Abstract
Objective:
To evaluate the efficacy and tolerability of cabozantinib in recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer.
Methods:
Patients with recurrent ovarian, fallopian or primary peritoneal tumors with at least 50% clear cell histomorphology, measurable disease, one or two prior regimens and ECOG performance status 0–2 received cabozantinib 60 mg orally once daily continuously, in 4-week cycles until disease progression or unacceptable toxicity. Primary endpoints were progression-free survival (PFS) at six months and complete or partial tumor response (as assessed by RECIST 1.1). Secondary endpoints included toxicity, PFS, and overall survival (OS).
Results:
Over 19 months, 13 patients were accrued. Fifty-four percent of patients were ≥60 years of age. Performance statuses of 0 and 1 comprised 8 and 5 patients. No objective tumor responses were seen. Three (23% [95% CI: 5%, 54%]) of 13 patients had PFS ≥ 6 months, including one patient who received cabozantinib for 23 cycles and was still on treatment as of the data cut-off date. Median PFS and OS were 3.6 and 8.1 months, respectively. There was one patient with a grade 5 event: a thromboembolic event considered possibly related to study therapy; patient’s cause of death was determined to be due to disease and protocol treatment. Four other patients had thromboembolic events (two grade 3 and one each grade 1 and grade 2). Other grade 3 or higher events reported in two or more patients were nausea, vomiting, fatigue, dyspnea, and dehydration.
Conclusions:
Cabozantinib demonstrated minimal activity in the second- and third-line treatments of clear cell ovarian, fallopian tube or primary peritoneal carcinoma.
Keywords: MET inhibition, Cabozantinib, Clear cell ovarian cancer, Anti-angiogenic therapy
INTRODUCTION
Ovarian clear cell carcinoma (OCCC) accounts for 5–10% of all epithelial ovarian cancers diagnosed in the US, and is characterized by relative resistance to platinum and/or taxane based chemotherapy and adverse prognosis [1, 2]. This is particularly evident in the setting of recurrent disease where the objective response to conventional chemotherapy is 9% in platinum sensitive and 1% in platinum resistant disease [3]. The relative chemoresistance of OCCCs is likely multifactorial in origin, with increased drug detoxification and export, decreased drug accumulation, low mitotic rate, and enhanced DNA repair reported as potential culprits [4–8].
Multiple lines of evidence indicate that gene amplification and protein overexpression of mesenchymal-epithelial transition factor (MET) receptor occur commonly in OCCCs suggesting that targeted inhibition of the MET pathway may be a promising treatment for OCCC [9–14]. Specifically, MET amplification has been reported in 24% of OCCCs by double in-situ hybridization, 28.5% of OCCCs by array-based comparative genomic hybridization (CGH) and 37% by real time quantitative PCR (>4 copies), which is significantly more frequent compared to other epithelial ovarian cancers[10]. Low-level gain of MET has been detected in 4 (40%) of 10 atypical endometrioses and 1 of 2 borderline clear cell adenofibromas (CCAFs), while high-level gain of MET has been detected in five (50%) of 10 atypical endometrioses [9, 14]. MET amplification is present in 2 of 8 OCCC cell lines and MET knockdown in these cell lines resulted in profound decline in cell proliferation and survival due to increased apoptosis and cellular senescence [10]. Overexpression of MET protein by immunohistochemistry is detected more frequently in OCCCs compared to other epithelial ovarian tumors (22% vs 0% exhibit ≥ +2 staining and 66% vs 21% exhibit ≥ +1 staining respectively) while overexpression of the MET ligand hepatocyte growth factor (HGF) has been observed in OCCCs and is much more frequent compared to serous ovarian tumors [9]. Of note, both MET protein overexpression by immunohistochemistry and MET gene amplification are associated with poor prognosis in OCCCs [9]. Additionally, overexpression of the IL6- STAT3-HIF pathway commonly occurs in OCCCs (significantly more compared to high-grade serous cancers); this pathway is known to activate MET promoter which contains hypoxia inducible factor-1 (HIF-1) binding sites and thus upregulates MET expression [11–13].
