Abstract
Polymerase (Pol) III dependent transcription controls the abundance of transfer RNAs, 5S ribosomal RNA and small non coding RNAs within cells, and is known to serve an essential role in the maintenance of intracellular homeostasis. However, its contribution to cancer progression has not been extensively explored. The present study demonstrated that the evolutionarily conserved MAF1 homolog, negative regulator of RNA Pol III (MAF1) may be closely associated with malignant potential and poor prognosis in colorectal cancer (CRC). Notably, immunohistochemical analysis of 146 CRC surgical specimens revealed that high expression levels of MAF1 were associated with advanced tumor depth, lymph node metastasis, distant metastasis and poor prognosis. In vitro loss of function assays revealed that MAF1 knockdown suppressed chemoresistance and migration of CRC cancer cells. Furthermore, detailed analysis of an independent CRC dataset (n=615) demonstrated that the prognostic impact of MAF1 gene expression was particularly marked in microsatellite instability (MSI)-positive patients, who benefit from immune checkpoint blockade. High expression levels of MAF1 were revealed to be an independent prognostic indicator in MSI-positive CRC. These findings suggested that MAF1 may have an essential role in CRC progression, particularly in MSI positive cases.
Keywords: MAF1, colorectal cancer, polymerase III, microsatellite instability, regulatory T cells
Introduction
Colorectal cancer (CRC) is the third most common type of cancer in men and the second most common in women world wide; every year, ~1.4 million new cases of CRC are diagnosed and it is responsible for ~700,000 cases of mortality (1,2). Although screening and multidisciplinary treatment has improved therapeutic outcomes in several countries, due to its high incidence, CRC remains a major healthcare challenge worldwide. Therefore, specific biomarkers for predicting treatment outcomes, as well as key target molecules responsible for cancer progression, need to be identified.
Transcriptional activity is frequently dysregulated in cancer, due to genomic and epigenetic alterations. Polymerase (Pol) II is responsible for the transcription of mRNAs, including oncogenes, and other Pol enzymes, including Pol I and III, are also involved in cancer progression via ribosomal RNA (rRNA) and transfer RNA (tRNA) biosynthesis (3,4). Although transcription of protein coding genes is Pol II dependent, oncogenes and tumor suppressors, including MYC, phosphoinositide 3 kinase and phosphatase and tensin homolog directly or indirectly activate or inhibit transcription by Pol I and III, thus resulting in altered rRNA production, which is required for rapid cell growth (5).
The present study focused on MAF1 homolog, negative regulator of RNA Pol III (MAF1). Although MAF1 has been demonstrated to inhibit Pol III dependent transcription, a certain number of Pol II dependent genes are also considered to be important targets of this gene (6,7). For example, previous studies demonstrated a conserved function of MAF1 in the maintenance of intracellular lipid pools, through regulation of fatty acid synthase and acetyl coA carboxylase expression (8,9). These findings suggest that MAF1 may not only be involved in a cell growth through rRNA and tRNA mediated post transcriptional regulation, but may also affect various biological and pathological processes, including malignant potential of cancer.
Previous studies have reported the tumor suppressive effect of MAF1 in certain solid malignancies (7,8,10). However, the clinical significance has not been investigated in CRC, which has a different genetic background and immune environment. The present study used surgical specimens and a large scale, multi layered open database to investigate the clinical significance of MAF1 in CRC. Furthermore, the association between MAF1 expression in cancer cells and tumor immunity, which is an important factor for predicting prognosis, was explored.
Materials and methods
Clinical samples
Primary CRC specimens were collected from 146 patients who underwent surgery at the Department of Gastroenterological Surgery, Osaka University (Suita, Japan) between January 2011 and December 2012. All patients were clearly diagnosed with CRC, based on the clinicopathological criteria described by the Japanese Society for Cancer of the Colon and Rectum (11). None of the patients received preoperative chemotherapy or radiotherapy. Specimens were fixed in 10% buffered formalin overnight at room temperature, processed through graded ethanol solutions and embedded in paraffin. The follow-up periods ranged between 1 month and 7 years, with a mean of 4 years. All data, including age, sex, tumor size and depth, lymphatic invasion, lymph node metastasis, vascular invasion, liver metastasis, peritoneal dissemination, distant metastasis and histological grade were obtained from clinical and pathological records. The present study was approved by the Research Ethics Committee of Osaka University (approval ID: 08226) and written informed consent was obtained from all patients included in this study.
