endpoint was hospitalization for a serious infection. Within serious infection hospitalizations, we defined sepsis as ≥2 system inflammatory response syndrome criteria. Using multivariable Cox and logistic regression, we investigated the association between LOF variants and hospitalization for infection and sepsis events, adjusting for sociodemographics, health behaviors, chronic medical conditions and select biomarkers.
Results
Among 10,924 Black participants, PCSK9 LOF variants were present in 244 (2.2%). Serious infection hospitalizations occurred in 779 participants (14 with PCSK9 variants and 765 without). The presence of PCSK9 variants was not associated with infection risk (adjusted HR 0.68; 95% CI: 0.38–1.25). Among participants hospitalized for a serious infection, the presence of PCSK9 variants was not associated with sepsis (adjusted OR 7.31; 95% CI = 0.91–58.7).
Conclusions
PCSK9 LOF variants are not associated with increased risk of hospitalization for a serious infection. Among those hospitalized for a serious infection, PCSK9 LOF variants was not associated with odds of sepsis.
Introduction
Sepsis is the syndrome of infection complicated by systemic inflammation and organ dysfunction. Low levels of low density lipoprotein cholesterol (LDL-C), a negative acute phase reactant, have been associated with organ failure and mortality in sepsis. Multiple clinical observations have displayed an association linking lower cholesterol levels with higher sepsis severity and mortality.[1–3] Moreover, in a previous study of the population-based REGARDS cohort we found an association between baseline low LDL-C and higher rates of future sepsis events.[4]
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a zymogen in the proprotein convertase family involved in the regulation of hepatic LDL receptors (LDL-Rs) and therefore affects LDL and LDL-C clearance. A single gene produces PCSK9, which autocleaves in the endoplasmic reticulum to a mature form that, upon release from cells, can bind LDL-Rs for endocytosis and lysosomal degradation; PCSK9 loss-of-function (LOF) variants result in no autocleavage of the protein, and the protein is not able to bind LDL-Rs. [5] Therefore, humans with a PCSK9 loss-of-function variant have increased LDL-R activity and higher LDL-C clearance rates. Three nonsense mutations have been shown to have significant effects on LDL-C levels: R46L, which is more common in Whites, and Y142X and C679X, which are more common in Blacks–other variants in PCSK9 do not tend to show consistent, significant reductions in LDL-C.[6] Overall, about 2% of Blacks have Y142X or C679X, which results in LDL-C levels that are approximately 40% lower than controls, while the R46L variant in Whites is associated with 21% lower LDL-C compared to controls.[7]
Given the associations between PCSK9 and LDL-C, and then LDL-C and immune response, there are plausible connections between PCSK9 variants and risk of hospitalization for serious infections and sepsis. For example, PCSK9’s LDL-R degrading function may decrease hepatocyte endotoxin clearance.[8] A murine study found that PCSK9 inhibition reduced sepsis mortality.[9] PCSK9 levels have been reported to be increased in septic patients, leading to decreased endotoxin clearance and increased rates of organ failure.[10] Walley et al. found better outcomes of septic shock in patients with lower serum PCSK9 protein levels.[11]
In this study, we sought to determine the association between the presence of PCSK9 LOF variants and risk of hospitalizations for community-dwelling Black adults. Among first-infection hospitalizations, we also sought to determine if PCSK9 LOF variants are associated with odds of sepsis. Given that our prior REGARDS investigation observed that lower serum LDL-C levels were associated with higher risk of sepsis and that a PCSK9 LOF variant results in lower serum LDL-C, we hypothesized that PCSK9 LOF variants would be associated with increased risk of developing future serious infections and sepsis.
Methods
Study design
We utilized data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) study cohort. The Institutional Review Board of the University of Alabama at Birmingham approved this study.
Data source—The REGARDS cohort
The REGARDS cohort is an ongoing longitudinal cohort of 30,239 community-dwelling adults in the United States.[12] REGARDS recruited White and Black adults 45 years of age or older during January 2003 through October 2007. [12] Approximately 55% of the cohort is female, 41% is Black, and 69% is aged 60 years and older. Baseline information collected from each participant included demographic, health behaviors, medical history, diet and exercise habits and medications. During baseline interview, REGARDS also collected blood and urine samples from each participant. [12] REGARDS contacted participants every six months to inquire about recent hospitalizations and illnesses. In the case of participant death, REGARDS interviewed proxies and conducted reviews of death certificates and medical records of the deceased in order to accurately document the cause of death. [12]
Identification of PCSK9 loss-of-function variants
We determined the presence of the PCSK9 variant using the Gentra Puregene Blood kit (Qiagen #158389). Extracted DNA was stored in aliquots at 4°C and -80°C. Analysis of PCSK9 variant presence focused on two nonsense variants in Blacks: Y142X (rs67608943) and C679X (rs28362286). We limited the analysis to Blacks because PCSK9 LOF nonsense variants in Whites do not have the same level of association with LDL-C levels as they do in Blacks (about 21% decrease in LDL-C in Whites versus about 40% decrease in Blacks).[7] Also, PCSK9 LOF variant status was known for fewer than 2,000 Whites in the REGARDS cohort.
