Abstract
A significant percentage (~30%) of estrogen receptor-α (ERα)-positive tumors become refractory to endocrine therapies; however, the mechanisms responsible for this resistance remain largely unknown. Chronic exposure to arsenic through foods and contaminated water has been linked to an increased incidence of several tumors and long-term health complications. Preclinical and population studies have indicated that arsenic exposure may interfere with endocrine regulation and increase the risk of breast tumorigenesis. In this study, we examined the effects of sodium arsenite (NaAsIII) exposure in ERα-positive breast cancer cells in vitro and in mammary tumor xenografts. The results revealed that acute (within 4 days) and long-term (10 days to 7 weeks) in vitro exposure to environmentally relevant doses reduced breast cancer 1 (BRCA1) and ERα expression associated with the gain of cyclin D1 (CCND1) and folate receptor 1 (FOLR1), and the loss of methylenetetrahydrofolate reductase (MTHFR) expression. Furthermore, long-term exposure to NaAsIII induced the proliferation and compromised the response of MCF7 cells to tamoxifen (TAM). The in vitro exposure to NaAsIII induced BRCA1 CpG methylation associated with the increased recruitment of DNA methyltransferase 1 (DNMT1) and the loss of RNA polymerase II (PolII) at the BRCA1 gene. Xenografts of NaAsIII-preconditioned MCF7 cells (MCF7NaAsIII) into the mammary fat pads of nude mice produced a larger tumor volume compared to tumors from control MCF7 cells and were more refractory to TAM in association with the reduced expression of BRCA1 and ERα, CpG hypermethylation of estrogen receptor 1 (ESR1) and BRCA1, and the increased expression of FOLR1. These cumulative data support the hypothesis that exposure to AsIII may contribute to reducing the efficacy of endocrine therapy against ERα-positive breast tumors by hampering the expression of ERα and BRCA1 via CpG methylation, respectively of ESR1 and BRCA1.
Keywords: arsenic, estrogen receptor, BRCA1, epigenetics, tamoxifen, breast cancer
Introduction
Inorganic arsenic is ubiquitously found in foods (i.e., rice and grains) (1,2) and drinking water (3–5). Chronic arsenic exposure through contaminated water has been linked to an increased incidence of several tumors (6,7) and long-term health complications at levels of exposure below safety limits (10 ppb) (8). Common human exposures to arsenic include inorganic trivalent arsenite (AsIII) and pentavalent arsenate (AsV). The AsIII form has potent estrogen-disrupting activities in connection with its affinity for the ligand-binding domain of the estrogen receptor-α (ERα). It also stimulates cell growth and the expression of the progesterone receptor (PR) (9). As the AsV form is enzymatically converted to AsIII, it provides a reservoir for ERα-binding metabolites (10) that may disrupt estrogen signaling and response to endocrine therapies based on antagonists of the ERα (11–13).
Approximately 70–80% of diagnosed breast tumors are ER-positive and they are treated with anti-estrogens, including tamoxifen (TAM). However, over time, a significant percentage (~30%) of these tumors become resistant to treatment with anti-estrogens (14,15). The reasons for this acquired resistance remain largely unknown. However, the loss of ERα expression has been linked to a poor response to endocrine therapy (16–18). The deregulation of ERα signaling associated with the drinking of water contaminated with arsenic has been reported both in men and women (19). Arsenic-induced genomic instability via the Fanconi anemia (FA)/breast cancer (BRCA) pathway disruption has been shown to directly contribute to arsenic carcinogenic effects (20). A previous study using rodent models (e.g., Sprague-Dawley rats) demonstrated that the in utero exposure to AsIII induced an increase in the number of mammosphere-forming cells, the branching of epithelial cells and density in the mammary gland of prepubertal offspring, and that these changes persisted into adulthood (21). Other studies using rodent models concluded that AsIII was a 'complete' transplacental carcinogen promoting the maternal dose-dependent induction of tumors in endocrine-related tissues (adrenal gland, ovary and uterus) in offspring (22,23). In a spontaneous mammary-tumor model (C3H/St mice), arsenic exposure was shown to abolish the anticancer effects of selenium and increase tumor growth rates and multiplicity (24). At the cellular level, in vitro studies have indicated that chronic exposure to low levels of arsenic induced the transformation of normal breast epithelial cells, and accelerated the growth of ERα-positive breast cancer cells (25,26). Exposure to AsIII has been shown to inhibit DNA mismatch repair, leading to genomic instability (27,28). In endocrine-responsive tissue (e.g., prostate), exposure to AsIII has been reported to induce the transition to a steroid receptor-independent tumor phenotype (29). These cumulative observations have raised the question of whether or not endocrine disruption associated with AsIII exposure contributes to breast carcinogenesis.
Epigenetics refers to changes in DNA methylation, histone post-translational modifications and the expression of non-coding RNAs (30). Maternal exposure to arsenic has been shown to alter DNA methylation in placental tissue (31), and to increase DNA methylation in children (32). Moreover, preclinical (33,34) and human (35) studies have demonstrated that arsenic causes the hypermethylation of tumor suppressor genes (i.e., p16INK4 and RASSF1) and a decrease in telomere length associated with genomic instability (36). Finally, exposure to AsIII has been found to induce cancer stem cell-like properties involving the epigenetic silencing of the let-7c via Ras/NF-κB pathways (37). Based on these observations, the main objective of this study was to investigate the effects of AsIII on BRCA1 and ESR1 (ERα) expression and CpG methylation, and response to TAM in cultured and xenografted MCF7 breast cancer cells.
Materials and methods
Cells and cell culture
Authenticated breast cancer MCF7 cells (Batch #62349993) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained at 37°C with 5% CO2 in Dulbecco's modified Eagle's/F12 medium (DMEM) from Corning Cellgro (Thermo Fisher Scientific, Pittsburgh, PA, USA) supplemented with 10% fetal calf serum (FCS; HyClone Laboratories Inc., Logan UT, USA) as previously described (38). Sodium arsenite (NaAsIII), TAM, genistein (GEN) and 17β-estradiol (E2) were obtained from Sigma-Aldrich (St. Louis, MO, USA). TAM and E2 were solubilized in stock solutions with ethanol, which was added to DMEM/F12 as the vehicle control. For cell proliferation experiments, the MCF7 cells (passage nos. 3–15) were seeded in 6-well plates at a density of 5×105 cells/well in triplicate overnight, and then switched to phenol-free media containing 10% charcoal-stripped FCS (HyClone Laboratories Inc.) for 3 days before the start of each treatment. For proliferation measurements, the cells were washed with ice-cold PBS and counted by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay (Promega, Madison, WI, USA). This assay is based on the conversion of the yellow tretrazolium dye MTT to purple formazan crystals by metabolically active cells. Briefly, 2×104 cells were seeded in 96-well tissue culture plates and maintained overnight. Six replicates were assigned to each experimental treatment. Following treatment, 15 µl of MTT dye solution were added to each well, and the plate was incubated for 4 h at 37°C. Solubilization/stop solution (100 µl) was added for 1 h at room temperature and the absorbance at 570/650 nm was recorded using a Synergy HT plate reader (Bio-Tek Instruments, Winooski, VT, USA). For flow cytometric analysis, trypsinized cells were washed in phosphate-buffered saline (PBS), treated with RNAse and stained with propidium iodide (50 µg/ml). Cell cycle distribution profiles were determined with a FACscan (BD Biosciences, Franklin Lakes, NJ, USA), using a CELLQuest program at the Flow Cytometry Laboratory of the Arizona Cancer Center, and analyzed with MODFIT.2 software.
