Abstract
Severe respiratory virus infections feature robust local host responses that contribute to disease severity. Immunomodulatory strategies that limit virus‐induced inflammation may be of critical importance, notably in the absence of antiviral vaccines. Here we examined the role of the pleiotropic cytokine, interleukin-6 (IL-6) in acute infection with pneumonia virus of mice (PVM), a natural rodent pathogen that is related to respiratory syncytial virus and that generates local inflammation as a feature of severe infection. In contrast to Influenza A, PVM is substantially less lethal in IL-6−/− mice than it is in wild-type, a finding associated with diminished neutrophil recruitment and reduced fluid accumulation in lung tissue. Ly6Chi proinflammatory monocytes are recruited in response to PVM via a CCR2-dependent mechanism, but they are not a major source of IL-6, nor do they contribute to lethal sequelae of infection. By contrast, alveolar macrophages (AMs) are readily infected with PVM in vivo; ablation of AMs results in prolonged survival in association with a reduction in virus-induced IL-6. Finally, as shown previously, administration of immunobiotic Lactobacillus plantarum to the respiratory tracts of PVM-infected mice promoted survival in association with diminished levels of IL-6. We demonstrated here that IL-6 suppression is a critical feature of the protective mechanism; PVM-infected IL-6−/− mice responded to low doses of L. plantarum and administration of IL-6 overcame L. plantarum-mediated protection in PVM-infected wild-type mice. Taken together, these results connect the actions of IL-6 to PVM pathogenesis and suggest cytokine blockade as a potential therapeutic modality in severe infection.
Keywords: Inflammation, Cytokines, Pneumoviridae, Lactobacillus
Introduction
Severe respiratory virus infections are notable for robust local host inflammatory responses that contribute to disease severity [1, 2]. Immunomodulatory strategies designed to limit virus‐induced inflammation may be of substantial therapeutic importance, particularly when no vaccines or broadly effective agents are available [3].
Our studies on inflammation and immunomodulatory strategies in vivo have utilized the natural rodent pathogen, pneumonia virus of mice (PVM). PVM is a virus of the family Pneumoviridae, genus, Orthopneumovirus, and is closely related to the human pathogen, Respiratory Syncytial Virus (RSV; [4, 5]). RSV does not replicate effectively in inbred mouse strains [6]. By contrast, PVM undergoes robust replication in mouse lung tissue and generates severe inflammatory disease in association with cytokine production and prominent leukocyte recruitment to the airways. Acute PVM infection in mice phenocopies the inflammatory responses characteristic of the most severe forms of RSV infection in human subjects and serves as a clinically relevant in vivo model for evaluating anti‐inflammatory and immunomodulatory therapeutic strategies [6, 7].
Among the individual cytokines with an impact on the pathogenesis of pneumovirus infection, CCL3 was shown to promote neutrophil recruitment; blockade of CCL3 or its receptor, CCR1, contributes improved survival in response to acute PVM infection [8, 9]. Other cytokine ligands and receptors implicated in promoting inflammatory pathology in PVM infection include cysteinyl leukotrienes, ChemR23, Fas/FasL, and CD8+ T cell-derived mediators [10–13].
Development of immunomodulatory strategies for the prevention of lethal sequelae of respiratory virus infection is currently an area of active exploration (reviewed in [14]). Towards this end, we and others have found that administration of otherwise benign Lactobacillus species directly to the respiratory mucosa of virus-infected mice has resulted in protection against lethal disease [15–19]. The mechanisms underlying this response, and, more broadly, the ways in which bacteria, bacterial components, and PRR-activating ligands administered to the respiratory tract promote protection against lethal virus infection remain to be fully elucidated [20–22]. We have found that Lactobacillus-mediated protection at the respiratory mucosa persists in the absence of B lymphocytes or antibodies, and likewise does not rely on IL-10, type I or type II interferon signaling [23, 24]. Nonetheless, Lactobacillus at the respiratory mucosa does result in suppression of a distinct cohort of virus-induced proinflammatory cytokines, most profoundly, interleukin (IL)-6 [25]. IL-6 is a pleiotropic cytokine produced by monocytes and macrophages, lymphocytes, endothelial cells and fibroblasts [26]; it is capable of cis- and trans-signaling, the latter via soluble signaling receptor gp130, and has both proinflammatory and anti-inflammatory activities (reviewed in [27–29]).
The role of IL-6 in chronic virus infections has been considered at length [30]. Among the most recent of these findings, Harker and colleagues [31] reported that IL-6 generated late in chronic murine LCMV infection generated T follicular helper cell responses that were critical and protective in nature. With respect to acute virus infections, IL-6 has been linked to severe RSV infection in adults and infants ([32, 33]; see Discussion), as a biomarker in severe pandemic H1N1 influenza A infection [34] and in association with fatal infection with influenza A H5N1 [35]. IL-6 was also among the cytokines expressed prominently in the lungs and airways of mice subjected to acute infection with influenza and found at levels correlating with clinical symptoms and lung pathology (reviewed in [36]). Interestingly, IL-6 deficiency had either no impact on survival [34] or resulted in poor outcomes, suggesting that this cytokine maintained a protective role. Specifically, Dienz and colleagues [37] reported that IL-6−/− mice succumbed to sublethal doses of Influenza A H1N1 in association with apoptosis of anti-viral neutrophils in the lung, while Lauder and colleagues [38] found that infection of IL-6−/− mice with Influenza A H1N1 resulted in infiltration of the lungs with monocytes in association with progression to pulmonary edema and respiratory distress due to an inefficient antiviral T cell response. Subsequent studies that focused on the roles of CD4+T cells, cytotoxic CD8+T cells and IL-27, IL-21 and antibody production, as well as lung repair mechanisms [39–42] all identified IL-6 as a protective factor in acute influenza infection.
By contrast, the studies presented here indicate that IL-6 plays an adverse role in acute respiratory infection with PVM. In direct contrast to what has been reported for acute influenza infection, PVM-infection is less lethal in IL-6-deficient compared to wild-type mice, an observation associated with diminished neutrophil recruitment and reduced fluid accumulation in lung tissue. A specific cellular source of IL-6 in PVM infection is identified as contributing to disease pathogenesis.
Materials and Methods
Mice.
Wild-type C57BL/6 mice (6 – 10 weeks old) were from the Jackson Laboratory, Bar Harbor, ME (JAX 000664) and Charles River Laboratories, Frederick, MD. IL-6−/− (JAX 002650) and Nr4a1−/− (JAX 006187) mice were from the Jackson Laboratory and maintained on site (14BS vivarium, NIAID/NIH). Ccr2−/− mice were from stocks maintained by NIAID/Taconic consortium. The National Institute of Allergy and Infectious Diseases Division of Intramural Research Animal Care and Use Committee, as part of the National Institutes of Health Intramural Research Program, approved all the experimental procedures as per protocol LAD 8E.
Viruses and virus infection.
Pneumonia virus of mice (PVM strain J3666) was prepared from mouse-passaged stocks stored in liquid nitrogen. In experiments described herein, mice were inoculated via the intranasal route while under isoflurane anesthesia with 50 µL PVM diluted 1:8000 or 1:10,000 to 0.25 or 0.20 TCID50 units / mouse, respectively, followed by evaluation of serial weights, survival, as well as specific outcomes at time points as indicated below. Recombinant PVM (rk2-PVM) encoding the fluorescent marker mKatushka2 (mKATE) was prepared and characterized as previously described [43]. Influenza A/HK/1/68 (H3N2; gift from J. Keicher, Symmune Therapeutics, Raleigh, NC) was provided as an egg-passaged stock; this was passaged three times in wild-type specific pathogen-free mice in our high-barrier facility prior to utilization in in vivo experiments. Mouse-passaged stocks were maintained at −80°C. Virus was administered intranasally in a total volume of 2.5 μL per nare (5 µL per mouse, diluted 1:2000, to 75 TCID50 units / mouse). Mice were evaluated by serial weights and survival.
Administration of Lactobacillus plantarum and recombinant IL-6 to the respiratory tract.
L. plantarum NCIMB 8826 (ATCC BAA-793) was grown overnight in Mann Rogosa Sharp (MRS) medium and administered live or as heat-inactivated (70°C for 30 min). Live cells were washed once in sterile PBS and stored at 1011 / mL in sterile PBS with 0.1% bovine serum albumin (BSA) at 4°C for no longer than 24 hours prior to administration; heat-inactivated cells were washed once in sterile PBS and stored at −20°C at 1011 / mL in PBS with 0.1% BSA. Mice inoculated with PVM on day 0 as described above received 50 µL inocula of live or heat-inactivated L. plantarum at 108 cells or cell equivalents per dose or diluent control (PBS with 0.1% BSA) on days 1 and 2. In some experiments, PVM-infected mice received a reduced dose of live L. plantarum at 107 cells in 50 µL on days 1 and 2 as indicated. In some experiments, PVM-infected mice treated with live L. plantarum as described also received recombinant murine IL-6 (R&D Systems), 25 µg / mouse or diluent (PBS with 0.1% BSA) in a 50 µL intranasal inoculation on days 4, 5, 6 and 7.
Bronchoalveolar lavage, lung tissue homogenates and histology.
