Abstract
Background:
Patients with pancreatic insufficient cystic fibrosis (PI-CF) meeting standard criteria for normal glucose tolerance display impaired β-cell secretory capacity and early-phase insulin secretion defects. We sought evidence of impaired β-cell secretory capacity, a measure of functional β-cell mass, among those with early glucose intolerance (EGI), defined as 1-hour oral glucose tolerance test (OGTT) glucose ≥155 mg/dL (8.6 mmol/L).
Methods:
A cross-sectional study was conducted in the Penn and CHOP Clinical & Translational Research Centers. PI-CF categorized by OGTT as normal (PI-NGT: 1-hour glucose <155 mg/dL and 2-hour <140 mg/dL [7.8 mmol/L]; n = 13), PI-EGI (1-hour ≥155 mg/dL and 2-hour <140 mg/dL; n = 13), impaired (PI-IGT: 2-hour ≥140 and <200 mg/dL [11.1 mmol/L]; n = 8), and diabetic (cystic fibrosis-related diabetes, CFRD: 2-hour ≥200 mg/dL; n = 8) participated. Post-prandial glucose tolerance and insulin secretion, and β-cell secretory capacity and demand were derived from mixed-meal tolerance tests (MMTTs), and glucose-potentiated arginine (GPA) tests, respectively.
Results:
PI-EGI had elevated post-prandial glucose with reduced early-phase insulin secretion during MMTT compared to PI-NGT (P < .05). PI-EGI also exhibited impaired acute insulin and C-peptide responses to GPA (P < .01 vs PI-NGT), measures of β-cell secretory capacity. Proinsulin secretory ratios were higher under hyperglycemic clamp conditions in PI-IGT and CFRD (P < .05 vs PI-NGT), and correlated with 1-hour glucose in PI-CF (P < .01).
Conclusions:
PI-CF patients with 1-hour OGTT glucose ≥155 mg/dL already manifest impaired β-cell secretory capacity with associated early-phase insulin secretion defects. Avoiding hyperglycemia in patients with EGI may be important for preventing excessive insulin demand indicated by disproportionately increased proinsulin secretion.
Keywords: cystic fibrosis-related diabetes, early glucose intolerance, insulin secretion, proinsulin secretory ratio, β-cell secretory capacity
1. INTRODUCTION
Cystic fibrosis (CF) is a life-threatening autosomal recessive disorder caused by severely impaired or absent function of the cystic fibrosis transmembrane regulator (CFTR), a protein that normally functions as an anion channel transporting bicarbonate and chloride across epithelial membranes.1 Loss of CFTR function results in multi-organ dysfunction with (1) exocrine pancreatic insufficiency typically commencing in infancy and leading to malabsorption and failure to thrive and (2) impaired respiratory secretion clearance and pulmonary inflammation contributing to susceptibility to sinopulmonary infections, progressive decline in lung function, and ultimately respiratory failure. With advances in CF nutrition and pulmonary care improving median survival well in to adulthood, cystic fibrosis-related diabetes (CFRD) has emerged as a common comorbidity affecting ~40% of adults with CF by age 30 years.2 This increased diabetes risk is more pronounced in the setting of pancreatic insufficiency,3 a finding suggested by the ability of CF patients with preserved pancreatic exocrine function to maintain normal β-cell secretory capacity relative to an impairment seen in those with pancreatic insufficiency.4
Highlighting its clinical relevance, CFRD is associated with deterioration in nutritional status, decline in pulmonary function, and increased mortality, which occurs despite early identification and treatment.5 Identification of patients with pancreatic insufficient CF (PI-CF) at high-risk of CFRD is critically important to developing therapeutic measures aimed at preserving β-cell function. Current criteria for evaluating glucose tolerance in CF acknowledge early glucose abnormalities and specify an isolated 1-hour glucose during oral glucose tolerance testing (OGTT) ≥200 mg/dL (11.1 mmol/L) as abnormal.6 We have recently reported impaired β-cell secretory capacity even in the setting of PI-CF and normal glucose tolerance (NGT), defined using the 1-hour OGTT glucose <200 mg/dL, compared to pancreatic sufficient CF (PS-CF) and healthy control subjects.4 In non-CF populations, 1-hour OGTT glucose above a threshold as low as 155 mg/dL (8.6 mmol/L) portends increased risk of developing type 2 diabetes,7–12 greater adverse cardiovascular disease outcomes,7,13 and increased mortality,7,14 and is associated with impaired β-cell function.15,16 In CF, higher 1-hour glucose is associated with a 4-to-5-fold increased risk for developing CFRD over the subsequent 5 years.17 Thus, improved discrimination of OGTT 1-hour glucose that identifies early defects in insulin secretion is needed to better understand the relationships between glucose intolerance and CF-relevant outcomes, and for developing interventions that may preserve pancreatic β-cell function and interrupt the progression to CFRD.
Based on these observations in non-CF and CF populations with elevated 1-hour OGTT, we hypothesized that the presence of 1-hour OGTT glucose ≥155 mg/dL in PI-CF would be associated with impaired early-phase insulin secretion during meal ingestion and impaired β-cell secretory capacity compared to PI-CF individuals with 1-hour OGTT glucose <155 mg/dL.
2. SUBJECTS AND METHODS
2.1. Subjects
Adolescents age ≥16 years and adults with a confirmed diagnosis of PI-CF, including positive sweat test or CFTR mutation analysis, and requirement for pancreatic enzyme replacement therapy,18 were invited to participate. Glucose tolerance was defined by fasting, 1-, and 2-hour glucose during 75-g OGTT6 performed for clinical indications within 3 months prior to study participation or based on a previously confirmed diagnosis of CFRD. Participants undergoing OGTT were categorized as having NGT for 1-hour glucose <155 mg/dL (8.6 mmol/L) and 2-hour glucose <140 mg/dL (7.8 mmol/L); early glucose intolerance (EGI) for 1-hour glucose ≥155 mg/dL and 2-hour glucose <140 mg/dL; impaired glucose tolerance (IGT) for 2-hour glucose ≥140 mg/dL and <200 mg/dL (11.1 mmol/L); CFRD for fasting glucose ≥126 mg/dL (7 mmol/L; with fasting hyperglycemia) or 2-hour glucose ≥200 mg/dL. The 1-hour glucose cutoff of 155 mg/dL was selected on the basis of non-CF studies associating this value with impaired β-cell function and increased risk for diabetes development. 7–12,15,16
Individuals with acute illness requiring a change in antibiotics or administration of oral or intravenous glucocorticoids within the previous 4 weeks, clinically symptomatic pancreatitis within the previous 12 months, prior lung or liver transplant, or significant kidney or liver dysfunction, as well as pregnant or nursing females, were excluded. The institutional review boards of the University of Pennsylvania (Penn) and Children’s Hospital of Philadelphia (CHOP) approved the study, and all participants gave written informed consent and assent (when age appropriate) to participate. Study procedures were performed over 2 study visits conducted in the Penn or CHOP Clinical and Translational Research Center as previously described.4
2.2. Continuous glucose monitoring
Continuous glucose monitoring (CGM) was performed as a dynamic assessment of glycemic control; CFRD subjects prescribed insulin therapy or repaglinide19 remained on treatment during CGM monitoring. Interstitial glucose was measured from a subcutaneously inserted sensor every 10 seconds with average values recorded every 5 minutes using a CGM system (CGMS iPro; Medtronic Minimed, Northridge, California). Subjects used a study glucometer (OneTouch Ultra; LifeScan, Milpitas, California) to monitor plasma glucose and calibrate the CGMS 4 times daily with no interval between readings exceeding 12 hours. Subjects removed the sensor after 72 hours, and downloaded data were included if ≥36 hours in duration and if based on adequate blood glucose calibrations.