Cabozantinib is an orally bioavailable multitargeted tyrosine kinase inhibitor whose primary targets are MET (IC50=1.8nM), VEGFR2/KDR (IC50=0.035nM) and RET (IC50=3.8 nM) [15]. Cabozantinib is currently FDA approved for treatment of medullary thyroid cancer and advanced clear cell renal cell carcinoma in patients who have received prior anti-angiogenic therapy. Apart from inhibition of MET on the cancer cells, which leads to block of proliferation, increase in apoptosis, decrease in epithelial mesenchymal transition and abrogation of metastasis, cabozantinib also exhibits potent antiangiogenic activity via inhibition of VEGFR2 and MET on endothelial cells, which acts synergistically to promote angiogenesis in several tumor models [16–18]. Furthermore, cabozantinib inhibition of the MET and AXL genes may help overcome development of resistance to VEGF inhibition [19]. Inhibition of angiogenesis is highly relevant for OCCCs where antiangiogenic agents have demonstrated activity [11, 20, 21]. Notably, in a phase II randomized discontinuation trial of XL184 in advanced ovarian cancer patients, there were 3 patients with clear cell ovarian cancer: one had a RECIST PR, one had a close to-PR response and the third had stable disease with decrease in her CA125 level [22]. Overall, in that study, cabozantinib exhibited an ORR of 21% in 70 patients with ovarian carcinoma (of whom 50% had platinum refractory/resistant disease). Furthermore, the overall disease control rate (CR + PR + SD) at week 12 was 50%, while throughout the study, 70% of the patients with ≥1 postbaseline scan had tumor regression. We therefore conducted a phase II trial of cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer.
METHODS
Study Design
This was a single-arm, three-stage, phase II trial of cabozantinib in women with recurrent, clear cell carcinoma of the ovary, fallopian tube, or peritoneum (NRG-GY-001). Eligible patients were required to have measurable disease and recurrent or progressive clear cell ovarian or fallopian or peritoneal cancer not solely based on CA-125. Primary tumors were required to exhibit at least 50% clear cell histomorphology or have a histologically documented recurrence with at least 50% clear cell histomorphology. Patients were required to have one prior platinum-based chemotherapeutic regimen for management of primary disease. Patients with platinum sensitive and resistant disease were eligible; patients were allowed but not required to have received one additional cytotoxic regimen for management of recurrent or persistent disease. Age ≥18, ECOG PS of 0,1 or 2 and adequate hematologic, renal, hepatic, thyroid and pancreatic function were required. Patients were not screened ahead of the trial enrollment for evidence of venous thrombosis of LE or pelvis or PEs. However, patients that were on therapeutic anticoagulation were specifically excluded from the study (i.e. they were ineligible). Prior MET inhibitor therapy and concomitant treatment with anticoagulants in therapeutic doses were not allowed. Primary objective was to evaluate the anti-tumor activity of cabozantinib based on the proportion of patients with progression-free survival (PFS) for at least 6 months and the proportion who have objective tumor response (complete or partial).
Protocol therapy
Patients received cabozantinib 60 mg orally once a day continuously, in 4-week cycles until disease progression or unacceptable toxicity. Tumor assessments were done with CT or MRI every 2 cycles, i.e. every 8 weeks until disease progression.
Statistical Methods
Primary endpoints were PFS at six months and complete or partial tumor response (as assessed by RECIST 1.1). Secondary endpoints included the nature, frequency and maximum degree of toxicity as assessed by CTCAE v4, PFS, and overall survival (OS).
The primary hypothesis of this study tested the proportion of patients with objective tumor response (complete or partial) (πr) and the proportion of patients with PFS for at least six months (πs). Based on historical data, the null hypothesis was that the proportion of patients with objective tumor response (πr) was ≤10% and the proportion of patients with PFS for at least six months (πs) was ≤15%, i.e., H0: πr ≤0.10 and πs ≤0.15. The alternative hypothesis was the complement of the null parameter space, and the study was powered to detect the following points in the alternative space: πr = .3 or πs =.4. The primary analysis included all eligible patients who received study drug. The outcome of patients who came off study treatment for reasons other than progression and received non-protocol therapy directed at their cancer prior to the six-month time point were considered treatment failure for the six-month PFS endpoint.