Identification of serious infection and sepsis events
The primary outcomes of the study were 1) first serious infection hospitalization, and 2) among first serious infection hospitalizations, the presence of sepsis. We used hospital admission and Emergency Department records to identify hospitalizations attributed to a serious infection.[13] For each hospitalization event, two abstractors independently reviewed all relevant medical records to verify the presence of serious infection at admission and infection as a primary reason for hospitalization. Abstractor agreement and additional physician-level review resolved any discordances.
Among first infection hospitalizations, we defined sepsis events as participants with a minimum of two Systemic Inflammatory Response Syndrome (SIRS) criteria that included 1) fever (temperature >38.3°C or <36°C), 2) heart rate >90 beats per minute, 3) tachypnea (>20 breaths per minute) or PCO2<32 mmHg, and 4) leukocytosis (white blood cell count >12,000 or <4,000 cells/mm3 or >10% band forms).[14] The diagnosis of SIRS was established from tests and vital signs obtained during the first 28 hours of hospitalization. In order to maintain consistency with prior REGARDS-sepsis analyses, we did not include organ dysfunction in the definition of sepsis.
We included hospitalization events reported between January 1, 2003 and December 31, 2012.
Participant demographics
Participant demographics assessed in the analysis include sociodemographics, health behaviors, chronic medical conditions, and select biomarkers. (Table 1) We identified alcohol intake according to the National Institute on Alcohol Abuse and Alcoholism classification.[15] We classified smoking status as smokers, past smokers, or current smokers. We observed presence of the following comorbidities: atrial fibrillation, chronic lung disease, coronary artery disease, deep vein thrombosis (self-reported), diabetes mellitus, chronic kidney disease, dyslipidemia, hypertension, obesity, stroke history (self-reported), and peripheral artery disease (self-reported). Dyslipidemia was defined as an LDL-C level > 130 mg/dL in accordance with guidelines from the American Association of Clinical Endocrinologists,[16] or participant reported use of lipid-lowering medications. We used biomarkers associated with lipid levels including LDL-C, HDL-C, serum total cholesterol, and triglycerides. HDL-C, serum total cholesterol, and triglycerides were directly measured from serum samples whereas LDL-C was calculated using the Friedewald formula from total cholesterol, HDL-C, and triglycerides. Other biomarkers included cystatin C and high sensitivity C-reactive protein.[17, 18]
Table 1. Baseline participant characteristics, stratified by presence or absence of a PCSK9 loss-of-function variant.
Includes 10,924 Black REGARDS participants.
| Characteristic | PCSK9 Variant Status | ||
|---|---|---|---|
| Present (n = 244) |
Absent (n = 10,680) |
P value | |
| Age (yrs)–mean (SD) | 64 (9.1) | 64 (9.2) | .52 |
| Gender (%) | .93 | ||
| Male | 38.1 | 38.4 | |
| Female | 61.9 | 61.6 | |
| Education (%) | .10 | ||
| Less than High School | 6.5 | 8.7 | |
| High School | 28.3 | 22.8 | |
| Some College | 32.6 | 29.6 | |
| College or more | 32.6 | 38.9 | |
| Income (%) | .31 | ||
| <$20k | 30.3 | 26.4 | |
| $20k-$34k | 28.3 | 26.4 | |
| $35-$74k | 22.1 | 25.8 | |
| ≥$75k | 6.6 | 9.2 | |
| Not Available | 12.7 | 12.3 | |
| Geographic Region (%) | .92 | ||
| Non-Stroke Belt or Buckle | 48.8 | 48.9 | |
| Stroke Belt | 34.4 | 33.5 | |
| Stroke Buckle | 16.8 | 17.7 | |
| Alcohol Use (%) | .36 | ||
| None | 76.1 | 72.0 | |
| Moderate | 21.4 | 25.5 | |
| Heavy | 2.6 | 2.5 | |
| Smoking (%) | .18 | ||
| Never | 47.7 | 45.3 | |
| Past | 32.1 | 37.5 | |
| Current | 20.2 | 17.2 | |
| Comorbidities (%) | |||
| Atrial Fibrillation | 5.9 | 7.8 | .27 |
| Chronic Lung Disease | 9.8 | 7.8 | .23 |
| Coronary Artery Disease | 11.2 | 15.6 | .07 |
| Deep Vein Thrombosis | 5.8 | 4.9 | .55 |
| Diabetes Mellitus | 34.0 | 30.5 | .23 |
| Chronic Kidney Disease | 13.5 | 12.1 | .49 |
| Dyslipidemia | 26.2 | 54.3 | < .001 |
| Hypertension | 70.1 | 71.4 | .66 |
| Obesity | 62.1 | 62.8 | .85 |
| Stroke | 7.8 | 4.9 | .09 |