2.3. Mixed-meal tolerance test
A standardized mixed-meal tolerance test (MMTT) was performed to assess post-prandial glucose tolerance and insulin secretion as previously described.4 After a 12-hour overnight fast, an antecubital or forearm venous catheter was placed for blood sampling. After at least 20 minutes of acclimatization to the catheter, baseline blood samples were taken at t = −10 and −1 minute before ingestion of an 820 kcal breakfast over 15 minutes starting at t = 0. The carbohydrate, fat, and protein composition of the meal were 47%, 40%, and 13% of the total energy content.20 Subjects took their prescribed dose of pancreatic enzyme replacement with the test meal. Participants with CFRD held any prescribed mealtime insulin or repaglinide. Additional blood samples were collected at t = 10, 15, 20, 30, 60, 90, 120, 150, 180, 210, and 240 minutes from the start of the meal.
2.4. Glucose-potentiated arginine test
The glucose-potentiated arginine (GPA) test was performed to assess β-cell secretory capacity and demand as previously described.4,21,22 After a 12-hour overnight fast, 1 catheter was placed in an antecubital vein for infusions, and 1 catheter was placed in a distal forearm or hand vein for blood sampling, with the hand placed in a heating pad to promote arterialization of the venous blood. After at least 20 minutes acclimatization to the catheters, baseline blood samples were taken at t = −5 and −1 minute before injection of 5 g of 10% arginine over 1-minute starting at t = 0. Additional blood samples were collected at t = 2, 3, 4, and 5 minutes after arginine injection. Beginning at t = 10 minutes, a hyperglycemic clamp technique23 using a variable rate infusion of 20% dextrose was performed to achieve a plasma glucose concentration of ~230 mg/dL (~13 mmol/L). Blood samples were taken for every 5 minutes to adjust the infusion rate and achieve the desired plasma glucose concentration. After 45 minutes of glucose infusion (at t = 55 minutes), a second 5-g arginine pulse was injected with identical blood sampling. A subsequent 2-hour period without glucose infusion allowed plasma glucose to return to the baseline. A second hyperglycemic clamp was then performed to achieve a plasma glucose concentration of ~340 mg/dL (~19 mmol/L). After 45 minutes of glucose infusion, a third 5-g arginine pulse was injected with identical blood sampling.
2.5. Biochemical analysis
Plasma glucose was measured in duplicate by the glucose oxidase method using an automated glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH). Samples were collected on ice into tubes containing EDTA and protease inhibitor cocktail (and for MMTT samples, DPP4 inhibitor; Sigma-Aldrich, St. Louis, Missouri), centrifuged at 4°C, separated, and frozen at −80°C for subsequent analysis. Plasma insulin, C-peptide, proinsulin, and glucagon were measured in duplicate by double antibody radioimmunoassays (Millipore, Billerica, Massachusetts). Active glucagon-like peptide 1 (GLP-1) and total glucose-dependent insulinotropic polypeptide (GIP) were measured in duplicate by enzyme-linked immunosorbent assays (Millipore).
2.6. Calculations and statistical analyses
2.6.1. Continuous glucose monitoring
Following international consensus guidelines on use of CGM,24 mean glucose, glucose SD, coefficient of variation (CV) and percent (%) time glucose >140 and >180 mg/dL were calculated from continuous interstitial glucose data, including data limited to the daytime period (defined as 06:00 to 24:00), using HypoCount software (version 1.1; PRECISE Center, University of Pennsylvania, Philadelphia, Pennsylvania). CV for glucose was calculated from the glucose SD divided by mean glucose.
2.6.2. Mixed-meal tolerance test
Insulin secretory rates (ISRs) were calculated from C-peptide values and derived by parametric deconvolution of C-peptide kinetics using a two-compartment model25 in WinSAAM software 3.0.8 (University of Pennsylvania, New Bolton Center, Kennett Square, Pennsylvania). Incremental areas under the curve (AUCs) for glucose, ISRs, GLP-1, and GIP were calculated with baseline values subtracted using the trapezoidal method in STATA 12 software (StataCorp LP, College Station, Texas).
2.6.3. GPA test
Acute islet cell responses were calculated as the difference between mean of the 2-, 3-, 4-, and 5-minute post-arginine values minus the mean of the pre-stimulus values under conditions of fasting (acute insulin [AIRarg], C-peptide [ACRarg], proinsulin [APRarg], and glucagon [AGRarg] responses), the ~230 mg/dL glucose clamp (glucose potentiation of arginine-induced insulin [AIRpot], C-peptide [ACRpot], and proinsulin [APRpot] release, and glucose inhibition of arginine-induced glucagon [AGRinh] release), and the ~340 mg/dL glucose clamp (maximum arginine-induced insulin [AIRmax], C-peptide [ACRmax], and proinsulin [APRmax] release, and minimum arginine-induced glucagon release [AGRmin]).21,23 The proinsulin-to-C-peptide ratio was calculated as the molar concentration of proinsulin divided by the molar concentration of C-peptide multiplied by 100.26 We also assessed the proinsulin secretory ratio (PISR) in response to each injection of arginine from the respective acute proinsulin:C-peptide responses to arginine.22,26 β-Cell sensitivity to glucose (PG50, plasma glucose at half-maximal insulin secretion) and peripheral insulin sensitivity (M/I, mean glucose infusion rate required during the 230 mg/dL glucose clamp [M] divided by the mean pre-stimulus insulin level [I] between 40 and 45 minutes of the glucose infusion) were calculated as previously described.4,22
2.6.4. Statistical analyses
The study was designed as a pilot powered to detect differences in β-cell secretory capacity between groups assuming a weighted SD ~1/3 of the mean27 and requiring ~10 subjects per group to provide 80% power. Data are reported as median and interquartile range, unless otherwise noted. Subject demographics were examined using the Kruskal-Wallis test for continuous variables and χ2 test for categorical variables. Comparison of results across study groups was performed with the nonparametric test for progressively worsening differences across ordered groups, nptrend, and when significant differences at P < .05 were found, pairwise comparisons were performed using the Mann-Whitney U test. Spearman correlation was performed to examine the relationship of PISRs and 1-hour OGTT glucose. All analyses were performed using STATA 12 (StataCorp LP). Significance was considered at P ≤ .05 (two-tailed).
