Abstract
Pancreatic cancer is an aggressive disease with a dismal prognosis in dire need of novel diagnostic and therapeutic approaches. The past decade has witnessed an explosion of data on the genetic alterations that occur in pancreatic cancer, as comprehensive next generation sequencing analyses have been performed on samples from large cohorts of patients. These studies have defined the genomic landscape of this disease and identified novel candidates whose mutations contribute to pancreatic tumorigenesis. They have also clarified the genetic alterations that underlie multistep tumorigenesis in precursor lesions and provided insights into clonal evolution in pancreatic neoplasia. In addition to these important insights into pancreatic cancer biology, these large scale genomic studies have also provided a foundation for the development of novel early detection strategies and targeted therapies. In this review, we discuss the results of these comprehensive sequencing studies of pancreatic neoplasms, with a particular focus on how their results will impact the clinical care of patients with pancreatic cancer.
Keywords: pancreatic cancer, pancreatic ductal adenocarcinoma, cancer genome, next generation sequencing, somatic mutation
I. Introduction
Pancreatic ductal adenocarcinoma is one of the most aggressive human cancer types and is the third leading cause of cancer-related deaths in the United States1. Despite intense research efforts in recent decades, only minor improvements to the generally dismal prognosis have been achieved: median survival under current chemotherapy regimens is still less than 12 months and overall 5-year survival rate only recently increased over 5%2. The majority of patients are diagnosed at an advanced, disseminated stage when surgical cure is no longer attainable. Even patients eligible for surgical resection frequently suffer from local recurrence, metachronous metastasis, and chemotherapy resistance. A better understanding of the molecular alterations driving pancreatic carcinogenesis is critical to enable new strategies for earlier diagnosis and novel targeted therapies.
Pancreatic ductal adenocarcinoma is the most common malignant neoplasm of the pancreas and can be histologically diagnosed by its characteristic pathologic features3: neoplastic gland-forming epithelial cells invade into the surrounding stroma and induce an extensive inflammatory and desmoplastic reaction. The development and implementation of next-generation sequencing (NGS) and modern bioinformatic technologies has allowed the generation of an enormous amount of genomic data which has refined our understanding in pancreatic ductal adenocarcinoma development 4-9. In this review, we focus on recent genomic insights into the molecular mechanisms driving pancreatic carcinogenesis, particularly focusing on tumor evolution and clinical applications.
II. Genetic landscape of pancreatic ductal adenocarcinoma
The malignant behavior of cancer cells is driven by somatic mutations in oncogenes and tumor suppressor genes 10,11. The accumulation of these genetic alterations significantly perturbs major signaling pathways, leading to the acquisition of a malignant phenotype. The most frequently altered driver genes in pancreatic ductal adenocarcinoma (the oncogene KRAS and the three tumor suppressor genes TP53, CDKN2A, SMAD4) have been thoroughly characterized in human tissue samples for some time (Table 1)12-15. However, recent comprehensive genomic analyses, including whole exome and whole genome sequencing studies, have provided a more detailed understanding of the complex genomic changes, orchestrated by a combination of chromosomal rearrangements, local copy number variations (CNV), and widespread single nucleotide variants (SNV) 4-9.
Table 1:
Mutated genes in recent comprehensive sequencing analyses of PDACs
| Driver gene | Signaling pathway | Mutation prevalence (%)* | Targeted therapy | Prognostic impact*** | |
|---|---|---|---|---|---|
| KRAS | KRAS signaling | 91 | |||
| TP53 | Cell cycle | 61 | |||
| CDKN2A | Cell cycle | 44** | CDK4/6 inhibitor | poor prognosis | Oshima M 2013 Ann Surg155 |
| SMAD4 | TGFβ signaling | 40** | poor prognosis | Blackford A 2009 Clin Cancer Res156 Oshima M 2013 Ann Surg155 |
|
| Candidate driver gene | |||||
| GATA6 | Wnt/Notch signaling | 17** | Gamma secretase inhibitor | improved prognosis | Zhong L 2011 PLoS one157 |
| ARID1A | Chromatin remodeling | 8 | |||
| RNF43 | Wnt/Notch signaling | 8** | Porcupine Inhibitor | improved prognosis | Dal Molin M 2015 Clin Cancer Res158 |
| TGFBR2 | TGFβ signaling | 6 | |||
| MAP2K4 | KRAS signaling | 5 | |||
| MLL3 | Chromatin remodeling | 5 | |||
| PIK3CA | KRAS signaling | 5** | mTOR Inhibitor | poor prognosis | |
| RBM10 | RNA processing | 4** | improved prognosis | Witkiewitz AK 2015 Nature5 | |
| ATM | DNA Repair | 4 | poor prognosis | Russell R 2015 Nat Commun159 Kamphues C 2015 Pancreas160 |
|
| ROBO2 | Axon Guidance | 3 | poor prognosis | Bailey P 2012 Nature5 | |
| SMARCA4 | Chromatin remodeling | 3 | |||
| PBRM1 | Chromatin remodeling | 3 | |||
| SLIT2 | Axon Guidance | 3 | |||
| KDM6A | Chromatin remodeling | 3 | poor prognosis | Bailey P 2012 Nature5 | |
| BRAF | KRAS signaling | 2 | BRAF inhibitor | Schultz NA 2012 Pancreas161 | |
| BRCA2 | DNA Repair | 2 | PARP inhibitor | Golan T 2017 Br J Cancer162 | |
Whole exome sequencing datasets (740 PDACs): Biankin AV 2012; Waddell N 2015; Witkiewitz AK 2015; Bailey P 2016
Excluded datasets of Biankin et al. and Bailey et al., which did not consider copy number variants (CNV)
Reported in expression/genomic databases (onclnk.com; TCGA) and selected publications
The average number of non-synonymous somatic mutations in pancreatic ductal adenocarcinoma cohorts ranges from 26-101, a subset of which affect major signaling pathways and collectively contribute to the malignant behavior of pancreatic ductal adenocarcinoma cells. In addition to the four frequently altered driver genes, a wide range of genes are mutated in only a small fraction of pancreatic ductal adenocarcinomas, including GATA6, ARID1A, RNF43, ATM, TGFBR2, MAP2K4, MLL3, PIK3CA, RBM10, ROBO2, SMARCA4, PBRM1, SLIT2, KDM6A, BRAF, BRCA2, among others (Table 1) 4-9. Some of these genes are also likely to be drivers of pancreatic tumorigenesis and reflect a degree of genetic heterogeneity among pancreatic cancers in different patients, superimposed on remarkable homogeneity demonstrated by the most frequently altered genes (KRAS, CDKN2A, TP53, and SMAD4). Mutations in some of these infrequently altered genes have been correlated with poor prognosis, providing evidence of their functional impact despite infrequent occurrence (Table 1) 4,5,7. Interestingly, in the event of KRAS wildtype pancreatic ductal adenocarcinoma, mutations in BRAF are a mutually exclusive hotspot, with similar oncogenic potential to drive pancreatic carcinogenesis 5. The majority of analyses of the pancreatic cancer genome have focused on mutations in the coding region of well characterized genes. However, a recent study identified commonly mutated regulatory regions in pancreatic ductal adenocarcinomas, suggesting that mutations outside of the coding region are also likely to have functional significance 16.