Cell lines and cell culture
The human CRC cell lines RKO, HT29, HCT 116, Colo205, SW480 and DLD 1 were purchased from the American Type Culture Collection (Manassas, VA, USA). These cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Waltham, MA, USA) at 37˚C in a humidified incubator containing 5% CO2.
RNA interference
Two types of MAF1-specific small inter fering RNA (siRNA; Sigma Aldrich; Merck KGaA) were used to knockdown MAF1 mRNA. MAF1 siRNAs, or the negative control siRNA, were transfected into RKO and HCT 116 cells, which were seeded at 2×105 cells/well in a 2 ml volume in 6-well flat-bottomed microtiter plates, at a final concentration of 50 nM using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.). These cells were maintained at 37˚C in a humidified incubator containing 5% CO2 for 48 h, according to the manufacturer's protocol. The sequences of siRNAs against MAF1 were as follows: #1, CCACGCUCAAUGAGU CCUUTT; #2, GGCUCAAGCGAAUCGUCUUTT. The MISSION® siRNA Universal Negative Control (cat. no. SIC001; Sigma Aldrich; Merck KGaA) was used as a negative control siRNA.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using TRIzol® RNA Isolation Reagents (Invitrogen; Thermo Fisher Scientific, Inc.) 48 h post-transfection as previously described (12). RNA quality was confirmed (RNA concentra tion >0.5 µg/µl and optical density 260/280=1.8 2.0). cDNA was synthesized from 10 ng total RNA using the ReverTra® Ace qPCR RT Master Mix (Toyobo Life Science, Osaka, Japan), according to the manufacturer's protocol. qPCR was performed using a LightCycler® 2.0 system (Roche Applied Science, Penzberg, Germany) and LightCycler® FastStart DNA Master SYBR Green I (Roche Applied Science). The amplification conditions were as follows: Initial denaturation at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 10 sec, annealing at 60°C for 10 sec and extension at 72°C for 10 sec. Data were normalized to the expression of GAPDH, which was used as an internal control for each experiment. The following primers were used: MAF1, sense 5' ctcacagctgactgtggagact 3', antisense 5' aacatgtgtttgtcgtctc ctg 3'; and GAPDH, sense 5' agccacatcgctcagacac 3' and anti sense 5' gcccaatacgaccaaatcc 3'.
Immunohistochemical staining
Protein expression levels of MAF1 were assessed by immunohistochemical staining of the 146 CRC specimens. The anti MAF1 rabbit antibody (cat. no. HPA058548; Atlas Antibodies AB, Bromma, Sweden) and VECTASTAIN® Elite ABC Rabbit Immunoglobulin G kit (cat. no. PK 6101; Vector Laboratories, Inc., Burlingame, CA, USA) were used for immunohistochemical staining, according to the manufacturer's protocol. Specimens were fixed in 10% buffered formalin overnight at room temperature, processed through graded ethanol solutions and embedded in paraffin. Tissue sections (3.5 µm) were prepared from paraffin-embedded blocks. Following antigen retrieval in 10 mM citrate buffer (pH 6.0) at 115°C for 15 min using Decloaking Chamber™ NxGen (Biocare Medical, Pacheco, CA, USA), and the slides were incubated overnight at 4°C with the primary antibody at 1:300 dilution, followed by incubation at room temperature for 30 min with the secondary antibody at 1:200 dilution. With reference to the Human Protein Atlas (image available from v18. proteinatlas.org/ENSG00000179632MAF1/tissue/esophagus#img), an intensity score of 2 was assigned to nuclei stained as intensely as normal esophageal mucosa, whereas unstained nuclei were assigned a score of 0. Nuclei that exhibited weaker staining than normal esophageal mucosa were assigned a score of 1. In the subsequent analysis, a score of 0 was defined as the MAF1-negative group, and a score of 1 or 2 was defined as the MAF1 positive group. Staining was reviewed by three independent pathologists without the knowledge of patient outcomes. The specimens were visualized on the light field of a confocal microscope BZ X710 (Keyence Corporation, Osaka, Japan) and were analyzed using a BZ X analyzer (v. 1.3.0.3; Keyence Corporation).