Promoter CpG methylation
Quantitative polymerase chain reaction (qPCR) analysis of human BRCA1 and ESR1 promoter CpG methylation was performed as previously described (38) with genomic DNA (DNeasy blood and tissue kit; Qiagen, Hilden, Germany) and bisulfonated with the Epitect bisulfite kit (Qiagen) using the following unmethylated (U)- and methylated (M)-specific primers (Sigma-Aldrich): BRCA1 U-sense, 5′-TTGGTTTTTGTGGTAATGGAAAAGTGT-3′ and U-antisense, 5′-CAAAAAATCTCAACAAACTCACACCA-3′; M-sense, 5′-TGGTAACGGAAAAGCG-3′ and M-antisense, 5′-ATCTCAACGAACTCACGC-3′; ESR1 U-sense, 5′-GGATATGGTTTGTATTTTGTTTGT-3′ and U-antisense, 5′-ACAAACAATTCAAAAACTCCAACT-3′; M-sense, 5′-GGTTTTTGAGTTTTTTGTTTTG-3′ and M-antisense, 5′-AACTTACTACTATCCAAATACACCTC-3′. The qPCR was carried out in a volume of 10 µl consisting of the following master mix: 5 µl of SYBER-Green mix (Thermo Fisher Scientific), 1 µl each of forward and reverse primers, 2 µl nuclease-free water, and 1 µl of bisulfonated genomic DNA. Data from qPCR of bisulfonated DNA were presented as the fold-change compared to the control of the ratio of CpG M/U, as previously described (38).
Chromatin immunoprecipitation assay
The Pierce magnetic chromatin immunoprecipitation (ChIP) kit (Pierce, Rockford, IL, USA) was used to analyze the occupancy of the BRCA1 promoter by DNA methyltransferase 1 (DNMT1) and RNA polymerase II (PolII) in MCF7 cells according to instructions provided by the manufacturer. Briefly, the cells were fixed in 1% paraformaldehyde for 10 min and neutralized with glycine. After 2 washes with cold PBS and protease inhibitors cocktail, cells were resuspended in membrane extraction buffer and prepared for DNA enzymatic digestion. Aliquots of digested chromatin were immunoprecipitated using antibodies against DNMT1 (Abcam Inc, Cambridge, MA, USA) and PolII (Thermo Fisher Scientific). qPCR was performed on aliquots of DNA obtained after the reversal of DNA-protein cross-links and purification through spin-filtration columns. Briefly, PCR amplification reactions were done at a final volume of 25 µl consisting of the following: 12.5 µl of SYBR-Green buffer, 1 µl each forward (5′-CTCCCATCCTCTGATTGTACCTTG AT-3′) and reverse (5′-CAGGAAGTCTCAGCGAGCTCAC-3′) oligonucleotides flanking exon-1a in the BRCA1 gene (39); 8.5 µl nuclease free water, and 2 µl DNA purified from the ChIP assay.
mRNA analyses
Total RNA was purified using RNeasy Mini kit as per the manufacturer's instructions (Qiagen) (38). The concentrations and quality of RNA were verified using the Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific). Equal amounts of total RNA (500 ng) were transcribed into cDNA using ISCRIPT supermix kit (Bio-Rad Laboratories, Hercules, CA, USA). Next, cDNA aliquots were analyzed by qPCR using the SYBR-Green PCR Reagents kit (Life Technologies/Thermo Fisher Scientific). Briefly, reactions were run at a final volume of 25 µl consisting of the following master mix: 12.5 µl of SYBR-Green mix, 1 µl each of forward and reverse primers, 9.5 µl nuclease-free water and 1 µl cDNA. The primer (Sigma-Aldrich) sequences were: ERα sense, 5′-CAAGCCCGCTCATGATCAA-3′ and antisense, 5′-CTGATCATGGAGGGTCAAATCCAC-3′; BRCA1 sense, 5′-AGCTCGCTGAGACTTCCTGGA-3′ and antisense, 5′-CAATTCAATGTAGACAGACGT-3′; cyclin D1 (CCND1 sense, 5′-ACAAACAGATCATCCGCAAACAC-3′ and anti-sense, 5′-TGTTGGGGCTCCTCAGGTTC-3′; folate receptor (FOLR1) sense, 5′-ATTCCTTGGTGCCACTGACC-3′ and antisense, 5′-ATAGAACCTCGCCACCTCCT-3′; methyltenetrahydrofolate reductase (MTHFR) sense, 5′-AAGCCTCTT CCTTTGTCGCA-3′ and antisense, 5′-AGGACCCTGGCTT TTCGATG-3′; and control glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense, 5′-ACCCACTCCTCCACCTTT-and antisense, 5′-CTCTTGTGCTCTTGCTGGG-3′. Amplification of GAPDH mRNA was used for the normalization of the transcript levels.
Western blot analysis
Western blot analysis was performed as previously described (38). Protein lysates were obtained from cells scraped in triplicates from 6-well plates and using Pierce RIPA buffer (Thermo Fisher Scientific), with 1% proteinase inhibitors. The protein concentration was calculated using a Nanodrop 1000 Spectrophotometer (Thermo Fisher Scientific). Immunoblotting was carried out with antibodies against BRCA1 (cat. no. 9010); GAPDH (cat. no. 2118) (both from Cell Signaling Technology, Beverly, MA, USA); and ERα (cat. no. sc-542) (Santa Cruz Biotechnology, Dallas, TX, USA). Immunocomplexes were detected using enhanced chemiluminescence (GE Healthcare Life Sciences, Little Chalfont, UK). Immunocomplexes for GAPDH were used as an internal control for the normalization of protein expression. Western blot analyses were carried out at least twice for each experiment. The quantification of immunocomplexes was carried out by densitometry performed using Kodak ID Image Analysis Software (Eastman Kodak Company, Rochester, NY, USA).
Mouse mammary xenografts
All in vivo mouse xenograft experiments were performed under the #07–029 protocol approved by the University of Arizona Institutional Animal Care and Use Committee approved on 02/22/2016. All procedures were performed in compliance with the standard operating procedures and relevant guidelines of the University of Arizona Animal Care. MCF7 cells (7.5–10×106 cells in 50 µl of Matrigel resuspension) pre-cultured for 4 weeks in control DMEM/F12 media plus 10% FCS (MCF7 Control) or DMEM/F12 plus 10% FCS with 1 µM NaAsIII (MCFAsIII) were injected into the left number-4 mammary fat pad of 4-week-old (19–22 g) ovariectomized (OVX) athymic rTac:NCr-Foxn1 nude female mice (Taconic Biosciences, Rensselaer, NY, USA) implanted with an estradiol pellet (0.72 mg, 60 days release; Innovative Research of America, Sarasota, FL, USA). After 30 days, the mice injected were with MCF7 control or MCF7NaAsIII cells were implanted with TAM pellets (5 mg, 60 days release; Innovative Research of America). Mice (10 animals/group × 4 experimental groups, 40 animals in total) were housed in conventional pathogen-free cages under a 12 h light/12 h dark cycle, at 20–22°C, and 50–55% humidity with free access to Teklad Global Rodent Diet (Harlan Laboratories, Madison, WI, USA) and tap water. The animals were sacrificed at 60 days after the start of TAM treatment. Tumor growth was measured once/week with a caliper until there were visible signs of tumor growth, then twice/week until the end of the study. Tumor volume was estimated using the following formula: [(width)2 × length]/2]. Tumor tissue was snap-frozen in liquid nitrogen and stored at −80°C for further analysis.