| Peripheral Artery Disease | 2.5 | 2.4 | .93 |
| Biomarkers | |||
| LDL Cholesterol mg/dL (mean, SD) | 84.8 (32.0) | 117 (36.2) | < .001 |
| HDL Cholesterol mg/dL (mean, SD) | 53.8 (16.0) | 53.4 (16.0) | .73 |
| Total Cholesterol mg/dL (mean, SD) | 161 (35.1) | 194 (41.0) | < .001 |
| Triglycerides mg/dL (mean, SD) | 111 (58.3) | 113 (73.5) | .59 |
| Estimated Glomerular Filtration Rate mL/min/1.73 m2 (mean, SD) | 87.8 (24.5) | 88.4 (23.6) | .73 |
| Cystatin C >1.12 mg/dL (%) | 23.7 | 27.8 | .16 |
| hsC-reactive protein >3.0 mg/dL (%) | 45.5 | 48.3 | .38 |
| Lipid Lowering Medication Use | |||
| Cholestyramine | 0.0 | 0.1 | .69 |
| Ezetimibe | 0.8 | 2.6 | .08 |
| Fibrates | 0.0 | 1.0 | .12 |
| Statins | 13.1 | 29.5 | <0.001 |
Hospital course variables consisted of Sequential Organ Failure Assessment (SOFA) scores for coagulation, central nervous system, and respiratory, renal, cardiovascular, and liver systems, culminating in a total SOFA score, and Mortality in Emergency Department Sepsis (MEDS) scores. We also noted the type of infection and intensive care unit (ICU) admission.
Data analysis
We determined differences in participant characteristics between those with and without PCSK9 variants using Chi-square tests for categorical variables, t-tests for parametric continuous variables, and Wilcoxon rank-sum tests for non-parametric continuous variables. To determine the independent association of PCSK9 variant presence with incident serious infection, we fit a series of Cox proportional hazards models, adjusting for sociodemographics, health behaviors, comorbid medical conditions, and biomarkers. We excluded dyslipidemia, LDL-C levels, and total cholesterol levels from these models because they are part of the PCSK9 pathway. To explore an alternate risk adjustment strategy, we fit an additional model adjusted for the REGARDS Sepsis Risk Score (SRS). The SRS is a previously derived scale characterizing 10-year risk of sepsis based upon the several biomarkers, sociodemographics, and health behaviors, and it uses these factors to define five categories of risk severity.[19]
Among first infection events, we performed logistic regression to examine the association between PCSK9 variant presence or absence and sepsis adjusted for sociodemographics, health behaviors, comorbidities, biomarkers, and 28-hour Sequential Organ Failure Assessment (SOFA) score. We also fit an alternate model adjusted for the SRS categories and SOFA score. We performed all analyses using Stata 14.1 (Stata, Inc., College Station, Texas).
Results
Among 12,514 Black participants, 10,934 consented to genetic testing, and 10,924 completed testing. Of these, 190 tested positive for the C679X variant and 54 tested positive for the Y142X variant; the overall prevalence of any PCSK9 LOF variant was 2.2% (Table 1). There were no statistically significant differences in sociodemographic factors or health behaviors (alcohol and smoking) between those with and without PCSK9 variants. Participants with PCSK9 LOF variants were less likely to have dyslipidemia when compared with other participants (26.2% vs 54.3%, P < .001) and had lower total cholesterol levels (mean values 161 vs 194 mg/dL, P < .001). There were no other biomarker differences between participants with and without the PCSK9 variants. Participants with PCSK9 LOF variants were less likely to report statin use.
There were a total of 779 hospitalizations for a serious infection, including 14 (5.74%) among participants with the variant and 765 (7.16%) among those without (Table 2, Fig 1). Respiratory infections were the most common type of serious infection. Hospital death, 30-day fatality, SOFA scores, and MEDS scores were similar between participants with and without a PCSK9 LOF variant. Participants with PCSK9 variants were more likely to be admitted to the intensive care unit (21.4% of infected variants vs. 7.3% of infected non-variants, p = 0.048). Among first infection hospitalizations, 447 (57.4%) met sepsis criteria. Among 367 lung infections, 217 (59.1%) met sepsis criteria. Among 169 kidney infections, 86 (51.2%) met sepsis criteria. Among 143 intrabdominal infections, 60 (45.5%) met sepsis criteria.