Scratch wound healing assay
Cells were seeded at a density of 5×105 cells/well in 6 well plates and were grown to confluence under standard conditions. Briefly, a scratch was generated in the cell layer using a 200 µl pipette tip, and the cells were cultured under standard conditions in DMEM supplemented with only 1% FBS to prevent proliferation. Plates were washed with DMEM supplemented with 1% FBS to remove non adherent cells prior to image capture. Images were captured at 0, 24 and 48 h after scratch generation using a confocal microscope BZ X710 (Keyence Corporation) and were analyzed using a BZ X analyzer (v. 1.3.0.3; Keyence Corporation). Cell migration was evaluated by measuring the average distance between the wound edges at 10 random areas.
Chemosensitivity assay
Cells were seeded at a density of 4×103 cells/well in 96 well plates and were precultured for 24 h. Subsequently, the cells were exposed to various concentrations of 5 fluouracil (5 FU; Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) and oxaliplatin (L OHP; Yakult Honsha Co., Ltd., Tokyo, Japan) at 37°C in a humidified incubator containing 5% CO2 for 72 h. The in vitro cytotoxic effects of 5 FU and L OHP were evaluated using the Cell Counting kit 8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer's protocol. The half maximal inhibi tory concentration values were calculated from the viability data of CRC cells treated with each concentration of L OHP. The results were analyzed using Bioconductor package 'drc' (https://cran.rproject.org/web/packages/drc/index.html) with default settings; this package uses non linear regression models.
Western blot analysis
Total proteins were extracted from cultured cells using radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Thermo Fisher Scientific, Inc.) and concentration was determined using the bicinchoninic acid method. Briefly, 15 µg proteins were separated by 10% SDS-PAGE and were electrob lotted onto polyvinylidene fluoride membranes (Merck KGaA, Darmstadt, Germany) at 300 mA for 60 min. After blocking with 3% skim milk at room temperature for 1 h, these membranes were incubated with primary antibodies against MAF1 (cat. no. HPA058548; Atlas Antibodies AB), poly (ADP ribose) polymerase (PARP), cleaved PARP, caspase 3, cleaved caspase 3 (apoptosis antibody sampler kit; cat. no. 9915; Cell Signaling Technology, Inc., Danvers, MA, USA) and actin (cat. no. A2066; Sigma Aldrich; Merck KGaA) at 4°C overnight. After incubating with secondary antibodies (1:100,000; cat. no. NA934; GE Healthcare, Little Chalfont, UK), the protein bands were detected using the Amersham Enhanced Chemiluminescence Prime Western Blotting Detection Reagent (GE Healthcare).
Data processing
Experiments were conducted in triplicate, and data are presented as the means ± standard deviation. The Cancer Genome Atlas (TCGA) mRNA expression data and clinical information were downloaded from the GDAC Firehose website (http://gdac.broadinstitute.org); the colorectal adenocarcinoma data set (COADREAD) (n=615) was used. Regulatory T cell (Treg) infiltration in each sample was calculated using CIBERSORT (http://cibersort.stanford.edu) (13,14). R programming language v3.5.0, JMP® Pro 13.2.1 (SAS Institute Inc., Cary, NC, USA) and Microsoft® Excel® Version 14.7.1 (Microsoft Corporation, Redmond, WA, USA) were used for analysis. Overall survival (OS) rates and relapse free survival (RFS) rates were calculated according to the Kaplan Meier method and were measured from the day of surgery. Differences between groups were estimated using χ2 test, Student's t test, one way analysis of variance followed by post hoc Dunnett test, or the log rank test. For univariate and multivariate analyses of clinicopathological factors, variables with P<0.05 in the univariate analysis were used in a subsequent multivariate analysis based on the Cox proportional hazards regression model. P<0.05 was considered to indicate a statistically significant difference.
