Abstract
CD4 T helper (Th) cells are organizers of the immune response, directing other immune cells to initiate and maintain effective humoral and cellular immunity. CD4 T cells differentiate into distinct Th effector or regulatory subsets in response to signals delivered to them during the course of infection. Ikaros is a transcription factor that is expressed in blood cells from the level of the hematopoietic stem cell. It is required for normal thymic T cell development and serves as a tumor suppressor as lack of Ikaros in developing lymphoid cells results in leukemia. In order to study the role of Ikaros in CD4 T cell differentiation and function, an Ikaros conditional knockout mouse was developed such that Ikaros expression was deleted specifically in mature T cells, thus avoiding defects observed in germline Ikaros mutant mice. Using this model system, we have shown that, in the absence of Ikaros, CD4 T cells are able to attain Th1, Th2 and Th17, but not iTreg, cell fates. However, they show enhanced expression of a cohort of pro-inflammatory cytokines, resulting in differentiation of Th17 cells with a phenotype that has been associated with autoimmunity and pathological inflammation. In addition, we define Ikaros as a repressor of the gene program associated with the response to type I interferons, another key pathway whose deregulation is linked to autoimmunity. Taken together, these data definitively define Ikaros as a critical regulator at the center of the inflammatory response in T cells and highlight a potential role in suppressing autoimmunity.
Introduction
CD4 T cells are required for elicitation of effective humoral and cellular immunity. Naive CD4 T cells have uniform differentiation potential and it is the nature of a pathogenic assault that elicits signals to drive acquisition of specific T helper (Th) cell phenotypes tailored to combat the infection. CD4 T cells can also attain an inducible regulatory T cell (iTreg) phenotype, which plays a role in down-regulation of the immune response to prevent dangerous inflammation. Cytokines, together with signals delivered through T cell receptor (TCR) complex and co-receptor, deliver the key instructive signals that drive the Th or iTreg lineage decision by, ultimately, changing programs of gene expression. Deregulation of the response to these cues can lead to autoimmunity and inflammatory diseases.
Ikaros is a highly conserved nuclear protein (greater than 95% at the amino acid level between mice and humans) that is expressed at high levels in CD4 Th cells, as well as in all blood cell lineages from the level of the hematopoietic stem cell (HSC) (1, 2). In humans, variants in the Ikaros gene sequence have been linked to inflammatory and autoimmune diseases including asthma (3), systemic lupus erythematosus (4, 5), type 1 diabetes (6, 7), Crohn’s disease (8) and systemic sclerosis (9). Its role in these diseases is unknown. Mice with a germline knockout mutation in the Ikaros gene (Ikaros null mice) display defects in HSC function as well as in development and/or function of all blood cell lineages studied to date (10–16). Studies using T cells from mice with germline mutations in the Ikaros gene have suggested that Ikaros is required for multiple aspects of CD4 Th cell development and function. In particular, they support a role for Ikaros in: 1) the Th1 vs. Th2 fate decision, whereby Ikaros null T cells cannot attain the Th2 but default to the Th1 fate (17), 2) differentiation of Th17 and iTreg cells, whereby differentiation of both is significantly decreased in the absence of Ikaros (18, 19) and 3) regulation of T cell proliferation whereby Ikaros deficient T cells display increased levels of proliferation and altered cell cycle kinetics, with a shorter time-frame to entry into S phase (20). While these studies provide evidence that Ikaros is an important regulator of CD4 T cell differentiation and function, it has been argued that use of Ikaros germline mutant models for these studies is contraindicated. This is due to the fact that Ikaros plays a critical role in regulating early T cell maturation processes that take place in the thymus. Specifically, lack of Ikaros results in greatly reduced numbers of thymocytes due to an impaired ability of progenitors cells to commit to the lymphoid lineage as well as defects in both positive and negative selection (14, 15, 21, 22). Adding yet another layer of complication, the germline Ikaros mutant mice that have been used to study Ikaros function in mature T cells develop T cell leukemia that arises in the thymus (15, 23, 24). Taken together, these phenotypes have led to concerns that Ikaros null “mature T cells” represent a population of cells that are so abnormal that they cannot be compared in function or differentiation potential to wild-type T cells.
Therefore, in order to define the function of Ikaros in CD4 T cells, we have generated an Ikaros conditional knockout mouse (hereafter designated Ikflox) and used it to delete Ikaros expression specifically in mature T cells. This would allow T cell commitment and thymic T cell maturation to occur with intact Ikaros expression. Using these mice, we have shown that, in the absence of Ikaros, mature CD4 T cells are able to attain Th1, Th2 and Th17, but not iTreg, cell fates. However, they show enhanced expression of a cohort of pro-inflammatory cytokines. This results in differentiation of Th17 cells with a phenotype that has been associated with autoimmunity and pathological inflammation, suggesting that Ikaros acts as a suppressor of this phenotype. We also show that, in contrast to what has been demonstrated using T cells from germline Ikaros mutant mice, lack of Ikaros starting at the level of the mature T cell results in their reduced ability to proliferate when activated via TCR/co-receptor pathways. Finally, using genome-wide transcriptomics analyses, we reveal that, in resting CD4 T cells, Ikaros is a repressor of the gene program associated with the response to type I interferon (IFN). Taken together, these data define Ikaros as a cell-intrinsic regulator of the inflammatory response in mature T cells.
Materials and Methods
Mice
The Ikflox mouse was generated in conjunction with the Mouse Biology Program at University of California, Davis. The Lck distal promoter Cre transgenic mouse (dLck-hcre3779) was obtained from The Jackson Laboratory. All mice were on the C57BL/6 inbred genetic background. Genotypes were determined by PCR analyses. Both male and female animals were used for experiments with no discernable difference in phenotype. All animal procedures were approved by the Boston University Institutional Animal Care and Use Committee.
Western blot analyses
Protein extracts were prepared from purified splenic CD4 T cells by cell lysis with 420mM NaCl lysis buffer (20mM Tris-HCl pH 7.5, 420mM NaCl, 1mM EDTA, 1% NP-40). Ten μg of protein extract were denatured and electrophoresed on 10 % SDS-polyacrylamide gels, followed by transfer to PVDF membranes. Membranes were probed with anti-Ikaros (clone 4E9, gift from Katia Georgopoulos) followed by, after stripping of the membrane, anti-Nucleolin (A300–711, Bethyl Laboratories Inc.). In both cases, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody after primary antibody incubation. SuperSignal™ West Femto (Thermo Fisher Scientific) was used to visualize HRP-conjugated antibodies. Light signal was detected with a Bio-Rad Universal Hood II and Molecular Imager®ChemiDoc™ XRS+ Camera (Bio-Rad). Densitometry analyses were performed using ImageJ software.
Thymocyte and splenocyte preparation
Thymuses or spleens from 6–8 week old mice were dissected using aseptic techniques and placed in a petri dish on ice with complete RPMI (RPMI 1640 medium supplemented with 10% fetal bovine serum, 50μM β-mercaptoethanol, 4mM L-glutamine, 50U/ml of penicillin and 50μg/ml streptomycin). They were then ground between two glass slides and red cells were lysed with RBC lysis buffer (Santa Cruz). After two series of washes with complete RPMI, cells were counted on a hemocytometer using trypan blue exclusion to identify live cells.
T cell cultures
Differentiation assays:
CD4 T cells were purified from splenocytes using the Dynabeads® FlowComp™ isolation kit (Invitrogen) according to manufacturer’s instructions. Purified CD4+ cells were plated with 3 μg/mL plate-bound anti-CD3ε (145–2C11, Biolegend) and 5 μg/mL soluble anti-CD28 (37.51, Bio-X-Cell) in complete RPMI. For differentiation the following were added: 1 ng/mL IL-12 (Peprotech) and 5 μg/mL anti-IL-4 (eBioscience) for Th1; 10 ng/mL IL-4 (Peprotech) and 5 μg/mL anti-IFNγ (Biolegend) for Th2; 2 ng/ml TGFβ (Biolegend), 50 U/ml IL-2 (Peprotech), 5 μg/ml anti-IFNγ and 5 μg/ml anti-IL-4 for iTreg; and 1 ng/mL TGFβ, 20 ng/ml IL-6 (Peprotech), 5 μg/ml anti-IFNγ and 5 μg/ml anti-IL-4 for Th17. Cells were re-stimulated with 20 ng/mL phorbol myristate acetate (PMA) and 400 ng/mL ionomycin, or plate-bound anti- CD3ε prior to intracellular cytokine staining or preparation of RNA. When cells were used for intracellular cytokine staining, they were also incubated with 1 μg/mL Brefeldin A (eBioscience) for 4 hours following an initial 2 hours of re-stimulation.
Proliferation and cell cycle analyses:
Splenocytes were loaded with 5μM carboxyfluoresceinsuccinimidyl ester (CFSE) and cultured with 0.5–8 μg/ml plate-bound anti-CD3ε and 5 μg/ml soluble anti-CD28. Prior to flow analyses, cells were stained with antibodies against CD4 (RM4–5, eBioscience) and CD8 (53–6.7, eBioscience) to identify T cells. For cell cycle analyses, CD4 T cells were purified from spleens and plated with anti-CD3ε/anti-CD28 as described above. In some experiments, 40 μM of the caspase-3 inhibitor Z-DEVD-FMK (ApexBio) was added to the culture. At 24, 48 and 72 hours, cells were harvested, fixed with 70% ethanol and stored overnight at −20°C. Staining was performed using a solution composed of 50 μg/ml propidium iodide, 3.8 mM sodium citrate and 10 μg/ml RNase A.