Statistical analysis
Data were analyzed by ANOVA as previously described (38). Post-hoc multiple comparisons among all means were conducted using Tukey's Test after main effects and interactions were found to be significant at P≤0.05. Data are presented as the means ± SEM and statistical differences highlighted with different letters for multiple comparisons (a>b>c, etc.) or asterisks when compared to the control.
Results
NaAsIII reduces the expression of BRCA1 via CpG hypermethylation in ERα-positive breast cancer cells
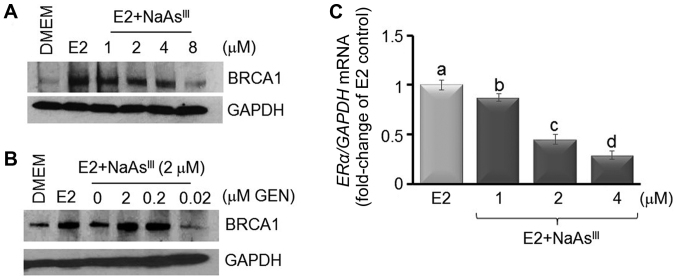
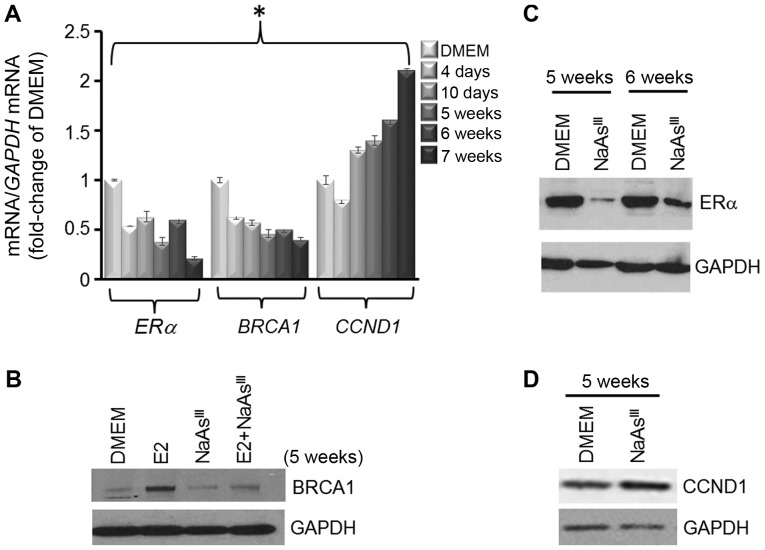
Previously (38–40), we reported that the expression of BRCA1 was stimulated by E2 in ER-positive MCF7 breast cancer cells (38). In this study, using western blot analysis (Fig. 1A), we observed that E2-induced BRCA1 expression was antagonized by NaAsIII, starting at the 1 µM concentration, and to a larger degree upon co-treatment with higher doses of NaAsIII (2 to 8 µM). As a control, we co-treated MCF7 cells with NaAsIII (2 µM) plus various doses (0.02, 0.2 and 2.0 µM) of the isoflavone GEN, which was found in our previous study to induce BRCA1 expression (38). Co-treatment with 0.2 and 2 µM GEN reversed the repressive effects of NaAsIII on BRCA1 expression (Fig. 1B). Based on the information that BRCA1 transcription is regulated by the ERα (40), changes in the expression of ERα mRNA were analyzed by qPCR in MCF7 cells treated for 72 h with various doses of NaAsIII. Compared to the E2 control, treatment with 1 µM NaAsIII decreased ERα mRNA expression by ~15%, which was further decreased (55–70%) by higher concentrations (2 and 4 µM) of NaAsIII (Fig. 1C). Based on these dose-response results, we examined the long-term effects of exposure to 1 µM NaAsIII, which approximates levels of AsIII measured in drinking water of populations residing in the US (41) and other geographical regions (42–44). MCF7 cells were cultured for various periods of time (4 days to 7 weeks) either as control DMEM cells or in the presence of 1 µM NaAsIII, which reduced the expression of ERα and BRCA1 mRNA (Fig. 2A). In parallel, the expression of CCDN1 was reduced by ~ 25% within 4 days post-treatment with NaAsIII, whereas the CCND1 levels were enhanced by longer exposure to NaAsIII. Western blot analysis confirmed that long-term (5 weeks) exposure to NaAsIII had repressive effects on E2-induced BRCA1 (Fig. 2B) and ERα (Fig. 2C), but induced the expression of CCND1.
Figure 1.
AsIII reduces the expression of BRCA1 and ERα. (A) MCF7 cells were cultured for 72 h in control DMEM, or DMEM plus E2 (10 nM) alone or various concentrations of NaAsIII as described in the Materials and methods. In (B) MCF7 cells were co-treated for 72 h with E2 plus 2 µM NaAsIII and various concentrations (0.02, 0.2 and 2.0 µM) of GEN. Bands are representative immunocomplexes for BRCA1 and internal standard GAPDH from two (n=2) separate experiments performed in duplicate. (C) Bars represent the means ± SEM of ERα mRNA expression (fold-change of E2 Control) from 2 separate experiments (n=2) performed in triplicate. Different letters indicate statistically significant multiple comparison (a>b>c>d) differences (P<0.05). AsIII, trivalent arsenite; BRCA1, breast cancer 1; ERα, estrogen receptor-α; E2, 17β-estradiol; NaAsIII, sodium arsenite; GEN, genistein.
Figure 2.
Long-term exposure to AsIII reduces expression of BRCA1 and ERα. MCF7 cells were cultured for various periods of time (4 days to 7 weeks) in control DMEM, or DMEM plus 1 µM NaAsIII. (A) Bars represent the means ± SEM of ERα, BRCA1 and CCND1 mRNA expression (fold-change of DMEM Control) from two separate experiments (n=2) performed in triplicate. Asterisk indicates statistically significant differences (P<0.05) compared to the DMEM control. (B-D) Bands are representative immunocomplexes for BRCA1, ERα, CCND1 and internal standard GAPDH from 2 (n=2) separate experiments performed in duplicate. AsIII, trivalent arsenite; BRCA1, breast cancer 1; ERα, estrogen receptor-α; NaAsIII, sodium arsenite; CCND1, cyclin D1.