The cellular pathways affected by somatic mutations in pancreatic ductal adenocarcinoma involve diverse cellular functions, including regulators of cell growth (G1/S-phase transition, KRAS signaling, hedgehog signaling, cell-cycle regulation), cell interaction with the extracellular environment (proteins involved in invasion/angiogenesis, integrins, cell adhesion molecules, TGFβ signaling, Wnt/Notch signaling, axon guidance pathways), and DNA repair mechanisms (DNA damage control, chromatin remodeling) 4-9. Some of these pathways are targetable and thus have significant clinical application as discussed below.
In addition to the detected single nucleotide variants (SNV), comprehensive next-generation sequencing (in particular whole genome sequencing) has helped to identify more complex structural changes in the pancreatic ductal adenocarcinoma genome 6,17,18. Although the functional role of structural rearrangements remains to be determined, they can affect tumor suppressors such as SMAD4 and CDKN2A, resulting in homozygous deletion or other inactivating alterations 17. Based on these structural variants, pancreatic ductal adenocarcinoma subtypes have been defined as stable, locally rearranged, scattered, and unstable 6. Crucially, deleterious germline and somatic mutations in BRCA1, BRCA2, PALB2, and other genes in the Fanconi anemia pathway are associated with the unstable subtype (>200 structural variation events), likely due to the resultant cancer’s inability to repair double stranded DNA breaks 19. In addition to these subtypes based specifically on chromosomal rearrangements, genome-wide analysis of somatic mutation types in over 100 pancreatic ductal adenocarcinomas identified four different mutational signatures 20. These signatures are related to age, APOBEC cytidine deaminases, BRCA1/2 alterations, and mismatch repair deficiency (MMR). Intriguingly, the signatures related to age and APOBEC enzymes were present in all pancreatic ductal adenocarcinomas, while the BRCA1/2 and mismatch repair deficiency signatures were only present in small subsets, presumably those with underlying alterations in these pathways 20.
In addition to categorization based on general patterns of genetic alterations or mutations in specific driver genes, comprehensive molecular analyses have also focused on classifying patterns of gene expression, resulting in novel classifications based on transcriptional signatures. These include the subtypes defined by Collisson et al. (classical, quasi-mesenchymal, exocrine) and those proposed by Bailey et al. (squamous, pancreas progenitor, ADEX, immunogenic) – these patterns were correlated with histologic features, clinical outcome, and chemo-sensitivity 4,21. However, some of the genes that define these subtypes are also expressed in non-neoplastic cells. The extent to which the gene expression patterns are intrinsic to the neoplastic cells (rather than a result of genes expressed in the desmoplastic stroma or infiltrating inflammatory cells) still needs to be defined.
Epigenetic changes contribute to altered gene expression in pancreatic ductal adenocarcinoma 22,23. The critical pancreatic ductal adenocarcinoma driver CDKN2A is frequently inactivated by promotor hypermethylation, demonstrating the impact of dysregulation conferred by epigenomic imprints 24,25. Methylation status may serve as a biomarker for the early detection of precursor lesions or pancreatic ductal adenocarcinomas 26-29. However, the clinical significance of the numerous aberrantly methylated candidates still needs to be determined; a recently created methylation database may help in this regard 30. In addition to alterations in the expression of specific genes, large-scale epigenomic reprogramming has recently been suggested to play a role in metastatic progression of pancreatic ductal adenocarcinoma 31.
In addition to the somatic mutations that play a critical role in driving pancreatic tumorigenesis in all pancreatic ductal adenocarcinoma patients, specific germline variants also contribute to the development of “familial” pancreatic ductal adenocarcinoma in ~10% of patients. Known genetic syndromes are associated with specific genetic alterations with an increased risk for pancreatic cancer, such as heredity breast/ovarian syndrome (BRCA 1/2, PALB2), Li-Fraumeni syndrome (TP53), Lynch syndrome (MLH1, MSH2, MSH6, PMS2), familial atypical multiple mole melanoma syndrome (CDKN2A), and Peutz Jeghers syndrome (STK11/LKB1) 32,33. Heterozygous germline alterations in the ATM gene also lead to an increased risk of pancreatic cancer 34. Remarkably, whole genome sequencing of germline DNA from 638 patients with familial pancreatic cancer did not reveal a single gene that accounts for most of the familial aggregation of the disease 35. Instead, a large number of genes with rare deleterious germline variants were identified. These candidates require further characterization to determine if they are true familial pancreatic cancer genes or rare private germline changes of no clinical significance. As such, the genetic basis of the majority (>80%) of familial pancreatic cancer cases has not yet been elucidated.
Recent large scale sequencing efforts have provided a vast quantity of data, which has helped to define molecular subtypes that can facilitate an individualized approach to pancreatic cancer therapy. However, significant challenges remain in the implementation of personalized medicine for patients with pancreatic ductal adenocarcinoma. The first challenge is the rarity of targetable mutations in most pancreatic ductal adenocarcinomas; simply put, most of the genes mutated in pancreatic ductal adenocarcinoma are not targetable with currently available drugs 36. Second, clinical trials of molecular targets involved in downstream signaling pathways affected by driver gene mutations have, to date, been disappointing 37,38. Third, although most pancreatic cancers are relatively uniform compared to other tumor types, tumor heterogeneity can potentially challenge targeted therapy approaches since mutations in small subclones can rapidly lead to therapeutic resistance driven by newly selected variants 39. These challenges are discussed in greater detail below.
III. The mutational sequence driving pancreatic carcinogenesis
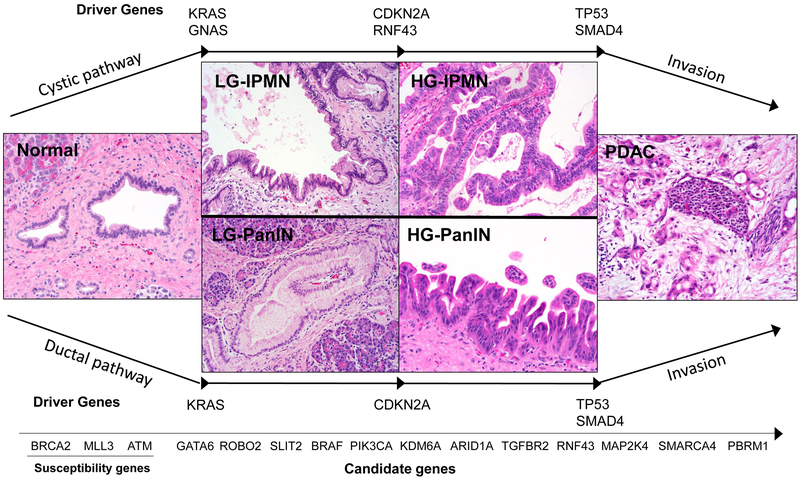
Because most patients with pancreatic ductal adenocarcinoma are diagnosed at an advanced stage of disease, novel approaches for early detection are greatly needed. A comprehensive understanding of the processes by which a normal pancreatic cell is transformed into a precursor lesion and then into an invasive carcinoma is critical to provide a rational foundation for early detection 40. Most pancreatic cancers are believed to develop from non-invasive precancerous lesions, histologically characterized either as pancreatic intraepithelial neoplasia (PanIN) when less than 5mm or intraductal papillary mucinous neoplasm (IPMN) when greater than 1cm41. Mucinous cystic neoplasms (MCN), a third type of precursor lesion, are less common and are characterized by the presence of ovarian-type stroma. These distinct precursor lesions represent three unique pathways in the development of pancreatic ductal adenocarcinoma, each with its own complement of somatic mutations 41,42. As precursor lesions progress, increasing grades of dysplasia are associated with accumulation of somatic mutations in key driver genes (Figure 1) 24,43,44. This conceptual model is based on data from multiple preceding studies that analyzed human precursor lesions with immunohistochemical and molecular techniques, demonstrating that most driver gene alterations occur in pre-invasive lesions 45-48.