3. RESULTS
3.1. Subject characteristics
Forty-two subjects with CF participated in the study: 13 PI-NGT, 13 PI-EGI, 8 PI-IGT, and 8 CFRD (Table 1). Three of the PI-EGI subjects had a 1-hour OGTT glucose ≥200 mg/dL (11.1 mmol/L) consistent with current Cystic Fibrosis Foundation criteria defining indeterminate glucose tolerance (PI-INDT).6 Age, sex distribution, and BMI were comparable across the groups (Table 1). Study participants were in stable pulmonary status, and their lung function, as represented by percent-predicted forced expiratory volume in 1 second (FEV1, % predicted), was similar across groups (Table 1). Half of the subjects were homozygous for the loss-of-function, F508del CFTR mutation. HbA1c was higher only in the group with CFRD (P < .01 vs PI-NGT; Table 1).
TABLE 1.
Subject characteristics
| PI-NGT | PI-EGI | PI-IGT | PI-CFRD | P-value | |
|---|---|---|---|---|---|
| Demographics | (n = 13) | (n = 13) | (n = 8) | (n = 8) | |
| Age, y | 19 (16–50) | 25 (18–44) | 27.5 (16–29) | 25.5 (19–43) | .27 |
| Sex, female | 7 (54) | 6 (46) | 4 (50) | 3 (38) | .91 |
| BMI, kg/m2 | 22 (18–31) | 24 (17–32) | 23 (20–34) | 23 (19–26) | .94 |
| FEV1, % predicted | 88 (54–113) | 75 (51–107) | 88 (57–112) | 82 (27–125) | .66 |
| CFTR mutation | |||||
| F508 del homozygous | 4 (31) | 8 (61) | 5 (63) | 4 (50) | .61 |
| F508 del heterozygous | 6 (46) | 4 (31) | 3 (37) | 3 (38) | |
| Other | 3 (23) | 1 (8) | 0 (0) | 1 (12) | |
| HbA1c, % | 5.5 (4.7–6.2) | 5.3 (5.1–5.9) | 5.6 (4.8–6.4) | 6.0** (5.5–10.2) | .012 |
| HbA1c, mmol/mol | 37 (28–44) | 34 (32–41) | 38 (29–46) | 42 (37–88) | |
| OGTT profile | (n = 13) | (n = 13) | (n = 8) | (n = 4) | |
| Fasting glucose, mg/dL | 82 (64–90) | 89 (73–104) | 83 (77–103) | 91 (69–139) | .11 |
| Fasting glucose, mmol/L | 4.6 (3.6–5.0) | 4.9 (4.1–5.8) | 4.6 (4.3–5.7) | 5.1 (3.8–7.7) | |
| 1-hour glucose, mg/dL | 128 (96–154) | 189** (162–216) | 182** (129–260) | 245** (233–399) | <.0001 |
| 1-hour glucose, mmol/L | 7.1 (5.3–8.5) | 10.5 (9.0–12.0) | 10.1 (7.2–14.4) | 13.6 (12.9–22.1) | |
| 2-hour glucose, mg/dL | 90 (66–116) | 98** (34–136) | 172** (151–196) | 289** (217–336) | <.0001 |
| 2-hour glucose, mmol/L | 5.0 (3.7–6.4) | 5.4 (1.9–7.5) | 9.5 (8.4–10.9) | 16.0 (12.0–18.6) | |
| CGMS profile | (n = 6) | (n = 6) | (n = 5) | (n = 5) | |
| Mean glucose, mg/dL | 108 (101–116) | 116 (111–121) | 117 (111–139) | 126* (123–192) | .014 |
| Mean glucose, mmol/L | 6.0 (5.6–6.4) | 6.4 (6.2–6.7) | 6.5 (6.2–7.7) | 7.0 (6.8–10.7) | |
| Daytimea mean glucose, mg/dL | 112 (102–117) | 115 (109–122) | 120 (115–143) | 129* (128–191) | .016 |
| Daytimea mean glucose, mmol/L | 6.2 (5.7–6.5) | 6.4 (6.0–6.8) | 6.7 (6.4–7.9) | 7.2 (7.1–10.6) | |
| Glucose SD, mg/dL | 18 (13–26) | 19 (17–22) | 33 (26–48) | 40* (31–49) | .005 |
| Glucose SD, mmol/L | 1.0 (0.7–1.4) | 1.1 (0.9–1.2) | 1.8 (1.4–2.7) | 2.2 (1.5–2.7) | |
| Daytimea glucose SD, mg/dL | 19 (13–26) | 17 (16–22) | 36* (26–54) | 42* (32–53) | .004 |
| Daytimea glucose SD, mmol/L | 1.1 (0.7–1.4) | 0.9 (0.9–1.2) | 2.0 (1.4–3.0) | 2.3 (1.8–2.9) | |
| Glucose CV, % | 0.17 (0.13–0.21) | 0.18 (0.14–0.20) | 0.28 (0.23–0.35) | 0.25* (0.25–0.32) | .009 |
| Daytimea glucose CV, % | 0.17 (0.13–0.21) | 0.15 (0.14–0.20) | 0.29* (0.22–0.38) | 0.28* (0.25–0.33) | .008 |
| Time >140 mg/dL (7.8 mmol/L), % | 7 (2–13) | 12 (10–13) | 22* (18–36) | 30* (28–80) | .003 |
| Daytimea >140 mg/dL (7.8 mmol/L), % | 8 (1–14) | 12 (5–17) | 25* (23–39) | 35* (33–83) | .002 |
| Time >180 mg/dL (10 mmol/L), % | 1 (0–4) | 1 (0–2) | 10* (5–11) | 10 (4–55) | .011 |
| Daytimea >180 mg/dL (10 mmol/L), % | 1 (0–6) | 1 (0–1) | 10* (6–12) | 11* (6–53) | .007 |
Abbreviations: CFRD, cystic fibrosis-related diabetes; CGMS, continuous glucose monitoring system; EGI, early glucose intolerance; FEV1, forced expiratory volume in 1 second; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; PI, pancreatic insufficient cystic fibrosis. Data are medians and ranges (min-max) for continuous variables and number and percentage (%) for categorical variables.