Mice were euthanized by cervical dislocation under isoflurane inhalation and subjected to bronchoalveolar lavage (BAL) with PBS with 0.1% BSA. BAL fluid was clarified by centrifugation and stored at −80°C prior to analysis. Whole lung tissue isolated from mice was immersed in 1 mL PBS with 0.1% BSA and subjected to blade homogenization and clarification by centrifugation at 4°C. Clarified homogenates were stored at −80°C prior to analysis. For histology, lungs were perfused with 10 ml of PBS, inflated with 0.8 mL of ice cold 10% phosphate-buffered formalin and fixed in 10 mL cold 10% phosphate-buffered formalin. H&E-stained sections were prepared by Histoserv, Inc. (Germantown, MD, USA). Photographs of the microscopic images were obtained with a DMI4000 light microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Retiga 2000R camera and analyzed using QCapture software (both from QImaging, Surrey, BC, Canada).
Lung wet-to-dry analysis.
Fluid accumulation in the lungs was evaluated quantitatively essentially as described [44, 45]. Briefly, whole lung tissue was removed from PVM-infected wild-type mice (both untreated and those treated with L. plantarum as described above) and also from PVM-infected IL-6−/− mice at sequential time points. Lung tissue weights (significant to 0.05 g, Denver Instruments top-loading balance, Model SI-403) were determined immediately after removal and again after fluid evaporation in a drying oven at 37°C for four days or more. The ratio of the wet lung weight to the dry lung weight at each time point was determined.
ELISAs.
Quantitative analysis of cytokine mediators in bronchoalveolar lavage (BAL) fluid, lung tissue homogenates, and serum was performed by ELISA (DuoSet, Quantikine; R & D Systems) as per manufacturers’ instructions. Mediator levels detected in lung tissue homogenates were normalized for total protein by BCA assay (Pierce).
Flow cytometry.
For preparation of single cell suspensions, PVM-infected wild-type and IL-6−/− mice were euthanized as noted above prior to perfusion with 8 mL PBS with 10 mM EDTA; lungs were removed from the body cavity and minced manually. Minced tissue was incubated in digestion buffer (RPMI 1640 with 5% fetal bovine serum (FBS), 20 µg/mL DNase I, and 40 µg/mL collagenase D) for 60 minutes at 37°C; tissue digests were filtered through 70 µm nylon mesh and erythrocytes were lysed with ACK buffer (Lonza). Cells were counted manually on a hemocytometer; live cells were identified by trypan blue exclusion (Lonza). For evaluation of myeloid subsets, cells (106 cells per 100 µL) were stained with live/dead Aqua or Blue viability dyes (20 min at 4°C; Invitrogen) followed by anti-CD16/32 (BD Bioscience) and fluorochrome-conjugated antibodies including: from eBioscience: anti-CD45 (clone 30-F11), anti-CD11c (clone N418), anti-MHCII-1-A/1-K (clone M5/114.15.2); from BD Biosciences: anti-Siglec F (clone E50–2440), anti-Gr1 (clone RB6–8C5). In studies that included identification of Ly6Chi and Ly6Clo monocytes, fluorochrome and biotin-conjugated antibodies included: from eBioscience: anti-CD45 (clone 30-F11), anti-MHC 11 (I-A/I-E; clone M5/114.15.2); from BD Biosciences: anti-CD45 (clone 30-F11), anti-CD19 (clone 1D3; biotinylated); anti-NK1.1 (clone PK136; biotinylated); anti-Siglec F (clone E50–2440); from BioLegend: anti-CD11b (clone M1/70); anti-Ly6G (clone 1A8); anti-Ly6C (clone HK1.4); anti-F4/80 (clone BM8); also from BD Bioscience, fluor-conjugated Streptavidin. Antibody staining was performed for 20 min at 4°C. Cell suspensions were then fixed in 4% paraformaldehyde in PBS prior to analysis on an LSR II flow cytometer (BD Bioscience). For evaluation of T lymphocytes in lung tissue of PVM-infected wild-type and IL-6−/− mice, single cell suspensions generated as described above were stained with live/dead Aqua (Invitrogen) followed by anti-CD16/32, anti-CD45 (clone 30-F11), anti-CD3 (clone eBIO500A2), anti-CD4 (clone RM4–5) and anti-CD8a (clone 53–6.7). After staining, cells were fixed and permeabilized (Thermo Fisher) and intracellular IL-17 was evaluated (clone TC11–18H10). For all analyses, compensation and analysis of flow cytometry data was performed using FlowJo 10.2 software (Tree Star, Ashland, OR). Gates were set using relevant “fluorescence minus one” or isotype controls.
Detection of PVM-infected monocyte and macrophage populations.
Mice were infected with rK2-PVM (50 µL with 400 TCID50 units/mouse) on day 0; lung tissue was collected for preparation of single cell suspensions on days 5, 6, and 7. Single cell suspensions were prepared as described above; all manipulations were carried out in the dark to limit quenching of the fluorescent tag. Cells were suspended at 106 per 100 µL and stained with live/dead Aqua and anti-CD16/32 as above. Monocytes and macrophage populations were identified using the protocol developed by Misharin et al. [46] with fluorochrome-conjugated antibodies including anti-CD24 (FITC; clone M1/69), anti-CD11b (PerCP/Cy5.5; clone M1/70), anti-Siglec F (BV421; clone E50–2440); anti-Ly6C (BV785; clone HK1.4); anti-MHCII (BV711; clone M5/114.15.2); anti-CD45 (BV605; clone 30-F11); anti-CD64 (APC; clone X54–5/7.1); and anti-CD11c (PE-Cy7; clone HL3); the virus-tag, mKATE is detected on the PE-Texas Red channel. Cells were fixed as described above and analysis was performed on an LSR Fortessa flow cytometer. Compensation and analysis of flow cytometry data was performed using FlowJo 10.2 software (Tree Star, Ashland, OR). Gates were set using relevant fluorescence minus one or isotype controls.
Depletion of alveolar macrophages with clodronate-filled liposomes.
Clodronate-filled liposomes (60 µL per mouse; Encapsula Nanosciences) were administered on day −1 prior to intranasal inoculation with PVM (day 0) and once again on day +4. Depletion of alveolar macrophages (AMs; live CD45+CD11c+SiglecF+ cells) from whole lung tissue after administration of clodronate-filled liposomes was determined by flow cytometry through day 15 as described above.
Virus titration by qPCR.
Virus was evaluated in whole lung and spleen tissue using a qPCR assay that targets the PVM small hydrophobic (SH) protein as previously described [15, 47]. Briefly, RNA was prepared from mouse lung tissue that had been immersed and stored in RNAlater (Ambion, Austin, TX). Isolated RNA (RNAzol, Tel-test, Friendswood, TX) was treated with DNase I to remove genomic DNA contaminants. Reverse transcription was performed using a first-strand cDNA synthesis kit (Roche) with random primers; a no reverse transcriptase control was included. The qPCR reactions were amplified in triplicate, with the ABI 2x TaqMan reagent, primer-probe mixes, and cDNA or plasmid standard in a 25-μL final volume (Applied Biosystems). Thermal cycling parameters for the ABI7500 absolute quantitation program (Applied Biosystems) include 50°C for 2 min, 95°C for 10 min, and 40 amplification cycles alternating 95°C for 15 sec and 60°C for 1 min. Custom design primer-probes include primers including: Primer 1 5′-GCC GTC ATC AAC ACA GTG TGT-3′; Primer 2 5′-GCC TGA TGT GGC AGT GCT T-3′; Probe 6FAM -CGC TGA TAA TGG CCT GCA GCA-TAMRA. The PVM SH gene (GenBank accession no. AY573815) cloned into pBACpak8 was used to generate a standard curve for absolute quantification. Experimental triplicate data points were interpolated to linear standard curves. A sample calculation from data generated by this method is included in reference [47]. The data from lung tissue RNA are normalized to absolute copies of GAPDH. This value is generated using commercially available mouse GAPDH primer-probes (Applied Biosystems catalog no. 4308313); values obtained are interpolated to a standard curve generated using a mouse GAPDH plasmid in pCMV pSport 6 (American Type Culture Collection cat no. 10539385), also as previously described [15, 47].
Statistics.
All quantitative findings were from multiple datasets. Findings were analyzed via algorithms (Log-Rank, ANOVA, Mann-Whitney U-test, Student’s t-test) within GraphPad PRISM 7.0. All error bars represent standard deviation (SD) unless otherwise indicated.
Results
Lactobacillus at the respiratory tract results in profound suppression of local inflammation and pulmonary edema.