Figure 1. Genetic alterations that drive pancreatic tumorigenesis.
Pancreatic ductal adenocarcinoma arises from two different types of precursor lesions: pancreatic intrapeithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN). Both pathways are defined by common low-risk precursors (LG-PanIN and LG-IPMN), which can give rise to more advanced precursors (HG-PanIN and HG-IPMN) at high risk for transformation to invasive cancer. In both pathways, activating mutations in oncogenes are early events, with KRAS mutation occurring in LG-PanIN and both KRAS and GNAS mutations occurring in LG-IPMN. Tumor suppressor gene loss (including CDKN2A, TP53, and SMAD4 in both pathways as well as RNF43 specifically in IPMNs) occurs in more advanced precursor lesions, often limited to HG-PanIN/HG-IPMN or associated invasive cancer. The timing of mutations in genes altered at low prevalence (“candidate genes”) in these pathways is less clear due to the infrequent occurrence of these mutations.
In PanIN lesions, somatic mutation in an oncogenic hotspot of KRAS is an early event that is almost universally found in PanINs (Figure 1). The activation of KRAS is subsequently followed by inactivation of tumor suppressors at higher grades of dysplasia, perhaps necessary to overcome oncogene-induced senescence 24,43,49-52. The prevalence of KRAS mutations in PanIN lesions is greater than 90% (even in low-grade PanIN lesions) and mutations in KRAS almost always occur at specific hotspot positions (codons 12, 13, 61), leading to the activation of intracellular effector pathways 50,53. Whole exome sequencing studies of PanIN lesions confirmed that CDKN2A may contribute in the transition to high-grade dysplasia, whereas aberrations in TP53 and SMAD4 occur late and predominantly drive invasiveness 24,43.
The genetic alterations driving the formation of IPMNs overlap with those driving PanINs, but with some significant differences. Notably, in contrast to PanINs, IPMNs often harbor activating mutations in the oncogene GNAS and inactivating mutations in the tumor suppressor gene RNF43 (Figure 1) 24,54. More than 90% of IPMNs harbor one or both oncogenic events (KRAS and GNAS), highlighting their combined role in tumor initiation of cystic precursors 54,55. As an oncogene, GNAS is usually targeted at a specific hotspot position (codon 201) 55,56. Missense mutations in GNAS lead to constitutively active G-protein signaling with elevated cAMP levels and increased protein kinase A activity, but the exact oncogenic function of GNAS still needs to be further clarified 56-59. As is seen in PanINs, after the initiating oncogene mutation, loss of tumor suppressor gene function mediates the progression of IPMNs. Loss of CDKN2A expression occurs in 10-50% of IPMNs and is found in both low-grade and high-grade IPMNs 54. TP53 alterations are usually confined to high-grade IPMNs and their associated invasive cancers (20-40%), suggesting a role for TP53 in malignant progression 54,60. The inactivation of SMAD4 is most commonly found in IPMN-associated invasive carcinoma 54. Whole exome sequencing has further identified RNF43 as an additional driver gene for IPMN, which is inactivated in 24% of cases analyzed 54,61. The protein product of the RNF43 gene functions as a tumor suppressor in the Wnt signaling pathway with intrinsic E3 ubiquitin activity and is commonly targeted by inactivating mutations combined with loss of heterozygosity (LOH) 62,63.
Mucinous cystic neoplasms are the third and least prevalent preinvasive pancreatic lesion and are distinguished by unique clinical and pathological features, including their distinctive ovarian stroma 64,65. Molecularly, MCNs resemble IPMNs, except that unlike IPMNs, MCNs do not typically harbor GNAS alterations. Whole exome sequencing of 8 MCNs identified KRAS (75%), RNF43 (50%) and TP53 (25%) as highly prevalent events in these lesions 61. Genetic changes accumulate with higher grade of dysplasia and invasive carcinomas associated with MCNs lesions can acquire all common driver gene alterations (KRAS, TP53, CDKN2A, SMAD4) 61.
The three most common precursor lesions that give rise to pancreatic ductal adenocarcinoma (PanIN, IPMN, and MCN) are each defined by a unique landscape of genetic alterations. Still, common themes among all three lesions include early oncogene mutations that initiate tumorigenesis and the later loss of tumor suppressor genes that drives tumor progression. This deep understanding of the genomic alterations in pancreatic cancer precursor lesions has major clinical implications, since early detection has the potential to drastically improve the clinical course of patients with pancreatic neoplasms. In particular, mutations in TP53 and SMAD4 are limited to high-risk lesions, including high-grade dysplasia and invasive cancer, and thus are optimal targets for novel strategies to detect pancreatic neoplasms at high risk for transformation to invasive carcinoma. In addition, further investigation in effector pathways of critical driver genes may clarify the individual roles of each alteration in the selective growth advantage and recalcitrant behavior of neoplastic cells, potentially identifying new methods to target these common aberrations early on 66.
IV. Clonal evolution leading to disease progression
The prevalence and distribution of genetic alterations in all types of cancer can be metaphorically represented as mountains and hills on the cancer genome landscape 67. The pancreatic cancer landscape is dominated by four mountains, all of which are altered in the majority of pancreatic ductal adenocarcinomas and are regarded as unequivocal drivers in pancreatic cancer initiation and progression. However, the majority of somatic mutations in pancreatic ductal adenocarcinoma occur in genes that are altered at low prevalence, only some of which are likely to be true driver genes 5,7,9. These less prevalent mutations accumulate during tumor growth and result in superimposed genetic heterogeneity, which may contribute to poor therapy response and resistance in individual cancers 68-70. Comprehensive analyses of multiple lesions from the same patient are currently underway in many tumor types, which will provide new insights into this heterogeneity in cancer evolution 71. These studies include multi-region and even single cell sequencing, as well as deep next generation sequencing and in situ mutation detection approaches, which will enable a more complete understanding of the evolutionary dynamics and heterogeneity in pancreatic cancer progression 72-76.
Nowell elegantly described the evolutionary process of tumor growth as an adaptive Darwinian process driven by somatic mutations with sequential subclonal selection 77. Due to the normal error rate of DNA replication, each cell division naturally results in approximately 3 somatic mutations, most of which affect intergenic regions without functional consequences 78,79. Increased mutation rate, induced by mutagenic exposure (exogenous) and/or genetic instability (endogenous), accelerates this system of Darwinian evolution by providing additional genetic variation on which selection can act 11,80,81. Remarkably, mathematical models incorporating stem cell division rates suggest that normal DNA replication errors (without additional genetic instability) can account for the somatic mutations identified in many tumor types 82.
At the initiation of neoplasia, a somatic mutation occurs to confer a selective growth advantage followed by clonal expansion. Then, throughout tumorigenesis, additional somatic mutations become clonally fixed because they provide survival advantage, such as increased proliferation, resistance to apoptosis, or evasion of the immune system79,83. The timing of selectively neutral passenger alterations is less clear – some likely occur before the initiating mutation while others accumulate during the process of clonal evolution 78,84,85.
The role of a mutation can be context dependent. A previously neutral mutation can become a driver, based on its micro-environmental influences, interdependence with other mutations, or location within the bulk tissue; similarly, the phenotype conferred by an early driver mutation may not be selected later in tumorigenesis 79,86,87. On the other hand, clonal expansion of a newly acquired beneficial mutation may be restrained by competition (clonal interference) with other clones for resources in the tumor microenvironment 88.