Results
High MAF1 expression is associated with advanced clinicopathological factors and poor prognosis
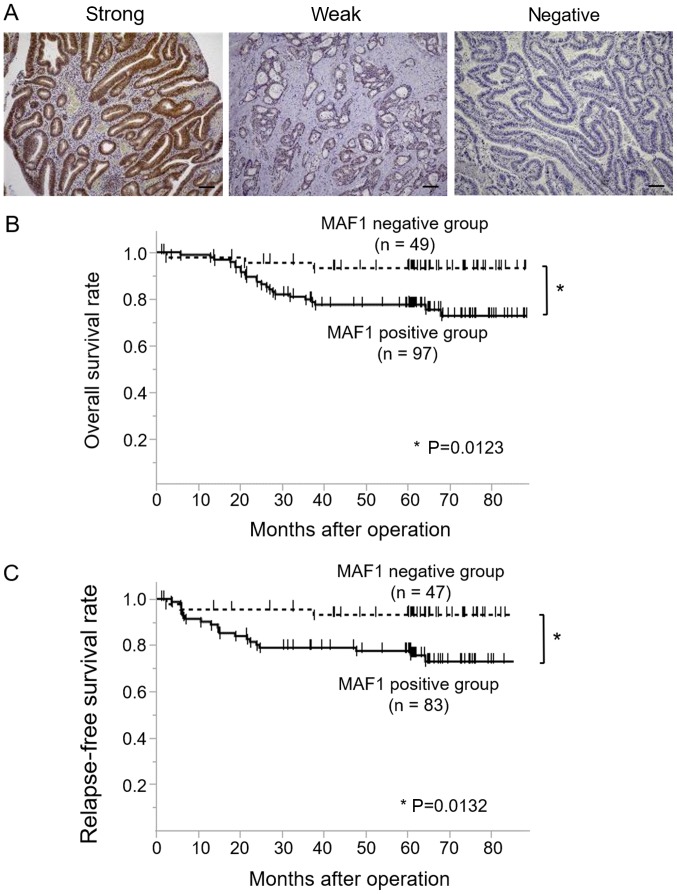
Of the 146 CRC cases, 97 cases were classified as MAF1-positive (52 cases with strong staining and 45 cases with weak staining), whereas 49 cases were classified as the MAF1 negative (Fig. 1A). A clinicopathological analysis demonstrated that high MAF1 expression was associated with tumor malignancy, including tumor depth, lymph node metastasis, distant metastasis and a poorer cancer stage (Table I). Kaplan Meier curves for OS (n=146) and RFS (n=130) revealed that the MAF1 positive group exhibited a significantly poorer prognosis (P=0.0123) and higher relapse rate (P=0.0132) compared with the MAF1 negative group (Fig. 1B and C). Univariate analysis indicated that tumor depth, lymph node metastasis, lymphatic invasion, venous invasion and MAF1 expression were prognostic factors for overall survival, whereas multivariate analysis revealed that tumor depth, lymph node metastasis and venous invasion were independent prognostic factors (Table II).
Figure 1.
Immunohistochemical analysis of MAF1 expression and its prognostic impact in CRC. (A) Representative cases of immunohistochemical staining with anti-MAF1 antibody. Examples of strong intensity, weak intensity and negative staining are presented. Scale bar, 100 µm. Negative staining was defined as the MAF1-negative group, whereas strong and weak staining was defined as the MAF1-positive group. Kaplan-Meier curves for (B) overall survival and (C) recurrence-free survival, according to MAF1 expression status in patients with CRC. CRC, colorectal cancer; MAF1, MAF1 homolog, negative regulator of RNA polymerase III.
Table I.
MAF1 expression and clinicopathological factors of patients with colorecetal cancer.
| Factor | Positive (n=97) Number (%) | Negative (n=49) Number (%) | P-value |
|---|---|---|---|
| Age (mean ± SD) | 63.8±1.35 | 67.8±1.56 | 0.0544 |
| Sex | |||
| Male | 60 (61.9) | 32 (65.3) | 0.6827 |
| Female | 37 (38.1) | 17 (34.7) | |
| Histological grade | |||
| tub1, tub2, pap | 87 (89.7) | 45 (91.8) | 0.6738 |
| por, muc | 10 (10.3) | 4 (8.2) | |
| Depth of tumor invasion | |||
| Tis, T1, T2 | 36 (37.1) | 31 (63.3) | 0.0027a |
| T3, T4 | 61 (62.9) | 18 (36.7) | |
| Lymph node metastasis | |||
| Absent | 61 (62.9) | 44 (89.8) | 0.0003a |
| Present | 36 (37.1) | 5 (10.2) | |
| Lymphatic invasion | |||
| Absent | 32 (33.0) | 23 (47.0) | 0.2139 |
| Present | 64 (66.0) | 25 (51.0) | |
| No data | 1 (1.0) | 1 (2.0) | |
| Venous invasion | |||
| Absent | 67 (69.1) | 39 (79.6) | 0.2890 |
| Present | 29 (29.9) | 9 (18.4) | |
| No data | 1 (1.0) | 1 (2.0) | |
| Distant metastasis | |||
| Absent | 83 (85.6) | 47 (95.9) | 0.0417a |
| Present | 14 (14.4) | 2 (4.1) | |
| Stage | |||
| 0, I, II | 57 (58.8) | 44 (89.8) | 0.0011a |
| IIIa, IIIb, IV | 40 (41.2) | 5 (10.2) | |
P<0.05. MAF1, MAF1 homolog, negative regulator of RNA polymerase III; muc, mucinous adenocarcinoma; pap, papillary adeno carcinoma; por, poorly differentiated adenocarcinoma; SD, standard deviation; Tis, tumor in situ; tub1, well differentiated tubular adeno carcinoma; tub2, moderately differentiated tubular adenocarcinoma.