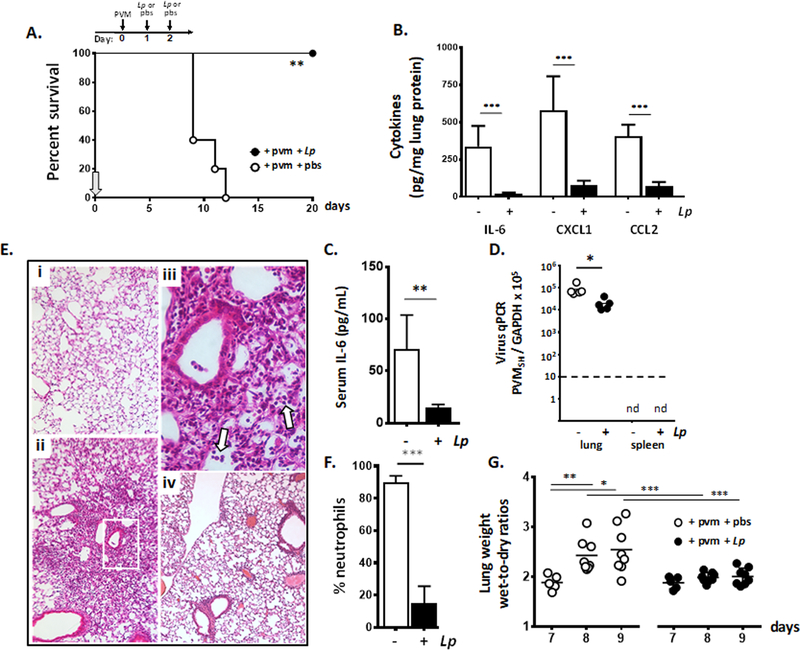
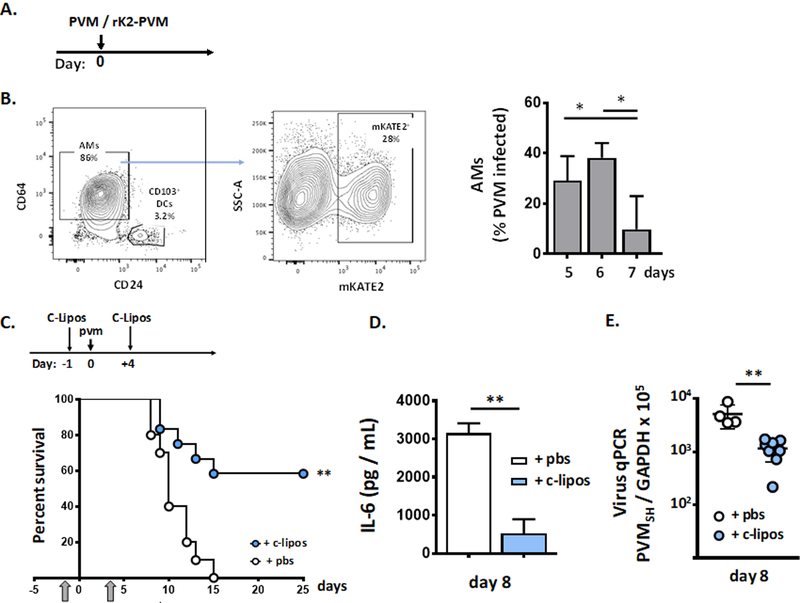
PVM is a natural rodent pathogen that replicates in mouse lung tissue and generates a severe lethal respiratory infection [5, 7]. Following the protocol shown in Fig. 1A, we showed that administration of L. plantarum directly to the respiratory tract on days 1 and 2 immediately after virus inoculation generated substantial protection against the lethal sequelae of PVM infection. Comparable to findings reported in earlier studies [25], survival was associated with profound suppression of virus-induced cytokines, notably reduction of local levels of interleukin (IL)-6, as well as CXCL1, and CCL2 shown here at 18, 7, and 6-fold respectively at day 8 of infection [Fig. 1B]). Survival was also associated with a significant reduction in IL-6 in systemic circulation at this time point [Fig. 1C] and a small but statistically significant reduction in virus recovery from lung tissue [Fig. 1D]. Of note, PVM infection remains localized to the lung, and does not result in viremia; no virus was detected in peripheral blood (data not shown) or spleen of untreated or L. plantarum-treated mice.
Fig. 1. Lactobacillus plantarum at the respiratory tract results in profound suppression of local inflammation.
A. Survival of wild-type mice inoculated intranasally with pneumonia virus of mice (PVM) at day 0 followed by intranasal inoculation with L. plantarum (Lp; 108 cells in 50 µL) or diluent (pbs) alone on days 1 and 2; arrow indicates the start of experiment on day 0, **p < 0.01 log-rank, n = 5 – 8 mice per group. B. Proinflammatory cytokines IL-6, CXCL1 and CCL2 detected in lung tissue homogenates of PVM-infected mice treated with L. plantarum or diluent control in A. (day 8); ***p < 0.001 one-way ANOVA, n = 5 mice per group. C. IL-6 detected in systemic circulation of PVM-infected mice treated with L. plantarum or diluent alone as in A.; day 8, **p < 0.01, Mann-Whitney U-test. D. Detection of virus in lung and spleen tissue by qPCR in PVM-infected mice treated with L. plantarum or diluent alone as in A.; *p < 0.05, Mann-Whitney U-test, nd, virus not detected, n = 5 mice per group; dashed line denotes lower limit of detection. E. Microscopic histology: (i.) original magnification 10x, lung tissue from uninfected control; (ii.), original magnification 10x, PVM infection followed by diluent as in A., day 8; (iii.) area within box in (ii.) is enlarged, original magnification 40x, arrows denote neutrophils in alveoli; (iv.), original magnification 10x, PVM infection followed by L. plantarum as in A. F. Neutrophil recruitment to the airways in PVM-infected mice treated with L. plantarum or diluent alone as in A.; day 8, ***p < 0.001 Student’s t-test, n = 5 mice per group. G. Wet-to-dry ratios determined for whole lungs from PVM-infected mice treated with L. plantarum or diluent alone as in A., *p < 0.05, **p < 0.01, ***p < 0.005, one-way ANOVA, n = 5 – 8 mice per group.
Microscopic images of lung tissue from PVM-infected mice are featured in Fig. 1E. Compared to lung tissue from uninfected mice (i.), PVM infection resulted in profound alveolitis with inflammatory cells within the airspaces evident throughout (ii.). The region within the box in (ii.) is shown at higher magnification in (iii.), in which neutrophil recruitment (white arrows) and early onset pulmonary edema within the airspaces was delineated. By contrast, lung tissue from PVM-infected mice that were treated with L. plantarum as in Fig. 1A display markedly reduced pathology, with alveoli that were largely clear of neutrophils and edema fluid (iv). Consistent with the tissue histology, BAL fluid leukocyte differential indicated a profound reduction in airway neutrophils among PVM-infected mice treated with L. plantarum vs. PVM-infected mice that were otherwise untreated [Fig. 1F]. Likewise, lungs from L. plantarum-treated PVM-infected mice maintained constant wet-to-dry ratios over time, consistent with minimal accumulation of edema fluid. This is in profound contrast to the wet-to-dry ratios determined for lungs from the otherwise untreated PVM-infected mice; these ratios increased over time with ratios on days 8 and 9 significantly higher than those determined for lungs from PVM-infected mice treated with L. plantarum at these same time points (***p < 0.001; [Fig. 1G]).
Critical adverse impact of IL-6 in primary pneumovirus infection.
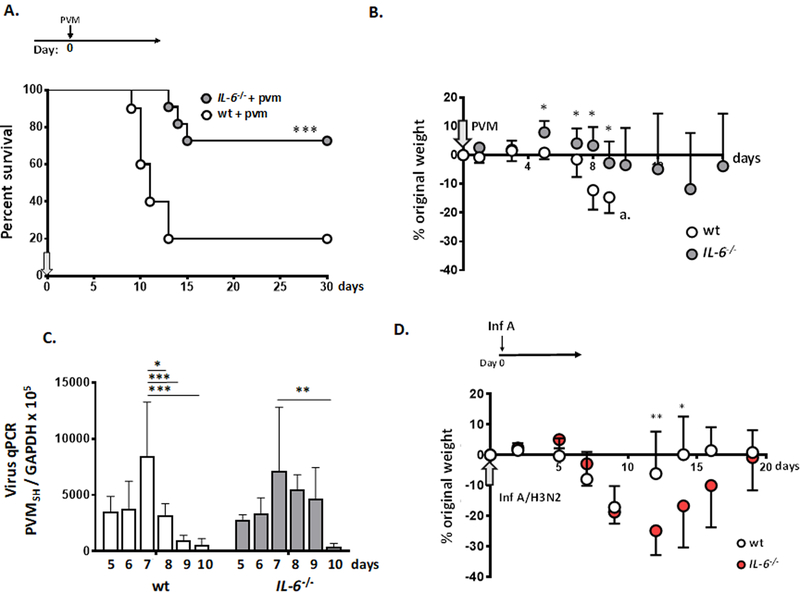
To explore the role of IL-6 in promoting inflammatory responses in acute PVM infection alone, in the absence of L. plantarum treatment, IL-6 gene-deleted (IL-6−/−) mice were inoculated at day 0 with PVM; weights and survival were evaluated at serial time points thereafter. As shown in Fig. 2A, 73% of the IL-6−/− mice survived long-term, as compared to only 18% of wild-type mice inoculated with a reduced inoculum of 0.2 TCID50 units per mouse (***p < 0.001). The corresponding weights for this group (days 0 through 16) are shown in Fig. 2B. Lung virus titer on days 5 – 10 of infection is shown in Fig. 2C. In both strains, virus titer reached a maximum at day 7 after inoculation, immediately prior to the onset of mortality in wild-type mice. Although there were some small differences noted between strains, both wild-type and IL-6-deficient mice cleared virus from the lungs, with PVM detected at only 6 – 7% respective peak levels by day 10.
Figure 2. Critical adverse impact of IL-6 in primary pneumovirus infection.
A. Survival of wild-type and IL-6 gene-deleted (IL6−/−) mice inoculated with PVM; ***p < 0.001 log rank, n = 10 – 11 mice per group. B. Weights of mice inoculated as in A., *p < 0.05, n = 5 – 11 mice per group; at the point indicated at a., too few mice remain to generate reliable weight data after this time point. C. Detection of virus in lung tissue by qPCR in PVM-infected wild-type and IL-6−/− mice on day 5 – day 10 after inoculation; *p < 0.05, **p < 0.01, ***p < 0.005, 2-way ANOVA; n = 4 – 5 mice per group. D. Wild-type and IL-6−/− mice were inoculated with Influenza A/HK/1/68 (H3N2; 75 TCID50 units / mouse, 2.5 µL per nare) on day 0 (at arrow); *p < 0.05; **p < 0.01 one-way ANOVA; n = 5 – 6 mice per group.