To evaluate the hypothesis stated above, a modification of the bivariate two-stage design of Sill et al [23] was utilized, which uses the numbers of patients’ progression-free at 6 months and the number with objective responses to make inferences about the efficacy of the study drug. An additional stage was added to the study—preceding the Sill design. The first stage target was ten patients but was allowed to range up to 14, and in this stage, if zero patients had a complete or partial response within four months of starting treatment the trial would be stopped, and the agent would be not considered worthy of further investigation in this patient population. If the study were to go to stage 2, the decision to stage 3 would be made on both six-month PFS and objective tumor response. The accrual targets (and allowable ranges) for stages 2 and 3 were 19 (15–22) and 31 (27–34), respectively. Assuming objective tumor response and 6 months-PFS were independent events, the probability of stopping at stage 1 was 0.26 under the null hypothesis stated above and was 0.004 under the joint alternative. Assuming a high degree of association between response and 6-month PFS (i.e., joint probability of response and PFS at 6 months equal to 0.90 × min{πr, πs}), these stage 1 stopping probabilities were 0.284 and 0.013 under the null and alternative, respectively.
Exact 95% confidence intervals (CIs) are presented for the percentages of patients with complete or partial response and with PFS ≥ 6 months. Kaplan-Meier plots are presented for PFS and OS.
RESULTS
Patient Accrual
The study opened on 4/1/2015 and accrued 13 of a planned overall total of 34 patients in 19 months. The first stage of accrual was completed, but a second stage was not warranted as the prespecified study criterion of at least one objective response was not reached. As shown in Table 1, more than half (54%) of patients were ≥60 years of age and 38% had a performance status of one. All patients had recurrent clear cell carcinoma. As of the data cut-off date of 5/23/2017, 12 of 13 patients have come off study treatment; 8 (67%) because of disease progression, 3 (25%) because of adverse event/side effects/complications while 1 patient withdrew after beginning protocol therapy.
Table 1.
Patient and Tumor Characteristics for All Eligible and Treated Patients
| Characteristic | Cabozantinib | |
|---|---|---|
| N | % | |
| Age (years) | ||
| 30–39 | 1 | 8 |
| 40–49 | 2 | 15 |
| 50–59 | 3 | 23 |
| 60–69 | 6 | 46 |
| 70–79 | 1 | 8 |
| Ethnicity | ||
| Hispanic or Latino | 1 | 8 |
| Non-Hispanic | 11 | 85 |
| Unknown/Not specified | 1 | 8 |
| Race | ||
| White | 10 | 77 |
| Black/African American | 1 | 8 |
| Asian | 1 | 8 |
| Native Hawaiian/Pacific Islander | 1 | 8 |
| Performance Status | ||
| 0 | 8 | 62 |
| 1 | 5 | 38 |
| Total | 13 | |
Efficacy Endpoints
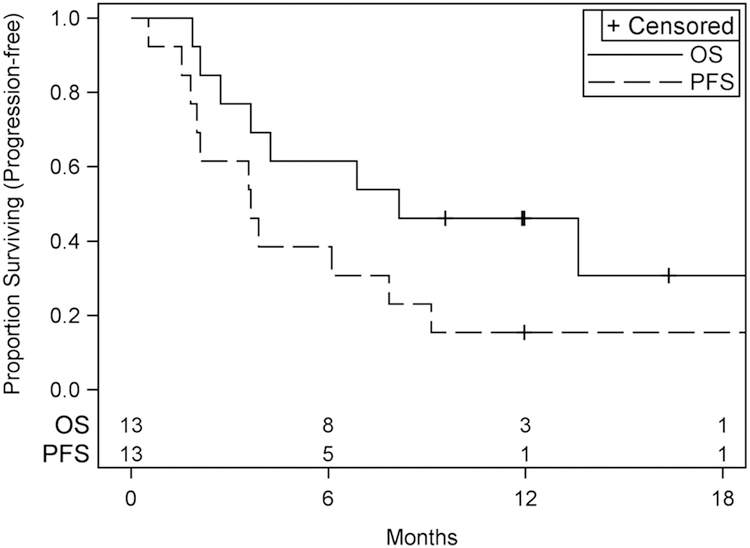
Table 2 shows the number of cycles of study therapy received, and Table 3 shows the results for the efficacy endpoints of the study. No objective tumor responses were seen, while at least one was required to go to stage 2. Three (23% [5%, 54%]) of 13 patients were alive and progression-free for at least six months, including one patient who received cabozantinib for 23 cycles and was still on treatment as of the data cut-off date of 5/23/2017. Median (95% CI) PFS was 3.6 (1.8, 7.9) months while median (95% CI) OS was 8.1 (2.7, >20.1) months. Kaplan-Meier plots of PFS and OS are presented in Figure 1.) Median follow-up for OS is 14 months.