IFNα treatment:
CD4 T cells were purified from the spleen as described above followed by incubation overnight with 10 ng/ml IL-7. The next day, cells were treated with 0, 1, 10 or 100 U/ml IFNα (Biolegend) for 4 hours, followed by preparation of RNA.
Staining cells for flow cytometry
In all cases, cells were analyzed by flow cytometry after staining on the BD FACSCalibur™ (BD Biosciences). Data were analyzed using FlowJo V10 software.
Surface marker staining:
Cells were incubated with 2 μg/mL Fc block (anti-CD32/anti-CD16, Biolegend) and rat serum, followed by staining with the following fluorochrome-conjugated antibodies (antibodies from Biolegend or eBioscience unless otherwise stated): anti- CD8 (53–6.7), CD4 (RM4–5), CD11b (M1/70), CD44 (IM7), B220 (RA3–6B2), CD62L (MEL-14), Gr-1 (RB6–8C5), IgM (II/41), TCRβ (H57–597).
Intracellular staining:
For cytokines, cells were fixed and permeabilized using the Intracellular Fixation & Permeabilization Buffer Set (eBioscience), followed by staining with fluorochrome-conjugated antibodies against IFNγ (XMG1.2) and IL-4 (11B11). For nuclear protein, cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience), followed by staining with fluorochrome-conjugated antibodies against: T-bet (eBio4B10), Foxp3 (FJK-16s), GATA3 (TWAJ), RORγt (B2D), Ikaros (2A9). For anti-phosphoSTAT1 analyses, each spleen was divided in half. Half was dissociated directly in 1.6% paraformaldehyde (Electron Microscopy Sciences), followed by permeabilization with 100% methanol overnight. The other half was prepared for culturing as described above. Splenocytes were plated with 100 U/ml IFNα for 30 minutes in a tissue culture incubator. They were then fixed and permeabilized with 100% methanol as described above. The next day, both sets of cells were stained with fluorochrome-conjugated antibodies against phospoSTAT1 (clone 4a, BD Biosciences) and Ikaros (2A9).
RNA Isolation and quantitative real-time RT-PCR (qRT-PCR) analyses
Total RNA was isolated from cells using the SV Total RNA Isolation System (Promega) or RNeasy MiniKit (Qiagen). cDNA was generated with Superscript Reverse Transcriptase (Invitrogen). qRT-PCR was performed using SSO-Advanced Universal SYBR Green Supermix (Bio-Rad) on a Bio-Rad MyiQ Real-Time PCR machine. Raw data obtained was analyzed using the Pfaffl method to normalize target gene data to that obtained with primers for the reference gene, HPRT. Primers were generated by IDT DNA Technologies. Sequences are available upon request.
Virus infection
Splenic CD4 T cells were purified and plated with anti-CD3ε/anti-CD28 as described above. After 16 hours, cells were infected with rVSV-eGFP (25) at an MOI of 10 for 24 hours. Following infection, cells were fixed with 4% paraformaldehyde, washed and analyzed by flow cytometry.
Microarray analyses
Microarray experiments and their subsequent analyses were performed at the Boston University Microarray Core Facility. Biotin labeling was performed using the WT Plus reagent kit (Affymetrix) according to the manufacturer’s instructions. The labeled, fragmented DNA was hybridized to the Affymetrix Mouse Gene 2.0 ST Array for 18 hours in a GeneChip Hybridization oven 640 at 45°C, followed by staining using an Affymetrix fluidics station 450. After staining, microarrays were immediately scanned using an Affymetrix GeneArray Scanner 3000 7G Plus. Microarray analyses were performed using the R environment for statistical computing (version 2.15.1). The raw expression data were quality assessed and subjected to background adjustment and normalization. Differential expression was assessed using the moderated (empirical Bayesian) ANOVA and t test implemented in the limma package (version 3.14.4). Nominal p values were corrected for multiple hypothesis testing using the Benjamini-Hochberg procedure to generate FDR-corrected p values (q values). FDR q < 0.25 are considered significant. GSEA analyses (default parameters with random seed 1234) was performed using the Entrez Gene versions of the Hallmark, Biocarta, KEGG, Reactome, Gene Ontology (GO), and transcription factor and microRNA motif gene sets obtained from the Molecular Signatures Database (MSigDB), version 6.0. The microarray data has been submitted to the NCBI Expression Omnibus under accession number GSE119067 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE119067).
Statistics
Statistical analyses for microarray experiments are described above. For all other analyses, two-tailed Student t-tests were performed. p-values greater than 0.05 were considered not significant (NS).
Results
A new tool for defining Ikaros in peripheral T cell differentiation and function
In order to study the function of Ikaros in mature T cells, an Ikaros conditional knockout mouse was generated using Cre/lox technology in conjunction with the Mouse Biology Project at UC Davis. Exon 8 (the last translated exon) and the 3’ untranslated region (UTR) of the Ikaros gene, Ikzf1, were flanked with loxP sites, such that this region of the gene is deleted upon expression of Cre-recombinase. We chose this strategy since it recapitulates that used to generate the Ikaros null mice (15). This method also precludes insertion of a loxP site into 3’ UTR sequences, which could destabilize the mRNA (Fig 1A). Ikflox mice were crossed to Lck (distal promoter)-Cre (hereafter referred to as Cre) transgenic mice, which targets expression of Cre-recombinase and, therefore, deletion of the loxP-flanked sequences within the floxed Ikaros allele to mature T cells (26), allowing thymic selection events to occur with wild-type levels of Ikaros. Western blot, intracellular flow and qRT-PCR analyses have demonstrated that Ikaros expression is efficiently deleted in Ikflox x Cre peripheral CD4 T cells, but not in immature double positive thymocytes (Fig. 1 B-D).
Figure 1. Characterization of Ikaros conditional knockout mice.

A. Schematic representation of floxed Ikzf1 locus showing placement of loxP sites. Not to scale. B. Western blot of Ikaros expression in splenic CD4 T cells from Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice. Two bands detected by anti-Ikaros antibody represent Ik-1 and Ik-2 isoforms. Densitometry readings for Ikaros bands normalized for levels of nucleolin (Nuc) expression are 1.2 (Cre-) and 0.26 (Cre+). C. qRT-PCR for Ikaros expression using cDNA from Cre+ (grey bar) and Cre- (white bar) thymuses or splenic CD4 T cells. Results are normalized to values for HPRT expression. n=3 p<.01 for spleen, NS for thymus. Error bars represent +/− SEM. D. Flow cytometric overlay histogram plots comparing levels of intracellular staining for Ikaros in thymus (gated on CD4+CD8+ cells or CD4 single positive cells) and spleen (gated on CD4+ T cells) of Cre+ (solid line) and Cre- (dashed line) mice. Representative of 10 independent experiments.
Characterization of thymocyte and splenocyte populations in Ikflox mice
Ikaros null mice have profound defects in thymic T cell maturation that result in decreased numbers of thymocytes, a dramatic increase in the percentage of CD4 single positive (SP) thymocytes and, by 5 weeks of age, outgrowth of leukemic T cells (15). Key to the success of our conditional knockout model is that T cell maturation in the thymus occurs normally.
Importantly, in Ikflox x Cre mice, double positive thymocytes express wild-type levels of Ikaros (Fig 1D). Deletion of Ikaros begins once thymocytes have transitioned into the single positive stage in the thymus, but is not observed in the majority of T cells until they reach the periphery (Fig. 1B-D). Because of this, numbers of thymocytes as well as CD4/CD8 thymic profiles from Ikflox x Cre mice are indistinguishable from their Ikflox (no Cre) counterparts (Fig. 2A). In the spleen, percentages and numbers of CD8 T cells are normal, whereas there is a slight, but significant increase in those of CD4 T cells (Fig. 2B). Percentages of B cells and myeloid cells were unaffected (Fig 2C).
Figure 2. Ikflox x dLck-Cre mice have normal thymocyte populations, but display slight alterations in cell populations in the spleen.

Representative flow cytometric analyses of CD4 and CD8 expression on the surface of thymocytes (A) and splenocytes (B) from Ikflox (no Cre) [Cre-] and Ikflox x dLck-Cre [Cre+] mice. Bar graphs represent average cellularity of thymus/spleen or percentage of cell subsets as labeled from four independent experiments. Error bars represent +/− SEM. *p=0.0058 C. Representative flow cytometric analyses delineating B (B220+IgM+) and myeloid (CD11b+Gr-1+ TCRβ-) cell populations in the spleens of Cre+ and Cre- mice. Bar graphs represent average percentage of cell subsets from four independent experiments. Error bars represent +/− SEM.