Interestingly, the responses to PVM infection are qualitatively different from those reported for influenza virus infection in mice, the latter defining a protective role for IL-6 [37–42]. To explore this further, we inoculated wild-type and IL-6−/− mice with Influenza A/HK/1/68 (H3N2) [Fig. 2D]. We found that, in contrast to PVM infection, IL-6−/− mice responded to infection with Influenza A with significantly more weight loss and with delayed recovery from an acute infection compared to their wild-type counterparts. This observation, that these two respiratory virus infections exhibited distinct and opposing responses to IL-6 deficiency is considered further in the Discussion.
Neutrophil recruitment and fluid accumulation in response to PVM infection were attenuated in IL-6−/− mice.
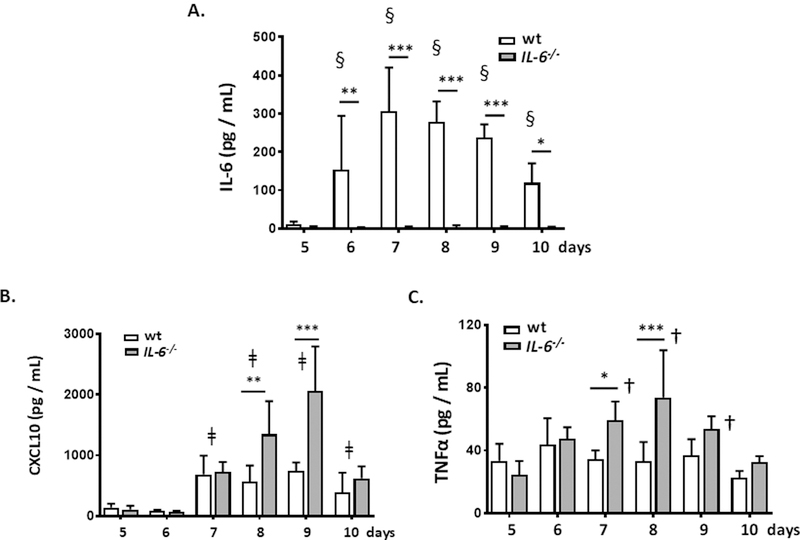
IL-6 has notable impact on priming and activation of neutrophilic leukocytes [48, 49]. We show here that PVM infection resulted in local production of IL-6, which was detected in the airways as early as day 6 of infection [Fig. 3A]. IL-6 levels in the airways reached a maximum at day 7 – 8 and were in decline by day 10. As to be expected, no IL-6 was detected in the airways of PVM-infected IL-6−/− mice. Other cytokines detected in the airways in response to PVM infection include CXCL10, TNFα, CXCL1, and CCL2, with levels of CXCL10, and TNFα, and, to a lesser extent, CCL2 in the airways of IL-6−/− mice exceeding those detected in the wild-type at days 7, 8 and/or 9 of infection [Fig. 3B and 3C, Suppl Fig. 1]. By contrast, relatively low levels of IL-17 were detected in the airways of wild-type and IL-6−/− mice, but no modulation in response to gene-deletion or to infection was observed [Suppl. Fig. 1].
Fig. 3. Differential expression of proinflammatory cytokines in the airways of PVM-infected wild-type and IL-6−/− mice.
A. Detection of immunoreactive IL-6 (pg/mL) in the airways (BAL fluid) of PVM-infected wild-type and IL-6−/− mice; *p < 0.05, **p < 0.01, ***p < 0.005, wild-type vs. IL-6−/− mice at time points indicated; §p < 0.01 all wild-type, later time points vs. day 5 as indicated. B. Detection of immunoreactive CXCL10 (pg/mL) in BAL fluid PVM-infected wild-type and IL-6−/− mice; **p < 0.01, ***p < 0.005, wild-type vs. IL-6−/− mice at time points indicated; ǂp < 0.01 wild-type later time points vs. day 5 as indicated and IL-6−/− later time points vs. day 5 as indicated. C. Detection of immunoreactive TNF-α (pg/mL) in BAL fluid from PVM-infected wild-type and IL-6−/− mice; *p < 0.05, ***p < 0.005, wild-type vs. IL-6−/− at time points indicated; †p < 0.05 IL-6−/− later time points vs. day 5 at time points indicated. For all panels, n = 5 mice per group, 2-way ANOVA.
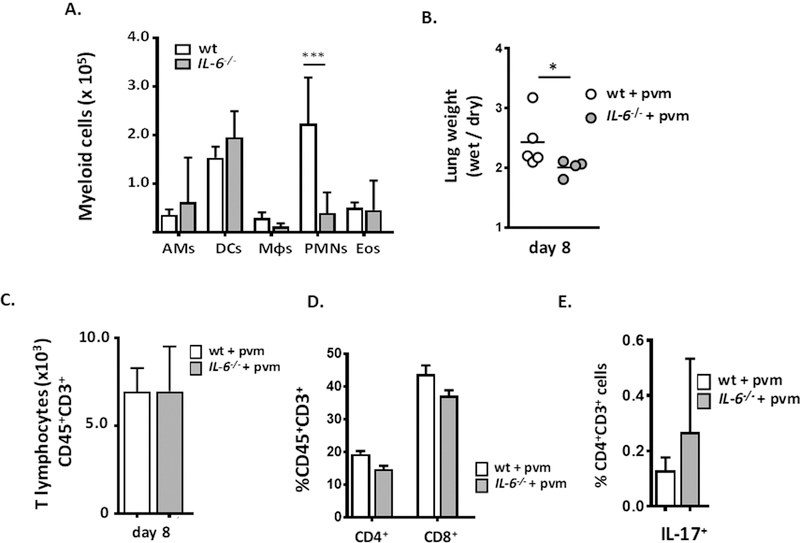
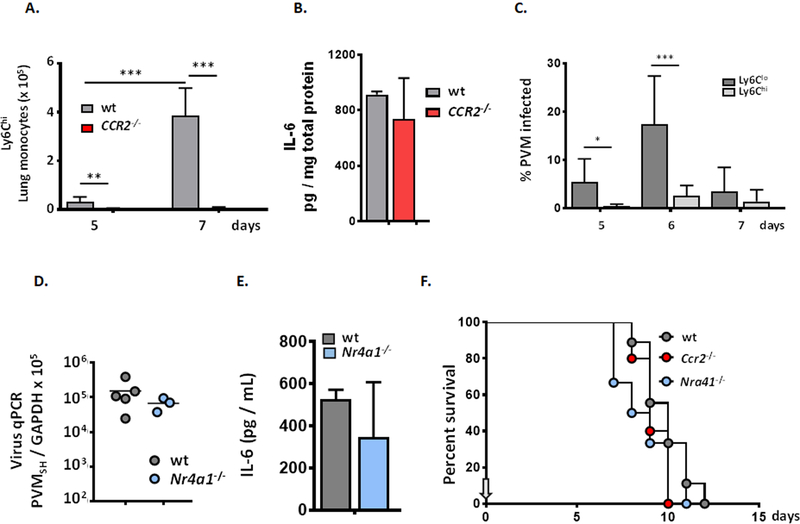
Flow cytometric evaluation of myeloid leukocyte subsets in whole lung tissue revealed a substantial, > 5-fold reduction in neutrophil recruitment to the lungs of PVM-infected IL-6−/− mice, from 2.2 ± 0.95 ×105 to 0.40 ± 0.16 ×105 total cells [***p < 0.001; Fig. 4A]. Likewise, the wet-to-dry weight ratio determined for lungs from wild-type mice was significantly higher than the value obtained for their IL-6-deficient counterparts [*p < 0.05; Fig. 4B].
Fig. 4. Neutrophil recruitment and fluid accumulation were attenuated in PVM-infected IL-6−/− mice.
A. Significantly fewer neutrophils (CD45+SiglecF−Gr1hi) were detected in single cell suspensions prepared from lung tissue of PVM-infected IL-6−/− mice compared to wild-type, day 8; ***p < 0.001, ANOVA, n = 5 – 7 mice per group. B. Wet-to-dry ratios determined for whole lungs from PVM-infected wild-type and IL-6−/− mice, *p < 0.05, n = 4 – 5 mice per group, Mann-Whitney U-test. C. No differential recruitment of T lymphocytes (CD45+CD3+) to the lungs of PVM-infected wild-type vs. IL-6−/− mice was observed, nor D. any differences in CD4+ and CD8+ T cells or E. Th17 cells.
T lymphocytes (CD3+ cells) were also recruited to the lungs in response to PVM infection [13, 24, 50]; in contrast to neutrophils, the numbers of T lymphocytes detected in lung single cell suspensions from wild-type and IL-6−/− mice were comparable to one another [Fig. 4C] as were the fractions of CD4+ and CD8+ T cells [Fig. 4D]; few to no IL-17+CD4+ (Th17) cells were detected in the lungs of PVM-infected wild-type or IL-6−/− mice [Fig. 4E], consistent with the aforementioned findings on immunoreactive IL-17 (Suppl. Fig. 1).
Alveolar macrophages (AMs) are a significant source of IL-6 in acute PVM infection.