In the case of pancreatic cancer development, the initiating event is likely a somatic mutation at a key oncogenic hotspot. As previously described, KRAS mutation occurs early in pancreatic tumorigenesis, as they are present in all grades of PanIN lesions. However, the proportion of cells with a KRAS mutation (also known as the variant allele frequency or VAF) increases gradually with increasing grade of dypslasia, possibly reflecting clonal expansion over time 50. This is also true for the other driver genes CDKN2A, TP53, and SMAD4, in which VAF increases from HG-PanIN to invasive carcinoma lesions 43,45,60. However, these allele frequencies should be interpreted with some caution, as the VAF is dependent on the neoplastic cellularity of the analyzed samples, which is exceedingly difficult to control for in such experiments.
Although the critical role of driver genes in pancreatic tumorigenesis is well established, the exact order and pace at which these alterations occur is intensely debated. Notta et al. recently challenged the stepwise progression model for pancreatic tumorigenesis, and inferred that a single catastrophic chromotrypsis event can lead to a simultaneous loss of multiple tumor suppressor genes18. These studies highlighted the importance of genomic instability, structural rearrangements, and polyploidization in pancreatic tumorigenesis18. Such a punctuated evolution pattern may at least partially explain the rapid progression of disease observed in some patients with pancreatic cancer 89. However, although such catastrophic chromosomal events may drive tumor progression in a subset of patients, it does not explain the typical stepwise accumulation of driver mutations typically found within most precursor lesions 90. In addition, recent whole exome studies with a cohort of 456 pancreatic ductal adenocarcinomas observed that such catastrophic patterns are uncommon, and when they do occur only rarely target known oncogenes or tumor suppressor genes 4,18. Ultimately, the pattern of evolution appreciated in a particular study is likely dependent on the type of alteration analyzed in the study, with point mutations accumulating sequentially and copy number alterations in a more punctuated manner 91.
Another evolutionary step in the fate of a cancer cell is dissemination and clonal expansion at distant sites. The degree of heterogeneity and the clonal composition of metastatic lesions can provide insights into the timing of metastatic progression 70,72,92,93. Such inter- and intra-metastatic genetic heterogeneity is of crucial clinical importance, as it underlies the development of therapeutic resistance, particularly to targeted therapies 68,69,94-96. Resistance to therapy occurs when treatment selects for an already-present resistant clone, which can expand once sensitive cells are killed.
Two complementary models of the time point of metastasis have been proposed: the “linear progression” model describes a stepwise accumulation of genetic changes until a cell acquires autonomous fitness to disconnect from the primary tumor at a late stage, and the “parallel progression” model infers early dissemination and independent accumulation of genetic changes in both the primary and metastatic site 68,93. Recently, multiple whole exome and whole genome sequencing studies in disseminated pancreatic cancer have analyzed genetic heterogeneity at metastatic sites, in comparison to the other metastases as well as to the primary tumor 72,92. In pancreatic ductal adenocarcinoma, mutations in well-established driver genes appear to be widely uniform within the primary tumor and among the distinct metastases. Further mathematical modeling based on these sequencing data suggests a broad window of time between tumor initiation and the acquisition of metastatic ability 92. These findings provide some support for the linear progression model predominating in pancreatic ductal adenocarcinomas, which infers late dissemination of specific metastatic subclones from the primary tumor. However, there is also marked genetic heterogeneity among metastases, conferred almost entirely by passenger mutations, likely due to neutral evolution after the acquisition of the driver mutations that confer malignancy 78,84.
Clonal evolution in cancer is a highly complex process which has only recently begun to be studied in detail. The resulting intratumoral heterogeneity may contribute to the emergence of therapeutic resistance. Future studies, perhaps even down to the single cell level, will more comprehensively characterize this heterogeneity, phylogenetic relationships, and clonal evolution, providing critical insights into tumor initiation and progression as well as a rational foundation for early detection and therapy in pancreatic cancer.
V. Exploiting molecular alterations in the clinical setting
Great efforts are being exerted to translate the many insights provided by recent next generation sequencing studies into clinical practice 97. Genetic alterations may be used to develop diagnostic markers for early detection, biomarkers for disease progression or therapeutic responsiveness, and novel targeted therapies. Molecular alterations are already used to select subsets of patients most likely to respond to targeted therapy in other tumor types. For example, only colorectal cancers without mutations in KRAS or BRAF respond to therapies targeting the epidermal growth factor receptor 98-102. As such, assays for these mutations are routinely performed before instituting these particular therapies. Molecular knowledge of pancreatic cancer progression generated by recent sequencing studies has significantly improved our understanding of the evolutionary processes that drive pancreatic ductal adenocarcinoma progression. However, genetic knowledge about pancreatic cancer has not had as much impact on clinical practice as it has for other tumor types 36. Here, we highlight a few specific examples of the clinical applicability of molecular alterations – these will almost certainly be expanded with further advances in sequencing technology and therapeutic targeting.
Perhaps one of the first clinical applications of molecular genetics will be in early detection. One major challenge in improving the overall-survival of pancreatic ductal adenocarcinoma patients lies in the fact that patients almost always present with advanced stage disease; at this stage, surgical cure is no longer possible, and patients literally have billions of cancer cells in their bodies. However, knowledge of precancerous lesions that precede the development of pancreatic ductal adenocarcinoma can provide a strategy for early detection and complete eradication of all neoplastic cells. Although precursor lesions likely take years to develop, the most common precursor lesion (PanIN) does not produce symptoms, and virtually all are undetectable by currently available imaging techniques 103-105. Mathematical models analyzing evolutionary patterns in metastatic lesions offers an estimated broad time-window (10 years) before an initiating mutation in the pancreas progresses to the parental founder cell of a pancreatic ductal adenocarcinoma 92. This suggests a broad period of time for early detection. As one considers early detection of precursor lesions, it is important to remember that most PanIN lesions as well as cystic precursors (IPMNs) carry a low risk for progression to invasive carcinoma. The benefits of early detection must be balanced against the risks of overtreatment 106-109.
Because of the low-incidence of pancreatic ductal adenocarcinomas in the general population (life-time risk 1.3%) and the high-cost of the most sensitive imaging techniques, population-wide screening has not been recommended thus far 110,111. Instead, most efforts have focused on identifying high-risk individuals (HRI) in whom there is an increased likelihood of detecting high-grade dysplasia or small pancreatic ductal adenocarcinomas by surveillance compared to the general population 103,112,113. The inherited germline alterations that predispose to pancreatic cancer include mutations in BRCA1, BRCA2, PALB2, CDKN2A, STK11/LKB1 (Peutz-Jeghers), ATM, TP53, PRSS1, and genes associated with mismatch repair defects (Lynch-Syndrome) 114. As previously discussed, it is anticipated that there are additional familial susceptibility genes for pancreatic ductal adenocarcinoma that have not yet been defined 35. Therefore, HRI have also been identified based on their cancer family history.
New screening methods incorporating molecular alterations are being investigated. These include molecular analysis of fine needle aspiration (FNA) samples of pancreatic cysts, as well as duodenal fluid collected endoscopically and circulating tumor DNA (ctDNA) (Table 2). Molecular analysis of cyst fluid can help to distinguish neoplastic precursors from benign cystic lesions 115. Mutations in KRAS or GNAS are present in >96% of patients with a mucin-producing premalignant neoplastic cyst (IPMN or MCN), and these mutations can be detected in cyst fluid with excellent sensitivity and specificity 55,115. Detection of such mutations can distinguish potentially harmful precursor lesions from benign cysts (such as serous cystadenomas), but at least to date, they do not reliably distinguish IPMNs with low-grade dysplasia from IPMNs with high-grade dysplasia. The assessment of ploidy by DNA cytometry may provide a simple method to suggest grade of dysplasia in IPMN cyst fluid 116. However, more accurate markers (such as mutations in late driver genes like TP53 and SMAD4) will be required to distinguish cysts requiring immediate clinical intervention from those that can be safely monitored.