Ikaros has been associated in activation and silencing of the genes encoding CD8 and CD4, respectively, in thymocytes (27, 28). T cells from Ikflox x Cre spleens do not have significantly altered expression of either, showing that Ikaros’ role in expression of CD4 and CD8 does not extend to the periphery (Fig. 2 and data not shown). Percentages of CD4+ effector memory (Tem, CD62LloCD44hi) and central memory (Tcm, CD62LhiCD44hi) cells (Fig 3A), as well as percentages of CD4 T cells expressing Foxp3 (Treg) and T-bet (Th1) do not differ in spleens of Ikflox x Cre and Ikflox (no Cre) mice (Fig 3B). There were negligible percentages of CD4 T cells expressing GATA3 (Th2) or RORγt (Th17) in spleens of either Ikflox x Cre or Ikflox (no Cre) mice (data not shown).
Figure 3. Ikflox x dLck-Cre mice show no evidence of increased memory or differentiated CD4 T cell subsets in the spleen.
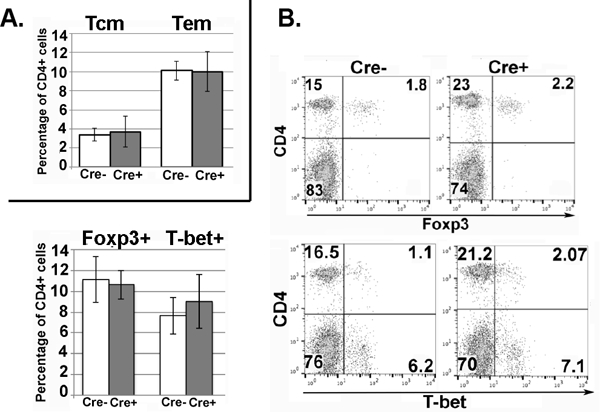
A. Splenocytes from Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice were analyzed by flow cytometry for surface markers CD4, CD62L and CD44 to identify central memory cells (Tcm, CD62L+CD44+) and effector memory cells (Tem, CD62L-CD44+) within CD4+ gated T cells. Bar graph shows average percentage of memory populations within the CD4+ T cells population from three independent experiments. Error bars represent +/− SEM. B. Representative flow cytometry plots showing expression of CD4/Foxp3 and CD4/T-bet within the splenocyte populations from Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice. Bar graph shows average percentage of each cell subset from three independent experiments. Error bars represent +/− SEM. All data shown are from analyses of mice 6–8 weeks of age.
Therefore, we can conclude that, in Ikflox x Cre mice, thymic T cell maturation is not perturbed and splenic T cell populations are similar to those observed in wild-type mice.
Ikflox x Cre T cells show decreased proliferation and survival upon TCR stimulation
T cells require signal delivered by pathogen through the TCR complex in order to undergo activation and differentiation events that drive their role in the immune response. In order to investigate how lack of Ikaros affects the ability of T cells to respond to TCR complex stimulation, splenocytes from Ikflox x Cre and as Ikflox (no Cre) mice were loaded with the fluorescent proliferation dye CFSE and plated in the presence of plate-bound αCD3 and soluble αCD28 (hereafter designated αCD3/αCD28). At the end of the assay, cells were stained with fluorescent antibodies against CD4 and CD8 to define the distinct T cell populations. Ikflox x Cre CD4 T cells did not proliferate as well as Ikflox (no Cre) CD4 T cells, lagging by about 2 cell divisions (Fig 4A). This held true over a range of αCD3 concentrations demonstrating that the observed decrease in proliferation was not due to an increased threshold of activation. Similar results were observed for Ikflox x Cre CD8 T cells (data not shown).
Figure 4. Ikaros positively regulates the proliferative response to TCR/co-receptor signal.

A. Splenocytes from Ikflox x dLck-Cre (dashed line) and Ikflox (no Cre) (solid line) mice were loaded with 5 μM CFSE and plated individually with 0.5, 2 or 8 μg/ml plate-bound αCD3/soluble αCD28 for 72 hours followed by flow analyses. Histograms show CFSE fluorescence within the CD4+ gated population. Results shown are representative of three independent experiments. B. CD4 T cells from spleens of from Ikflox x dLck-Cre [Cre+] and from Ikflox (no Cre) [Cre-] mice were plated with 2 μg/ml plate-bound αCD3/soluble αCD28. 48 hours post-plating, cells were fixed in ethanol, followed by propidium iodide staining and flow analyses. Bar graph shows average percentages of cells from four independent experiments that fall in the following categories: Sub-G0 (<2N DNA), G0/G1 (2N DNA) and S/G2/M (>2N DNA). Error bars represent +/− SEM. * p<0.001, **p<0.05. C. Numbers of cells in Cre+ (solid lines) and Cre- (dashed lines) CD4 T cell activation cultures at 0, 24, 48 and 72 hours post-plating. Three independent experiments are shown. D. CD4 T cells from spleens of Cre+ and Cre- mice were plated and analyzed as described in (B). To some cultures, caspase inhibitor was added (+ inhibitor). Cell cycle profiles are from cells cultured for 48 hours. Numbers show percentages of cells in Sub-G0 (<2N DNA), G0/G1 (2N DNA), S and G2/M (>2N DNA). Results shown are representative of four independent experiments.
Next, CD4 T cells purified from the spleens of Ikflox x Cre and Ikflox (no Cre) mice were plated with αCD3/αCD28 for 24–72 hours in order to perform cell cycle analyses. At the end of the culture period, cells were permeabilized and stained with propidium iodide, a fluorescent dye that intercalates into DNA thereby allowing quantification of DNA content. By 48 hours post-activation, Ikflox x Cre CD4 T cell cultures showed decreased percentages of cells entering S phase (p<0.02) and increased percentages of cells with sub-G0 amounts of DNA (p<0.01) as compared to Ikflox (no Cre) cultures (Fig 4B). Therefore, we can conclude that the reduced proliferative capacity of Ikflox x Cre CD4 T cells is the result of their decreased ability to progress beyond G0/G1 and their increased propensity to die. Taken together, these defects resulted in an inability of Ikflox x Cre cultures to increase in number over time (Fig 4C). The defect in survival, but not proliferation, could be reduced by addition of the caspase-3 inhibitor Z-DEVD-FMK to the activation cultures (Fig 4D), showing that reduced proliferative capacity of Ikflox x Cre CD4 T cells was not solely due to their increased propensity to die when stimulated. It is important to note that Ikflox x Cre CD4 T cells show normal up-regulation of CD25 and CD40L upon activation (Fig 5A) proving that the defects observed upon TCR stimulation are not due to grossly abnormal cells that cannot be activated.
Figure 5. Early analyses of CD4 T cells post-TCR activation show normal activation but upregulated expression of select cytokines in the absence of Ikaros.

A. Representative flow cytometric analyses of splenocytes from Ikflox x dLck-Cre (Cre+) and Ikflox (Cre-) mice plated with 2μg/ml plate-bound αCD3/soluble αCD28 for 24 hours showing expression of the activation markers CD25 and CD40L within the CD4 T cell subset. Boxed numbers in upper right quadrant of the plots shows calculated percentage of CD4 T cells expressing the given marker. Bar graph shows average percentage of CD4 T cells expressing CD25 and CD40L from five independent experiments. Error bars represent +/− SEM. B. qRT-PCR of genes encoding GM-CSF (Csf2), IL-2 (Il2), IFNγ (Ifng), CD25 (Il2ra) and TNFα (Tnf) in Cre+ and Cre- CD4 T cell cultures that had been activated for 24 hours. Values were first normalized to HPRT and then to expression in Cre- cultures (making Cre- cultures equivalent to “1”). Error bars represent +/− SEM. * p<0.001, **p<0.05
Thus, we can conclude that Ikaros is required for viability and proliferation of CD4 T cells after activation, but is not required for early activation events that promote up-regulation of CD25 and CD40L.
Early cytokine response is upregulated in the absence of Ikaros
Cytokines are the mediators of the CD4 T cell response. Their expression is upregulated at the level of gene expression upon TCR/co-receptor signaling. Therefore, expression of cytokine genes was examined following activation (24 hours with αCD3/αCD28) of purified CD4 T cells from the spleens of Ikflox x Cre and Ikflox (no Cre) mice. Ikflox x Cre cultures showed increased expression of a select set of cytokine genes including those encoding IL-2 (Il2; p=0.033), IFNγ (Ifng; p=4.2 × 10^−4), TNFα (Tnf; p=0.036) and GM-CSF (Csf2; p=1.37 × 10^−5) (Fig 5B). Many of these fall into the category of proinflammatory effectors. There was no significant difference in expression of the genes encoding IL-4 (Il4) or IL-17A (Il17a), suggesting that Ikaros deficiency does not impact expression of all cytokine genes (Fig 5B).
Expression of the activation-induced gene encoding IL-2Rα (Il2ra) was also unaffected in the absence of Ikaros (Fig 5B), confirming the normal up-regulation of CD25 discussed above (Fig. 5A) These data suggest that lack of Ikaros predisposes cells to enhanced expression of cytokines linked to inflammation at early stages of activation.