In previous studies, we found that AMs were targets of PVM infection and that AMs isolated from PVM-infected mice generated IL-6 in ex vivo culture [51]. However, we had not previously explored the role of AMs as a source of specific cytokines in PVM infection in vivo. As shown in Fig. 5A, PVM-infected AMs were isolated from mice inoculated with fluorescent-tagged virus; detection of mKATE+ AMs reached a maximum (38 ± 6% of mKATE+CD45+CD11c+ CD11b−CD64+CD24− cells) at day 6 after inoculation [Fig. 5B]. AMs were depleted by administration of clodronate liposomes one day prior to and again four days after inoculation with PVM. AM depletion resulted in improved survival in response to PVM infection in wild-type mice (0 vs. 58%, **p < 0.01; [Fig. 5C]). Prolonged survival was associated with a substantial reduction in levels of IL-6 in the airways [Fig. 5D] and a small but significant reduction in virus titer [Fig. 5E].
Fig. 5. Alveolar macrophages (AMs) are a significant source of IL-6 in acute PVM infection.
A. Wild-type mice were inoculated on day 0 with recombinant PVM (rK2-PVM) that has incorporated the fluorescent protein mKATE2 within the virus genome. B. mKATE2-positive AMs (CD45+CD11c+CD64+CD24-/lo) were identified by flow cytometry on days 5, 6 and 7 of infection, *p < 0.05, Student’s t test, n = 5 – 6 mice per group. C. On day −1, mice received 60 µL of clodronate-filled liposomes (+ c-lipos) or diluent via intranasal inoculation, PVM on day 0, followed by a second dose of clodronate-filled liposomes or diluent on day +4. AM depletion is associated with prolonged survival in response to acute PVM infection; **p < 0.01, log rank, n = 10 – 12 mice per group. D. Prolonged survival was accompanied by diminished levels of IL-6 in BAL fluid; **p < 0.01, Mann-Whitney U-test, n = 5 – 8 mice per group, and E. diminished virus titer as determined by qPCR; n = 5 – 8 mice per group, *p < 0.01, Mann-Whitney U-test.
Ly6Chi inflammatory monocytes are recruited to the lungs but are not a significant source of IL-6 in PVM infection.
Inflammatory (Ly6ChiCCR2+) monocytes are typically recruited from circulation in response to signals from pathogen infection and have also been characterized as a prominent source of proinflammatory cytokines [52–54]. As shown in Fig. 6A, Ly6Chi inflammatory monocytes were recruited to the lungs in response to PVM infection in wild-type mice but were not detected in the lungs in mice devoid of the critical chemokine receptor, CCR2. Interestingly, the absence of proinflammatory monocytes had no impact on virus titer (data not shown) and no impact on the level of virus-induced IL-6 in lung tissue [Fig. 6B]. In contrast to Ly6Chi cells, non-classical, or “patrolling” Ly6Clo monocytes [55, 56] are significant targets of PVM-infection; at peak, 17.5 ± 10% of these cells were mKATE+ [Fig. 6C]. Nonetheless, PVM infection in Nr4a1−/− mice, which are devoid of patrolling monocytes [57], display similar virus titers in lung tissue [Fig. 6D] and the levels of virus-induced IL-6 in the airways were indistinguishable from those detected in PVM-infected wild-type mice [Fig. 6E]. Survival rates in PVM-infected Ccr2−/− and Nr4a1−/− strains were indistinguishable from one another (9.2 ± 0.84 vs. 8.8 ± 1.8 days, respectively) and were indistinguishable from that determined for PVM-infected wild-type mice (9.9 ± 1.3 days; [Fig. 6F]).
Fig. 6. Ly6Chi inflammatory monocytes are recruited to the lungs but are not a significant source of IL-6 in PVM infection.
A. Acute PVM infection results in recruitment of Ly6Chi inflammatory monocytes; recruitment of these cells is blocked in mice devoid of the chemokine receptor, CCR2 (Ccr2−/− mice); ***p < 0.001, ANOVA, n = 5 mice per group. B. CCR2 gene-deletion and absence of Ly6Chi monocytes has no impact on levels of virus-induced IL-6, n = 3 – 5 mice per group. C. Ly6Clo patrolling monocytes, but not Ly6Chi inflammatory monocytes, are major targets of PVM infection; *p < 0.05, ANOVA, n = 5 – 6 mice per group. D. Virus titer in lung tissue remains unchanged in Nr4a1−/− mice which are devoid of Ly6Clo monocytes. E. Absence of Ly6Clo monocytes has no impact on local production of IL-6; n = 4 – 5 mice per group. F. Ccr2 and Nra41 gene-deletions have no impact on survival in response to PVM infection when compared to one another and to wild-type control mice, n = 5 – 9 mice per group.
Suppression of PVM-induced IL-6 is a critical element of Lactobacillus-mediated protection at the respiratory tract.
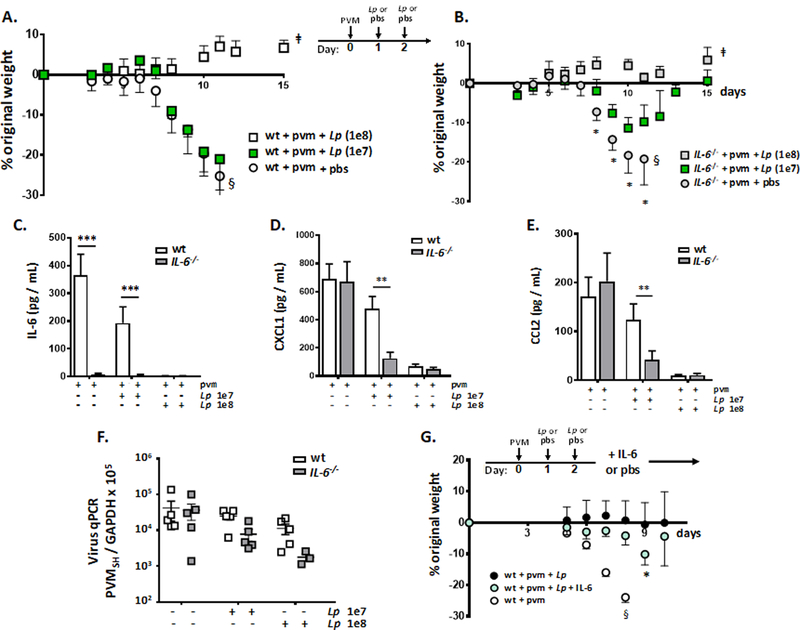
We have shown clearly that administration of immunobiotic Lactobacillus at the respiratory tract results in survival in response to lethal PVM infection in association with profound suppression of virus-induced IL-6, among other proinflammatory cytokines (Fig. 1, [25]). We have also shown here in Fig. 2A that PVM infection is substantially less lethal in mice devoid of IL-6 than it is in IL-6-sufficient, wild-type mice, a finding that suggests that IL-6 is a central mediator of lethal inflammation in this infection. Finally, we show here that suppression of virus-induced IL-6 is a critical element of the mechanism of Lactobacillus-mediated protection at the respiratory tract. As shown in Fig. 7A, wild-type mice were fully protected in response to administration of live L. plantarum to the respiratory tract at the standard dose of 108 cfu/mouse. However, PVM-infected wild-type mice did not respond to treatment with a 10-fold lower inoculum, at 107 cfu L. plantarum /mouse; weight loss was indistinguishable from that of wild-type mice that received diluent alone (pbs). By contrast, IL-6−/− mice were partially protected, with limited weight loss in response to the lower inoculum of L. plantarum (107 cfu/mouse, *p < 0.05; [Fig. 7B]). As shown in Fig. 7C, the lower inoculum of L. plantarum (107 cfu/mouse) resulted in minimal suppression of IL-6 in PVM-infected wild-type mice, compared to the standard (108 cfu/mouse) dose. Similarly, in Fig. 7D and Fig. 7E, the lower inoculum of L. plantarum resulted in more effective suppression of CXCL1 and CCL2, respectively, in IL-6−/− mice compared to the wild-type. The mechanism(s) linking IL-6 to these cytokines, directly or indirectly, remains unclear, although, Ahuja and colleagues have reported on cross-talk between IL-6 and CXCL1 in acute kidney injury in mice [49]. Of note, no differences in virus titer were determined at any L. plantarum inoculum [Fig. 7F]. In a parallel experiment, PVM-infected mice were treated with L. plantarum (days 1 and 2) and then treated with recombinant IL-6 or diluent control (days 4 – 7). Although recombinant IL-6 did not fully overcome the protective effects of L. plantarum, significant weight loss among the L. plantarum-treated mice was observed (*p < 0.05; [Fig. 7G]).
Fig. 7. Suppression of PVM-induced IL-6 is a critical element of Lactobacillus-mediated protection.