Table 2. Infection type and hospital course among n = 779 first serious infection hospitalizations.
SOFA = Sequential Organ Failure Assessment. MEDS = Mortality in Emergency Department Sepsis. ICU-intensive care unit.
| Variable | PCSK9 variant present (n = 14) |
PCSK9 variant absent (n = 765) |
P value |
|---|---|---|---|
| Infection Type (n = 779) | < .001 | ||
| Lung | 7 (50.0%) | 311 (40.7%) | |
| Kidney | 2 (14.3%) | 150 (19.6%) | |
| Abdominal | 0 (0.0%) | 120 (15.7%) | |
| Skin | 1 (7.1%) | 102 (13.3%) | |
| Sepsis | 4 (28.6%) | 35 (4.6%) | |
| Other | 0 (0.0%) | 47 (6.1%) | |
| Hospital Course Variables | |||
| SOFA Score (median, IQR) | 1 (1–2) | 1 (0–2) | .26 |
| MEDS score (median, IQR) | 11 (6–15) | 9 (6–11) | .35 |
| ICU admission (n = 779) | 3 (21.4%) | 56 (7.3%) | .05 |
| Outcomes | |||
| Sepsis Hospital Death | 1 (7.1%) | 46 (6.0%) | .86 |
| 30-day case fatality | 4 (28.6%) | 145 (19.0%) | .37 |
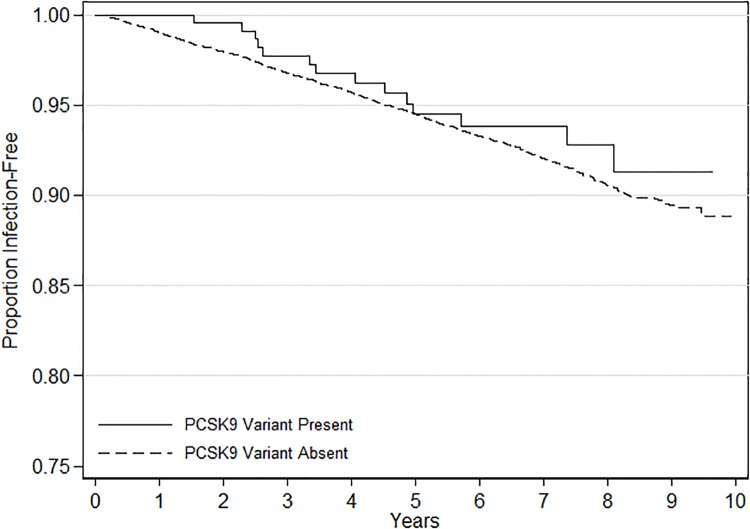
Fig 1. Kaplan-Meier survival curve for incident serious infection hospitalizations, stratified by presence or absence of PCSK9 variant.
Analysis limited to blacks. Log-rank test p = 0.47.
On multivariable analysis, there was no association between the presence of a PCSK9 variant and incident first serious infection; adjusted HR 0.68, 95% CI: 0.38–1.25 (Table 3). We observed similar associations after risk adjustment using the REGARDS Sepsis Risk Score. There were no associations with incident first infection when limited to the C679X variant (adjusted HR 0.79; 95% CI 0.42–1.48) or Y142X variant (0.30; 0.04–2.11). When limiting the analysis to first serious infection events, the presence of a PCSK9 LOF variant was not associated with odds of sepsis; adjusted OR 7.31 (95% CI: 0.91–58.7).
Table 3. Association of PCSK9 loss-of-function variants and risk of serious infection and sepsis hospitalizations.
Serious infection risk estimated by Cox proportional hazards analysis. Odds of sepsis among serious infection hospitalizations estimated by logistic regression. CI = confidence interval, HR = hazard ratio, OR = odds ratio.