P ≤ .05 vs PI-NGT
P < .01 vs PI-NGT.
Daytime is defined as 06:00 to 24:00.
By definition, 1-hour OGTT glucose was higher in PI-EGI compared to PI-NGT (P < .01; Table 1). As expected, 1-hour OGTT glucose was also higher in PI-IGT and CFRD groups compared to PI-NGT (P < .01 for all comparisons; Table 1). By definition 2-hour OGTT glucose was <140 mg/dL (7.8 mmol/L) in PI-EGI and in PI-NGT, but comparatively higher in PI-EGI than in PI-NGT (P < .01). Similarly, 2-hour OGTT glucose was higher in the PI-IGT and CFRD groups compared to subjects with PI-NGT (P < .01 for all comparisons; Table 1). Four of the eight subjects with CFRD were recent diagnoses based on 2-hour OGTT glucose ≥200 mg/dL. Of these 4 recent diagnoses, 1 had fasting hyperglycemia and 1 had been chronically treated (~5 years) with insulin administered prior to continuous overnight enteral nutrition but was not known to have daytime hyperglycemia. Four subjects had established diagnoses of CFRD; 2 were treated with the oral anti-diabetic agent repaglinide, and 1 had fasting hyperglycemia and was managed with an insulin pump.
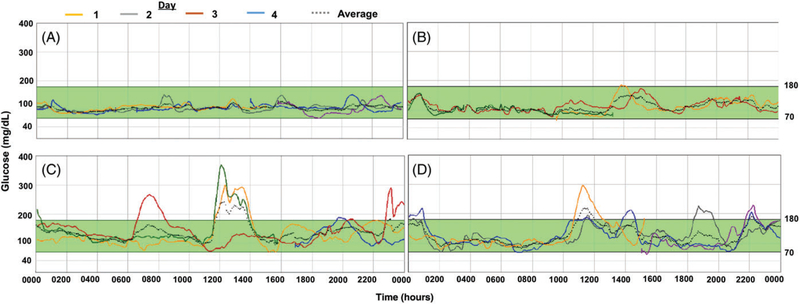
Under free-living conditions captured by CGM, progressively higher mean glucose, glucose SD, glucose CV and times spent with glucose over 140 and 180 mg/dL (10 mmol/L) were present in PI-CF with worsening OGTT-defined glucose tolerance (Table 1; Figure 1). However, PI-EGI did not demonstrate higher glucose or glucose variability than PI-NGT.
FIGURE 1.
Continuous glucose monitoring (CGM) for >72 hours in representative individual subjects with PI-CF. PI-CF subjects were categorized by an oral glucose tolerance test (OGTT) as normal (NGT: 1-hour glucose <155 mg/dL [8.6 mmol/L] and 2-hour glucose <140 mg/dL [7.8 mmol/L]), early glucose tolerance (EGI: 1-hour glucose ≥155 mg/dL and 2-hour glucose <140 mg/dL), impaired (IGT: 2-hour glucose ≥140 mg/dL and <200 mg/dL [11.1 mmol/L]), or diabetic (CFRD: 2-hour glucose ≥200 mg/dL). (A) 17-year-old female with PI-NGT. (B) 20-year-old female with PI-EGI. (C) 29-year-old male with PI-IGT. (D) 27-year-old female with CFRD, controlled by diet
3.2. Insulin and incretin secretion during the MMTT
During the first 30 minutes after meal ingestion, plasma glucose (AUCglu) was higher and insulin secretion (AUCISR) lower only in subjects with CFRD (P < .05 vs PI-NGT for both; Table 2; Figure 1A,B). However, insulin secretion relative to the plasma glucose excursion (AUCISR/AUCglu) worsened across PI glucose tolerance groups and was already reduced in the PI-EGI group compared to PI-NGT (P = .04; Table 2). GLP-1 (AUCGLP-1) and GIP (AUCGIP) secretion by 30 minutes after meal ingestion were similar across groups (Table 2).
TABLE 2.
Mixed-meal tolerance test (MMTT) responses during the first 30 and 180 minutes post-ingestion
| PI-NGT (n = 13) | PI-EGI (n = 13) | PI-IGT (n = 8) | PI-CFRD (n = 8) | P-value | |
|---|---|---|---|---|---|
| 30 minutes | |||||
| AUCglu, mg min/dL | 349 (120–499) | 487 (227–568) | 484 (355–713) | 740** (565–800) | .004 |
| AUCglu, mmol min/L | 19.4 (6.7–27.7) | 27.0 (12.6–31.5) | 26.9 (19.7–39.6) | 41.1 (31.4–44.4) | |
| AUCISR, mU/L | 52 (26–98) | 42 (16–53) | 29 (22–53) | 13* (2–32) | .018 |
| AUCISR, pmol/L | 361 (181–681) | 292 (111–368) | 201 (152–368) | 90 (14–222) | |
| AUCISR/AUCglu, mU min/mg | 0.01 (0.009–0.02) | 0.008* (0.006–0.01) | 0.005* (0.004–0.01) | 0.002* (0.001–0.004) | <.0001 |
| AUCGLP-1, pmol min/L | 29 (16–47) | 30 (12–83) | 38 (22–85) | 47 (29–66) | .37 |
| AUCGIP, pg min/mL | 795 (379–1410) | 758 (666–1573) | 662 (575–834) | 1102 (652–1624) | .67 |
| 180 minutes | |||||
| AUCglu, mg min/dL | 3855 (2372–5197) | 5506* (4858–7990) | 7904** (6177–9247) | 16 128** (12 435–20 350) | <.0001 |
| AUCglu, mmol min/L | 214 (132–288) | 306 (270–443) | 439 (343–513) | 895 (690–1129) | |
| AUCISR, mU/L | 1966 (909–2130) | 1069 (713–1513) | 1300 (1050–1808) | 1311 (1171–1684) | .72 |
| AUCISR, pmol/L | 13 654 (6313–14 793) | 7424 (4952–10 508) | 9029 (7292–12 557) | 9105 (8133–11 695) | |
| AUCISR/AUCglu, mU min/mg | 0.04 (0.03–0.07) | 0.02* (0.01–0.03) | 0.02** (0.01–0.03) | 0.01** (0.006–0.01) | <.0001 |
| AUCGLP-1, pmol min/L | 326 (149–476) | 191 (103–422) | 603 (255–827) | 550 (305–764) | .07 |
| AUCGIP, pg min/mL | 18 721 (14 824–25 375) | 20 305 (12 411–28 675) | 14 627 (10 201–20 446) | 22 363 (16 622–25 774) | .92 |
Abbreviations: AUC, incremental area under the curve for glucose (glu); CFRD, cystic fibrosis-related diabetes; EGI, early glucose intolerance; GIP, glucose-dependent insulinotropic polypeptide; GLP, glucagon-like peptide-1; IGT, impaired glucose tolerance; ISR, insulin secretory rate; NGT, normal glucose tolerance; PI, pancreatic insufficient cystic fibrosis. Data are medians and interquartile ranges.