A. Serial weights of PVM-infected wild-type mice treated with full dose L. plantarum, Lp (1e8), at 108 cells in 50 µL, with a reduced dose Lp (1e7) in the same volume, or diluent (pbs) on days 1 and 2 as indicated; ǂp < 0.01, [wt + pvm + pbs] vs. [wt + pvm + Lp (1e8)] on days 8, 10, and 11. No differences were observed between groups [wt + pvm + pbs] vs. [wt + pvm + Lp (1e7)]; n = 5 – 10 mice per group, 2-way ANOVA; §too few mice remain to generate reliable weight data after this point, final survival 0%. B. Serial weights of PVM-infected IL-6−/− mice treated with Lp or diluent as in A. Similar to A., **p < 0.01, [IL-6−/− + pvm + pbs] vs. [IL-6−/− + pvm + Lp (1e8)] on days 8, 10 and 11. However, here, *p < 0.05 for [IL-6−/− + pvm + pbs] vs. [IL-6−/− + pvm + Lp (1e7)] as indicated; 2-way ANOVA, n = 5 – 10 mice per group; §too few mice remain to generate reliable weight data after this point, final survival 40%. C. Detection of IL-6 in the airways (BAL fluid) of PVM-infected wild-type and IL-6−/− mice treated with diluent, or Lp (1e7 or 1e8) as in A. or B.; ***p < 0.005, n = 5 mice per group, ANOVA. D. Detection of CXCL1 in BAL fluid of mice as in C.; **p < 0.01, n = 5 mice per group, ANOVA. E. Detection of CCL2 in BAL fluid of mice as in C., **p < 0.01, n = 5 mice per group, ANOVA. F. Virus titer in lung tissue from PVM infected wild-type or IL-6−/− mice detected by qPCR treated with diluent or Lp (1e7 or 1e8) as in A. or B. G. Wild-type mice inoculated with PVM on day 0 followed by Lp or diluent (pbs) on days 1 and 2 as in Fig. 1A; mice were then inoculated intranasally with recombinant mouse IL-6 (25 µg / 50 µL) on days 4 – 7 accompanied by serial weights; *p < 0.05 [wt + pvm + Lp + IL-6] vs. [wt + pvm + Lp + pbs]; §too few mice remaining in this group to generate reliable weight after this point, final survival 0%.
Discussion
In our ongoing work, we have focused on a role for immunomodulatory therapy for treatment and prophylaxis of acute respiratory virus infection. Specifically, we and others found that the benign or lethal nature of a given respiratory virus infection relates directly to the nature and extent of the virus-induced cytokine storm [5, 7, 58, 59]. Using the endogenous rodent pathogen, PVM, we have found that virus-induced inflammation, once initiated, is not readily suppressed, even upon timely addition of agents capable of halting virus replication [8, 9].
Identifying the appropriate pathways for inhibition is not a straightforward matter. Nonetheless, we have shown that full and sustained protection against lethal inflammatory sequelae of PVM infection can be generated using preparations of the benign probiotic bacterium, L. plantarum, administered directly to the respiratory tract [15, 23–25, 60]. The impact of Lactobacillus species resident in and introduced into the lung microenvironment is an area of ongoing and active exploration [61]. The impact of L. plantarum administered to the respiratory mucosa may be similar that described for inhaled immuno-stimulants such as OM-85 BV (Broncho-Vaxom; [62, 63]); however, unlike the single species L. plantarum administered in this study, OM-85 BV is an alkaline extract prepared from 21 distinct bacterial strains, including Gram-negative pathogens such as H. influenzae and B. pertussis and the mycoplasma M. pneumoniae [58]. With respect to mechanism, we have shown that L. plantarum exerts its impact largely via coordinate engagement of the pattern recognition receptors TLR2 and NOD2 [25, 60]. Furthermore, an extensive microarray analysis of lung tissue from PVM-infected mice has shown clearly that L. plantarum administered directly to the respiratory tract results in profound suppression of a distinct cohort of virus-induced proinflammatory mediators, including, among others, IL-6 [25], although until now, no distinct role for any single cytokine had emerged. With this study, we have uncovered both a central role for IL-6 in promoting lethal inflammation in acute pneumovirus infection and clarified the molecular mechanism of L. plantarum in circumventing this response.
As noted earlier, PVM is a virus of the same family (Pneumoviridae), and same genus, (Orthopneumovirus) as the human pathogen, respiratory syncytial virus [RSV; 4, 5, 7]. The inflammatory nature of human RSV has been documented in numerous studies [2, 64, 65]. Of specific note, Walsh and colleagues [32] found that adults requiring hospitalization for severe RSV infection exhibited extensive virus shedding in association with elevated levels of mucosal IL-6. Likewise, Tabarani and colleagues [33] identified elevated levels of nasopharyngeal IL-6 as a factor correlating with severe disease in a study of >800 RSV-infected pediatric subjects. Yet, and perhaps paradoxically, both infants and adults with the −174C/C promoter polymorphism, a genotype that predicts reduced IL-6 production, are at higher risk for severe RSV disease [66, 67]. IL-6 has also been identified as an immunomodulatory cytokine in the lungs of RSV-challenged BALB/c mice. Among these findings, Pribul and colleagues [68] reported diminished release of IL-6 along with other proinflammatory cytokines (CCL3, TNF, IFNα) in AM-depleted mice subjected to RSV challenge. More recently, Pyle and colleagues [40] reported that IL-6 was critical for resolution of RSV-induced immunopathology in BALB/c mice, a feature that was dependent on induction of IL-27 and maturation of regulatory T cells.
Moving forward, one hopes that these findings may ultimately be translated into workable therapeutic strategies, particularly in settings where no effective vaccines are available. Anti-cytokine and anti-cytokine signaling therapeutics are now used for a wide variety of inflammatory conditions where blockade of single pathways has been proven to be effective [69–71]. Inflammation associated with acute respiratory virus infection has been perceived as the result of “cytokine storm,” with cross-amplification and redundancy at multiple levels [58, 59]. While it may not be immediately clear whether cytokine blockade will be useful in this setting, the contributions of individual cytokines have already been examined in complex acute inflammatory conditions, notably acute respiratory distress syndrome (ARDS; reviewed in [72]), including a specific antagonist to TNFR1, which is currently undergoing clinical evaluation [73].
In this paper, we have focused on IL-6 and show that the actions of this cytokine alone may be critical in promoting lethal sequelae of acute pneumovirus infection in vivo. IL-6 is produced by fibroblasts, monocytes and macrophages and interacts with numerous target cells largely due to its capacity for trans-signaling by soluble gp130 (reviewed in [74, 75]). It is critical to note that elimination of IL-6 may be remarkably effective in PVM infection due to the ability of this cytokine to prime and activate neutrophils [48, 49]. We note that Cortjens and colleagues [76] have presented data suggesting that the lethal sequelae of PVM infection are not directly dependent on neutrophils in the airways. However, our findings indicate that neutrophils are critical intermediaries in acute pneumovirus infection [8, 9, 15].
Monocytes and macrophages are major sources of proinflammatory mediators in respiratory virus infection [77–79]. We show here that classical Ly6Chi monocytes were recruited to the lungs in response to PVM infection, and that this response is absent in mice devoid of the chemokine receptor, CCR2. Interestingly, there were no differences in the level of total IL-6, nor do we observe any differential survival in PVM-infected Ccr2−/− mice compared to their wild-type counterparts. These results suggest that Ly6Chi monocytes recruited in response to PVM have limited direct impact in this infection and/or there is an as yet-uncharacterized immediate and direct compensation in their absence. By contrast, we showed that AMs are not only a target of PVM infection but a substantial source of IL-6 in vivo; elimination of AMs results in prolonged survival in response to PVM infection in association with a reduction in total IL-6 in lung tissue and airways. However as noted earlier, AMs are only one of many potential sources of this cytokine. While CD8+ T lymphocytes have been implicated as a source of critical inflammatory mediators in acute PVM infection [13], we showed that PVM-infected lymphocyte-deficient Rag1−/− mice generated no TNFα but maintained IL-6 in the airways at levels that were indistinguishable from their PVM-infected wild-type counterparts [24]; these findings indicate that lymphocytes are not a significant source of IL-6 in acute PVM infection. Nonetheless, we need to consider the possibility that IL-6 may be released directly or indirectly from epithelial cells and/or fibroblasts, respectively, in conjunction with acute infection. Toward this end, we are exploring respiratory virus infection and the impact of L. plantarum in mouse tracheal epithelial cell cultures [80]), as important interactions linking virus and L. plantarum to IL-6 signaling may be revealed in this setting (Mai, Percopo and Rosenberg, unpublished findings).
Likewise, while we have shown previously that AMs produce IL-6 in response to PVM infection ex vivo [51], we cannot rule out the possibility that ablation of AMs may have indirect effects on other sources of this proinflammatory cytokine in vivo. We were intrigued to note that the role of IL-6 in PVM infection is strikingly different from that observed in response to acute infection with Influenza A. Specifically, Dienz and colleagues [37] and Lauder and colleagues [38] found that Influenza A H1N1 A/PR/8/34 infection in IL-6−/− mice resulted in more severe outcomes, with mechanisms including loss of neutrophil-mediated virus clearance and failure to mount an antiviral T cell response, respectively; of note, Paquette and colleagues [34] found that IL-6 gene-deletion had no impact on disease severity in mice infected with Influenza A H1N1pdm. In our hands, IL-6−/− mice infected with Influenza A/HK/1/68 H3N2 also lost more weight overall and experienced delayed recovery from a sublethal virus inoculum. When considering both Influenza A and PVM infections in mice, we note that the former results in acute depletion of AMs [81, 82], while loss of AMs is not observed in PVM infection. A closer evaluation of the role of AMs as both an independent source and a target of IL-6 in both pneumovirus and influenza infections might help to explain some of these divergent responses. It is also intriguing to note that the lethal sequelae Influenza A can be limited by Lactobacillus at the respiratory mucosa [16 – 19], results that suggest that AMs and IL-6 are not unique or universal targets of this immunomodulatory strategy.