Table 2.
Cycles of Study Therapy
| Number of cycles given |
Cabozantinib | |
|---|---|---|
| N | % | |
| 1 | 1 | 8 |
| 2 | 4 | 31 |
| 3 | 1 | 8 |
| 4 | 3 | 23 |
| 9 | 2 | 15 |
| 10 | 1 | 8 |
| 23† | 1 | 8 |
| n | 13 | |
| Mean | 6 | |
| Median | 4 | |
Patient who has received 23 cycles is still on treatment.
Table 3.
Efficacy Endpoints
| Efficacy Endpoint | Cabozantinib | |
|---|---|---|
| N | % | |
| Best Objective Tumor Response | ||
| Stable Disease (≥6 weeks) | 7 | 54 |
| Disease progression | 4 | 31 |
| Not evaluable | 2 | 15 |
| Complete or Partial Response | 0 | 0 |
| Progression-Free Survival | ||
| ≥6 months | 3 | 23 |
| Median (95% CI), months | 3.6 (1.8, 7.9) | |
| Overall Survival | ||
| Median (95% CI), months | 8.1 (2.7, >20.1) | |
Figure 1:
PFS and Overall Survival in NRG-GY001
Adverse Events
For all treated patients, Table 4 displays adverse events (AEs) that have occurred during the study by system organ class (SOC), and Supplement Table 1 displays AEs by system organ class and term restricted to terms in which at least one grade 3 event was reported. CTC version 4.0 was used. There was one patient with a grade 5 event: a thromboembolic event that was considered possibly related to study therapy; the patient’s cause of death was determined to be due to disease and protocol treatment. There were four other patients with thromboembolic events (two grade 3 and one each grade 1 and grade 2).
Table 4.
Distribution of Patients by Highest Grade Adverse Event By System Organ Class For All Reported Adverse Events without Regard to Attribution
| Cabozantinib (n=13) | |||||
|---|---|---|---|---|---|
| No. and (%) of Patients by Grade | |||||
| 1 | 2 | 3 | 4 | 5 | |
| System Organ Class | |||||
| Overall Highest Grade | 1 (7.7) |
3 (23.1) |
7 (53.8) |
1 (7.7) |
1 (7.7) |
| Blood and Lymphatic System Disorders | 5 (38.5) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Cardiac Disorders | 1 (7.7) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Ear and Labyrinth Disorders | 2 (15.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Endocrine Disorders | 1 (7.7) |
2 (15.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Eye Disorders | 1 (7.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Gastrointestinal Disorders | 3 (23.1) |
5 (38.5) |
4 (30.8) |
0 (0.0) |
0 (0.0) |
| General Disorders and Administration Site Conditions | 5 (38.5) |
4 (30.8) |
3 (23.1) |
0 (0.0) |
0 (0.0) |
| Infections and Infestations | 0 (0.0) |
2 (15.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Injury, Poisoning and Procedural Complications | 1 (7.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Investigations | 6 (46.2) |
3 (23.1) |
2 (15.4) |
0 (0.0) |
0 (0.0) |
| Metabolism and Nutrition Disorders | 3 (23.1) |
6 (46.2) |
2 (15.4) |
1 (7.7) |
0 (0.0) |
| Musculoskeletal and Connective Tissue Disorders | 5 (38.5) |
2 (15.4) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
| Nervous System Disorders | 3 (23.1) |
7 (53.8) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
| Psychiatric Disorders | 1 (7.7) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Renal and Urinary Disorders | 0 (0.0) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Respiratory, Thoracic and Mediastinal Disorders | 4 (30.8) |
2 (15.4) |
2 (15.4) |
0 (0.0) |
0 (0.0) |
| Skin and Subcutaneous Tissue Disorders | 5 (38.5) |
1 (7.7) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
| Vascular Disorders | 1 (7.7) |
5 (38.5) |
3 (23.1) |
0 (0.0) |
1 (7.7) |
Adverse events were graded with CTCAE version 4
The most commonly reported events were fatigue, nausea, vomiting, dysgeusia, abdominal pain, increased alkaline phosphatase, oral mucositis, increased aspartate aminotransferase, hypertension, and anorexia, all of which were reported in more than half of the patients. Grade 3 or higher events reported in two or more patients were nausea, vomiting, thromboembolic event, fatigue, dyspnea, and dehydration.