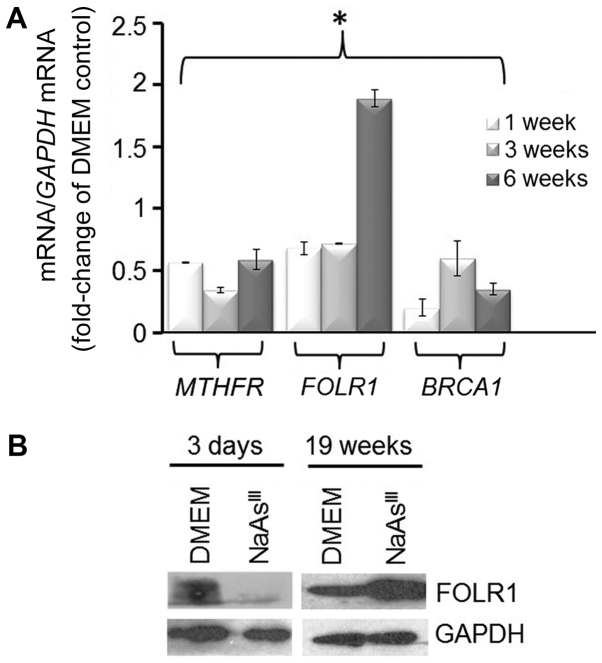
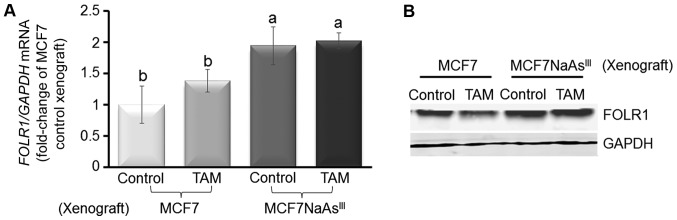
It has previously been documented (45) that AsIII treatment decreases the expression of MTHFR, an enzyme involved in one-carbon metabolism. Analysis of MTHFR expression by RT-qPCR (Fig. 3A) showed that 1 to 6 weeks exposure of MCF7 cells to 1 µM NaAsIII reduced markedly (~50%) MTHFR mRNA. The treatment with NaAsIII had a biphasic effect on expression of FOLR1 mRNA, which was reduced at 1 and 3 weeks, but induced at 6 weeks, of exposure. FOLR1 participates in cellular uptake of 5-methyltetrahydrofolate into cells, and its overexpression has been linked to poor prognosis in particular in triple-negative breast cancers (TNBC) (46). As an additional control, we confirmed the repressive effects on BRCA1 mRNA expression by treatment of the MCF7 cells with NaAsIII by RT-qPCR. As another control, we also examined the expression of FOLR1 protein and found that exposure to NaAsIII reduced its expression within 3 days, although it had a stimulatory effect long-term (19 weeks) (Fig. 3B).
Figure 3.
Long-term exposure to AsIII induces the expression of FOLR1. (A) Bars represent the means ± SEM of MTHFR, FOLR1 and BRCA1 mRNA expression (fold-change of DMEM Control) from 2 separate experiments (n=2) performed in triplicate. Asterisk indicates statistically significant differences (P<0.05) compared to the DMEM control. (B) Bands are representative immunocomplexes for FOLR1 and internal standard GAPDH from two (n=2) separate experiments performed in duplicate. AsIII, trivalent arsenite; BRCA1, breast cancer 1; MTHFR, methylenetetrahydrofolate reductase; FOLR1, folate receptor 1.
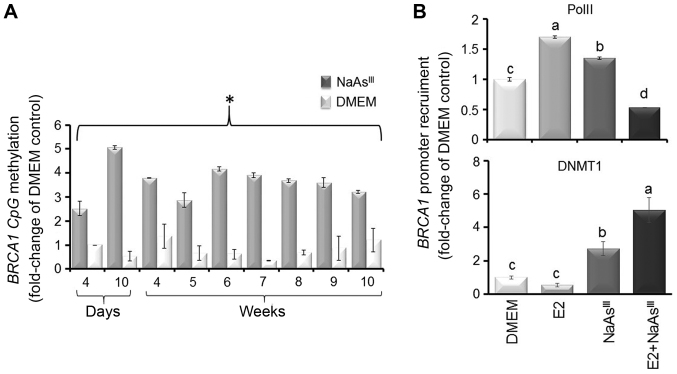
One mechanism through which NaAsIII may lower BRCA1 expression is epigenetic silencing involving DNA methylation. The analysis of bisulfonated genomic DNA prepared from the MCF7 cells revealed that exposure to 1 µM NaAsIII from 4 days to 10 weeks brought about an increase (2.5- to 5-fold) in BRCA1 CpG methylation (Fig. 4A), which was associated at 6 days post-treatment with a reduction in the recruitment of PolII to the BRCA1 promoter and increased occupancy by DNMT1 (Fig. 4B). These results suggested that the NaAsIII-dependent downregulation of BRCA1 was associated with the reduced transcription and recruitment of DNA-modifying enzymes (i.e., DNMT1) to the BRCA1 gene.
Figure 4.
AsIII induces BRCA1 CpG methylation. MCF7 cells were cultured in control DMEM or DMEM plus 1 µM NaAsIII. Bars represent the means ± SEM of fold-change of DMEM Control for (A) BRCA1 CpG methylation (4 days to 10 weeks) and (B) PolII and DNMT1 recruitment (6 days) by ChIP assay at the BRCA1 gene from 2 separate experiments (n=2) performed in triplicate. (A) Asterisk or (B) different letters indicates statistically significant multiple comparison (a>b>c>d) differences (P<0.05) compared to the DMEM control. AsIII, trivalent arsenite; BRCA1, breast cancer 1; NaAsIII, sodium arsenite; PolII, polymerase II.
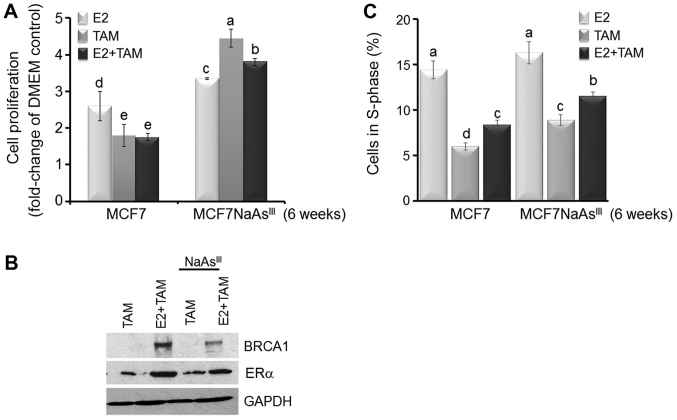
NaAsIII disrupts the response to TAM in MCF7 cells in culture and in mouse mammary tumor xenografts
The observed reduction in ERα expression depicted in Figs. 1 and 2 raised the question as to whether NaAsIII exposure influences E2-induced cell proliferation and response to TAM. The results presented in Fig. 5 indicated that treatment of the MCF7 cells with TAM for 72 h reduced E2-induced cell growth. Conversely, in the MCF7 cells pre-treated for 6 weeks with 1 µM NaAsIII, treatment with TAM increased cell proliferation (Fig. 5A). The results of western blot analysis indicated that pre-treatment with NaAsIII for 6 weeks antagonized E2-induced BRCA1 expression, while it reduced ERα expression, a known target for TAM (Fig. 5B). The analysis of cell cycle distribution by flow cytometry revealed that a larger percentage of cells co-treated for 6 weeks with NaAsIII plus TAM or E2 plus TAM were positioned in the S-phase of the cell cycle compared to the control MCF7 cells (Fig. 5C). These cumulative results suggested that long-term exposure to environmentally relevant doses (1 µM) of NaAsIII increased the resistance of MCF7 cells to TAM through the downregulation of ERα.