Effect of conditional deletion of Ikaros on Th1 and Th2 lineage specification
CD4 T cells differentiate into T helper subsets after antigenic stimulation through TCR. The cytokines present in their microenvironment are the major determinants in the decision to commit to a specific lineage, as they promote expression of lineage-specific master transcription factors (29). Th1 and Th2 cells were the first two identified T helper subsets. They are flip sides of a coin, expressing factors that drive their own differentiation while repressing the alternative fate. Previous reports have suggested that in CD4 T cells that do not express Ikaros, Th1 lineage differentiation is enhanced whereas that of the Th2 lineage is prohibited (17). This has been attributed, in part, to the inability to repress expression of Tbx21, the gene encoding T-bet, in the absence of Ikaros (17, 30). However, as stated previously, these experiments used CD4 T cells from animals with abnormal thymic T cell development that developed T cell leukemia.
In order to definitively assess how lack of Ikaros in mature T cells impacts their ability to differentiate into Th1 and Th2 lineage cells, CD4 T cells from Ikflox x Cre and Ikflox (no Cre) mice were activated with αCD3/αCD28 under Th1 (IL-12, anti-IL-4) and Th2 (IL-4, anti-IFNγ) polarizing conditions. First, the ability of cells to respond to differentiation cues early in their differentiation process was assessed by examining expression of T-bet and GATA-3, the Th1 and Th2 lineage master regulators respectively, by intracellular staining followed by flow cytometry at 24, 48 and 72 hours post-initiation of culture. Since deletion of Ikaros expression is not complete within the CD4 T cell population, differentiation potential of Ikaros-positive and Ikaros-negative cells in Ikflox x Cre cultures were tracked.
There was no significant difference in levels of Th1 specification in Ikflox x Cre and Ikflox (no Cre) CD4 polarization cultures as assayed by percentage of cells expressing T-bet at 72 hours post-initiation of culture (Fig 6A). However, the Ikaros-deficient sub-population within the Ikflox x Cre cultures did show delayed differentiation, with significant differences in percentages of T-bet positive cells at 24 and 48 hour time points (Fig 6A). Inclusion of a cell viability dye in the stainings ensured that the observed delay was not due to increased cell death in these cultures.
Figure 6. Lack of Ikaros has limited effects on Th1 and Th2 differentiation.

A. CD4 T cells from Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice were plated with αCD3/soluble αCD28 under Th1 or Th2 polarizing conditions. Cells were analyzed for expression of T-bet, GATA3 and Ikaros by flow cytometry at 24, 48 and 72 hours post-culture. The three lines in the plots represent levels of expression of transcription factors in 1) the Cre- culture (solid line), 2) the Ikaros-positive sub-population in the Cre+ culture (dashed line) and 3) the Ikaros-negative sub-population in the Cre+ culture (dotted line). Results shown are the average of three independent experiments. Error bars represent +/− SEM. p values represent comparisons to values in Cre- culture. p* < 0.01, ** p<0.05. B. Flow cytometry plots are representative of three independent experiments. Quantitated data are shown in line graph. Error bars represent +/− SEM. Cre- (solid line), Ik+ Cre+ (dashed line), Ik- Cre+ (dotted line). p values represent comparisons to values in Cre- culture. * p < 0.01. C. CD4 T cells in Th1 and Th2 cultures were re-stimulated with PMA and ionomycin in the presence of Brefeldin A, followed by intracellular cytokine staining and flow cytometric analyses. Data shown are representative of three independent experiments. The bar graph quantitates the result of qRT-PCR analyses of genes encoding IFNγ (Ifng) and IL-4 (Il4) in these cultures.. (making Cre- cultures equivalent to “1”). Error bars represent +/− SEM. n=3 **p<0.05
The early stages of Th2 lineage commitment, as assessed by levels of GATA-3 staining, also did not proceed normally. The percentage of cells expressing GATA-3 was significantly reduced in Ikflox x Cre compared to Ikflox (no Cre) cultures (Fig 6A). This was true for both the Ikaros-positive and Ikaros-negative sub-populations. Previous reports using germline Ikaros mutant mouse models have presented data suggesting a crucial role for Ikaros in suppression of T-bet expression during Th2 differentiation (17, 30). Therefore, we examined levels of T-bet to determine if this defect underlies the decreased Th2 specification observed in Ikflox x Cre cultures. However, there is no difference in T-bet expression in Ikflox x Cre as compared to Ikflox (no Cre) cultures at 24 hours, when GATA-3 expression in the culture is already reduced, suggesting that this cannot be the sole underlying cause (Fig 6B).
Interestingly, up-regulation of T-bet expression is observed in both Ikflox x Cre and Ikflox (no Cre) Th2 cultures by 48 hours, suggesting that this is a normal occurrence during Th2 differentiation (Fig 6B). However, by 72 hours, cells in Ikflox (no Cre) cultures had successfully down-regulated T-bet expression, while those in Ikflox x Cre cultures, both Ikaros-positive and Ikaros-negative subsets, showed sustained expression (Fig 6B). Taken together, these data demonstrate that increased T-bet expression cannot explain the reduction in Th2 specification observed at 24 and 48 hours in Ikflox x Cre cultures, and it also suggests that the defect is not cell-autonomous.
After 6–7 days in Th1 and Th2 polarizing cultures, cells were re-stimulated with PMA and ionomycin, and examined for expression of the signature Th1 and Th2 cytokines, IFNγ and IL-4 respectively, at both the protein and mRNA levels. Ikflox x Cre Th1 cultures showed no significant difference in expression of IFNγ compared to Ikflox (no Cre) Th1 cultures (Fig 6C). The same was true for IL-4 in Th2 cultures. There was a significant increase in Ifng expression in Ikflox x Cre Th2 cultures, although percentages of cells expressing IFNγ were much less than observed in bone fide Th1 cultures (Fig 6B, C).
Taken together these data show that whereas lack of Ikaros does not significantly impact Th1 differentiation, it does result in decreased Th2 specification within the first 72 hours of polarization. We hypothesize that in Ikflox x Cre Th2 cultures, a microenvironment is created by inclusion of Ikaros-negative T cells that results in an inability to repress expression of T-bet and IFNγ, which together impact early events in Th2 differentiation. Yet, at least under conditions of optimal Th2 differentiation as used here, this does not result in skewing of cells towards the Th1 lineage, as demonstrated by lack of reduction in the percentage of cells that are competent to secrete IL-4 at day 6 of culture.
Effect of conditional deletion of Ikaros on Th17/iTreg axis
Th17 and inducible regulatory T (iTreg) cells are closely related CD4 T cell subsets with diametrically opposed functions. They both depend on TGFβ for their differentiation, suggesting that they use overlapping molecular pathways. Presence of the inflammatory cytokine IL-6 in the microenvironment promotes down-regulation of the transcription factor Foxp3, the master regulator of the iTreg cell fate, blocking iTreg and driving Th17 specification (31, 32). Studies using T cells from germline Ikaros mutant mice have reported a role for Ikaros in iTreg differentiation (19, 33), but differ in their assessment of the importance of Ikaros for Th17 differentiation (18, 33).
To study how lack of Ikaros in mature T cells impacts their ability to differentiate into iTreg and Th17 cells, purified splenic CD4 T cells from Ikflox x Cre and Ikflox (no Cre) mice were cultured with αCD3/αCD28 under iTreg (TGFβ, IL-2 anti-IL4, anti-IFNγ) or Th17- (TGFβ, IL-6, anti-IL4, anti-IFNγ) differentiation conditions. First, the ability of cells to respond to differentiation cues early in development was assessed by examining expression of RORγt and Foxp3, the Th17 and Treg lineage master regulators respectively, by intracellular staining followed by flow cytometry at 24, 48 and 72 hours post-initiation of culture. Th17 specification was unaffected by lack of Ikaros whereas iTreg differentiation was significantly decreased (Fig 7A). Unlike the defect in Th2 specification described above, the defect in iTreg differentiation was cell-intrinsic, being observed only in Ikaros-deficient cells in Ikflox x Cre cultures (Fig 7A). A previous report has suggested that Ikaros represses expression of IL-21, a driver of RORγt expression and Th17 differentiation, which could underlie the defect observed in iTreg differentiation (33). However, we did not observe abnormal expression of RORγt in Ikflox x Cre iTreg cultures (Fig 7B). This, together with the observation that the defect in iTreg differentiation is only seen within the Ikaros-negative sub-population in Ikflox x Cre cultures is strong evidence that increased secretion of IL-21, or any other cytokine or factor, by Ikaros-negative cells could not underlie the phenotype.
Figure 7. Ikaros is required for iTreg differentiation and suppresses the pathological Th17 phenotype.