Table 2:
Potential applications of next generation sequencing to diagnosis of pancreatic neoplasms
| Specimen | Procedure | Evidence | Sensitivity* | Specificity* |
|---|---|---|---|---|
| Tumor biopsy | EUS-FNA | Kameta E 2016 Onco Lett163 Young G 2013 Cancer Cytopathol164 Bournet B 2015 J Clin Gastroenterol165 |
80-95% | 95-100% |
| Cyst fluid (IPMN) | EUS-FNA | Springer S 2015 Gastroenterology115 Wu J 2011 Sci Trans Med55 |
75-96% | 92-98% |
| Pancreatic juice | Endoscopic juice collection | Yu J 2016 Gut89 Sadakari Y 2014 Clin Trans Gastro117 Kanda M 2013 Clin Gastroent Hepat50 |
60-85% | 80-100% |
| Blood | Circulating tumor DNA | Bettegowda C 2014 Sci Trans Med121 Sausen M 2015 Nature120 Pietrasz D 2016 Clin Cancer Res166 |
80-90% | 90-100% |
EUS, Endoscopic ultrasound; FNA, Fine needle aspiration
Sensitivity/specificity to detect oncogenic hotspot mutations (KRAS, GNAS)
Genetic alterations from cystic and non-cystic precursor lesions can also be detected in duodenal fluid collected after secretin stimulation, albeit with less sensitivity and specificity than in analysis of directed FNA samples 117,118. As KRAS and GNAS occur at the lowest grades of dysplasia, they are unlikely to be markers for high-risk lesions. However, detection of TP53 mutations in duodenal fluid samples can be an indication of worrisome high-risk lesions such as high-grade PanINs, high-grade IPMNs, and small invasive pancreatic ductal adenocarcinomas, which could have otherwise escaped diagnostic imaging criteria 60. More recently, a novel digital NGS technique was reported that detected even low-abundance mutations down to 0.1-1% mutation prevalence 119. In this study, mutations in TP53 and SMAD4 were frequently identified in duodenal fluids from patients with pancreatic ductal adenocarcinoma, distinguishing them from a control population and even from patients with IPMNs with high specificity. Interestingly, high-risk mutations could be detected months prior a definitive clinical diagnosis of pancreatic ductal adenocarcinoma, highlighting the promise of this technique to facilitate earlier diagnosis. Still, such techniques need to be validated in large cohorts before broad implementation, even in high-risk individuals.
In addition to analysis of cyst and duodenal fluid, another form of so-called “liquid biopsy” can be collected from peripheral blood. Circulating tumor DNA (ctDNA) is readily detectible in patients with metastatic pancreatic cancer but also in almost half of the patients with localized disease 120,121. However, the sensitivity of ctDNA in the detection of non-invasive lesions is limited. Still, this approach can be used to detect invasive cancers earlier and to follow patients with known cancer 120,121. Mutations detected in ctDNA prior to intervention predicted mutations found in the resected tumor, demonstrating that ctDNA represents a surrogate for genetic alterations in the tumor 121. Thus, sequencing of ctDNA can be used to non-invasively detect mutations that confer therapeutic resistance, and it can also predict recurrence earlier than CT scan and is correlated with survival among metastatic and resected patients 122,123.
Despite the low prevalence of targetable mutations identified in pancreatic ductal adenocarcinoma, it is worthwhile to consider some of the therapeutic targets that have been identified. To date, few agents have obtained approval from the United States Food and Drug Administration (FDA): the use of the EGFR inhibitor erlotinib demonstrated a survival benefit in pancreatic ductal adenocarcinoma patients in combination with Gemcitabine versus Gemcitabine alone 124,125. However, targeting major genomic events in pancreatic cancer, such as oncogenic KRAS signaling, have been disappointing until now 126,127,128.
Still, deeper understanding of altered signaling pathways has suggested other targeted treatment strategies. For example, promising pre-clinical results demonstrated treatment response to porcupine inhibitors (Wnt/Notch signaling) in pancreatic ductal adenocarcinomas with loss-of-function mutations in RNF43; this approach is currently being investigated in a clinical trial (NCT01351103) 62. In addition, patients with alterations in BRCA-mediated repair of DNA double strand breaks (BRCA2, PALB, ATM) are sensitive to DNA-crosslinking agents (MitomycinC, Cisplatin, Carboplatin) and poly ADP-ribose polymerase (PARP) inhibitors, which induce additional DNA damage that affect cancer cells selectively 129,130. Pre-clinical success was also reported by CDK4/6 inhibitors in p16INK4A deficient cell lines and may represent actionability for at least a subset of CDKN2A deficient pancreatic ductal adenocarcinomas 131. In addition, promising results of BRAF-inhibitors in metastatic melanoma, which obtained FDA approval, may be transitioned into pancreatic ductal adenocarcinoma trials of BRAF-mutated tumors 132. Finally, although uncommon (occurring only in about 1% of pancreatic ductal adenocarcinomas), defects in DNA mismatch repair may indicate a subset of pancreatic ductal adenocarcinomas that are specifically responsive to immunotherapy. A potential mechanism suggested in other tumor types is that increased immune cell infiltration occurs due to neo-antigens as a result of a higher mutational load 133-135. Clinical trials for immune checkpoint inhibitor therapies in pancreatic ductal adenocarcinoma are currently underway (NCT02907099; NCT02305186).
Currently, several centers have implemented a new approach to clinical trials of targeted therapy. In large scale randomized “basket” trials (such as IMPaCT), the efficacy of genotype-guided therapies with existing targeting agents are currently being investigated in pancreatic cancer (along with many other tumor types) based on individual tumor genotype36,136,137. Such studies of genotype-guided therapy in pancreatic ductal adenocarcinoma will be required to implement personalized medicine for patients with pancreatic cancer.
In summary, to date, signaling pathways of the four main pancreatic ductal adenocarcinoma driver genes are not druggable. Thus, current strategies for molecularly targeted therapies in pancreatic cancer focus on mutations that occur at lower prevalence. In order to define which of the low prevalence mutations are best targeted, a deeper pathological and cellular understanding of specific gene mutations is crucial. In addition, mutations can improve survival by providing biomarkers for early detection, as survival of pancreatic ductal adenocarcinoma patients is highly correlated with their stage at diagnosis. Although genetic alterations can be detected in multiple types of bio-specimens, including blood and duodenal fluid, the precise clinical applications of such assays have not yet been standardized.
VI. Future directions
The cellular and biological implications of the vast majority of genetic changes found in individual pancreatic ductal adenocarcinomas are still not well understood. Our molecular knowledge is predominantly based on sequencing studies at a single time point, complicating our ability to perform in-depth analysis of the evolutionary trajectory of disease progression. This is particularly challenging to investigate in human specimens because in the follow-up of operatively resected patients, no additional tumor samples are typically harvested for routine clinical care. Serial biopsies for research studies can overcome this hurdle, but such analyses can be augmented by reproducible experimental models to assess the molecular alterations and downstream consequences in a longitudinal fashion. As our knowledge grows, the following questions need to be addressed: what process is driven by each driver gene mutation: invasion, proliferation, or dissemination? Which of the infrequently altered genes are truly drivers? To answer these pressing questions, the functional impact of specific somatic mutations on cellular signaling and other phenotypes also needs to be more completely characterized.