Figure 5.
AsIII antagonizes the TAM-dependent inhibition of proliferation. MCF7 cells and MCF7 cells pre-treated for 6 weeks in the presence of 1 µM NaAsIII (MCF7NaAsIII) were cultured for 72 h in control DMEM, or DMEM plus E2 (10 nM), TAM (1 µM), or their combination. (A) Bars represent the means ± SEM of quantitation (fold-change of DMEM Control) of proliferation determined by MTT assay from 2 separate experiments (n=2) with 5 replicates. (B) Bands are representative immunocomplexes for BRCA-1, ERα and internal standard GAPDH from 2 (n=2) separate experiments performed in duplicate. (C) Bars represent the means ± SEM of percentage cells in S-phase measured by flow cytometry from two separate experiments (n=2) with 5 replicates. In (A) and (C) different letters represent statistically significant multiple comparison (a>b>c, etc.) differences (P<0.05). AsIII, trivalent arsenite; BRCA1, breast cancer 1; NaAsIII, sodium arsenite; TAM, tamoxifen; E2, 17β-estradiol.
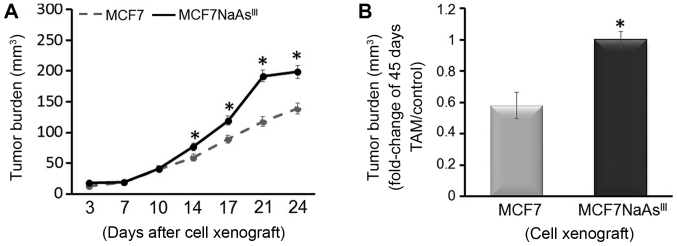
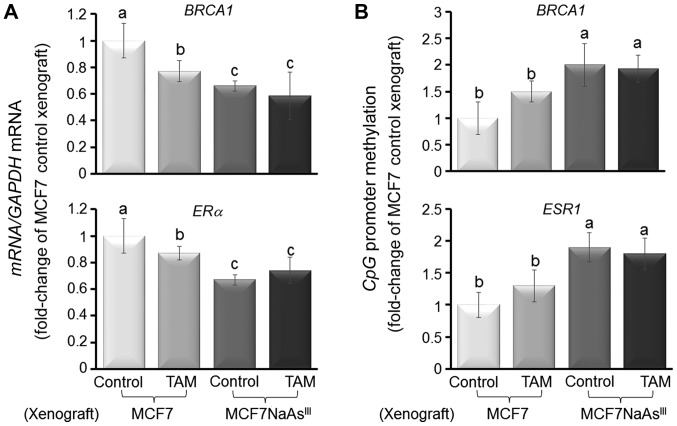
To further investigate the influence of NaAsIII exposure on tumor development, we injected control MCF7 cells or MCF7 cells pre-treated with 1 µM NaAsIII for 4 weeks (MCF7 NaAsIII) into the cleared mammary fat pad of 4-week-old OVX athymic rTac:NCr-Foxn1 nude female mice also implanted with an E2 pellet. We then monitored tumor growth for 24 days and noted a higher tumor volume for mice injected with MCF7 NaAsIII compared to mice xenografted with control MCF7 cells (Fig. 6A). Subsequently, the xenografted mice were implanted with a TAM pellet and tumors were allowed to grow for an additional 45 days. Mammary tumors that originated from xenografted MCF7 NaAsIII cells were more refractory (~40%) to TAM treatment compared with tumors that developed from control MCF7 cells (Fig. 6B). The resilience of MCF7 NaAsIII tumors to TAM was coupled with the reduced expression of BRCA1 and ERα mRNA (Fig. 7A), and increased CpG methylation of the respective genes (i.e., BRCA1 and ESR1) (Fig. 7B). As a control, we measured the expression of FOLR1 mRNA (Fig. 8A) and FOLR1 protein (Fig. 8B), which were increased (~1.0-fold) in mammary tumors from xenografted MCF7 NaAsIII cells compared to tumors that developed from control MCF7 cells. Taken together, the results of the tumor xenograft experiments indicated that exposure to NaAsIII conferred the resistance of mammary tumors to TAM and that this resilience was associated with the hypermethylation of BRCA1 and ESR1, the reduced expression of BRCA1 and ERα, and increased levels of FOLR1 mRNA and tumor burden.
Figure 6.
AsIII promotes growth of MCF7 cell mammary xenografts and antagonizes the anti-proliferative effects of TAM. MCF7 and MCF7 cells pre-cultured for 4 weeks in DMEM plus 1 µM NaAsIII (MCF7NaAsIII) were xenografted into the mammary fat pad of OVX nude mice implanted with E2 pellets as described in the Materials and methods. Tumors were allowed to grow for 24 days, after which mice were implanted with TAM pellets. (A) Tumor burden (mm3) was measured up to 24 days post-xenograft. (B) Tumor burden (mm3 fold-change of TAM/Control) was measured at 45 days after the implantation of TAM pellets. Bars are the means ± SEM from 5 animals/group from 2 separate experiments (n=10). Asterisks represent statistically significant differences (P<0.05) compared to MCF7 control xenografts. TAM, tamoxifen; NaAsIII, sodium arsenite.
Figure 7.
AsIII induces BRCA1 and ESR1 CpG methylation in MCF7 cell mammary tumor xenografts. Bars are from 5 animals/group from 2 separate experiments (n=10) and represent the means (fold-change of MCF7 Control xenograft) ± SEM for (A) BRCA1 and ERα mRNA expression; (B) BRCA1 and ESR1 CpG methylation. Different letters represent statistically significant multiple comparison (a>b>c) differences (P<0.05). AsIII, trivalent arsenite; BRCA1, breast cancer 1; ESR1, estrogen receptor 1; ERα, estrogen receptor-α.
Figure 8.
AsIII induces expression of FOLR1 in MCF7 cell mammary tumor xenografts. (A) FOLR1 mRNA expression in MCF7 and MCF7NaAsIII cell mammary tumor xenografts. Bars are from 5 animals/group from two separate experiments (n=10) and represent means (fold-change of MCF7 Control xenograft) ± SEM. Different letters represent statistically significant multiple comparison (a>b) differences (P<0.05). (B) Bands are representative immunocomplexes for FOLR1 and internal standard GAPDH from 2 (n=2) separate experiments performed in duplicate. AsIII, trivalent arsenite; BRCA1, breast cancer 1; FOLR1, folate receptor 1.