|
Serious Infection Hospitalizations (N = 10,924) |
No. Events | No. Non-Events |
Events Per 1,000 Person-Years (95%CI) |
Crude HR (95% CI) |
Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
| PCSK9 variant present | 14 (5.7%) | 230 (94.3%) | 9.8 (5.8–16.5) | 0.82 (0.48–1.40) | 0.84 (0.49–1.42) | 0.68 (0.38–1.25) | 0.83 (0.49–1.40) |
| PCSK9 variant absent | 765 (7.2%) | 9,915 (92.8%) | 11.9 (11.1–12.8) | Ref. | Ref. | Ref. | Ref. |
| Total | 779 (7.1%) | 10,145 (92.9%) | 11.9 (11.1–12.7) | - | - | - | - |
|
Sepsis Within Serious Infection Hospitalizations (N = 779) |
No. Events | No. Non-events |
Events Per 1,000 Person-Years (95%CI) |
Crude OR (95% CI) |
Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
| PCSK9 variant present | 11 (2.5%) | 3 (0.9%) | N/A | 3.28 (0.91–11.84) | 3.34 (0.91–12.19) | 7.31 (0.91–58.7) | 3.22 (0.89–11.6) |
| PCSK9 variant absent | 404 (97.4%) | 361 (99.2%) | N/A | Ref. | Ref. | Ref. | Ref. |
| Total | 415 (53.3%) | 364 (46.7%) | N/A | - | - | - | - |
Model 1. Adjusted for demographics, smoking, and alcohol use
Model 2. Adjusted for model 1 + comorbidities + biomarkers
Model 3. Adjusted for sepsis risk score categories
Discussion
In this study of 10,924 Blacks in the REGARDS cohort, we found no association between PCSK9 LOF variants and the incidence of serious infections. Among first infection hospitalizations, we also did not observe significant associations between the presence of PCSK9 LOF variants and odds of sepsis.
The literature linking PCSK9, LDL-C, and infection shows conflicting results. Some studies suggest that higher cholesterol and/or PCSK9 function are associated with better response to some infections[4, 20–23] while others reported lower risk and/or better outcomes with lower PCSK9 and/or LDL-C.[8–11, 24] It is important to consider the distinctions of these studies. Select studies involved murine models.[9] Walley, et al. demonstrated that LDL-R and PCSK9 are critical regulators of endotoxin clearance in sepsis.[8, 10, 11] However, Berger, et al. found that PCSK9 LOF mutations did not affect lipopolysaccharide-induced mortality and failed to find a role for LDL-R in the process at all. [25] Walley’s studies used human hepatic cells and only demonstrated increased hepatic clearance via LDL-R of endotoxin above a threshold level commonly present in human sepsis. In contrast, Berger, et al. used wild type mice or LDL-R-/- or Pcsk9+/- mice. This may be one reason for the discrepancy between these studies.
Lagrost, et al. evaluated total cholesterol without stratifying by LDL-C and HDL-C,[20] which is important given reported associations between HDL-C and infection and sepsis outcomes.[26, 27] LDL-C and PCSK9 have been reported to have a protective association against parasitic or viral infections, but most sepsis cases are thought to be due to bacterial pathogens.[28] Most importantly, many studies of PCSK9 and sepsis used circulating PCSK9 plasma levels gathered during sepsis events instead of at baseline.[1, 10, 11] Our study used genetic analysis to assess the presence of PCSK9 genetic variation, baseline LDL-C levels, and risk of future infection and sepsis events.
Our analysis conceptualized that the presence of a PCSK9 LOF variant would lead to decreased levels of PCSK9 protein, leading to decreased LDL-C and increased long-term rates of infection or sepsis. Our previous studies demonstrate that low cholesterol levels (specifically LDL-C) may increase sepsis risk.[4, 29] Barter, et al. found that CETP inhibitors lead to drastic elevations in HDL, reductions in LDL, and increased all-cause mortality.; death from infection comprised nearly half of the deaths.[30] However, the lack of an independent association between PCSK9 LOF variant presence and infection risk may suggest that an alternate pathway also likely links LDL-C with sepsis risk. For example, HDL binds endotoxin and/or lipotechoic acid from bacteria and returns it to the liver for elimination.[21] This process requires multiple steps, and perturbations in the pathway could include reduced HDL-C function or dysfunction, impairment in clearance via scavenger receptors, or other steps in the process. Moreover, during critical illness, impaired lipid absorption through the gut leads to lipolysis and increased triglyceride levels while cholesterol-containing lipoprotein levels plummet, with non-survivors having lower LDL-C, HDL-C, and lysophosphatidylcholine than survivors.[31] Therefore, the low LDL-C may be in part a symptom of impaired lipid absorption. Inflammation may also play a role in the connection between cholesterol and sepsis risk. In an acute inflammatory response, the composition of lipoproteins changes, increasing LDL oxidative susceptibility, fostering HDL proinflammatory dysfunction, and altering of the levels of both LDL-C and HDL-C.[32] If anti-inflammatory drugs are given, the lipid profile returns towards normal.[33] In addition, PCSK9 itself may act in inflammation. Some researchers observed PCSK9 induction in response to TNF-alpha, which has been implicated in chronic inflammation.[34] A recent study supports the role of PCSK9 in atherosclerotic inflammation, finding that PCSK9 promotes secretion of inflammatory cytokines in macrophages in vitro while in vivo PCSK9 silencing decreases vascular inflammation and reduces pro-inflammatory cytokine expression in apolipoprotein E knockout mice.[35] Ricci and colleagues found a similar effect in humans and report that PCSK9 influences induction of multiple pro-inflammatory cytokines.[36]