In conclusion, we have examined the role of IL-6 in promoting pathology in acute respiratory virus infection with PVM, a natural rodent pathogen that generates local inflammation in lung tissue. We showed here that IL-6 has a critical negative impact in PVM infection. Among our results, we found that PVM was substantially less lethal in IL-6-deficient than in wild-type mice and was associated with reduced neutrophil recruitment to the lungs. Likewise, administration of immunobiotic L. plantarum to the respiratory mucosa results in profound suppression of PVM-induced IL-6, and L. plantarum is significantly more effective in protecting against weight loss and virus-induced inflammation in IL-6-deficient mice. Ly6Chi proinflammatory monocytes were recruited in response to PVM, but they were not a significant source of IL-6 and did not contribute to the lethal sequelae of infection. By contrast, AMs were readily infected with PVM in vivo; ablation of AMs resulted in prolonged survival in association with a reduction in virus-induced IL-6 in lung tissue. The results of this work connect the actions of IL-6 to the immunomodulatory sequelae of L. plantarum at the respiratory tract and suggest that cytokine blockade may be a strategy to consider in severe pneumovirus infections.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the caretakers and technicians of the 14BS Animal Facility, NIAID / NIH, for their ongoing assistance with our research program.
Abbreviations
- AM
alveolar macrophage
- ARDS
acute respiratory distress syndrome
- Lp
Lactobacillus plantarum
- PRR
pattern recognition receptor
- PVM
pneumonia virus of mice
- RSV
respiratory syncytial virus
- SD
standard deviation
- TCID
tissue culture infectious dose
Footnotes
This work was supported by the NIAID Division of Intramural Research (Z01-AI000943–14 to HFR).
References
- 1.Schwarze J, and Mackenzie KJ 2013. Novel insights into immune and inflammatory responses to respiratory viruses. Thorax 68: 108–111. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee S, and Lukacs NW 2013. Innate immune responses to respiratory syncytial virus infection. Curr. Top. Microbiol. Immunol 372: 139–154. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg HF, and Domachowske JB 2012. Inflammatory responses to respiratory syncytial virus infection and the development of immunomodulatory pharmacotherapeutics. Curr. Med. Chem 19: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Easton AJ, Domachowske JB, and Rosenberg HF 2006. Pneumonia virus of mice, In Cane P, ed., Perspectives in Medical Virology, Elsevier Press, Inc., pp. 299–320. [Google Scholar]
- 5.Rosenberg HF, and Domachowske JB 2008. Pneumonia virus of mice: severe respiratory infection in a natural host. Immunol. Lett 118: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bem RA, Domachowske JB, and Rosenberg HF 2011. Animal models of human respiratory syncytial virus disease. Am. J. Physiol. Lung Cell Mol. Physiol 301: L148–L156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyer KD, Garcia-Crespo KE, Glineur S, Domachowske JB, and Rosenberg HF 2012. The pneumonia virus of mice (PVM) model of acute respiratory infection. Viruses 4: 3494–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonville CA, Easton AJ, Rosenberg HF, and Domachowske JB 2003. Altered pathogenesis of severe pneumovirus infection in response to combined antiviral and specific immunomodulatory agents. J. Virol 77: 1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonville CA, Lau VK, DeLeon JM, Gao JL, Easton AJ, Rosenberg HF, and Domachowske JB 2004. Functional antagonism of chemokine receptor CCR1 reduces mortality in acute pneumovirus infection in vivo. J. Virol 78: 7984–7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bondue B, Vosters O, De Nadaj P, Glineur S, De Hanau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, and Parmentier M 2011. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog 7: e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonville CA, Rosenberg HF, and Domachowske JB 2006. Ribavirin and cysteinyl leukotriene-1 receptor blockade as treatment for severe bronchiolitis. Antiviral Res 69: 53–59. [DOI] [PubMed] [Google Scholar]
- 12.van den Berg E, van Woensel JB, Bos AP, Bem RA, Altemeier WA, Gill SE, Martin TR, and Matute-Bello G 2011. Role of the Fas/FasL system in a model of RSV infection in mechanically ventilated mice. Am. J. Physiol. Lung Cell Mol. Physiol 301: L451–L460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh KB, Teijaro JR, Brock LG, Fremgen DM, Collins PL, Rosen H, and Oldstone MB 2014. Animal model of respiratory syncytial virus: CD8+T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J. Virol 88: 6281–6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pizzolla A, Smith JM, Brooks AG, Reading PC 2017. Pattern recognition receptor immunomodulation of innate immunity as a strategy to limit the impact of influenza virus. J. Leukoc. Biol 101: 851–861. [DOI] [PubMed] [Google Scholar]
- 15.Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, and Rosenberg HF 2011. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol 186: 1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, Yasui H, and Kiso Y 2010. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunompharmacol 10: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 17.Tomosada Y, Chiba E, Zelaya H, Takahashi T, Tsukida K, Kitazawa H, Alvarez S, and Villena J 2013. Nasally administered Lactobacillus rhamnossus strains differentially modulate respiratory antiviral immune responses and induced protection against respiratory syncytial virus infection. BMC Immunol 14: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park MK, Ngo V, Kwon YM, Lee YT, Yoo S, Cho YH, Hong SM, Hwang HS, Ko EJ, Jung YJ, Moon DW, Jeong EJ, Kim MC, Lee YN, Jang JH, Oh JS, Kim CH, and Kang SM 2013. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS One 8: e75368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung YJ, Lee YT, Ngo VL, Cho YH, Ko EJ, Hong SM, Kim KH, Jang JH, Oh JS, Park MK, Kim CH, Sun J, and Kang SM 2017. Heat-killed Lactobacillus casei confers broad protection against Influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep 7: 17360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaufmann SHE, Dorhoi A, Hotchkiss RS, and Bartenschlager R 2018. Host-directed therapies for bacterial and viral infections. Nat. Revs. Drug Discovery 17: 35–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfefferle PI, Prescott SL, and Kopp M 2013. Microbial influenza on tolerance and opportunities for intervention with prebiotics / probiotics and bacterial lysates. J. Allergy Clin. Immunol 131: 1453–1463. [DOI] [PubMed] [Google Scholar]
- 22.Kearney SC, Dziekiewicz M, and Feleszko W 2015. Immunoregulatory and immunostimulatory responses of bacterial lysates in respiratory infections and asthma. Ann. Allergy Asthma Immunol 114: 364–369. [DOI] [PubMed] [Google Scholar]
- 23.Percopo CM, Dyer KD, Garcia-Crespo KE, Gabryszewski SJ, Shaffer AL 3rd, Domachowske JB, and Rosenberg HF 2014. B cells are not essential for Lactobacillus-mediated protection against lethal pneumovirus infection. J. Immunol 192: 5265–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percopo CM, Ma M, and Rosenberg HF 2017. Administration of immunobiotic Lactobacillus plantarum delays but does not prevent lethal pneumovirus infection in Rag1−/− mice. J. Leukoc. Biol 102: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Percopo CM, Rice TA, Brenner TA, Dyer KD, Luo JL, Kanakabandi K, Sturdevant DE, Porcella SF, Domachowske JB, Keicher JD, and Rosenberg HF 2015. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antiviral Res 121: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabay C 2006. Interleukin-6 and chronic inflammation. Arthritis Res. Ther 8 Suppl 2: S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheller J, Chalaris A, Schmidt-Arras D, and Rose-John S 2011. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et Biophysica Acta 1813: 878–888. [DOI] [PubMed] [Google Scholar]
- 28.Garbers C, Aparicio-Siegmund S, and Rose-John S 2015. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr. Opin. Immunol 34: 75–82. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, and Kishimoto T 2012. Targeting interleukin-6: all the way to treat autoimmune and inflammatory diseases. Int. J. Biol. Sci 8: 1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluethmann H, Rothe J, Schultze N, Tkachuk M, and Koebel P 1994. Establishment of the role of IL-6 and TNF receptor 1 using gene knockout mice. J. Leukoc. Biol 56: 565–570. [DOI] [PubMed] [Google Scholar]
- 31.Harker JA, Lewis GM, Mack L, and Zuniga EI 2011. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh EE, Peterson DR, Kalkanoglu AE, Lee FE, and Falsey AR 2013. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J. Infect. Dis 207: 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabarani CM, Bonville CA, Suryadevara M, Branigan P, Wang D, Huang D, Rosenberg HF, and Domachowske JB 2013. Novel inflammatory markers, clinical risk factors and virus type associated with severe respiratory syncytial virus infection. Pediatr. Infect. Dis. J 32: e437–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paquette SG, Banner D, Zhao Z, Fang Y, Huang SS, Leon AJ, Ng DC, Almansa R, Martin-Loeches I, Ramirez P, Socias L, Loza A, Blanco J, Sansonetti P, Rello J, Andaluz D, Shum B, Rubino S, de Lejarazu RO, Tran D, Delogu G, Fadda G, Krajden S, Rubin BB, Bermejo-Martin JF, Kelvin AA, and Kelvin DJ 2012. Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLoS One 7: e38214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, and Cheng AF 2001. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J. Med. Virol 63: 242–246. [DOI] [PubMed] [Google Scholar]
- 36.Van Reeth K 2000. Cytokines in the pathogenesis of influenza. Vet. Microbiol 74: 109–116. [DOI] [PubMed] [Google Scholar]