Discussion
The loss of ERα expression has been linked to a poor response to endocrine therapy (16–18). Drinking water contaminated with arsenic has been linked to the disruption of ERα signaling (19) and arsenic exposure has been shown to contribute to genomic instability through the disruption of BRCA1-regulated DNA repair (20). Arsenic may accelerate cancer growth (24) and confer a steroid receptor-independent phenotype (29). These cumulative observations suggest arsenic exposure may interfere with endocrine regulation and prompted our investigation into whether or not AsIII contributes to resistance to TAM therapy through the silencing of BRCA1 and ESR1. In this study, we first examined the in vitro effects of NaAsIII in ERα-positive breast cancer cells and found that acute (within 4 days) and long-term (10 days to 7 weeks) exposure to environmentally relevant doses of AsIII reduced BRCA1 expression. Furthermore, NaAsIII compromised ERα expression and the in vitro response of MCF7 cells to treatment with TAM. In normal breast epithelial cells, the BRCA1 and ESR1 (encoding for ERα) genes are regulated through a positive feedback loop in which ERα induces expression of BRCA1 in the presence of E2 (40). In turn, BRCA1 transcriptionally activates the ESR1 gene (47). This crosstalk between BRCA1 and ERα is thought to favor DNA repair controlled by BRCA1 before cells progress through division under the proliferative pressure of estrogens. Conversely, in BRCA1 mutation and sporadic breast tumors, the reduced expression of BRCA1, also termed 'BRCAness', is usually associated with the reduced expression of ERα and resistance to TAM (48). Our cell culture data suggested that exposure to NaAsIII may compromise BRCA1 expression and confer resistance to antagonists of the ERα such as TAM. The results of this study are in agreement with those of a previous study (49) showing that environmentally relevant doses of NaAsIII (~1–5 µM) reduced the expression of the ERα.
A mechanism that may contribute to the NaAsIII-dependent loss of BRCA1 is epigenetic silencing via CpG methylation, which has been documented in sporadic breast tumors, particularly in those that are more invasive (i.e., TNBC) compared to lobulo-alveolar breast cancers (50). In this study, we documented that in MCF7 cells both the short- (4 days) and long- (10 days to 10 weeks) term in vitro exposure to NaAsIII induced BRCA1 CpG methylation was associated with the increased recruitment of DNMT1 and the loss of PolII at the BRCA1 gene. These observations are in accordance with those of a previous study reporting promoter hypermethylation and silencing of other DNA repair (MLH1 and MSH2) genes in arsenic-exposed populations (51). The reprogramming of DNA methylation elicited by NaAsIII has been previously linked to increased growth rate (52). In keeping with these earlier reports, in this study, we noted that MCF7 treated for 6 weeks with NaAsIII displayed increased proliferative capacity and were refractory to TAM.
The injection of NaAsIII-preconditioned MCF7 cells into the mammary fat pad of nude mice provided in vivo evidence that the prior exposure to NaAsIII may alter the behavior of ERα-positive breast cancer cells. Xenografted MCF7NaAsIII cells produced a larger tumor volume compared to control MCF7 cells and were more refractory to treatment with TAM. We attributed this resilience of MCF7NaAsIII to TAM, at least in part, to the reduced expression of ERα associated with the CpG hypermethylation of ESR1. The reduced expression of ERα in MCF7NaAsIII tumors was paralleled by the lower expression and hypermethylation of BRCA1, further supporting the hypothesis that exposure to NaAsIII may contribute to breast tumorigenesis by hampering DNA repair capacity controlled by BRCA1 and altering the crosstalk between BRCA1 and ERα.
In agreement with previous findings (45), we noted that the expression of MTHFR in MCF7 cells treated in vitro with NaAsIII was markedly downregulated. Thus, exposure to inorganic arsenic may deplete the pool of methyl groups and interfere with folate metabolism with consequences on DNA synthesis and repair. The reduced expression of MTHFR has been previously associated with breast cancer development (53). Conversely, in this study, we noted in MCF7 cells in culture that exposure to NaAsIII had a biphasic effect on the expression of FOLR1, a membrane-bound protein involved in transport of folate into cells. Short-term exposure to NaAsIII reduced FOLR1 expression, whereas a stimulatory effect on FOLR1 levels was observed after long-term exposure. The upregulation of FOLR1 was confirmed in mammary tumors that developed from xenografted MCF7NaAsIII cells. The upregulation of FOLR1 has been interpreted as an adaptive response triggered by cellular depletion of methyl groups by metabolism of NaAsIII (45). Moreover, recent studies reported that the increased expression of FOLR1 was associated with a higher risk of recurrence in patients with TNBC (54), which were significantly enriched in FOLR1 compared to ERα- and human epidermal growth factor receptor 2-positive breast tumors (46). Whereas it remains unknown whether NaAsIII affects expression of MTHFR and FOLR1 through epigenetic mechanisms, a possible translational implication of our data is that breast cancer patients exposed to NaAsIII and undergoing treatment with TAM may benefit from combination therapy with anti-FOLR1 agents (54).
Taken together, the data of the present study provide novel in vitro and mammary tumor xenograft evidence that exposure to inorganic trivalent arsenic, such as NaAsIII may increase resistance to endocrine therapy based on TAM through reduction in BRCA1 and ERα expression. Future studies with ERα-positive breast cancer patients residing in geographical regions at high risk of exposure to AsIII are warranted to investigate whether the dysregulation of CpG hypermethylation of BRCA1 and ESR1 causes persistent genomic instability (55), and variations in efficacy of therapies based on antagonists of the ERα. As DNA methylation changes are potentially reversible, they may offer a novel target for combination therapies of ER-positive breast tumors with epigenetic drugs.
Acknowledgments
The authors wish to acknowledge the support of The Experimental Mouse Shared Resource Core of the University of Arizona Cancer Center.
Abbreviations
- AsIII
trivalent arsenite
- AsV
pentavalent arsenate
- BRCA1
breast cancer 1
- CCND1
cyclin D1
- ChIP
chromatin immunoprecipitation
- DMEM/F12
Dulbecco's modified Eagle's/F12 medium
- DNMT1
DNA methyltransferase 1
- E2
17β-estradiol
- ERα
estrogen receptor-α
- FCS
fetal calf serum; FOLR1, folate receptor 1
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GEN
genistein
- M
methylated-specific primers
- MTHFR
methylenetetrahydrofolate reductase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NaAsIII
sodium arsenite
- OVX
ovariecromized
- PBS
phosphate-buffered saline
- PolII
RNA polymerase II
- PR
progesterone receptor
- qPCR
quantitative polymerase chain reaction
- TAM
tamoxifen
- TNBC
triple-negative breast cancers
- U
unmethylated-specific primers
Funding
This study was supported by a Pilot Project grant from the National Cancer Institute of the National Institutes of Health under the award for the Partnership of Native American Cancer Prevention U54CA143924 (UACC); Cancer Biology Training Grant T32CA009213; and Cancer Center Support Grant P30CA023074.
Availability of data and materials
All data generated during this study are included in this published article.
Authors' contributions
OIS and DFR conceived the study and drafted the manuscript. MGD contributed to laboratory experiments, data analysis, and writing of the manuscript. OIS conducted cellular and molecular measurements with cell lines and tumor xenografts. BS and GDPM contributed to designing and performing the xenograft experiments and review of data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All mouse xenograft experiments were performed under the #07–029 protocol approved by the University of Arizona Institutional Animal Care and Use Committee approved on 02/22/2016. All procedures were performed in compliance with the standard operating procedures and relevant guidelines of the University of Arizona Animal Care.
Patient consent for publication
Not applicable
Competing interests
The authors declare they have no competing interests.