An important related question is whether PCSK9 inhibitors may influence infection risk. Two prior placebo-controlled Phase 3 studies reported minor infection as one of the most common adverse events in patients treated with the PCSK9 inhibitor Evolocumab.[37, 38] These findings contrast with our finding that PCSK9 LOF variants did not alter infection hospitalization risk. Assuming the validity of the prior studies, our current results suggest that a PCSK9 inhibitor may increase infection risk through mechanisms other than downregulation of hepatic LDL-Rs. For example, PCSK9 is expressed in other tissues including the intestine and kidneys, circulating PCSK9 does not uniformly affect LDL-Rs in all organ systems, and the ability of PCSK9 to bind to other receptors is still debated.[39] Proposed receptors include apolipoprotein E receptor 2, cluster of differentiation 36, LDL-R related protein 1, and very low-density lipoprotein receptor; these are present in various organs, including the central nervous system, hepatocytes, macrophages, adipocytes, and intestinal cells.[5] It is possible that PCSK9 variants and/or PCSK9 inhibitors interfere with these and other targets in addition to their effect on LDL-C, and that improperly cleaved PCSK9 variant proteins may have their own yet-undiscovered effects. Because much of the detail of PCSK9’s function beyond LDL-R downregulation is yet unknown, additional studies are needed to clarify the pathways of PCSK9 inhibitors and their effects on infection risk.
Limitations
We limited the analysis to presence and absence of the Y142X or C679X variants in PCSK9 in Blacks. We did not assess other genomic variants. We did not assess these variants in Whites due to the emphasis of REGARDS on genetic risk in Blacks. REGARDS is not a surveillance study, and thus complete ascertainment of all infection or sepsis events is unlikely. By design, the REGARDS cohort includes only Blacks and Whites, and this study limited further only to Blacks. Therefore, these results may not generalize to other ethnic groups. There is potential for participation bias because some individuals did not consent to genetic testing. Infection events in REGARDS were based upon clinical documentation, not culture, laboratory or diagnostic imaging. We did not consider the causal organism of infection.
While we used multivariable adjustment to account for confounders, sample size was relatively modest, leading to some uncertain statistical inferences as evidenced by select odds ratios with wide confidence intervals. Because of the limited sample size, we opted not to further stratify the analysis by individual PCSK9 variant. While we had data on the presence or absence of chronic medical conditions, we did not have information on measures of severity. The focus of our study was on community-acquired sepsis, so we did not include cases of sepsis that occurred during hospital stay. The absence of an association between PCDK9 variants and cardiovascular disease risk was likely due to the limited sample size.
In our prior study using REGARDS data, we found no association between HDL-C and sepsis hospitalizations.[4] In contrast Madsen, et al. observed a U-shaped relationship between HDL-C and serious infection hospitalization in the Copenhagen General Population Study and the Copenhagen City Heart Study.[40] The latter study used a different cohort and identified infections using discharge diagnoses. In contrast, in REGARDS we identified sepsis event through structured review of clinical findings. The findings of the current analysis regarding PCKS9 variants and sepsis risk may differ if replicated in a different cohort.
Conclusion
Among Blacks in the REGARDS cohort, we found no association between PCSK9 LOF variant presence and risk of serious infection hospitalization. Among first serious infection hospitalizations, there was no association between PCSK9 variant presence and the odds of sepsis. Other mechanisms may be responsible for links between LDL-C and sepsis risk.
Data Availability
This study uses data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. In order to abide by its obligations with NIH/NINDS and the Institutional Review Board of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to access the REGARDS data through this process. Requests for data access may be sent to regardsadmin@uab.edu.
Funding Statement
This study was supported by award R01-NR012726 from the National Institute for Nursing Research, UL1-RR025777 from the National Center for Research Resources, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement U01-NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Mr. Moore received grant support from R25-CA4788, the Cancer Prevention and Control Training Program grant, funded by the National Cancer Institute, National Institutes of Health. Ms. Mitchell received grant support from NIH T35HL007473 from the National Institute of Health.