A. CD4 T cells from Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice were plated with αCD3/soluble αCD28 under Th17 or iTreg polarizing conditions. Cells were analyzed for expression of RORγt, Foxp3 and Ikaros by flow cytometry at 24, 48 and 72 hours post-culture. The three lines in the plots represent levels of expression of transcription factors in 1) the Cre- culture (solid line), 2) the Ikaros-positive sub-population in the Cre+ culture (dashed line) and 3) the Ikaros-negative sub-population in the Cre+ culture (dotted line). Results shown are the average of three independent experiments. Error bars represent +/− SEM. p values represent comparisons to values in Cre- culture. *p < 0.005. B. Flow cytometric analyses of RORγt expression as assayed by intracellular staining in 3-day Th17 (dashed line) and iTreg (solid line) Cre+ and Cre- cultures. Data shown are representative of two independent experiments. C. qRT-PCR of gene encoding IL-17A (Il17a) in PMA and ionomycin re-stimulated Th17 cultures. Each of the four lines represents an independent experiment consisting of one Cre+ and one Cre- culture. Values for levels of each transcript were first normalized to HPRT and then plotted as fold increased relative to expression in Cre- cultures (making Cre- cultures equivalent to “1”). D. Flow plots showing expression of T-bet and RORγt in 3-day Th17 cultures as assessed by intracellular staining. Data shown are representative of three independent experiments. E. qRT-PCR of genes encoding GM-CSF (csf2) and IFNγ (ifng) in PMA and ionomycin re-stimulated Th17 cultures. Each of the four lines represents an independent experiment consisting of one Cre+ and one Cre- culture. Values for levels of each transcript were first normalized to HPRT and then plotted as fold increased relative to expression in Cre- cultures (making Cre- cultures equivalent to “1”).
After 3–4 (for Th17 cultures) or 6–7 (for iTreg cultures) days in polarizing cultures, cells were analyzed for expression of cytokines after re-stimulation with PMA and ionomycin. Expression of the gene encoding the hallmark Th17 cytokine IL-17A was not significantly different in Ikflox x Cre and Ikflox (no Cre) Th17 differentiation cultures (Fig 7C). These data, together with the normal up-regulation of RORγt in Ikflox x Cre Th17 cultures at all time points (Fig 7A), shows that lack of Ikaros does not impact specification into the Th17 lineage.
Co-expression of T-bet with RORγt in Th17 cells has been linked to a pathological phenotype in some forms of autoimmune disease (34–37). Since lack of Ikaros leads to the inability to suppress expression of T-bet in Th2 cells (Fig, 5B), we hypothesized that Ikaros-deficient Th17 cells could have this pathological phenotype. Indeed, a larger percentage of RORγt+ cells also expressed T-bet in Ikflox x Cre as compared to Ikflox (no Cre) Th17 cultures (Fig. 7D). High-level expression of the cytokine GM-CSF and an “exTh17” phenotype defined by expression of IFNγ are additional hallmarks of pathological Th17 cells associated with autoimmune syndromes (38–43). In PMA and ionomycin re-stimulated Ikflox x Cre Th17 cultures, there was significantly increased expression of the genes encoding GM-CSF (p=.0375) and IFNγ (p=.018) relative to that expressed in Ikflox (no Cre) counterparts (Fig 7E). In conclusion, although lack of Ikaros does not impact Th17 specification, it causes a shift in the qualitative nature of the Th17 cell response towards a pathological phenotype associated with autoimmunity.
Deregulation of gene programs associated with inflammation and autoimmunity in the absence of Ikaros
In order to more comprehensively examine the effect of loss of Ikaros in CD4 T cells, we undertook global analyses of gene expression in purified resting (freshly isolated) and activated (αCD3/αCD28 for 24 hours) CD4 T cells from spleens of Ikflox x Cre and Ikflox (no Cre) mice. Gene-set enrichment analyses (GSEA) of the microarray data reveals the over-representation of genes associated with inflammation and autoimmunity in activated Ikaros-deficient CD4 T cells (Table 1). Interestingly, up-regulation of gene sets and genes associated with the response to type I IFN is also observed in resting (freshly isolated) Ikflox x Cre CD4 T cells (Table 2 and Fig 8A, B) (44).
Table 1. In the absence of Ikaros, activated CD4 T cells display up-regulation of pathways linked to inflammation and autoimmunity.
Results of GSEA analyses of microarray data for Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre] CD4 T cells activated with αCD3/soluble αCD28 for 24 hours. In total, 1876 gene sets were significantly enriched in Cre+ relative to Cre- CD4 T cells (FDR q<0.25). Examples of enhanced biological processes, functions and pathways are shown. ES: Enrichment score; NES: Normalized enrichment score.
| Gene Set Name | Gene Set Size |
ES | NES | Nominal p value |
FDR q value |
|---|---|---|---|---|---|
| Hallmark TNFA Signaling via NFKB | 192 | 0.67 | 2.72 | <0.001 | 0.0000 |
| Hallmark IL2 STAT5 signaling | 196 | 0.59 | 2.41 | <0.001 | 0.0000 |
| Hallmark Interferon Gamma Response | 182 | 0.59 | 2.41 | <0.001 | 0.0000 |
| Hallmark Inflammatory Response | 189 | 0.58 | 2.37 | <0.001 | 0.0000 |
| GO Response to Interferon Gamma | 104 | 0.62 | 2.36 | <0.001 | 0.0000 |
| Biocarta Cytokine Pathway | 18 | 0.89 | 2.34 | <0.001 | 0.0000 |
| KEGG Type I Diabetes Mellitus | 31 | 0.76 | 2.29 | <0.001 | 0.0001 |
| Biocarta Inflammatory Pathway | 25 | 0.77 | 2.20 | <0.001 | 0.0005 |
| KEGG Graft versus Host Disease | 23 | 0.77 | 2.21 | <0.001 | 0.0006 |
| KEGG Allograft Rejection | 25 | 0.77 | 2.18 | <0.001 | 0.0010 |
| GO Response to Interferon Beta | 20 | 0.79 | 2.11 | <0.001 | 0.0022 |
| Hallmark Interferon Alpha Response | 87 | 0.58 | 2.08 | <0.001 | 0.0030 |
| GO Negative Regulation of Viral Genome Replication | 35 | 0.68 | 2.06 | <0.001 | 0.0037 |
| KEGG Autoimmune Thyroid Disease | 27 | 0.69 | 2.02 | <0.001 | 0.0057 |
Table 2. In the absence of Ikaros, resting CD4 T cells up-regulate expression of pathways linked to the response to type I Interferons.
Results of GSEA analyses of microarray data for freshly isolated Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre] CD4 T cells. In total, 152 gene sets were significantly enriched in Cre+ relative to Cre- CD4 T cells (FDR q<0.25). Examples of enhanced biological processes, functions and pathways are shown. ES: Enrichment score; NES: Normalized enrichment score.
| Gene Set Name | Gene Set Size |
ES | NES | Nominal p value |
FDR q value |
|---|---|---|---|---|---|
| Hallmark Interferon Alpha Response | 87 | 0.63 | 2.23 | <0.001 | 0.0033 |
| Reactome Interferon Alpha Beta Signaling | 39 | 0.73 | 2.25 | <0.001 | 0.0055 |
| GO Response to Type I Interferon | 41 | 0.67 | 2.14 | <0.001 | 0.0244 |
| Reactome Interferon Signaling | 115 | 0.55 | 2.06 | <0.001 | 0.0349 |
| GO Response to Interferon Alpha | 16 | 0.78 | 2.04 | <0.001 | 0.0358 |
| Reactome RIG MDA5 Mediated Induction of IFN Alpha Beta Pathway | 53 | 0.59 | 1.99 | <0.001 | 0.0541 |
| GO Response to Interferon Beta | 20 | 0.72 | 1.94 | <0.001 | 0.0756 |
Figure 8. Ikaros is required for suppression of interferon stimulated gene (ISG) expression in T cells.
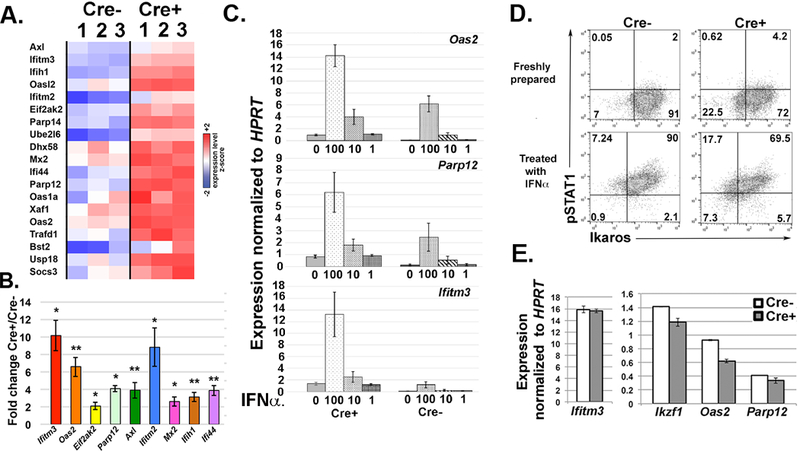
A. Heat map comparing expression levels of ISGs from transcriptome analyses of freshly isolated CD4 splenic T cells from Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice. Results from three independent samples of each are shown. All gene shown were enhanced an average 1.4-fold or greater in Cre+ samples and differences were statistically significant (FDRq<0.25, p ≤ 0.02). B. qRT-PCR analyses of representative ISGs to validate differences in resting CD4 T cells. Values for levels of each transcript were first normalized to HPRT. Ratios of normalized Cre+ to Cre- values are shown. n=5 Error bars represent +/− SEM. *p ≤ 0.01, **p<0.005 C. qRT-PCR analyses showing expression of the three ISGs Oas2, Parp12 and Ifitm3 in Cre+ and Cre- splenic CD4 T cells that had been treated with 0, 1, 10 or 100 U of IFNα for 4 hours. Bar graph shows average results from three independent experiments. Results are normalized to values for HPRT expression. Error bars represent +/− SEM. n=3 D. Flow plots showing expression of Ikaros and phospho-STAT1 (pSTAT1) in freshly prepared splenocytes from Cre+ and Cre- mice. Splenocytes treated with IFNα served as a positive control for pSTAT1 staining. In the plots representing Cre+ splenocytes, T cell populations would fall into the Ikaros-negative quadrants (upper and lower left on plots). E. qRT-PCR analyses showing expression of the three ISGs Oas2, Parp12 and Ifitm3 in peritoneal macrophages harvested from Cre+ and Cre- mice. Results are normalized to values for HPRT expression. Error bars represent +/− SEM.