DISCUSSION
Cabozantinib demonstrated minimal activity in the second- and third-line treatment of recurrent clear cell ovarian, fallopian tube and primary peritoneal cancer. No objective tumor responses by RECIST 1.1 were seen among the 13 patients enrolled in the first stage, and the study did not go to the second stage as the prespecified study criterion of at least one objective response was not reached. Interestingly, one patient received cabozantinib for 23 cycles and remained on protocol therapy as of the date of data cut-off. Similarly, in the previously reported phase II randomized discontinuation trial of cabozantinib in advanced ovarian cancer patients, 1 out of 3 patients OCCC had a partial response by RECIST. Genomic studies of the tumors of these patients may help elucidate the etiology of these exceptional responses to cabozantinib.
Cabozantinib has shown excellent responses and is currently FDA approved for medullary thyroid cancer and advanced clear cell renal cell carcinoma (CCRC); cabozantinib was also recently shown to improve OS and PFS in previously treated hepatocellular carcinoma. [23] Medullary thyroid cancer commonly exhibits RET mutations (in more than 50% of sporadic cases and in nearly all inherited cases of this disease) and increased coexpression of MET and its ligand HGF; these alterations may explain the increased activity of cabozantinib in this disease as both RET and MET tyrosine kinases are primary targets of cabozantinib [24]. Clear cell renal cell carcinoma exhibits considerable similarity in gene expression profiles with clear cell tumors of other organs, including clear cells of the ovary or uterus. Given the similarity between renal and ovarian CCC gene expression profiles, it has been suggested that novel agents that are active in metastatic CCRC cancer may also have activity in OCCC. However, our study indicates that the activity of cabozantinib in OCCC was clearly inferior to that in CCRC; similarly, sunitinib, which has shown good activity and is FDA approved in CCRC, showed minimal activity in OCCC [25]. Specifically, as reported in GOG-254, the objective response to sunitinib in OCCC was 6.7%, with 16.7% PFS6 rate and median PFS of only 2.7 months. Both GOG-254 and NRG-GY001 support the notion that despite the similarities in gene expression profiles between OCCC and CCRC, treatment inferences from CCRC do not necessarily apply to OCCC. Important differences between OCCC and CCRC such as the the prevalence of VHL mutations in CCRC as opposed to the absence of these mutations in OCCC, the increased incidence of abnormalities in the PI3K/mTOR/AKT pathway in OCCC (mutations/amplifications of PI3K pathway genes are observed in 33%−45% of OCCCs [26]) and the increased incidence of ARID1A mutations in OCCC (observed in 46% of OCCCs [27]) may explain the differences in treatment outcomes between the OCCC and CCRC. Of note, activation of the PI3K pathway has been associated with resistance to MET inhibition [28, 29], suggesting that combinatorial strategies with PI3K/mTOR inhibitors may be necessary in OCCC.
The safety/toxicity profile of cabozantinib was similar to that observed in larger phase III studies of cabozantinib in various tumor types. However, a signal for increased incidence of thromboembolic events was observed in this study [5 (38.4%)of 13 patients] compared to the 7.3% incidence observed in the large phase III METEOR trial in CCRC [30] where a similar dose of cabozantinib (60mg daily) was used. This may be a coincidence given the small sample size of our study or may reflect the fact that thromboembolic events occur more frequently in OCCCs compared with epithelial ovarian carcinomas of other histologies [31].