P < .05 vs PI-NGT;
P < .01 vs PI-NGT.
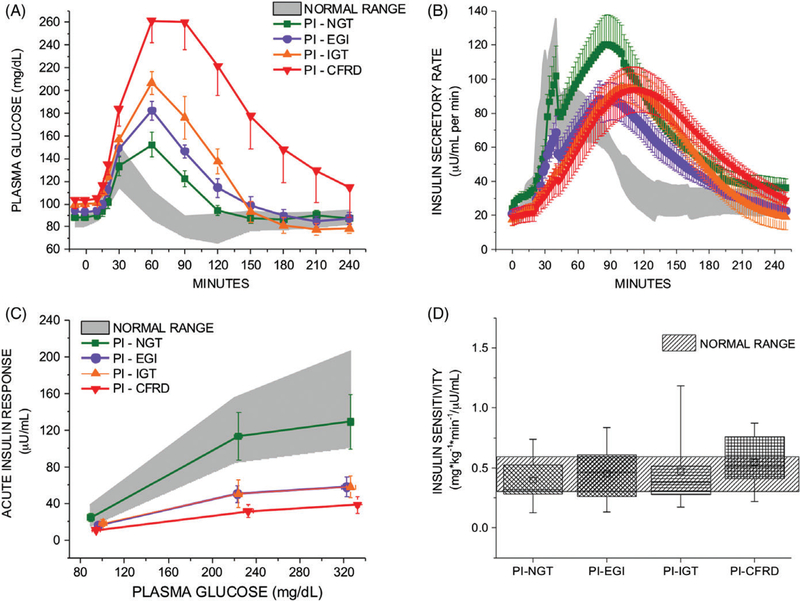
Over 180 minutes, higher plasma glucose excursion became evident in PI-EGI subjects (P = .01 vs PI-NGT) and progressed in severity among those with PI-IGT and CFRD (P < .01 vs PI-NGT; Table 2; Figure 2A). With the increasing plasma glucose, AUCISR/AUCglu was lower in the PI-EGI (P < .05 vs PI-NGT), PI-IGT (P < .01 vs PI-NGT) and CFRD (P < .01 vs PI-NGT) groups and progressed in severity as glucose tolerance worsened across PI (P < .0001; Table 2; Figure 2B). GLP-1 and GIP secretion were similar across groups (Table 2).
FIGURE 2.
Plasma glucose (A) and insulin secretory rates (B) in response to the mixed-meal tolerance test (MMTT) in subjects with pancreatic insufficient CF (PI-CF). Impaired mixed-meal tolerance (A) and early-phase insulin secretion (B) were consistent with the oral glucose tolerance test categorization (NGT, normal glucose tolerance; EGI, early glucose intolerance; IGT, impaired glucose tolerance; CFRD, CF-related diabetes). Acute insulin responses to arginine as a function of the pre-stimulus plasma glucose concentration (C) derived from the glucose-potentiated arginine test indicate a downward shift consistent with reduced β-cell secretory capacity in PI-EGI that is similar as for PI-IGT, progressing further in CFRD, with no change in β-cell sensitivity to glucose (PG50). Insulin sensitivity (D) calculated as M/I from the ~230 mg/dL (13 mmol/L) glucose clamp was not different across the groups of PI subjects. In A, B, and C data are given as mean ± SE, and in D as median and interquartile range (box) and mean (open squares) and range (error bars). Normal ranges are provided as the 95% CI for data derived from healthy control subjects4
3.3. Glucose and islet cell hormonal responses during the GPA test
Fasting plasma glucose prior to GPA testing was 93 (88–99) mg/dL (5.2 [4.9–5.5] mmol/L) and not different across the groups. Prestimulus plasma glucose during the ~230 mg/dL clamp was 225 (221–232) mg/dL (12.5 [12.3–12.9] mmol/L) and during the ~340 mg/dL clamp was 328 (319–336) mg/dL (18.2 [17.7–18.6] mmol/L); neither pre-stimulus glucose condition was different across the groups.
Fasting islet cell hormone concentrations were similar among the groups (Table 3). Acute insulin responses (AIRs) under fasting, ~230 and 340 mg/dL hyperglycemic clamp conditions (AIRarg, AIRpot, and AIRmax, respectively) were progressively lower with worsening OGTT- defined glucose intolerance (P < .01 for all; Table 3). Under hyperglycemic clamp conditions, PI-EGI had lower AIRs than PI-NGT (AIRpot: P < .01 and AIRmax: P < .05; Table 3), while AIRpot and AIRmax were not significantly different between PI-EGI and PI-IGT (Table 3; Figure 2C).
TABLE 3.