- 37.Dienz O, Rud JG, Eaton SM, Lanthier PA, Burg E, Drew A, Bunn J, Suratt BT, Haynes L, and Rincon M 2012. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol 5: 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauder SN, Jones E, Smart K, Bloom A, Williams AS, Hindley JP, Ondondo B, Taylor PR, Clement M, Fielding C, Godkin AJ, Jones SA, and Gallimore AM 2013. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol 43: 2613–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Longhi MP, Wright K, Lauder SN, Nowell MA, Jones GW, Godkin AJ, Jones SA, and Gallimore AM 2008. Interleukin-6 is crucial for recall of influenza-specific memory CD4 T cells. PLoS Pathog 4: e1000006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyle CJ, Uwadiae FI, Swieboda DP, and Harker JA 2017. Early IL-6 signaling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog 13: e1006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang ML, Wang CT, Yang SJ, Leu CH, Chen SH, Wu CL, and Shiau AL 2017. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep 7: 43829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang R, Masters AR, Fortner KA, Champagne DP, Yanguas-Casas N, Silberger DJ, Weaver CT, Haynes L, and Rincon M 2016. IL-6 promotes the differentiation of a subset of naïve CD8+ T cells into IL-21 producing B helper CD8+ T cells. J. Exp. Med 213: 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyer KD, Drummond RA, Rice TA, Percopo CM, Brenner TA, Barisas DA, Karpe KA, Moore ML, Rosenberg HF 2015. Priming of the respiratory tract with immunobiotic Lactobacillus plantarum limits infection of alveolar macrophages with recombinant pneumonia virus of mice (rK2-PVM). J. Virol 90: 979–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuyama H, Amaya F, Hashimoto S, Ueno H, Beppu S, Mizuta M, Shime N, Ishizaka A, and Hashimoto S 2008. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: an experimental study. Respir. Research 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, and Nakajima H 2001. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am. J. Respir. Crit. Care Med 163: 762–769. [DOI] [PubMed] [Google Scholar]
- 46.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, and Perlman H 2013. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol 49: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Percopo CM, Dyer KD, Karpe KA, Domachowske JB, and Rosenberg HF 2014. Eosinophils and respiratory virus infection: a dual-standard curve qRT-PCR-based method for determining virus recovery from mouse lung tissue. Methods Mol. Biol 1178: 257–266. [DOI] [PubMed] [Google Scholar]
- 48.Wright HL, Cross AL, Edwards SW, and Moots RJ 2014. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology 53: 1321–1331. [DOI] [PubMed] [Google Scholar]
- 49.Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, He Z, Edelstein CL, and Faubel S 2012. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am. J. Physiol. Renal. Physiol 303: F864–F872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frey S, Krempl CD, Schmitt-Graff A, and Ehl S 2008. Role of T cells in virus control and disease after infection with pneumonia virus of mice. J. Virol 82: 11619–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigaux P, Killoran KE, Qiu Z, and Rosenberg HF Depletion of alveolar macrophages prolongs survival in response to acute pneumovirus infection. Virology 422: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Epelman S, Lavine KJ, and Randolph GJ 2014. Origin and functions of tissue macrophages. Immunity 41: 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauvau G, Chorro L, Spaulding E, and Soudja SM 2014. Inflammatory monocyte effector mechanisms. Cell. Immunol 291: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J, Zhang L, Yu C, Yang XF, and Wang H 2014. Monocyte and macrophage differentiation: circulation inflammatory monocyte and biomarker for inflammatory diseases. Biomark. Res 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas G, Tacke R, Hedrick CC, and Hanna RN 2015. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol 35: 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray PJ 2018. Immune regulation by monocytes. Semin. Immunol 35: 12–18. [DOI] [PubMed] [Google Scholar]
- 57.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, and Hedrick CC 2011. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat. Immunol 12: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong JP, Viswanathan S, Wang M, Sun L-Q, Clark GC, and D’Elia RV 2017. Current and future developments in the treatment of virus-induced hyper-cytokinemia. Future Med. Chem 9: 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guo XJ and Thomas PG 2017. New fronts emerge in the influenza cytokine storm. Semin. Immunopathol 39: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice TA, Brenner TA, Percopo CM, Ma M, Keicher JD, Domachowske JB, and Rosenberg HF 2016. Signaling via pattern recognition receptors NOD2 and TLR2 contributes to immunomodulatory control of lethal pneumovirus infection. Antiviral Res 132: 131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosas-Salazar C, Shilts MH, Tovchigrechko A, Schobel S, Chappell JD, Larkin EK, Gebretsadik T, Halpin RA, Helson KE, Moore ML, Anderson LJ, Peebles RS Jr., Das SR, and Hartert TV 2018. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J. Allergy Clin. Immunol 142: 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bessler WG, vor dem Esche U, and Masihi N 2010. The bacterial extract OM-85 BV protects mice against influenza and Salmonella infection. Int. Immunopharmacol 10: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 63.De Benedetto F, and Sevieri G 2013. Prevention of respiratory tract infections with bacterial lysate OM-85 bronchomunal in children and adults: a state of the art. Mult. Resp. Med 8: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell CD, Unger SA, Walton M, and Schwarze J 2017. The Human Immune Response to Respiratory Syncytial Virus Infection. Clin. Microbiol. Rev 30: 481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ascough S, Paterson S, and Chiu C 2018. Induction and subversion of human protective immunity: contrasting influenza and respiratory syncytial virus. Front. Immunol 9: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gentile DA, Doyle WJ, Zeevi A, Piltcher O, and Skoner DP 2003. Cytokine gene polymorphisms moderate responses to respiratory syncytial virus in adults. Hum. Immunol 64: 93–98. [DOI] [PubMed] [Google Scholar]
- 67.Gentile DA, Doyle WJ, Zeevi A, Howe-Adams J, Kapadia S, Trecki J, and Skoner DP 2003. Cytokine gene polymorphisms moderate illness severity in infants with respiratory syncytial virus infection. Hum. Immunol 64: 338–344. [DOI] [PubMed] [Google Scholar]
- 68.Pribul PK, Harker J, Wang B, Wang H, Tregoning JS, Schwarze J, and Openshaw PJ 2008. Alveolar macrophages are a major determinant of early responses to viral lung infection but do not influence subsequent disease development. J. Virol 82: 4441–4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rawla P, Sunkara T, and Raj JP 2018. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J. Inflamm. Res 16: 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shrimanker R, and Pavord ID 2017. Interleukin-5 inhibitors for severe asthma: rationale and future outlook. BioDrugs 31: 93–103. [DOI] [PubMed] [Google Scholar]
- 71.Rivellese F, Lobasso A, Barbieri L, Liccardo B, De Paulis A, and Rossi FW 2018. Novel therapeutic approaches in rheumatoid arthritis: role of janus kinase inhibitors. Curr. Med. Chem, in press. [DOI] [PubMed]
- 72.Boyle AJ, McNamee JJ, and McAuley DF 2014. Biological therapies in the acute respiratory distress syndrome. Expert Opinion Biol. Ther 14: 969–981. [DOI] [PubMed] [Google Scholar]
- 73.Proudfoot A, Bayliffe A, O’Kane CM, Wright T, Serone A, Bareille PJ, Brown V, Hamid UI, Chen Y, Wilson R, Cordy J, Morley P, de Wildt R, Elborn S, Hind M, Chilvers ER, Griffiths M, Summers C, and McAuley DF 2018. Novel anti-tumor necrosis factor receptor-1 (TNFR1) domain antibody prevents pulmonary inflammation in experimental acute lung injury. Thorax 73: 723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schaper F, and Rose-John S 2015. Interleukin-6: biology, signaling and strategies of blockade. Cytokine and Growth Factor Revs 26: 475–487. [DOI] [PubMed] [Google Scholar]
- 75.Tanaka T, Narazaki M, and Kishimoto T 2014. IL-6 in inflammation, immunity and disease. CSH Perspectives in Biology 6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cortjens B, Lutter R, Boon L, Bem RA, and van Woensel JB 2016. Pneumovirus-induced lung disease in mice is independent of neutrophil driven inflammation. PLoS One 11: e0168779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cole SL, Dunning J, Kok WL, Benam KH, Benlahrech A, Repapi E, Martinez FO, Drumright L, Powell TJ, Bennett M, Elderfield R, Thomas C, MOSAIC Investigators, Dong T, McCauley J, Liew FY, Taylor S, Zambon M, Barclay W, Cerundolo V, Openshaw PJ, McMichael AJ, and Ho LP 2017. M1-like monocytes are a major immunological determinant of severity in previously healthy adults with life-threatening influenza. JCI Insight 2: e91868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan M, Hibbs ML, and Chen W 2016. The contributions of lung macrophage and monocyte heterogeneity to influenza pathogenesis. Immunol. Cell Biol 2016: 1–11. [DOI] [PubMed] [Google Scholar]
- 79.Coates BM, Staricha KL, Koch CM, Cheng Y, Shumaker DK, Budinger DRS, Perlman H, Misharin AV, and Ridge KM 2018. Inflammatory monocytes drive influenza A virus-mediated lung injury in juvenile mice. J. Immunol 200: 2391–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.You Y, and Brody SL 2012. Chapter 9: Culture and differentiation of mouse tracheal epithelial cells. In: Randell SH, Fulcher L, eds. Epithelial Cell Culture Protocols, 2nd Edition. Methods in Mol. Biol. Vol 945, pp. 123–143. [DOI] [PubMed] [Google Scholar]
- 81.Ghoneim HE, Thomas PG, and McCullers JA 2013. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol 191: 1250–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wein AN, Dunbar PR, McMaster SR, Li ZT, Denning TL, and Kohlmeier JE 2018. IL-36-gamma protects against severe influenza infection by promoting lung alveolar macrophage survival and limiting viral replication. J. Immunol, 201: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.