Overall, cabozantinib demonstrated minimal activity in the second- and third-line treatments of persistent or recurrent OCCC; alternative strategies are urgently needed for patients with this histologic subtype of ovarian, fallopian tube or primary peritoneal cancer.
Supplementary Material
RESEARCH HIGHLIGHTS.
NRG-GY001 was a phase II study of cabozantinib in clear cell ovarian cancer
No objective responses were seen but 3 of 13 patients had PFS ≥6 months
Grade ≥3 AEs were thromboembolism, nausea, vomiting, fatigue, dyspnea, dehydration
Acknowledgments
This study was supported by National Cancer Institute grants to NRG Oncology (1 U10 CA180822) and NRG Operations (U10CA180868).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: CWRU Case Comprehensive Cancer Center LAPS, Froedtert and the Medical College of Wisconsin, Avera Cancer Institute, Cancer Research for the Ozarks NCORP, Dana-Farber/Partners Cancer Care LAPS, Franciscan Research Center – Northwest Medical Plaza, Heartland Cancer Research NCORP, Maine Medical Center – Scarborough Campus, New Mexico Minority Underserved NCORP, University of Iowa/Holden Comprehensive Cancer Center and University of Oklahoma Health Sciences Center LAPS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Dr. Panagiotis Konstaintinopoulos served on the Advisory Board of Merck, Pfizer and Vertex as a consultant.
Dr. William Brady received travel expenses to FDA meeting in Bethesda from Advaxis, Inc. He is also a member of the Data and Safety Monitoring Board for Ultragenyx.
Dr. John Farley served on the Advisory Board for Clovis Oncology as a consultant.
Dr. David Gershenson is the Chair of the Rare Tumor Committee for NRG Oncology. He also received monies to travel to the semi-annual NRG Oncology Meeting.
All other co-authors have no conflicts of interest to declare.
REFERENCES
- 1.del Carmen MG, Birrer M, Schorge JO. Clear cell carcinoma of the ovary: a review of the literature. Gynecol Oncol 2012; 126:481–490. [DOI] [PubMed] [Google Scholar]
- 2.Groen RS, Gershenson DM, Fader AN. Updates and emerging therapies for rare epithelial ovarian cancers: one size no longer fits all. Gynecol Oncol 2015; 136:373–383. [DOI] [PubMed] [Google Scholar]
- 3.Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol 2007; 105:404–408. [DOI] [PubMed] [Google Scholar]
- 4.Itamochi H, Kigawa J, Akeshima R, Sato S, Kamazawa S, Takahashi M, et al. Mechanisms of cisplatin resistance in clear cell carcinoma of the ovary. Oncology 2002; 62:349–353. [DOI] [PubMed] [Google Scholar]
- 5.Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet Gynecol 2002; 100:281–287. [DOI] [PubMed] [Google Scholar]
- 6.Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci 2008; 99:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajihara H, Yamada Y, Kanayama S, Furukawa N, Noguchi T, Haruta S, et al. Clear cell carcinoma of the ovary: potential pathogenic mechanisms (Review). Oncol Rep 2010;23: 1193–1203. [DOI] [PubMed] [Google Scholar]
- 8.Schaner ME, Ross DT, Ciaravino G, Sorlie T, Troyanskaya O, Diehn M, et al. Gene expression patterns in ovarian carcinomas. Mol Biol Cell 2003; 14:4376–4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto S, Tsuda H, Miyai K, Takano M, Tamai S, Matsubara O. Gene amplification and protein overexpression of MET are common events in ovarian clear-cell adenocarcinoma: their roles in tumor progression and prognostication of the patient. Mod Pathol 2011; 24:1146–1155. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita Y, Akatsuka S, Shinjo K, Yatabe Y, Kobayashi H, Seko H, Kajiyama H, Kikkawa F, Takahashi T, Toyokuni S. Met is the most frequently amplified gene in endometriosis-associated ovarian clear cell adenocarcinoma and correlates with worsened prognosis. PLoS One 2013; 8:e57724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anglesio MS, George J, Kulbe H, Friedlander M, Rischin D, Lemech C, et al. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res 2011;17: 2538–2548. [DOI] [PubMed] [Google Scholar]