Fasting islet cell hormones, acute responses, proinsulin to C-peptide and proinsulin secretory ratios (PISRs) during the glucose-potentiated arginine (GPA) test
| PI-NGTa (n = 13) | PI-EGIb (n = 13) | PI-IGTc (n = 7) | PI-CFRDd (n = 8) | P-value | |
|---|---|---|---|---|---|
| Fasting insulin, μU/mL | 8.5 (5.2–9.1) | 8.6 (7.2–9.7) | 8.4 (5.7–13.3) | 7.6 (6.5–9.3) | .97 |
| Fasting insulin, pmol/L | 59.0 (36.1–63.2) | 59.7 (50.0–67.4) | 58.3 (39.6–92.4) | 52.8 (45.1–64.6) | |
| AIRarg, μU/mL | 23.0 (15.7–29.7) | 14.3 (9.8–19.6) | 14.3 (13.0–26.1) | 10.0** (8.7–15.1) | .015 |
| AIRarg, pmol/L | 159.7 (109.0–206.3) | 99.3 (68.1–136.1) | 99.3 (90.3–181.3) | 69.5 (60.4–104.9) | |
| AIRpot, μU/mL | 75.5 (67.7–152.1) | 46.6** (21.3–56.1) | 40.6* (26.3–48.3) | 27.3** (21.5–34.2) | .001 |
| AIRpot, pmol/L | 524.3 (470.2–1056.3) | 323.6 (147.9–389.6) | 282.0 (182.7–335.4) | 189.6 (149.3–237.5) | |
| AIRmax, μU/mL | 86.5 (69.8–163.9) | 54.9* (29.6–71.6) | 42.6 (38.0–82.2) | 30.1** (22.3–48.5) | .003 |
| AIRmax, pmol/L | 600.7 (484.8–1138.3) | 381.3 (205.6–497.3) | 295.9 (263.9–570.9) | 209.0 (154.9–336.8) | |
| Fasting C-peptide, ng/mL | 1.1 (0.9–1.2) | 0.8 (0.7–1.2) | 1.0 (0.5–1.1) | 0.7 (0.5–1.2) | .10 |
| Fasting C-peptide, nmol/L | 0.4 (0.3–0.4) | 0.3 (0.2–0.4) | 0.3 (0.2–0.4) | 0.2 (0.2–0.4) | |
| ACRarg, ng/mL | 1.0 (0.8–1.3) | 0.7* (0.5–0.8) | 0.6* (0.6–0.8) | 0.7** (0.3–0.7) | .006 |
| ACRarg, nmol/L | 0.3 (0.3–0.4) | 0.2 (0.2–0.3) | 0.2 (0.2–0.3) | 0.2 (0.1–0.2) | |
| ACRpot, ng/mL | 4.1 (3.0–4.6) | 1.7** (1.2–2.2) | 1.7** (1.5–2.6) | 1.3** (0.9–1.9) | .001 |
| ACRpot, nmol/L | 1.4 (0.1–1.5) | 0.6 (0.4–0.7) | 0.6 (0.5–0.9) | 0.4 (0.3–0.6) | |
| ACRmax, ng/mL | 4.0 (2.7–5.6) | 2.4** (1.4–2.6) | 1.9** (1.8–2.1) | 1.6** (1.0–2.3) | .001 |
| ACRmax, nmol/L | 1.3 (0.9–1.9) | 0.8 (0.5–0.9) | 0.6 (0.6–0.7) | 0.5 (0.3–0.8) | |
| Fasting glucagone, pg/mL | 52 (37–62) | 49 (43–58) | 45 (30–56) | 51 (38–76) | .67 |
| AGRarge, pg/mL | 33 (24–60) | 24 (18–34) | 35 (18–50) | 34 (17–55) | .99 |
| AGRinhe, pg/mL | 24 (17–42) | 29 (15–36) | 29 (23–30) | 39 (15–49) | .59 |
| AGRmine, pg/mL | 20 (16–42) | 20 (10–30) | 18 (7–38) | 27 (17–40) | .70 |
| Fasting proinsulin, pmol/L | 10.9 (8.6–13.0) | 10.3 (5.7–11.7) | 9.6 (2.0–15.2) | 4.7 (3.1–14.5) | .31 |
| APRarg, pmol/L | 5.9 (3.0–7.3) | 3.0 (2.3–4.4) | 4.4 (1.5–8.0) | 1.6 (0.3–4.4) | .11 |
| APRpot, pmol/L | 20.6 (19.3–26.4) | 13.8** (9.7–18.5) | 13.9* (12.3–15.5) | 8.2** (5.3–20.3) | <.0001 |
| APRmax, pmol/L | 17.7 (12.2–20.9) | 14.0 (9.1–16.8) | 13.8 (10.5–19.2) | 11.3 (7.1–15.3) | .06 |
| Fasting proinsulin: C-peptide ratio, % | 3.3 (1.5–3.8) | 2.9 (1.6–3.7) | 2.8 (1.3–3.2) | 1.8 (1.7–2.8) | .29 |
| PISR fasting, % | 1.6 (0.9–1.9) | 1.6 (0.7–2.2) | 1.7 (0.7–2.9) | 1.4 (0.1–1.9) | .67 |
| PISR 230 mg/dL (13 mmol/L), % | 1.7 (1.5–2.0) | 2.7 (1.6–2.9) | 2.3 (2.1–3.2) | 1.9 (1.4–2.2) | .64 |
| PISR 340 mg/dL (19 mmol/L), % | 1.3 (1.0–1.5) | 1.8 (1.4–2.4) | 1.9* (1.7–2.2) | 2.3* (1.4–2.8) | .01 |
Abbreviations: CFRD, cystic fibrosis-related diabetes; EGI, early glucose intolerance; IGT, impaired glucose tolerance; NGT, normal glucose tolerance; PI, pancreatic insufficient cystic fibrosis. Data are medians and interquartile ranges.
P < .05 vs PI-NGT;
P < .01 vs PI-NGT.
n = 12 for AIRarg, AGRarg, AGRpot, APRarg, PISR, fasting, PISR, 340 mg/dL (19 mmol/L).
n = 11 for AIRmax.
n = 6 for ACRpot, AIRpot, AGRpot, APRpot.
n = 7 for APRmax.
1:1 conversion from pg/mL to ng/L.
Similarly, C-peptide results were consistent with reduced β-cell secretory capacity. Acute C-peptide responses (ACRarg, ACRpot, and ACRmax) were progressively lower in PI-CF groups as glucose tolerance worsened. Under all conditions, PI-EGI had lower ACRs compared to PI-NGT (P < .05 for all comparisons; Table 3).
No differences in acute glucagon responses (AGRarg, AGRinh, and AGRmin) were observed across the groups (Table 3).
The acute proinsulin responses during fasting (APRarg) did not differ significantly across groups (Table 3). While APRpot was lower in PI-EGI compared to PI-NGT (P < .01) and progressed in severity as glucose tolerance worsened (Table 3), during the ~340 mg/dL hyperglycemic clamp, APRmax was only lower by trend across the groups (P = .06; Table 3).
No differences in fasting proinsulin-to-C-peptide ratios or the PISR were present under fasting or ~230 mg/dL hyperglycemic clamp conditions across the groups. Under the ~340 mg/dL hyperglycemic clamp condition, with worsening glucose tolerance, PISR was progressively higher, and PISR was specifically higher in PI-IGT and CFRD vs PI-NGT (P < .05 for both comparisons; Table 3).
β-Cell sensitivity to glucose, measured as PG50 (Figure 2C), and insulin sensitivity, measured as M/I (Figure 2D), were not different across the PI-CF groups.
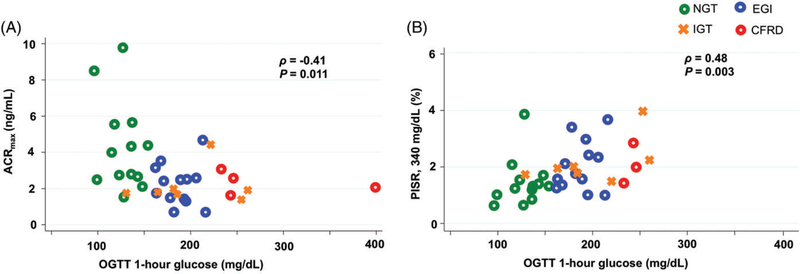
The 1-hour OGTT glucose was significantly inversely correlated with measures of β-cell secretory capacity (AIRmax: ρ = −0.39, P = .021; ACRmax: ρ = −0.41, P = .011; Figure 3A) and directly correlated with PISR under ~340 mg/dL hyperglycemic clamp conditions (ρ = 0.48, P = .003; Figure 3B).