Table II.
Univariate and multivariate analyses of overall survival in patients with colorectal cancer (Cox regression model).
| Factors | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value | |
| Age (≤65/>65 years) | 0.59 | 0.26-1.28 | 0.1855 | - | - | - |
| Sex (male/female) | 1.35 | 0.60-3.28 | 0.4769 | - | - | - |
| Histology grade (muc, por/tub1, tub2, pap) | 2.65 | 0.88-6.54 | 0.0778 | - | - | - |
| Depth of tumor invasion (T3, T4/Tis, T1, T2) | 11.8 | 3.50-73.4 | <0.0001a | 7.76 | 1.95 52.7 | 0.0021a |
| Lymph node metastasis (positive/negative) | 5.28 | 2.42-12.1 | <0.0001a | 2.84 | 1.10 8.30 | 0.0303a |
| Lymphatic invasion (positive/negative) | 3.34 | 1.27-11.5 | 0.0123a | 0.49 | 0.13 2.10 | 0.3138 |
| Venous invasion (positive/negative) | 5.13 | 2.32-11.8 | <0.0001a | 2.46 | 1.05 6.09 | 0.0389a |
| MAF1 expression (positive/negative) | 4.12 | 1.43-17.4 | 0.0062a | 1.94 | 0.64 8.43 | 0.2666 |
P<0.05. CI, confidence interval; MAF1, MAF1 homolog, negative regulator of RNA polymerase III; muc, mucinous adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; RR, relative risk; Tis, tumor in situ; tub1, well differentiated tubular adenocarcinoma; tub2, moderately differentiated tubular adenocarcinoma.
MAF1 expression is associated with the malignant potential of CRC cells
To elucidate how MAF1 contributes to the malig nant potential of CRC, in vitro loss of function assays were performed using RNA interference. HCT 116 and RKO CRC cell lines were used for MAF1knockdown experiments, since they exhibited relatively high expression levels of MAF1 in the CRC cell lines investigated (Fig. 2A). Knockdown efficiency was confirmed using RT-qPCR and western blotting (Fig. 2B). Subsequently, MAF1 knockdown significantly inhibited the migratory ability of HCT 116 and RKO cells (Fig. 2C), whereas proliferation rate was not affected (data not shown). To further investigate the involvement of MAF1 in CRC progression, the association between drug resistance of cancer cells and MAF1 expression was explored. The results from the chemosensitivity assay revealed that knockdown of MAF1 significantly improved chemosensitivity to L-OHP, which is one of the standard chemotherapy drugs used to treat CRC (Fig. 3A), whereas chemosensitivity to 5 FU was not affected (data not shown). Western blotting was performed to confirm the enhanced induction of apoptosis in MAF1 knockdown cells treated with L OHP. Knockdown of MAF1 markedly enhanced the expression of cleaved PARP and caspase 3 in L OHP treated cells, thus demonstrating that MAF1 may be critically involved in the avoidance of CRC cell apoptosis following exposure to cytotoxic agents (Fig. 3B).
Figure 2.
MAF1 is involved in the migratory ability of CRC cells. (A) Western blotting of MAF1 in CRC cell lines. (B) MAF1 expression levels in HCT 116 and RKO cells transfected with negative control siRNA or siRNAs against MAF1. mRNA expression levels were detected by reverse transcription quantitative polymerase chain reaction and were normalized to GAPDH. *P<0.001 (upper panel). Protein expression levels were detected by western blotting (lower panel). (C) Representative images of the scratch wound healing assay post transfection of HCT 116 and RKO cells with negative control siRNA or siRNAs against MAF1 (upper panel; magnification, ×100). Distance between wound edges at the indicated time points (normalized to distance at 0 h); the average of 10 dif ferent areas was obtained (lower panel). *P<0.05, **P<0.001 vs. negative control. CRC, colorectal cancer; MAF1, MAF1 homolog, negative regulator of RNA polymerase III; siRNA, small interfering RNA.