- 12.Eckerich C, Zapf S, Fillbrandt R, Loges S, Westphal M, Lamszus K. Hypoxia can induce c-Met expression in glioma cells and enhance SF/HGF-induced cell migration. Int J Cancer 2007; 121:276–283. [DOI] [PubMed] [Google Scholar]
- 13.Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003; 3:347–361. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Tsuda H, Miyai K, Takano M, Tamai S, Matsubara O. Accumulative copy number increase of MET drives tumor development and histological progression in a subset of ovarian clear-cell adenocarcinomas. Mod Pathol 2011; 25:122–130. [DOI] [PubMed] [Google Scholar]
- 15.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10: 2298–2308. [DOI] [PubMed] [Google Scholar]
- 16.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2012; 2:270–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Zhau HE, Osunkoya AO, Iqbal S, Yang X, Fan S, et al. Vascular endothelial growth factor regulates myeloid cell leukemia-1 expression through neuropilin-1-dependent activation of c-MET signaling in human prostate cancer cells. Mol Cancer 2010; 9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You WK, Sennino B, Williamson CW, Falcon B, Hashizume H, Yao LC, et al. VEGF and c-Met blockade amplify angiogenesis inhibition in pancreatic islet cancer. Cancer Res 2011; 71:4758–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci USA 2014; 111:13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alifrangis C, Thornton A, Fotopoulou C, Krell J, Gabra H. Response to sunitinib (Sutent) in chemotherapy refractory clear cell ovarian cancer. Gynecol Oncol Rep 2016; 18:42–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauh-Hain JA, Penson RT. Potential benefit of Sunitinib in recurrent and refractory ovarian clear cell adenocarcinoma. Int J Gynecol Cancer 2008; 18:934–936. [DOI] [PubMed] [Google Scholar]
- 22.Vergote IB, Smith DC, Berger R, Kurzrock R, Vogelzang NJ, Sella A, Wheler J, Lee Y, Foster PG, Weitzman R, Buckanovich RJ. A phase 2 randomised discontinuation trial of cabozantinib in patients with ovarian carcinoma. Eur J Cancer 2017;83: 229–236. [DOI] [PubMed] [Google Scholar]
- 23.Abou-Alfa GK, Meyer T, Cheng A, El-Khoueiry AB, Rimassa L, Ryoo B, Cicin I, Merle P, Park J, Blanc J, Bolondi L, Klümpen HJ, Chan SL, Dadduzio V, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib (C) versus placebo (P) in patients (pts) with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: Results from the randomized phase III CELESTIAL trial. In: ASCO GI Cancer San Francisco, CA; 2018. [Google Scholar]
- 24.Hart CD, De Boer RH. Profile of cabozantinib and its potential in the treatment of advanced medullary thyroid cancer. Onco Targets Ther 2013; 6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan J, Brady WE, Brown J, Shahin MS, Rose PG, Kim JH, et al. A phase II evaluation of sunitinib (SU11248) in the treatment of persistent or recurrent clear cell ovarian carcinoma: An NRG Oncology/Gynecologic Oncology Group (GOG) study. In: Society of Gynecologic Oncology Meeting 2015 Chicago; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol 2009; 174:1597–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 2010; 363:1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji F, Liu X, Wu Y, Fang X, Huang G. Overexpression of PI3K p110alpha contributes to acquired resistance to MET inhibitor, in MET-amplified SNU-5 gastric xenografts. Drug Des Devel Ther 2015; 9:5697–5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi J, McTigue MA, Rogers A, Lifshits E, Christensen JG, Janne PA, et al. Multiple mutations and bypass mechanisms can contribute to development of acquired resistance to MET inhibitors. Cancer Res 2011; 71:1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015; 373:1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duska LR, Garrett L, Henretta M, Ferriss JS, Lee L, Horowitz N. When ‘never-events’ occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol Oncol 2009; 116:374–377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.