Studies of genetically engineered mouse models (GEMMs) have largely supported the insights from human samples. For example, pancreas-specific expression of mutant KRAS alone leads to the development of PanIN-like structures and with some latency to subsequent pancreatic ductal adenocarcinoma development 138. When targeting additional drivers (CDKN2A, TP53, SMAD4), pancreatic ductal adenocarcinoma development with rapid progression can be reproduced, with a higher prevalence of invasion and metastasis that mimics the clinical course of human disease 139-141. Future models could support the functional role of low-prevalence mutations found in recent large-scale sequencing attempts, such as ATM, MLL3, Axon-guidance genes (ROBO1/2, SLIT), KDM6A, GLI3 and others.
GEMMs also provide a tool for clinically important questions, as they can be used as a model for drug testing prior to clinical trials 142. EGFR- and VEGF-inhibitors were successfully tested in GEMMs that recapitulated therapeutic responses found in patients 143,144. However, results in GEMMs are not always indicative of clinical success in human pancreatic cancer patients. For example, the Hedgehog-signaling inhibitor IPI-926 showed an exceptional response with improved drug delivery and survival in a GEMM system, but results in human clinical trials were disappointing 145,146.
A promising new development is the use of three-dimensional (organoid) culture systems, which enable the analysis of local growth and invasiveness of human cancer cells over time 147. There are major advantages of this system over two-dimensional culture and animal models, as organoids enable time-lapse imaging and spatial characterization during pancreatic cancer cell invasion 148. The flexibility offered by the growth of human organoids directly from dissociated primary tissue or even from biopsies offers an extremely valuable methodology for examining the biologic behavior of individual patient samples, with a much higher success rate than establishment of traditional two-dimensional cell cultures 149. For example, such three-dimensional cultures can be used as a surrogate for treatment response.
Genetic changes can be induced in in vitro cell models, including two-dimensional cell culture as well as organoids. Attempts to model the pancreatic cancer progression sequence in cultured human cells have confirmed the malignant potential of specific drivers 148,150,151. Recent developments in genome engineering using CRISPR/Cas9 (Clustered regularly interspaced palindromic regions/CRISPR-associated proteins) gene editing system have facilitated improved genomic modification of cultured cells by avoiding the use of unnatural exogenous or leaky transgene expression systems 152. In this case, the functional impact on pancreatic carcinogenesis can be accurately analyzed following each genetic hit. The CRISPR/Cas9 technology may also improve the process of developing new animal models 153,154.
Modern experimental developments in modeling pancreatic carcinogenesis enable us to understand the biological consequences of genetic alterations found in recent large-scale sequencing studies. In order to augment data from in silico analyses of the functional impact of mutations, a combination of studies in in vitro and in vivo models will highlight the most promising genetic alterations to be further explored for the development of novel clinically relevant approaches, including new strategies for early diagnosis and targeted therapy of pancreatic cancer.
Acknowledgements
The authors acknowledge the following sources of support: NIH/NCI P50 CA62924; NIH/NIDDK K08 DK107781; Sol Goldman Pancreatic Cancer Research Center; Buffone Family Gastrointestinal Cancer Research Fund; Kaya Tuncer Career Development Award in Gastrointestinal Cancer Prevention; AGA-Bernard Lee Schwartz Foundation Research Scholar Award in Pancreatic Cancer; Sidney Kimmel Foundation for Cancer Research Kimmel Scholar Award; AACR-Incyte Corporation Career Development Award for Pancreatic Cancer Research; Rolfe Pancreatic Cancer Foundation; Joseph C Monastra Foundation; The Gerald O Mann Charitable Foundation (Harriet and Allan Wulfstat, Trustees)
References
- 1.Cancer Facts & Figures. American Cancer Society 2017. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 4.Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47–52. [DOI] [PubMed] [Google Scholar]
- 5.Witkiewicz AK, McMillan EA, Balaji U, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun 2015;6:6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012;491:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Tsutsumi S, Kawaguchi T, et al. Whole-exome sequencing of human pancreatic cancers and characterization of genomic instability caused by MLH1 haploinsufficiency and complete deficiency. Genome Res 2012;22:208–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008;321:1801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 11.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature 2009;458:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn SA, Schutte M, Hoque AT, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science 1996;271:350–3. [DOI] [PubMed] [Google Scholar]
- 13.Redston MS, Caldas C, Seymour AB, et al. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res 1994;54:3025–33. [PubMed] [Google Scholar]
- 14.Caldas C, Hahn SA, Hruban RH, et al. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res 1994;54:3568–73. [PubMed] [Google Scholar]
- 15.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988;53:549–54. [DOI] [PubMed] [Google Scholar]
- 16.Feigin ME, Garvin T, Bailey P, et al. Recurrent noncoding regulatory mutations in pancreatic ductal adenocarcinoma. Nat Genet 2017;49:825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy SJ, Hart SN, Halling GC, et al. Integrated Genomic Analysis of Pancreatic Ductal Adenocarcinomas Reveals Genomic Rearrangement Events as Significant Drivers of Disease. Cancer Res 2016;76:749–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Notta F, Chan-Seng-Yue M, Lemire M, et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 2016;538:378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceccaldi R, Sarangi P, D’Andrea AD. The Fanconi anaemia pathway: new players and new functions. Nat Rev Mol Cell Biol 2016;17:337–49. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov LB, Nik-Zainal S, Wedge DC, et al. Signatures of mutational processes in human cancer. Nature 2013;500:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collisson EA, Sadanandam A, Olson P, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med 2011;17:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nones K, Waddell N, Song S, et al. Genome-wide DNA methylation patterns in pancreatic ductal adenocarcinoma reveal epigenetic deregulation of SLIT-ROBO, ITGA2 and MET signaling. Int J Cancer 2014;135:1110–8. [DOI] [PubMed] [Google Scholar]
- 23.Tan AC, Jimeno A, Lin SH, et al. Characterizing DNA methylation patterns in pancreatic cancer genome. Mol Oncol 2009;3:425–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoda W, Chianchiano P, Griffin JF, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol 2017;242:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang B, Li Y, Qi G, et al. Clinicopathological Significance of CDKN2A Promoter Hypermethylation Frequency with Pancreatic Cancer. Sci Rep 2015;5:13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kisiel JB, Raimondo M, Taylor WR, et al. New DNA Methylation Markers for Pancreatic Cancer: Discovery, Tissue Validation, and Pilot Testing in Pancreatic Juice. Clin Cancer Res 2015;21:4473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi JM, Guzzetta AA, Bailey VJ, et al. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin Cancer Res 2013;19:6544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent A, Omura N, Hong SM, et al. Genome-wide analysis of promoter methylation associated with gene expression profile in pancreatic adenocarcinoma. Clin Cancer Res 2011;17:4341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsubayashi H, Canto M, Sato N, et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res 2006;66:1208–17. [DOI] [PubMed] [Google Scholar]
- 30.Nagpal G, Sharma M, Kumar S, et al. PCMdb: pancreatic cancer methylation database. Sci Rep 2014;4:4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald OG, Li X, Saunders T, et al. Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nat Genet 2017;49:367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts NJ, Klein AP. Genome-wide sequencing to identify the cause of hereditary cancer syndromes: with examples from familial pancreatic cancer. Cancer Lett 2013;340:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruijs MW, Verhoef S, Rookus MA, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. J Med Genet 2010;47:421–8. [DOI] [PubMed] [Google Scholar]