References
- 1.Wilson RF, Barletta JF, Tyburski JG. Hypocholesterolemia in sepsis and critically ill or injured patients. Crit Care. 2003;7(6):413–4. 10.1186/cc2390 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiarla C, Giovannini I, Siegel JH. The relationship between plasma cholesterol, amino acids and acute phase proteins in sepsis. Amino Acids. 2004;27(1):97–100. 10.1007/s00726-004-0064-x . [DOI] [PubMed] [Google Scholar]
- 3.Vermont CL, den Brinker M, Kakeci N, de Kleijn ED, de Rijke YB, Joosten KF, et al. Serum lipids and disease severity in children with severe meningococcal sepsis. Crit Care Med. 2005;33(7):1610–5. . [DOI] [PubMed] [Google Scholar]
- 4.Guirgis FW, Donnelly JP, Dodani S, Howard G, Safford MM, Levitan EB, et al. Cholesterol levels and long-term rates of community-acquired sepsis. Crit Care. 2016;20(1):408 10.1186/s13054-016-1579-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon DL, Trankle C, Buckley L, Parod E, Carbone S, Van Tassell BW, et al. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10(5):1073–80. 10.1016/j.jacl.2016.07.004 . [DOI] [PubMed] [Google Scholar]
- 6.Huang CC, Fornage M, Lloyd-Jones DM, Wei GS, Boerwinkle E, Liu K. Longitudinal association of PCSK9 sequence variations with low-density lipoprotein cholesterol levels: the Coronary Artery Risk Development in Young Adults Study. Circ Cardiovasc Genet. 2009;2(4):354–61. 10.1161/CIRCGENETICS.108.828467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JC, Boerwinkle E, Mosley TH Jr., Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72. 10.1056/NEJMoa054013 . [DOI] [PubMed] [Google Scholar]
- 8.Topchiy E, Cirstea M, Kong HJ, Boyd JH, Wang Y, Russell JA, et al. Lipopolysaccharide Is Cleared from the Circulation by Hepatocytes via the Low Density Lipoprotein Receptor. PLoS One. 2016;11(5):e0155030 Epub 2016/05/14. 10.1371/journal.pone.0155030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwivedi DJ, Grin PM, Khan M, Prat A, Zhou J, Fox-Robichaud AE, et al. Differential Expression of PCSK9 Modulates Infection, Inflammation, and Coagulation in a Murine Model of Sepsis. Shock. 2016;46(6):672–80. 10.1097/SHK.0000000000000682 . [DOI] [PubMed] [Google Scholar]
- 10.Boyd JH, Fjell CD, Russell JA, Sirounis D, Cirstea MS, Walley KR. Increased Plasma PCSK9 Levels Are Associated with Reduced Endotoxin Clearance and the Development of Acute Organ Failures during Sepsis. J Innate Immun. 2016;8(2):211–20. Epub 2016/01/13. 10.1159/000442976 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walley KR, Thain KR, Russell JA, Reilly MP, Meyer NJ, Ferguson JF, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6(258):258ra143 Epub 2014/10/17. 10.1126/scitranslmed.3008782 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. Epub 2005/07/02. NED2005025003135 [pii] 10.1159/000086678 . [DOI] [PubMed] [Google Scholar]
- 13.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. . [DOI] [PubMed] [Google Scholar]
- 14.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. Epub 2013/12/18. 10.4161/viru.27372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician. 2009;80(1):44–50. . [PubMed] [Google Scholar]
- 16.Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract. 2017;23(Suppl 2):1–87. 10.4158/EP171764.APPGL . [DOI] [PubMed] [Google Scholar]
- 17.Powell TC, Donnelly JP, Gutierrez OM, Griffin RL, Safford MM, Wang HE. Cystatin C and long term risk of community-acquired sepsis: a population-based cohort study. BMC nephrology. 2015;16:61 Epub 2015/04/24. 10.1186/s12882-015-0055-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HE, Shapiro NI, Safford MM, Griffin R, Judd S, Rodgers JB, et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7):e69232 Epub 2013/08/13. 10.1371/journal.pone.0069232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HE, Donnelly JP, Griffin R, Levitan EB, Shapiro NI, Howard G, et al. Derivation of Novel Risk Prediction Scores for Community-Acquired Sepsis and Severe Sepsis. Critical care medicine. 2016;44(7):1285–94. 10.1097/CCM.0000000000001666 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagrost L, Girard C, Grosjean S, Masson D, Deckert V, Gautier T, et al. Low preoperative cholesterol level is a risk factor of sepsis and poor clinical outcome in patients undergoing cardiac surgery with cardiopulmonary bypass. Crit Care Med. 2014;42(5):1065–73. 10.1097/CCM.0000000000000165 . [DOI] [PubMed] [Google Scholar]
- 21.Feingold KR, Grunfeld C. Lipoproteins: are they important components of host defense? Hepatology. 1997;26(6):1685–6. 10.1002/hep.510260647 . [DOI] [PubMed] [Google Scholar]