FIGURE 3.
Relationship between oral glucose tolerance test (OGTT) 1-hour glucose and β-cell secretory capacity (ACRmax; A) and proinsulin secretory ratio (PISR; B) during the ~340 mg/dL (19 mmol/L) hyperglycemic clamp
4. DISCUSSION
This study evaluated insulin secretion across the spectrum of OGTT- defined glucose abnormalities in PI-CF with specific attention to isolated elevation of 1-hour OGTT glucose ≥155 mg/dL (8.6 mmol/L). Individuals with such early glucose intolerance (PI-EGI) in the setting of CF manifest impaired mixed-meal tolerance with reduced early phase insulin secretion and reduced β-cell secretory capacity that related to disproportionately elevated proinsulin-to-C-peptide secretion during hyperglycemia to ~340 mg/dL. Importantly, β-cell secretory capacity provides a measure of functional β-cell mass, and disproportionate proinsulin secretion reflects increased β-cell secretory demand.21 These defects were similar to those present in individuals with PI-IGT as defined by 2-hour OGTT glucose ≥140 mg/dL (7.8 mmol/L), and were, as expected, worse in those with CFRD. Thus, the disproportionate proinsulin-to-C-peptide secretion evidences release of immature β-cell granules that can be seen with endoplasmic reticulum stress during the exposure of a reduced β-cell mass to hyperglycemia.28 Here we show that even an only slightly elevated 1-hour glucose ≥155 mg/dL in PI-CF indicates the presence of a lower functional β-cell mass that is under increased demand for insulin secretion. We therefore suggest that the compromised β-cell function that accompanies these subtle glucose differences should be considered when targeting dietary and/or pharmacological interventions intended to reduce (1) pancreatic β-cell stress and (2) ultimately, CFRD development.
We previously reported that patients with PS-CF have similar β-cell secretory capacity as healthy control subjects with normal PISRs during hyperglycemia, while PI-CF subjects with a 1-hour OGTT glucose <200 mg/dL exhibited reduced β-cell secretory capacity, increased PISRs during hyperglycemia, and impaired early-phase insulin secretion in response to meal ingestion.4 In that study, the 1-hour OGTT glucose was inversely correlated with measures of β-cell secretory capacity.4 Using an expanded cohort of PI-CF subjects, here we report that using a lower 1-hour OGTT glucose cutoff of ≥155 mg/dL to define EGI already identifies individuals exhibiting marked insulin secretory defects. The small sample of PI-EGI participants with 1-hour OGTT ≥200 mg/dL (Cystic Fibrosis Foundation defined indeterminate glucose tolerance) in the current study precludes comparison with those having 1-hour OGTT ≥155 to <200 mg/dL; however, similar impairment in β-cell secretory capacity would be expected based on the similar impairment seen in the PI-EGI and PI-IGT groups. Consistent with this notion, in non-CF populations individuals with a 1-hour OGTT glucose ≥155 mg/dL but a 2-hour OGTT glucose <140 mg/dL are as likely to develop diabetes as those with IGT defined by a 2-hour OGTT glucose >140 mg/dL.8,9 Identification of insulin secretion defects in the setting of subtle glucose abnormalities offers a potential opportunity to protect β-cells prior to the development of more advanced glucose intolerance. Thus, the identification of EGI may be as important as identification of IGT in PI-CF, and represent similar underlying pathophysiologic defects in pancreatic β-cell function. Nevertheless, the implications of these findings for CF-specific outcomes such as nutritional status and pulmonary function require further study.
Reductions in β-cell secretory capacity and ISRs in PI-EGI did not readily translate into differences in glycemic control under free-living conditions examined by CGM. The reason for the worse glucose tolerance in PI-IGT compared to PI-EGI is also unclear given similarities in β-cell secretory capacity, but the smaller number of IGT participants studied may have limited detection of subtle differences in β-cell secretory capacity. More likely, further delayed insulin secretory dynamics beyond the first 30 minutes of nutrient ingestion seen during the MMTT may explain the greater impairment in oral glucose tolerance. Another possible explanation for the differences in glucose tolerance across pre-diabetic PI-CF groups could be differences in nutrient digestion and absorption within PI subjects. These potential differences in nutrient digestion and absorption between PI glucose tolerance groups require further study. Clinically, patients with PI-CF can vacillate between conditions of PI-EGI and PI-IGT before ultimately progressing to CFRD, but the mechanisms underlying this transition remain to be resolved. A limitation to the CGM data is that it was not controlled for physical activity or dietary intake, information that should be considered in future studies. The small CFRD group size may have limited detection of differences in the β-cell secretory capacity for CFRD vs PI-EGI and PI-IGT. However, these differences approach statistical significance, supporting worsening β-cell secretory capacity once CFRD ensues. Future longitudinal studies should clarify the temporal risk for developing CFRD after detection of either PI-EGI or PI-IGT. Moreover, understanding the relevance of these early glucose tolerance abnormalities and insulin secretion defects to CF-relevant outcomes such as lung function and nutritional status is crucial and requires examination in a larger, longitudinal cohort.
We previously reported evidence for generalized islet loss based on reductions in both β- and α-cell secretory responses during the GPA test in PI-CF compared to PS-CF subjects and healthy controls.4 Here, we show that α-cell glucagon responses to arginine under fasting (when in fact the glucagon response is maximal) and both hyperglycemic clamp conditions are similar in all PI groups. In contrast to the increased glucagon secretion reported in type 2 diabetes,29,30 enhanced glucagon secretion does not contribute to the progression of glucose intolerance in PI-CF. In addition, we did not detect worsening of insulin sensitivity across the groups of PI-CF subjects. Therefore, the physiologic defect in maintaining glucose homeostasis in CF appears restricted to progressive deficiency in islet β-cell insulin secretion.
In conclusion, impaired early-phase insulin secretion and β-cell secretory capacity are present with EGI defined by 1-hour OGTT glucose as low as 155 mg/dL. The disproportionately increased proinsulin-to-C-peptide secretion provides evidence for increased insulin secretory demand during exposure to hyperglycemia and raises concern for compounding the underlying insulin secretion defects. Future studies should assess the clinical implications of these early islet β-cell defects and address whether dietary and/or pharmacological interventions that reduce pancreatic β-cell stress in PI-EGI may delay progression to CFRD.