Figure 3.
MAF1 inhibition sensitizes colorectal cancer cells to the chemotherapeutic agent L OHP. (A) Dose response curves for viability of HCT 116 and RKO cells. Each bar represents the means ± standard error of the mean of samples measured in triplicate. *P<0.05, **P<0.001 vs. negative control. IC50 values in each cell line are shown. (B) Western blotting images of PARP and caspase 3 cleavage following L OHP treatment with or without MAF1 knockdown. HCT 116 and RKO cells were treated with 5 and 20 µM oxaliplatin for 72 h post transfection with negative control siRNA or siRNA against MAF1. IC50, half maximal inhibitory concentration; L OHP, oxaliplatin; MAF1, MAF1 homolog, negative regulator of RNA polymerase III; PARP, poly (ADP ribose) polymerase; siRNA, small interfering RNA.
High MAF1 expression is associated with poor prognosis in CRC with microsatellite instability (MSI)
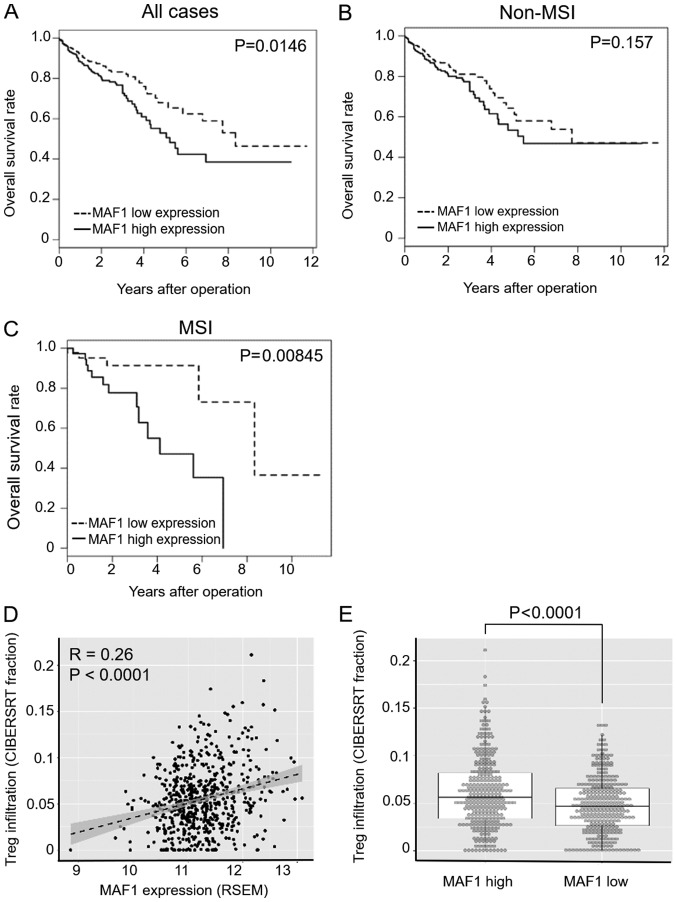
To further evaluate the clinical significance and prognostic impact of MAF1 expression in CRC, the present study analyzed a large scale CRC dataset from TCGA (n=615), which consisted of 87 MSI positive cases and 528 non MSI cases. Since Pol III activity is closely associated with immune response in various cell types (15,16), this study focused on MAF1 expression and MSI status, which strongly affects activation of tumor immunity. 'MSI high' samples were considered MSI positive, whereas all other samples were categorized in the non MSI group; this is because of the differences in clinical and immunological behaviors between MSI high tumors and other tumors (17,18). In the subgroup analysis using MSI status information, the prognostic impact of MAF1 was significantly higher in MSI positive cases (P=0.00845) compared with all cases (P=0.0146) or non MSI cases (P=0.157) (Fig. 4A C). Multivariate analysis of MSI positive cases (n=87) indicated that high MAF1 expression was an independent prognostic factor for overall survival (Table III). To further investigate the association between MAF1 expression and the immune microenvironment, bioinformatics analysis was performed using CIBERSORT (13,14). CIBERSORT is a computational program used to predict the relative contribution of each immune cell type in a mixed cell population using transcriptome data (13,14). A mild correlation between MAF1 expression and Treg infiltration was detected (Fig. 4D), and the extent of Treg infiltration was significantly higher in the MAF1 high expression group in the TCGA dataset (P<0.0001; Fig. 4E).