- 22.Mbikay M, Mayne J, Seidah NG, Chretien M. Of PCSK9, cholesterol homeostasis and parasitic infections: possible survival benefits of loss-of-function PCSK9 genetic polymorphisms. Med Hypotheses. 2007;69(5):1010–7. 10.1016/j.mehy.2007.03.018 . [DOI] [PubMed] [Google Scholar]
- 23.Liberale L, Montecucco F, Casetta I, Seraceni S, Trentini A, Padroni M, et al. Decreased serum PCSK9 levels after ischaemic stroke predict worse outcomes. Eur J Clin Invest. 2016;46(12):1053–62. 10.1111/eci.12692 . [DOI] [PubMed] [Google Scholar]
- 24.Kahdemi F, Momtazi-Borojeni AA, Reiner Z, Banach M, Rasadi KA, Sahebkar A. PCSK9 and infection: a potentially useful or dangerous association? J Cell Physiol. 2017. 10.1002/jcp.26040 . [DOI] [PubMed] [Google Scholar]
- 25.Berger JM, Loza Valdes A, Gromada J, Anderson N, Horton JD. Inhibition of PCSK9 does not improve lipopolysaccharide-induced mortality in mice. J Lipid Res. 2017;58(8):1661–9. 10.1194/jlr.M076844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grion CM, Cardoso LT, Perazolo TF, Garcia AS, Barbosa DS, Morimoto HK, et al. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Invest. 2010;40(4):330–8. 10.1111/j.1365-2362.2010.02269.x . [DOI] [PubMed] [Google Scholar]
- 27.von Eckardstein AaKDe. High density lipoproteins. Handb Exp Pharmacol. 2015;224:483–526. 10.1007/978-3-319-09665-0_15 [DOI] [PubMed] [Google Scholar]
- 28.Martin GS. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev Anti Infect Ther. 2012;10(6):701–6. 10.1586/eri.12.50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guirgis FW, Dodani S, Leeuwenburgh C, Moldawer L, Bowman J, Kalynych C, et al. HDL inflammatory index correlates with and predicts severity of organ failure in patients with sepsis and septic shock. PLoS One. 2018;13(9):e0203813 Epub 2018/09/15. 10.1371/journal.pone.0203813 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. The New England journal of medicine. 2007;357(21):2109–22. Epub 2007/11/07. 10.1056/NEJMoa0706628 . [DOI] [PubMed] [Google Scholar]
- 31.Green P, Theilla M, Singer P. Lipid metabolism in critical illness. Curr Opin Clin Nutr Metab Care. 2016;19(2):111–5. 10.1097/MCO.0000000000000253 . [DOI] [PubMed] [Google Scholar]
- 32.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–96. . [DOI] [PubMed] [Google Scholar]
- 33.Feingold KR, Grunfeld C. The Effect of Inflammation and Infection on Lipids and Lipoproteins In: De Groot LJ, Chrousos G, Dungan K, Feingold KR, Grossman A, Hershman JM, et al. , editors. Endotext. South Dartmouth (MA)2000. [Google Scholar]
- 34.Ruscica M, Ricci C, Macchi C, Magni P, Cristofani R, Liu J, et al. Suppressor of Cytokine Signaling-3 (SOCS-3) Induces Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Expression in Hepatic HepG2 Cell Line. J Biol Chem. 2016;291(7):3508–19. 10.1074/jbc.M115.664706 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang ZH, Peng J, Ren Z, Yang J, Li TT, Li TH, et al. New role of PCSK9 in atherosclerotic inflammation promotion involving the TLR4/NF-kappaB pathway. Atherosclerosis. 2017;262:113–22. 10.1016/j.atherosclerosis.2017.04.023 . [DOI] [PubMed] [Google Scholar]
- 36.Ricci C, Ruscica M, Camera M, Rossetti L, Macchi C, Colciago A, et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci Rep. 2018;8(1):2267 10.1038/s41598-018-20425-x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–19. 10.1056/NEJMoa1316222 . [DOI] [PubMed] [Google Scholar]
- 38.Raal FJ, Hovingh GK, Blom D, Santos RD, Harada-Shiba M, Bruckert E, et al. Long-term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open-label TAUSSIG study. The Lancet Diabetes & Endocrinology. 2017;5(4):280–90. 10.1016/s2213-8587(17)30044-x [DOI] [PubMed] [Google Scholar]
- 39.Cariou B, Le May C, Costet P. Clinical aspects of PCSK9. Atherosclerosis. 2011;216(2):258–65. 10.1016/j.atherosclerosis.2011.04.018 . [DOI] [PubMed] [Google Scholar]
- 40.Madsen CM, Varbo A, Tybjaerg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J. 2018;39(14):1181–90. Epub 2017/12/12. 10.1093/eurheartj/ehx665 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study uses data from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. In order to abide by its obligations with NIH/NINDS and the Institutional Review Board of the University of Alabama at Birmingham, REGARDS facilitates data sharing through formal data use agreements. Any investigator is welcome to access the REGARDS data through this process. Requests for data access may be sent to regardsadmin@uab.edu.