ACKNOWLEDGEMENTS
We are indebted to the CF subjects for their participation, to the nursing and dietary staff of the Penn and CHOP Clinical & Translational Research Centers for their subject care and technical assistance, to Dr Heather Collins of the University of Pennsylvania Diabetes Research Center for performance of the radioimmunoassays, to Samir Sayed of the Children’s Hospital of Philadelphia’s Translational Core Laboratory for performance of the enzyme-linked immunosorbent assays, and to Huong-Lan Nguyen of the Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism for laboratory assistance. This work was supported by grants from the Cystic Fibrosis Foundation (to A.K. and M.R.R.), Public Health Services Research Grants R01 DK97830 (to A.K. and M.R.R.), K23 DK107937 (to S.S.), UL1 TR000003 (Penn and CHOP Clinical & Translational Research Centers), P30 DK19525 (University of Pennsylvania Diabetes Research Center), and T32 DK007314 (University of Pennsylvania Training Grant in Diabetes, Endocrine and Metabolic Diseases), and the Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism.
Funding information
Cystic Fibrosis Foundation; National Institute of Diabetes and Digestive and Kidney Diseases; Human Metabolism Resource of the University of Pennsylvania Institute for Diabetes, Obesity & Metabolism; University of Pennsylvania Training Grant in Diabetes, Endocrine and Metabolic Diseases, Grant/Award Number: T32 DK007314; University of Pennsylvania Diabetes Research Center, Grant/Award Number: P30 DK19525; Penn and CHOP Clinical & Translational Research Centers, Grant/Award Number: UL1 TR000003; Public Health Services Research Grants, Grant/Award Numbers: K23 DK107937, R01 DK97830
Footnotes
Conflict of interest
No potential conflicts of interest relevant to this article exist and all authors have reviewed and approved submission of this manuscript.
REFERENCES
- 1.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med 2015;372(4):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care 2009;32(9):1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr 2005;146(5):681–687. [DOI] [PubMed] [Google Scholar]
- 4.Sheikh S, Gudipaty L, De Leon DD, et al. Reduced beta-cell secretory capacity in pancreatic-insufficient, but not pancreatic-sufficient, cystic fibrosis despite normal glucose tolerance. Diabetes 2017;66(1): 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran A, Becker D, Casella SJ, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes Care 2010;33(12):2677–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moran A, Brunzell C, Cohen RC, et al. Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33(12):2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pareek M, Bhatt DL, Nielsen ML, et al. Enhanced predictive capability of a 1-hour oral glucose tolerance test: a prospective population- based cohort study. Diabetes Care 2018;41(1):171–177. [DOI] [PubMed] [Google Scholar]
- 8.Fiorentino TV, Marini MA, Andreozzi F, et al. One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. J Clin Endocrinol Metab 2015;100(10):3744–3751. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008; 31(8):1650–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care 2009;32(2):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: observations from the 24-year follow-up of the Israel study of glucose intolerance, obesity and hypertension. Diabetes Res Clin Pract 2016;120:221–228. [DOI] [PubMed] [Google Scholar]
- 12.Oka R, Aizawa T, Miyamoto S, Yoneda T, Yamagishi M. One-hour plasma glucose as a predictor of the development of type 2 diabetes in Japanese adults. Diabet Med 2016;33(10):1399–1405. [DOI] [PubMed] [Google Scholar]
- 13.Succurro E, Marini MA, Arturi F, et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009;207(1): 245–249. [DOI] [PubMed] [Google Scholar]
- 14.Bergman M, Chetrit A, Roth J, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts mortality: observations from the Israel study of glucose intolerance, obesity and hypertension. Diabet Med 2016;33(8):1060–1066. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi C, Miccoli R, Trombetta M, et al. Elevated 1-hour postload plasma glucose levels identify subjects with normal glucose tolerance but impaired beta-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV Study. J Clin Endocrinol Metab 2013;98(5):2100–2105. [DOI] [PubMed] [Google Scholar]
- 16.Jagannathan R, Sevick MA, Li H, et al. Elevated 1-hour plasma glucose levels are associated with dysglycemia, impaired beta-cell function, and insulin sensitivity: a pilot study from a real world health care setting. Endocrine 2016;52(1):172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheikh S, Putt ME, Forde KA, Rubenstein RC, Kelly A. Elevation of one hour plasma glucose during oral glucose tolerance testing. Pediatr Pulmonol 2015;50(10):963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell PM, Rosenstein BJ, White TB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153(2):S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballmann M, Hubert D, Assael BM, et al. Repaglinide versus insulin for newly diagnosed diabetes in patients with cystic fibrosis: a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 2018;6(2): 114–121. [DOI] [PubMed] [Google Scholar]
- 20.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57(3):678–687. [DOI] [PubMed] [Google Scholar]
- 21.Seaquist ER, Robertson RP. Effects of hemipancreatectomy on pan-creatic alpha and beta cell function in healthy human donors. J Clin Invest 1992;89(6):1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guldstrand M, Ahren B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab 2003;284(3):E557–E565. [DOI] [PubMed] [Google Scholar]
- 23.Ward WK, Halter JB, Beard JC, Porte D Jr. Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol 1984;246(5, pt 1):E405–E411. [DOI] [PubMed] [Google Scholar]
- 24.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40(12):1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toffolo G, Breda E, Cavaghan MK, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indexes of beta-cell function during graded up&down glucose infusion from C-peptide minimal models. Am J Physiol Endocrinol Metab 2001;280(1):E2–E10. [DOI] [PubMed] [Google Scholar]
- 26.Loopstra-Masters RC, Haffner SM, Lorenzo C, Wagenknecht LE, Hanley AJ. Proinsulin-to-C-peptide ratio versus proinsulin-to-insulin ratio in the prediction of incident diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetologia 2011;54(12):3047–3054. [DOI] [PubMed] [Google Scholar]
- 27.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. Improvement in beta-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013;62(8):2890–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seaquist ER, Kahn SE, Clark PM, Hales CN, Porte D Jr, Robertson RP. Hyperproinsulinemia is associated with increased beta cell demand after hemipancreatectomy in humans. J Clin Invest 1996;97(2):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unger RH, Orci L. Glucagon and the A cell: physiology and pathophysiology (first two parts). N Engl J Med 1981;304(25):1518–1524. [DOI] [PubMed] [Google Scholar]
- 30.Unger RH, Orci L. Glucagon and the A cell: physiology and pathophysiology (second of two parts). N Engl J Med 1981;304(26): 1575–1580. [DOI] [PubMed] [Google Scholar]