- 34.Roberts NJ, Jiao Y, Yu J, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2:41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts NJ, Norris AL, Petersen GM, et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov 2016;6:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodoky G, Timcheva C, Spigel DR, et al. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs 2012;30:1216–23. [DOI] [PubMed] [Google Scholar]
- 38.Rinehart J, Adjei AA, Lorusso PM, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol 2004;22:4456–62. [DOI] [PubMed] [Google Scholar]
- 39.Anagnostou V, Smith KN, Forde PM, et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov 2017;7:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014;74:3381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basturk O, Hong SM, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 2015;39:1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosoda W, Wood LD. Molecular Genetics of Pancreatic Neoplasms. Surg Pathol Clin 2016;9:685–703. [DOI] [PubMed] [Google Scholar]
- 43.Murphy SJ, Hart SN, Lima JF, et al. Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology 2013;145:1098–109.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res 2000;6:2969–72. [PubMed] [Google Scholar]
- 45.Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res 2000;60:2002–6. [PubMed] [Google Scholar]
- 46.Luttges J, Reinecke-Luthge A, Mollmann B, et al. Duct changes and K-ras mutations in the disease-free pancreas: analysis of type, age relation and spatial distribution. Virchows Arch 1999;435:461–8. [DOI] [PubMed] [Google Scholar]
- 47.Brat DJ, Lillemoe KD, Yeo CJ, et al. Progression of pancreatic intraductal neoplasias to infiltrating adenocarcinoma of the pancreas. Am J Surg Pathol 1998;22:163–9. [DOI] [PubMed] [Google Scholar]
- 48.Wilentz RE, Geradts J, Maynard R, et al. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res 1998;58:4740–4. [PubMed] [Google Scholar]
- 49.Matthaei H, Wu J, Dal Molin M, et al. GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as “incipient IPMNs”. Am J Surg Pathol 2014;38:360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012;142:730–3.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee KE, Bar-Sagi D. Oncogenic KRas suppresses inflammation-associated senescence of pancreatic ductal cells. Cancer Cell 2010;18:448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caldwell ME, DeNicola GM, Martins CP, et al. Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer. Oncogene 2012;31:1599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011;11:761–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amato E, Molin MD, Mafficini A, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 2014;233:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011;3:92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep 2011;1:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Hayre M, Vazquez-Prado J, Kufareva I, et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 2013;13:412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson CH, McIntyre RE, Arends MJ, et al. The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc(Min/+) mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene 2010;29:4567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chetty R, Serra S, Salahshor S, et al. Expression of Wnt-signaling pathway proteins in intraductal papillary mucinous neoplasms of the pancreas: a tissue microarray analysis. Hum Pathol 2006;37:212–7. [DOI] [PubMed] [Google Scholar]
- 60.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013;11:719–30.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu J, Jiao Y, Dal Molin M, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 2011;108:21188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang X, Hao HX, Growney JD, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A 2013;110:12649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koo BK, Spit M, Jordens I, et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 2012;488:665–9. [DOI] [PubMed] [Google Scholar]
- 64.Griffin JF, Page AJ, Samaha GJ, et al. Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: A large single institution series. Pancreatology 2017;17:490–6. [DOI] [PubMed] [Google Scholar]
- 65.Noe M, Brosens LA. Pathology of Pancreatic Cancer Precursor Lesions. Surg Pathol Clin 2016;9:561–80. [DOI] [PubMed] [Google Scholar]
- 66.Spira A, Yurgelun MB, Alexandrov L, et al. Precancer Atlas to Drive Precision Prevention Trials. Cancer Res 2017;77:1510–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood LD, Parsons DW, Jones S, et al. The genomic landscapes of human breast and colorectal cancers. Science 2007;318:1108–13. [DOI] [PubMed] [Google Scholar]
- 68.McGranahan N, Favero F, de Bruin EC, et al. Clonal status of actionable driver events and the timing of mutational processes in cancer evolution. Sci Transl Med 2015;7:283ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bozic I, Reiter JG, Allen B, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife 2013;2:e00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010;467:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alizadeh AA, Aranda V, Bardelli A, et al. Toward understanding and exploiting tumor heterogeneity. Nat Med 2015;21:846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makohon-Moore AP, Zhang M, Reiter JG, et al. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet 2017;49:358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yates LR, Gerstung M, Knappskog S, et al. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med 2015;21:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Waters J, Leung ML, et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 2014;512:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Navin N, Kendall J, Troge J, et al. Tumour evolution inferred by single-cell sequencing. Nature 2011;472:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nowell PC. The clonal evolution of tumor cell populations. Science 1976;194:23–8. [DOI] [PubMed] [Google Scholar]
- 78.Bozic I, Antal T, Ohtsuki H, et al. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci U S A 2010;107:18545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greaves M, Maley CC. Clonal evolution in cancer. Nature 2012;481:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu S, Powers S, Zhu W, et al. Substantial contribution of extrinsic risk factors to cancer development. Nature 2016;529:43–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loeb LA. Human cancers express mutator phenotypes: origin, consequences and targeting. Nat Rev Cancer 2011;11:450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015;347:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014;505:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams MJ, Werner B, Barnes CP, et al. Identification of neutral tumor evolution across cancer types. Nat Genet 2016;48:238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McFarland CD, Korolev KS, Kryukov GV, et al. Impact of deleterious passenger mutations on cancer progression. Proc Natl Acad Sci U S A 2013;110:2910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer 2016;16:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gatenby RA, Cunningham JJ, Brown JS. Evolutionary triage governs fitness in driver and passenger mutations and suggests targeting never mutations. Nat Commun 2014;5:5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marusyk A, Tabassum DP, Altrock PM, et al. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature 2014;514:54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu J, Blackford AL, Dal Molin M, et al. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut 2015;64:1783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reiter JG, Iacobuzio-Donahue CA. Pancreatic cancer: Pancreatic carcinogenesis - several small steps or one giant leap? Nat Rev Gastroenterol Hepatol 2016;14:7–8. [DOI] [PubMed] [Google Scholar]
- 91.Rode A, Maass KK, Willmund KV, et al. Chromothripsis in cancer cells: An update. Int J Cancer 2016;138:2322–33. [DOI] [PubMed] [Google Scholar]
- 92.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer 2009;9:302–12. [DOI] [PubMed] [Google Scholar]
- 94.Barber LJ, Sandhu S, Chen L, et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol 2013;229:422–9. [DOI] [PubMed] [Google Scholar]
- 95.Diaz LA Jr., Williams RT, Wu J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 2012;486:537–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Misale S, Di Nicolantonio F, Sartore-Bianchi A, et al. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov 2014;4:1269–80. [DOI] [PubMed] [Google Scholar]
- 97.Dreyer SB, Chang DK, Bailey P, et al. Pancreatic Cancer Genomes: Implications for Clinical Management and Therapeutic Development. Clin Cancer Res 2017;23:1638–46. [DOI] [PubMed] [Google Scholar]
- 98.Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016;17:1426–34. [DOI] [PubMed] [Google Scholar]
- 99.Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34. [DOI] [PubMed] [Google Scholar]