Next, we wanted to determine the magnitude of up-regulation of ISGs in Ikaros-deficient T cells as compared to what is observed during a bona fide type I IFN response. To activate the type I IFN response, CD4 T cells from Ikflox x Cre and Ikflox (no Cre) mice were treated with varying concentrations of IFNα for 4 hours, followed by preparation of RNA. In some instances, levels of over-expression in Ikflox x Cre resting T cells approximated those observed in 10U/ml (Oas2 and Parp12) or 100U/ml (Ifitm3) IFNα-stimulated Ikflox (no Cre) CD4 T cells, as shown by levels of three of the top de-regulated ISGs (Fig 8C).
In order to determine if increased expression of ISGs was due to the presence of active IFN signaling due to an inflammatory environment in the Ikflox x Cre mice, we undertook two approaches. First, we examined Ikflox x Cre T cells for the presence of phospho-STAT1, a downstream activator of both type I and type II IFNs using flow cytometry (45). Intracellular staining with Ikaros antibody was performed concurrently to identity the T cells (which would be the Ikaros-negative sub-population). Whereas we could detect phospho-STAT1 in splenocytes activated with IFNα ex vivo for 30 minutes, no phospho-STAT1 was present in freshly isolated Ikflox x Cre splenocytes that were fixed immediately upon isolation (Fig 8D). Secondly, we examined expression of ISGs in macrophages from Ikflox x Cre mice, which would have intact Ikaros expression. We observed no enhanced ISG expression relative to what is observed in macrophages from Ikflox (no Cre) mice (Fig 8E). Taken together, these data show that the increased ISG expression in freshly isolated Ikflox x Cre CD4 T cells occurs in the absence of active IFN signaling and is not due an inflammatory environment in the mouse, which would also impact ISG expression in the macrophages.
Increased expression of the ISG program would be expected to decrease the ability of a cell to be infected by virus. To determine if Ikflox x Cre CD4 T cells are more resistant to viral infection, CD4 T cells from spleens of Ikflox x Cre and Ikflox (no Cre) mice were infected with the recombinant virus rVSV-eGFP) (25) for 24 hours followed by flow cytometric analyses. Cultures treated with IFNα were significantly less infected, demonstrating that signals provided by type I IFN reduced infection rates in our model system (Fig 9). Ikflox x Cre CD4 T cells were significantly less infected than their Ikflox (no Cre) counterparts (Fig 9). Significantly, the percentage of infected cells in the Ikflox x Cre cultures was not significantly different than those in IFNα -treated Ikflox (no Cre) cultures, showing that the level of infection was similar to that observed in cells undergoing a strong induction of the type I IFN-induced gene program.
Figure 9. Lack of Ikaros renders cells comparably resistant to infection by virus as wild-type cells treated with IFNα.
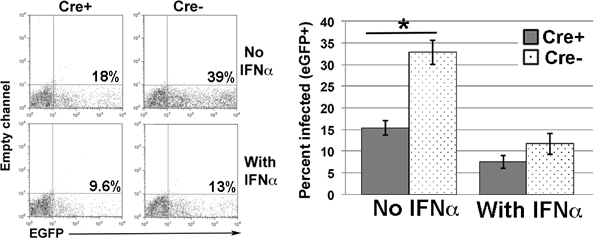
CD4 T cells, purified from the spleens of Ikflox x dLck-Cre [Cre+] and Ikflox (no Cre) [Cre-] mice, were cultured with or without 10 U/ml IFNα, followed by infection with the recombinant virus rVSV-eGFP. 24 hours after infection, cells were analyzed for expression of eGFP by flow cytometry. Shown are representative plots from two independent experiments, with two cultures/condition per experiment. Bar graph shows compilation of all data. Error bars represent +/− SEM. There was no significant difference in percentage of cells infected in Cre+ cultures without IFNα vs. Cre- cultures with IFNα (p=0.2615). n=4 *p=0.0016. Note: For both Cre+ and Cre- cultures, addition of IFNα resulted in a significant decrease in percentage of cells infected relative to that observed in cultures with no IFNα (for Cre+ cultures, p=0.011; for Cre- cultures, p=0.00125).
Taken together, these data show that lack of Ikaros induces a robust de-repression of the ISG program in T cells in the absence of outside IFNα signals.
Discussion
Although it has been known for more than a decade that Ikaros is a critical factor for regulation of early developmental decisions at the level of the HSC as well as in T cell maturation in the thymus, its role in mature T cells is less well defined. Our new conditional knockout Ikaros mouse model has now given us the tools to define an important role for Ikaros in T cells as repressor of inflammatory gene programs, both at the level of inflammatory cytokine gene expression and gene programs induced by type I IFNs. Deregulation of these programs in the absence of Ikaros leads to altered Th phenotypes and crippled proliferative responses.
Our data demonstrate that peripheral T cells from mice with germline Ikaros null mutations have different phenotypes from those in which Ikaros is deleted after thymocyte maturation. Whereas CD4 T cells from Ikaros null mice are unable to differentiate into the Th2 and Th17 lineages, CD4 T cells from Ikflox x dLck- Cre mice show no defect in up-regulation of RORγt in response to Th17 differentiation signals, and, while up-regulation of GATA-3 is decreased, Th2 specification can proceed. Ikflox x dLck- Cre Th2 and Th17 cultures also express wild-type levels of Il4 and IL17a, respectively. These data suggest that Th2 and Th17 lineage differentiation circuits are intact and do not depend on Ikaros. Lack of Ikaros has also been associated with a hyper-proliferative phenotype in CD4 T cells from Ikaros germline knockout mice. However, our data suggest a contrary role with lack of Ikaros resulting in decreased proliferation after T cell activation. The confounding factors of the development of T cell leukemia as well as the defects in both thymic positive and negative selection observed in germline Ikaros null mice, which does not occur in our model system, likely contributes to these disparate phenotypes.
Interestingly, data from studies using T cells from mice with germline mutations in sequences within the DNA binding domain of Ikaros leading to high level expression of “dominant negative” Ikaros proteins demonstrated that they are able to differentiate into Th2 cells ex vivo (30), and even show enhanced Th17 differentiation (33). These studies, together with results reported here, support the contention that although there is much to be learned by the study of germline Ikaros null mice, particularly as regards understanding mechanisms of human disease that are the result of inherited genetic mutations, they are likely not the best choice for parsing out the mechanistic role of Ikaros in mature T cells.
Although, in our model, Ikaros-deficient T cells are able to attain a Th17 fate, the Th17 cells that develop are qualitatively different than their wild-type counterparts. First, a large percentage of cells in the Ikflox x dLck- Cre cultures co-express RORγt and T-bet, which has been associated with Th17 cells with pathological tendencies. Secondly, cells in Ikflox x dLck- Cre cultures display significantly increased expression of the genes encoding IFNγ and GM-CSF, cytokines linked to Th17-driven autoimmune and inflammatory diseases such as autoimmune encephalomyelitis, diabetes, autoimmune myocarditis and colitis (38–43, 46). The molecular pathways that underlie development of pathological Th17 cells are largely unknown, although IL-23 signaling has been shown to be important. IL-23 induces expression of both IFNγ and GM-CSF in Th17 cells, which likely contributes to this role (39, 43, 47). Our Th17 cultures did not contain IL-23, yet lack of Ikaros has similar consequences. This suggests that IL-23-signaling in Th17 cells may impinge on Ikaros function, which should be further explored. Enhanced expression of IFNγ and GM-CSF is not relegated to the Th17 lineage and can be observed as early as 24 hours post-activation in non-polarized Ikflox x dLck- Cre CD4 T cell cultures. In addition, genome-wide gene expression analyses reveal that, in 24-hour activated Ikaros-deficient CD4 T cell cultures, there is global enhancement of gene sets associated with inflammation and autoimmunity, suggesting that this de-regulation occurs early and is not dependent on a particular Th cell differentiation program. Taken together, these data define Ikaros as a guardian of the inflammatory phenotype in T cells.