References
- 1.Ayotte JD, Belaval M, Olson SA, Burow KR, Flanagan SM, Hinkle SR, Lindsey BD. Factors affecting temporal variability of arsenic in groundwater used for drinking water supply in the United States. Sci Total Environ. 2015;505:1370–1379. doi: 10.1016/j.scitotenv.2014.02.057. [DOI] [PubMed] [Google Scholar]
- 2.Sorg TJ, Chen AS, Wang L. Arsenic species in drinking water wells in the USA with high arsenic concentrations. Water Res. 2014;48:156–169. doi: 10.1016/j.watres.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Agusa T, Trang PT, Lan VM, Anh DH, Tanabe S, Viet PH, Berg M. Human exposure to arsenic from drinking water in Vietnam. Sci Total Environ. 2014;488–489:562–569. doi: 10.1016/j.scitotenv.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Dummer TJ, Yu ZM, Nauta L, Murimboh JD, Parker L. Geostatistical modelling of arsenic in drinking water wells and related toenail arsenic concentrations across Nova Scotia, Canada. Sci Total Environ. 2015;505:1248–1258. doi: 10.1016/j.scitotenv.2014.02.055. [DOI] [PubMed] [Google Scholar]
- 5.George CM, Sima L, Arias MH, Mihalic J, Cabrera LZ, Danz D, Checkley W, Gilman RH. Arsenic exposure in drinking water: An unrecognized health threat in Peru. Bull World Health Organ. 2014;92:565–572. doi: 10.2471/BLT.13.128496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, Navas-Acien A. Arsenic exposure and cancer mortality in a US-based prospective cohort: The strong heart study. Cancer Epidemiol Biomarkers Prev. 2013;22:1944–1953. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saint-Jacques N, Parker L, Brown P, Dummer TJ. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health. 2014;13:44. doi: 10.1186/1476-069X-13-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentry PR, Clewell HJ, III, Greene TB, Franzen AC, Yager JW. The impact of recent advances in research on arsenic cancer risk assessment. Regul Toxicol Pharmacol. 2014;69:91–104. doi: 10.1016/j.yrtph.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Stoica A, Pentecost E, Martin MB. Effects of arsenite on estrogen receptor-alpha expression and activity in MCF-7 breast cancer cells. Endocrinology. 2000;141:3595–3602. doi: 10.1210/endo.141.10.7704. [DOI] [PubMed] [Google Scholar]
- 10.Ren X, McHale CM, Skibola CF, Smith AH, Smith MT, Zhang L. An emerging role for epigenetic dysregulation in arsenic toxicity and carcinogenesis. Environ Health Perspect. 2011;119:11–19. doi: 10.1289/ehp.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentry PR, McDonald TB, Sullivan DE, Shipp AM, Yager JW, Clewell HJ., III Analysis of genomic dose-response information on arsenic to inform key events in a mode of action for carcinogenicity. Environ Mol Mutagen. 2010;51:1–14. doi: 10.1002/em.20505. [DOI] [PubMed] [Google Scholar]
- 12.Kijima I, Itoh T, Chen S. Growth inhibition of estrogen receptor-positive and aromatase-positive human breast cancer cells in monolayer and spheroid cultures by letrozole, anastrozole, and tamoxifen. J Steroid Biochem Mol Biol. 2005;97:360–368. doi: 10.1016/j.jsbmb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Khanjani N, Jafarnejad AB, Tavakkoli L. Arsenic and breast cancer: A systematic review of epidemiologic studies. Rev Environ Health. 2017;32:267–277. doi: 10.1515/reveh-2016-0068. [DOI] [PubMed] [Google Scholar]
- 14.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 15.Schiff R, Massarweh S, Shou J, Osborne CK. Breast cancer endocrine resistance: how growth factor signaling and estrogen receptor coregulators modulate response. Clin Cancer Res. 2003;9(1 Pt 2):447S–454S. [PubMed] [Google Scholar]
- 16.Johnston SR, Saccani-Jotti G, Smith IE, Salter J, Newby J, Coppen M, Ebbs SR, Dowsett M. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res. 1995;55:3331–3338. [PubMed] [Google Scholar]
- 17.Kuukasjärvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol. 1996;14:2584–2589. doi: 10.1200/JCO.1996.14.9.2584. [DOI] [PubMed] [Google Scholar]
- 18.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz A, Chervona Y, Hall M, Kluz T, Gamble MV, Costa M. Sex-specific patterns and deregulation of endocrine pathways in the gene expression profiles of Bangladeshi adults exposed to arsenic contaminated drinking water. Toxicol Appl Pharmacol. 2015;284:330–338. doi: 10.1016/j.taap.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peremartí J, Ramos F, Marcos R, Hernández A. Arsenic exposure disrupts the normal function of the FA/BRCA repair pathway. Toxicol Sci. 2014;142:93–104. doi: 10.1093/toxsci/kfu159. [DOI] [PubMed] [Google Scholar]
- 21.Parodi DA, Greenfield M, Evans C, Chichura A, Alpaugh A, Williams J, Martin MB. Alteration of mammary gland development and gene expression by in utero exposure to arsenic. Reprod Toxicol. 2015;54:66–75. doi: 10.1016/j.reprotox.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP. Carcinogenic effects of 'whole-life' exposure to inorganic arsenic in CD1 mice. Toxicol Sci. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: Inorganic arsenic is a transplacental carcinogen in mice. Toxicol Appl Pharmacol. 2004;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Schrauzer GN, White DA, McGinness JE, Schneider CJ, Bell LJ. Arsenic and cancer: Effects of joint administration of arsenite and selenite on the genesis of mammary adenocarcinoma in inbred female C3H/St mice. Bioinorg Chem. 1978;9:245–253. doi: 10.1016/S0006-3061(78)80005-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Hock JM, Sullivan C, Fang G, Cox AJ, Davis KT, Davis BH, Li X. Activation of the p38 MAPK/Akt/ERK1/2 signal pathways is required for the protein stabilization of CDC6 and cyclin D1 in low-dose arsenite-induced cell proliferation. J Cell Biochem. 2010;111:1546–1555. doi: 10.1002/jcb.22886. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Tokar EJ, Waalkes MP. Arsenic-induced cancer cell phenotype in human breast epithelia is estrogen receptor-independent but involves aromatase activation. Arch Toxicol. 2014;88:263–274. doi: 10.1007/s00204-013-1131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong D, Ortega J, Kim C, Huang J, Gu L, Li GM. Arsenic Inhibits DNA Mismatch Repair by Promoting EGFR Expression and PCNA Phosphorylation. J Biol Chem. 2015;290:14536–14541. doi: 10.1074/jbc.M115.641399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharjee P, Banerjee M, Giri AK. Role of genomic instability in arsenic-induced carcinogenicity. A review. Environ Int. 2013;53:29–40. doi: 10.1016/j.envint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Benbrahim-Tallaa L, Webber MM, Waalkes MP. Acquisition of androgen independence by human prostate epithelial cells during arsenic-induced malignant transformation. Environ Health Perspect. 2005;113:1134–1139. doi: 10.1289/ehp.7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardenas A, Houseman EA, Baccarelli AA, Quamruzzaman Q, Rahman M, Mostofa G, Wright RO, Christiani DC, Kile ML. In utero arsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics. 2015;10:1054–1063. doi: 10.1080/15592294.2015.1105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou WC, Chung YT, Chen HY, Wang CJ, Ying TH, Chuang CY, Tseng YC, Wang SL. Maternal arsenic exposure and DNA damage biomarkers, and the associations with birth outcomes in a general population from Taiwan. PLoS One. 2014;9:e86398. doi: 10.1371/journal.pone.0086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui X, Wakai T, Shirai Y, Hatakeyama K, Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci. 2006;91:372–381. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez T, Brocher J, Stopper H, Hock R. Sodium arsenite modulates histone acetylation, histone deacetylase activity and HMGN protein dynamics in human cells. Chromosoma. 2008;117:147–157. doi: 10.1007/s00412-007-0133-5. [DOI] [PubMed] [Google Scholar]