- 100.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626–34. [DOI] [PubMed] [Google Scholar]
- 101.Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705–12. [DOI] [PubMed] [Google Scholar]
- 102.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65. [DOI] [PubMed] [Google Scholar]
- 103.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142:796–804; quiz e14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008;1:306–16. [PMC free article] [PubMed] [Google Scholar]
- 105.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006;4:766–81; quiz 665. [DOI] [PubMed] [Google Scholar]
- 106.Crippa S, Pergolini I, Rubini C, et al. Risk of misdiagnosis and overtreatment in patients with main pancreatic duct dilatation and suspected combined/main-duct intraductal papillary mucinous neoplasms. Surgery 2016;159:1041–9. [DOI] [PubMed] [Google Scholar]
- 107.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97. [DOI] [PubMed] [Google Scholar]
- 108.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery 2012;152:S4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62:339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang W, Chen S, Brune KA, et al. PancPRO: risk assessment for individuals with a family history of pancreatic cancer. J Clin Oncol 2007;25:1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012;16:771–83. [DOI] [PubMed] [Google Scholar]
- 113.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010;16:5028–37. [DOI] [PubMed] [Google Scholar]
- 114.Hruban RH, Canto MI, Goggins M, et al. Update on familial pancreatic cancer. Adv Surg 2010;44:293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klein F, Denecke T, Faber W, et al. DNA Cytometry for Differentiation Between Low- and Medium-grade Dysplasia in Intraductal Papillary Mucinous Neoplasms. Anticancer Res 2017;37:735–40. [DOI] [PubMed] [Google Scholar]
- 117.Sadakari Y, Kanda M, Maitani K, et al. Mutant KRAS and GNAS DNA Concentrations in Secretin-Stimulated Pancreatic Fluid Collected from the Pancreatic Duct and the Duodenal Lumen. Clin Transl Gastroenterol 2014;5:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eshleman JR, Norris AL, Sadakari Y, et al. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol 2015;13:963–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yu J, Sadakari Y, Shindo K, et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sausen M, Phallen J, Adleff V, et al. Clinical implications of genomic alterations in the tumour and circulation of pancreatic cancer patients. Nat Commun 2015;6:7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 2016;8:346ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol 2015;26:1715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang JP, Wu CY, Yeh YC, et al. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open-label, prospective trial. Oncotarget 2015;6:18162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960–6. [DOI] [PubMed] [Google Scholar]
- 126.Ledford H Cancer: The Ras renaissance. Nature 2015;520:278–80. [DOI] [PubMed] [Google Scholar]
- 127.Javle MM, Shroff RT, Xiong H, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer 2010;10:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430–8. [DOI] [PubMed] [Google Scholar]
- 129.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther 2011;10:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 2009;361:123–34. [DOI] [PubMed] [Google Scholar]
- 131.Heilmann AM, Perera RM, Ecker V, et al. CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers. Cancer Res 2014;74:3947–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348:124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chantrill LA, Nagrial AM, Watson C, et al. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res 2015;21:2029–37. [DOI] [PubMed] [Google Scholar]
- 137.Cowley MJ, Chang DK, Pajic M, et al. Understanding pancreatic cancer genomes. J Hepatobiliary Pancreat Sci 2013;20:549–56. [DOI] [PubMed] [Google Scholar]
- 138.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003;4:437–50. [DOI] [PubMed] [Google Scholar]
- 139.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 2003;17:3112–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83. [DOI] [PubMed] [Google Scholar]
- 141.Bardeesy N, Aguirre AJ, Chu GC, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A 2006;103:5947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gopinathan A, Morton JP, Jodrell DI, et al. GEMMs as preclinical models for testing pancreatic cancer therapies. Dis Model Mech 2015;8:1185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Miyabayashi K, Ijichi H, Mohri D, et al. Erlotinib prolongs survival in pancreatic cancer by blocking gemcitabine-induced MAPK signals. Cancer Res 2013;73:2221–34. [DOI] [PubMed] [Google Scholar]
- 144.Singh M, Lima A, Molina R, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nat Biotechnol 2010;28:585–93. [DOI] [PubMed] [Google Scholar]
- 145.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Catenacci DV, Junttila MR, Karrison T, et al. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol 2015;33:4284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 2014;345:1247125. [DOI] [PubMed] [Google Scholar]
- 148.Huang L, Holtzinger A, Jagan I, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 2015;21:1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boj SF, Hwang CI, Baker LA, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015;160:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee J, Snyder ER, Liu Y, et al. Reconstituting development of pancreatic intraepithelial neoplasia from primary human pancreas duct cells. Nat Commun 2017;8:14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Inagawa Y, Yamada K, Yugawa T, et al. A human cancer xenograft model utilizing normal pancreatic duct epithelial cells conditionally transformed with defined oncogenes. Carcinogenesis 2014;35:1840–6. [DOI] [PubMed] [Google Scholar]
- 152.Chiou SH, Winters IP, Wang J, et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev 2015;29:1576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014;157:1262–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang H, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013;153:910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oshima M, Okano K, Muraki S, et al. Immunohistochemically detected expression of 3 major genes (CDKN2A/p16, TP53, and SMAD4/DPC4) strongly predicts survival in patients with resectable pancreatic cancer. Ann Surg 2013;258:336–46. [DOI] [PubMed] [Google Scholar]
- 156.Blackford A, Serrano OK, Wolfgang CL, et al. SMAD4 gene mutations are associated with poor prognosis in pancreatic cancer. Clin Cancer Res 2009;15:4674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhong Y, Wang Z, Fu B, et al. GATA6 activates Wnt signaling in pancreatic cancer by negatively regulating the Wnt antagonist Dickkopf-1. PLoS One 2011;6:e22129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dal Molin M, Zhang M, de Wilde RF, et al. Very Long-term Survival Following Resection for Pancreatic Cancer Is Not Explained by Commonly Mutated Genes: Results of Whole-Exome Sequencing Analysis. Clin Cancer Res 2015;21:1944–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Russell R, Perkhofer L, Liebau S, et al. Loss of ATM accelerates pancreatic cancer formation and epithelial-mesenchymal transition. Nat Commun 2015;6:7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kamphues C, Bova R, Bahra M, et al. Ataxia-telangiectasia-mutated protein kinase levels stratify patients with pancreatic adenocarcinoma into prognostic subgroups with loss being a strong indicator of poor survival. Pancreas 2015;44:296–301. [DOI] [PubMed] [Google Scholar]
- 161.Schultz NA, Roslind A, Christensen IJ, et al. Frequencies and prognostic role of KRAS and BRAF mutations in patients with localized pancreatic and ampullary adenocarcinomas. Pancreas 2012;41:759–66. [DOI] [PubMed] [Google Scholar]
- 162.Golan T, Sella T, O’Reilly EM, et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer 2017;116:697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kameta E, Sugimori K, Kaneko T, et al. Diagnosis of pancreatic lesions collected by endoscopic ultrasound-guided fine-needle aspiration using next-generation sequencing. Oncol Lett 2016;12:3875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Young G, Wang K, He J, et al. Clinical next-generation sequencing successfully applied to fine-needle aspirations of pulmonary and pancreatic neoplasms. Cancer Cytopathol 2013;121:688–94. [DOI] [PubMed] [Google Scholar]
- 165.Bournet B, Selves J, Grand D, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with a KRAS mutation assay using allelic discrimination improves the diagnosis of pancreatic cancer. J Clin Gastroenterol 2015;49:50–6. [DOI] [PubMed] [Google Scholar]
- 166.Pietrasz D, Pecuchet N, Garlan F, et al. Plasma Circulating Tumor DNA in Pancreatic Cancer Patients Is a Prognostic Marker. Clin Cancer Res 2017;23:116–23. [DOI] [PubMed] [Google Scholar]