Using germline Ikaros mutant mice, we and others have reported increased expression of T-bet in CD4 T cells cultured under Th2 polarizing conditions. It has been reported that Ikaros may be involved in directly repressing expression of Tbx21 during Th2 differentiation. Interestingly, we observed that T-bet up-regulation is a normal event early in Th2 differentiation and lack of Ikaros does not impact levels or frequency of T-bet expression up to 48 hours post-initiation of Th2 differentiation. Rather Ikaros’ role appears to be evident later in the process, when sustained T-bet expression is observed in Ikflox x dLck- Cre cultures as opposed to its down-regulation in Ikflox (no Cre) cultures. In spite of this, Ikaros-deficient CD4 T cells can attain the Th2 fate, albeit at a reduced rate, as there is no effect on expression of IL-4 at later time points. While cells in Ikflox x dLck- Cre Th2 cultures also express increased levels of IFNγ compared to Ikflox (no Cre) cultures, the frequency of IFNγ-secreting cells is significantly lower than that observed in bona fide Th1 cultures. However, taking into consideration that T-bet expression is upregulated in response to IFNγ (48), our data suggest that the inability to repress IFNγ production (whose expression is enhanced by 24 hours post-activation in Ikflox x dLck-Cre activation cultures) is the likely mechanism underlying sustained T-bet expression in Ikflox x dLck- Cre Th2 cultures. This model is supported by our previous report showing that restoration of Ikaros expression using retroviral transduction was able to suppress expression of IFNγ in Ikaros null T cells, but was unable to alter expression of Tbx21 (49).
Consistent with what has been reported using CD4 T cells from Ikaros germline mutant mice, we observe a significantly reduced ability of Ikflox x dLck- Cre CD4 T cells to differentiate into iTreg cells. Due to incomplete efficiency of Ikaros deletion within the CD4 T cell population in the spleen, our conditional knockout model has provided insight into the mechanism underlying this defect. Since decreased iTreg differentiation was observed in Ikaros-negative, but not Ikaros-positive, cells in iTreg differentiation cultures, this points to a cell-intrinsic defect rather than increased secretion of cytokines that would suppress the iTreg program. It has been reported that iTregs can have unstable expression of Foxp3, resulting in generation of pro-inflammatory exTreg cells that can be destructive (50). Our data cannot distinguish between reduced iTreg differentiation vs. reduced iTreg stability in the absence of Ikaros. Since Ikaros associates with chromatin remodeling complexes (51), it is reasonable to hypothesize that differentiation induced programs may be unstable in its absence. Future studies are needed to differentiate between these two mechanisms.
In a quest to define the mechanism underlying the pro-inflammatory phenotype observed in Ikflox x dLck- Cre CD4 T cells, global gene expression analyses were performed. These studies utilized freshly isolated CD4 T cells from spleens of Ikflox x Cre and Ikflox (no Cre) mice. Therefore, they would be a mixture of naive, memory, Treg and differentiated Th cell subsets. Ikflox x dLck- Cre and Ikflox (no Cre) CD4 T cell populations are equivalent based on assessment of defined markers for these populations. Therefore, in order to capture the most differences and not rely on a sorting strategy based on expression of surface markers whose expression could be altered in the absence of Ikaros, we chose this approach. Gene set enrichment analyses (GSEA) of these data revealed that the most significant differences in freshly isolated Ikflox x Cre and Ikflox (no Cre) CD4 T cells were in expression of gene sets associated with the response to type I IFNs. Enhanced expression of IFN-stimulated genes (ISGs) in Ikflox x dLck- Cre CD4 T cells occurred in the absence of type I IFN signaling in the T cells or an overt inflammatory environment in the animals, suggesting that Ikaros functions as a repressor of this gene program in the absence of activating signal. Could there be a link between the inappropriate activation of a type I IFN response in resting Ikaros-deficient T cells and the pro-inflammatory phenotype of Ikaros-deficient activated T cells? Studies with innate immune cells have provided conflicting results with some studies reporting that type I IFNs restrain pro-inflammatory pathways and others demonstrating that they enhance these pathways. Reports supporting the latter include a mouse model of RSV infection in the lung, where it was discovered that type I IFNs serve to amplify the pro-inflammatory response, including production of IFNγ, GM-CSF and TNFα (52). In addition, a recent report has shown that, in human macrophages, type I IFNs prime the chromatin associated with pro-inflammatory cytokines to enable robust transcriptional responses to weak signals (53). Therefore, there are intriguing data that serve as a precedent to investigate this possibility further.
Why would T cells need to limit expression of ISGs? The response to type I IFNs results in establishment of an antiviral state, which is characterized by expression of proteins that interfere with the viral life cycle. These include proteins involved in RNA degradation, translational inhibition and apoptosis (54). Therefore, not surprisingly, expression of ISGs can result in anti-proliferative and pro-apoptotic phenotypes. We have shown that Ikflox x dLck- Cre CD4 T cells proliferate less and die more than their wild-type counterparts when activated. We hypothesize that the elevated expression of ISGs in Ikaros-deficient resting T cells contributes to this phenotype. ISG expression also must be controlled after the immune response has peaked since persistent type I IFN signaling can impair host immunity, leading to virus persistence (55–57). Taken together, it is clear that a productive adaptive immune response requires a tightly controlled response to type I IFNs and our data support the conclusion that Ikaros is required for this preventative roadblock.
Ikaros gene variants have been associated with autoimmune and inflammatory diseases in humans. Our data have revealed likely mechanisms that could underlie this. However, Ikflox x dLck- Cre mice do not display overt symptoms of spontaneous autoimmunity. It is important to note that we have not yet conducted targeted studies to address this (i.e. looked for autoantibodies). Also, most models of spontaneous autoimmunity require time to develop and our studies have focused on young mice (<3 months of age). Future studies will more carefully investigate this.
In conclusion, we have identified Ikaros as the center of a complex web of events important for a regulated immune response. Ikaros deficiency results in an inability of CD4 T cells to attain an iTreg fate as well as drives the T cell response down an inflammatory pathway, providing insight into how its activity is crucial to maintain T cell homeostasis and prevent autoimmune and inflammatory diseases. We also have defined Ikaros as a repressor of the type I IFN-stimulated gene program, another key pathway whose deregulation is linked to autoimmunity. It will be important translate these findings into in vivo mouse models of autoimmunity as well as define these roles for Ikaros in human cells and disease.
Acknowledgements
The authors would like to acknowledge Dr. Anna Belkina for help with cell cycle analyses; Dr. John Connor, Dr. John Ruedas, and Alexandra Soucy for the generous gift of the rVSV-eGFP virus and for carrying out the infections; Dr. Hans Dooms for providing the protocol and antibody used for the anti-phosphoSTAT1 studies; and Dr. Yuriy Alekseyev and Dr. Adam Gower from the Microarray and Sequencing Core Facility for the microarray analyses and countless hours of help.
This work was supported by National Institutes of Health grants AI113522 and AI133228 to SW. PA was supported by the Immunology Training Program (ITP) training grant funded by the National Institutes of Health (T32 A1007309).