- 35.Lu G, Xu H, Chang D, Wu Z, Yao X, Zhang S, Li Z, Bai J, Cai Q, Zhang W. Arsenic exposure is associated with DNA hyper-methylation of the tumor suppressor gene p16. J Occup Med Toxicol. 2014;9:42. doi: 10.1186/s12995-014-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang TC, Schmitt MT, Mumford JL. Effects of arsenic on telomerase and telomeres in relation to cell proliferation and apoptosis in human keratinocytes and leukemia cells in vitro. Carcinogenesis. 2003;24:1811–1817. doi: 10.1093/carcin/bgg141. [DOI] [PubMed] [Google Scholar]
- 37.Jiang R, Li Y, Zhang A, Wang B, Xu Y, Xu W, Zhao Y, Luo F, Liu Q. The acquisition of cancer stem cell-like properties and neoplastic transformation of human keratinocytes induced by arsenite involves epigenetic silencing of let-7c via Ras/NF-κB. Toxicol Lett. 2014;227:91–98. doi: 10.1016/j.toxlet.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Romagnolo DF, Donovan MG, Papoutsis AJ, Doetschman TC, Selmin OI. Genistein prevents BRCA1 CpG methylation and proliferation in human breast cancer cells with activated aromatic hydrocarbon receptor. Curr Dev Nutr. 2017;1:e000562. doi: 10.3945/cdn.117.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papoutsis AJ, Borg JL, Selmin OI, Romagnolo DF. BRCA-1 promoter hypermethylation and silencing induced by the aromatic hydrocarbon receptor-ligand TCDD are prevented by resveratrol in MCF-7 cells. J Nutr Biochem. 2012;23:1324–1332. doi: 10.1016/j.jnutbio.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Jeffy BD, Hockings JK, Kemp MQ, Morgan SS, Hager JA, Beliakoff J, Whitesell LJ, Bowden GT, Romagnolo DF. An estrogen receptor-alpha/p300 complex activates the BRCA-1 promoter at an AP-1 site that binds Jun/Fos transcription factors: Repressive effects of p53 on BRCA-1 transcription. Neoplasia. 2005;7:873–882. doi: 10.1593/neo.05256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lothrop N, Wilkinson ST, Verhougstraete M, Sugeng A, Loh MM, Klimecki W, Beamer PI. Home Water Treatment Habits and Effectiveness in a Rural Arizona Community. Water. 2015;7:1217–1231. doi: 10.3390/w7031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hough RL, Fletcher T, Leonardi GS, Goessler W, Gnagnarella P, Clemens F, Gurzau E, Koppova K, Rudnai P, Kumar R, et al. Lifetime exposure to arsenic in residential drinking water in Central Europe. Int Arch Occup Environ Health. 2010;83:471–481. doi: 10.1007/s00420-010-0519-1. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A, Adak P, Gurian PL, Lockwood JR. Arsenic exposure in US public and domestic drinking water supplies: A comparative risk assessment. J Expo Sci Environ Epidemiol. 2010;20:245–254. doi: 10.1038/jes.2009.24. [DOI] [PubMed] [Google Scholar]
- 44.Raessler M. The Arsenic Contamination of Drinking and Groundwaters in Bangladesh: Featuring Biogeochemical Aspects and Implications on Public Health. Arch Environ Contam Toxicol. 2018;75:1–7. doi: 10.1007/s00244-018-0511-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Ramos R, López- Carrillo L, Albores A, Hernández-Ramírez RU, Cebrian ME. Sodium arsenite alters cell cycle and MTHFR, MT1/2, and c-Myc protein levels in MCF-7 cells. Toxicol Appl Pharmacol. 2009;241:269–274. doi: 10.1016/j.taap.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Necela BM, Crozier JA, Andorfer CA, Lewis-Tuffin L, Kachergus JM, Geiger XJ, Kalari KR, Serie DJ, Sun Z, Moreno-Aspitia A, et al. Correction: Folate receptor-α (FOLR1) expression and function in triple negative tumors. PLoS One. 2015;10:e0127133. doi: 10.1371/journal.pone.0127133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosey AM, Gorski JJ, Murray MM, Quinn JE, Chung WY, Stewart GE, James CR, Farragher SM, Mulligan JM, Scott AN, et al. Molecular basis for estrogen receptor alpha deficiency in BRCA1-linked breast cancer. J Natl Cancer Inst. 2007;99:1683–1694. doi: 10.1093/jnci/djm207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lips EH, Mulder L, Oonk A, van der Kolk LE, Hogervorst FB, Imholz AL, Wesseling J, Rodenhuis S, Nederlof PM. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer. 2013;108:2172–2177. doi: 10.1038/bjc.2013.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakareangrit W, Thiantanawat A, Visitnonthachai D, Watcharasit P, Satayavivad J. Sodium arsenite inhibited genomic estrogen signaling but induced pERα (Ser118) via MAPK pathway in breast cancer cells. Environ Toxicol. 2016;31:1133–1146. doi: 10.1002/tox.22122. [DOI] [PubMed] [Google Scholar]
- 50.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8:R38. doi: 10.1186/bcr1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bhattacharjee P, Sanyal T, Bhattacharjee S, Bhattacharjee P. Epigenetic alteration of mismatch repair genes in the population chronically exposed to arsenic in West Bengal, India. Environ Res. 2018;163:289–296. doi: 10.1016/j.envres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Riedmann C, Ma Y, Melikishvili M, Godfrey SG, Zhang Z, Chen KC, Rouchka EC, Fondufe-Mittendorf YN. Inorganic Arsenic-induced cellular transformation is coupled with genome wide changes in chromatin structure, transcriptome and splicing patterns. BMC Genomics. 2015;16:212. doi: 10.1186/s12864-015-1295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zara-Lopes T, Gimenez-Martins AP, Nascimento-Filho CH, Castanhole-Nunes MM, Galbiatti-Dias AL, Padovani-Júnior JA, Maniglia JV, Francisco JL, Pavarino EC, Goloni-Bertollo EM. Role of MTHFR C677T and MTR A2756G polymorphisms in thyroid and breast cancer development. Genet Mol Res. 2016;15:gmr8222. doi: 10.4238/gmr.15028222. [DOI] [PubMed] [Google Scholar]
- 54.Ginter PS, McIntire PJ, Cui X, Irshaid L, Liu Y, Chen Z, Shin SJ. Folate receptor alpha expression is associated with increased risk of recurrence in triple-negative breast cancer. Clin Breast Cancer. 2017;17:544–549. doi: 10.1016/j.clbc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Mauro M, Caradonna F, Klein CB. Dysregulation of DNA methylation induced by past arsenic treatment causes persistent genomic instability in mammalian cells. Environ Mol Mutagen. 2016;57:137–150. doi: 10.1002/em.21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this published article.