References
- 1.Georgopoulos K, Winandy S, and Avitahl N. 1997. The role of the Ikaros gene in lymphocyte development and homeostasis. Annu Rev Immunol 15: 155–176. [DOI] [PubMed] [Google Scholar]
- 2.Molnar A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, Jenkins NA, Bruns G, and Georgopoulos K. 1996. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. Journal of immunology 156: 585–592. [PubMed] [Google Scholar]
- 3.Igartua C, Myers RA, Mathias RA, Pino-Yanes M, Eng C, Graves PE, Levin AM, Del-Rio-Navarro BE, Jackson DJ, Livne OE, Rafaels N, Edlund CK, Yang JJ, Huntsman S, Salam MT, Romieu I, Mourad R, Gern JE, Lemanske RF, Wyss A, Hoppin JA, Barnes KC, Burchard EG, Gauderman WJ, Martinez FD, Raby BA, Weiss ST, Williams LK, London SJ, Gilliland FD, Nicolae DL, and Ober C. 2015. Ethnic-specific associations of rare and low-frequency DNA sequence variants with asthma. Nat Commun 6: 5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunninghame Graham DS, Morris DL, Bhangale TR, Criswell LA, Syvanen AC, Ronnblom L, Behrens TW, Graham RR, and Vyse TJ. 2011. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with systemic lupus erythematosus. PLoS Genet 7: e1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han JW, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu JH, Cai ZM, Huang W, Zhao GP, Xie HF, Fang H, Lu QJ, Xu JH, Li XP, Pan YF, Deng DQ, Zeng FQ, Ye ZZ, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou FS, Du WH, Cheng YL, Yang JQ, Shen SK, Li J, Sheng YJ, Zuo XX, Zhu WF, Gao F, Zhang PL, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu KJ, Li Y, Hu DY, Lu WS, Huang JL, Liu SX, Li H, Ren YQ, Wang ZX, Yang CJ, Wang PG, Zhou WM, Lv YM, Zhang AP, Zhang SQ, Lin D, Li Y, Low HQ, Shen M, Zhai ZF, Wang Y, Zhang FY, Yang S, Liu JJ, and Zhang XJ. 2009. Genome-wide association study in a Chinese Han population identifies nine new susceptibility loci for systemic lupus erythematosus. Nat Genet 41: 1234–1237. [DOI] [PubMed] [Google Scholar]
- 6.Davidson A, and Diamond B. 2001. Autoimmune diseases. N Engl J Med 345: 340–350. [DOI] [PubMed] [Google Scholar]
- 7.Swafford AD, Howson JM, Davison LJ, Wallace C, Smyth DJ, Schuilenburg H, Maisuria-Armer M, Mistry T, Lenardo MJ, and Todd JA. 2011. An allele of IKZF1 (Ikaros) conferring susceptibility to childhood acute lymphoblastic leukemia protects against type 1 diabetes. Diabetes 60: 1041–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Consortium NIG, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van Gossum A, Zelenika D, Franchimont D, Hugot JP, de Vos M, Vermeire S, Louis E, Belgian-French IBDC, Wellcome C Trust Case Control, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, and Daly MJ. 2008. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 40: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorlova O, Martin JE, Rueda B, Koeleman BP, Ying J, Teruel M, Diaz-Gallo LM, Broen JC, Vonk MC, Simeon CP, Alizadeh BZ, Coenen MJ, Voskuyl AE, Schuerwegh AJ, van Riel PL, Vanthuyne M, van ‘t Slot R, Italiaander A, Ophoff RA, Hunzelmann N, Fonollosa V, Ortego-Centeno N, Gonzalez-Gay MA, Garcia-Hernandez FJ, Gonzalez-Escribano MF, Airo P, van Laar J, Worthington J, Hesselstrand R, Smith V, de Keyser F, Houssiau F, Chee MM, Madhok R, Shiels PG, Westhovens R, Kreuter A, de Baere E, Witte T, Padyukov L, Nordin A, Scorza R, Lunardi C, Lie BA, Hoffmann-Vold AM, Palm O, Garcia de la Pena P, Carreira P, Spanish Scleroderma G, Varga J, Hinchcliff M, Lee AT, Gourh P, Amos CI, Wigley FM, Hummers LK, Nelson JL, Riemekasten G, Herrick A, Beretta L, Fonseca C, Denton CP, Gregersen PK, Agarwal S, Assassi S, Tan FK, Arnett FC, Radstake TR, Mayes MD, and Martin J. 2011. Identification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategy. PLoS Genet 7: e1002178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumortier A, Kirstetter P, Kastner P, and Chan S. 2003. Ikaros regulates neutrophil differentiation. Blood 101: 2219–2226. [DOI] [PubMed] [Google Scholar]
- 11.Lopez RA, Schoetz S, DeAngelis K, O’Neill D, and Bank A. 2002. Multiple hematopoietic defects and delayed globin switching in Ikaros null mice. Proceedings of the National Academy of Sciences of the United States of America 99: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichogiannopoulou A, Trevisan M, Friedrich C, and Georgopoulos K. 1998. Ikaros in hemopoietic lineage determination and homeostasis. Semin Immunol 10: 119–125. [DOI] [PubMed] [Google Scholar]
- 13.Nichogiannopoulou A, Trevisan M, Neben S, Friedrich C, and Georgopoulos K. 1999. Defects in hemopoietic stem cell activity in Ikaros mutant mice. The Journal of experimental medicine 190: 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban JA, and Winandy S. 2004. Ikaros null mice display defects in T cell selection and CD4 versus CD8 lineage decisions. Journal of immunology 173: 4470–4478. [DOI] [PubMed] [Google Scholar]
- 15.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, and Georgopoulos K. 1996. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity 5: 537–549. [DOI] [PubMed] [Google Scholar]
- 16.Wu L, Nichogiannopoulou A, Shortman K, and Georgopoulos K. 1997. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity 7: 483–492. [DOI] [PubMed] [Google Scholar]
- 17.Quirion MR, Gregory GD, Umetsu SE, Winandy S, and Brown MA. 2009. Cutting edge: Ikaros is a regulator of Th2 cell differentiation. Journal of immunology 182: 741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong LY, Hatfield JK, and Brown MA. 2013. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T cell development. J Biol Chem 288: 35170–35179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agnihotri P, Robertson NM, Umetsu SE, Arakcheeva K, and Winandy S. 2017. Lack of Ikaros cripples expression of Foxo1 and its targets in naive T cells. Immunology 152: 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, and Georgopoulos K. 1999. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity 10: 333–343. [DOI] [PubMed] [Google Scholar]
- 21.Urban JA, Brugmann W, and Winandy S. 2009. Cutting Edge: Ikaros null thymocytes mature into the CD4 lineage with reduced TCR signal: A study using CD3{zeta} immunoreceptor tyrosine-based activation motif transgenic mice. Journal of immunology 182: 3955–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winandy S, Wu L, Wang JH, and Georgopoulos K. 1999. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. The Journal of experimental medicine 190: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winandy S, Wu P, and Georgopoulos K. 1995. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell 83: 289–299. [DOI] [PubMed] [Google Scholar]
- 24.Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, Lawson GW, Bensinger SJ, Farnham PJ, Witte ON, and Smale ST. 2013. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nature immunology 14: 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitlow ZW, Connor JH, and Lyles DS. 2006. Preferential translation of vesicular stomatitis virus mRNAs is conferred by transcription from the viral genome. Journal of virology 80: 11733–11742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Q, Strong J, and Killeen N. 2001. Homeostatic competition among T cells revealed by conditional inactivation of the mouse Cd4 gene. The Journal of experimental medicine 194: 1721–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harker N, Naito T, Cortes M, Hostert A, Hirschberg S, Tolaini M, Roderick K, Georgopoulos K, and Kioussis D. 2002. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol Cell 10: 1403–1415. [DOI] [PubMed] [Google Scholar]
- 28.Naito T, Gomez-Del Arco P, Williams CJ, and Georgopoulos K. 2007. Antagonistic interactions between Ikaros and the chromatin remodeler Mi-2beta determine silencer activity and Cd4 gene expression. Immunity 27: 723–734. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Yamane H, and Paul WE. 2010. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, and Wells AD. 2010. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. J Biol Chem 285: 2545–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, Reid SP, Levy DE, and Bromberg JS. 2009. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. Journal of immunology 182: 259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, and Dong C. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 29: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heller JJ, Schjerven H, Li S, Lee A, Qiu J, Chen ZM, Smale ST, and Zhou L. 2014. Restriction of IL-22-producing T cell responses and differential regulation of regulatory T cell compartments by zinc finger transcription factor Ikaros. Journal of immunology 193: 3934–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grifka-Walk HM, and Segal BM. 2017. T-bet promotes the accumulation of encephalitogenic Th17 cells in the CNS. J Neuroimmunol 304: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, and Stockinger B. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nature immunology 12: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB, Lee YK, Weaver CT, Yagi R, and Lazarevic V. 2014. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-gamma-producing T helper 17 cells. Immunity 40: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Weiner J, Liu Y, Smith AJ, Huss DJ, Winger R, Peng H, Cravens PD, Racke MK, and Lovett-Racke AE. 2009. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. The Journal of experimental medicine 206: 1549–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, and Dittel BN. 2007. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. Journal of immunology 178: 39–48. [DOI] [PubMed] [Google Scholar]
- 39.El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, and Rostami A. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature immunology 12: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, and Becher B. 2011. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature immunology 12: 560–567. [DOI] [PubMed] [Google Scholar]
- 41.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, and Romagnani S. 2007. Phenotypic and functional features of human Th17 cells. The Journal of experimental medicine 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bending D, De la Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, and Cooke A. 2009. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. The Journal of clinical investigation 119: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, and Powrie F. 2010. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fensterl V, Chattopadhyay S, and Sen GC. 2015. No Love Lost Between Viruses and Interferons. Annual review of virology 2: 549–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platanias LC 2005. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nature reviews. Immunology 5: 375–386. [DOI] [PubMed] [Google Scholar]
- 46.Sonderegger I, Iezzi G, Maier R, Schmitz N, Kurrer M, and Kopf M. 2008. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. The Journal of experimental medicine 205: 2281–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gaffen SL, Jain R, Garg AV, and Cua DJ. 2014. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nature reviews. Immunology 14: 585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, and O’Shea JJ. 2001. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proceedings of the National Academy of Sciences of the United States of America 98: 15137–15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Umetsu SE, and Winandy S. 2009. Ikaros Is a Regulator of Il10 Expression in CD4+ T Cells. Journal of immunology 183: 5518–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, and Bluestone JA. 2009. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nature immunology 10: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, Kingston R, and Georgopoulos K. 1999. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity 10: 345–355. [DOI] [PubMed] [Google Scholar]
- 52.Goritzka M, Durant LR, Pereira C, Salek-Ardakani S, Openshaw PJ, and Johansson C. 2014. Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. Journal of virology 88: 6128–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park SH, Kang K, Giannopoulou E, Qiao Y, Kang K, Kim G, Park-Min KH, and Ivashkiv LB. 2017. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nature immunology 18: 1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sadler AJ, and Williams BR. 2008. Interferon-inducible antiviral effectors. Nature reviews. Immunology 8: 559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng L, Ma J, Li J, Li D, Li G, Li F, Zhang Q, Yu H, Yasui F, Ye C, Tsao LC, Hu Z, Su L, and Zhang L. 2017. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. The Journal of clinical investigation 127: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, Schreiber RD, de la Torre JC, and Oldstone MB. 2013. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, Aronow BJ, Karp CL, and Brooks DG. 2013. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340: 202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]