Figure 4.
Prognostic impact of MAF1 expression in MSI tumors and involvement of MAF1 in the immune microenvironment. Kaplan Meier curves of overall survival in (A) all colorectal cancer cases (n=615), (B) non MSI cases (n=528) and (C) MSI positive cases (n=87). (D) Correlation between MAF1 expression and Treg infiltration in The Cancer Genome Atlas datasets. Dashed line and gray area indicate linear regression line and 95% confidence interval, respectively (correlation coefficient, R=0.26, P<0.0001). (E) Treg infiltration fraction was calculated using CIBERSORT; Treg infiltration fraction in MAF1 high and low expression groups is shown. Median of the expression in all cases was used as a cut off level. MAF1, MAF1 homolog, negative regulator of RNA poly merase III; MSI, microsatellite instability; Treg, regulatory T cell.
Table III.
Univariate and multivariate analyses of overall survival in MSI cases using The Cancer Genome Atlas dataset (Cox regression model).
| Factor | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value | |
| Age (≤65/>65 years) | 0.33 | 0.05-1.19 | 0.0962 | - | - | - |
| Sex (male/female) | 0.24 | 0.06-0.76 | 0.0131a | 0.21 | 0.05 0.69 | 0.0079a |
| Depth of tumor invasion (T3, T4/T1, T2) | 3.21 | 0.65-58.2 | 0.1797 | - | - | - |
| Lymph node metastasis (negative/positive) | 0.82 | 0.30-2.61 | 0.7124 | - | - | - |
| MAF1 expression (low/high) | 0.25 | 0.07-0.70 | 0.0075a | 0.22 | 0.06 0.64 | 0.0046a |
P<0.05. CI, confidence interval; MAF1, MAF1 homolog, negative regulator of RNA polymerase III; RR, relative risk; Tis, tumor in situ.
Discussion
The present used two independent datasets and demonstrated that high MAF1 expression may be closely associated with cancer progression in patients with CRC. The results of clinicopathological analysis revealed that high MAF1 expression was highly associated not only with tumor depth, but also with lymph node and distant metastasis, thus suggesting that MAF1 may contribute to metastatic ability. In addition, in vitro analyses revealed that MAF1 inhibition significantly suppressed the migratory ability of CRC cells. MAF1 knockdown also rendered cancer cells more sensitive to a chemotherapeutic agent. In the present analysis, MAF1 was significantly highly expressed in patients with stage III and IV cancer; these patients have been reported to benefit from standard chemotherapy (19). Therefore, the MAF1 gene may be a useful biomarker, as well as therapeutic target, for patients receiving chemotherapy. However, further in vivo investigation is required to fully confirm the improvement of chemoresistance to L OHP by knockdown of MAF1 expression.
High MAF1 expression was not considered an independent prognostic indicator in all patients with CRC, whereas analysis of an MSI positive population clearly demonstrated that MAF1 was a significant independent prognostic indicator. These find ings suggested that the prognostic value of MAF1 expression may be particularly important in MSI positive patients.
Although MAF1 expression has been reported to suppress cancer proliferation through negative regulation of Pol III mediated transcription in some cell lines, the malignant potential of MAF1, including its effects on chemoresistance, have not been sufficiently explored. Cellular stress, which can cause growth arrest, also induces cell dormancy, leading to stress tolerance and cell survival, depending on cellular context (20,21). Notably, MAF1 has been reported to be required for maintaining a cell dormancy like state and subsequent cell survival under nutrient starvation in Plasmodium falciparum (22). Furthermore, previous reports have revealed that MAF1 can directly or indirectly regulate Pol II dependent genes (7,8,23), indicating that MAF1 may control various cellular functions, including the malignant potential of cancer cells. However, since this study did not identify direct targets of the MAF1 gene that regulate cell mobility and survival, further exploration is required to determine the direct involvement of MAF1 in the metastatic process of CRC.
The present results obtained from clinical samples indicated an oncogenic role for the MAF1 gene in CRC; however, other reports have demonstrated a tumor suppressive role of MAF1 in other malignancies, with the exception of CRC (8,10). To explain this discrepancy, this study focused on the immune microenvironment, which differs markedly among tissues. Growing evidence has suggested that both tumor antigenicity and the immune environment have a marked influence on the prognosis of patients with cancer (24,25). In addition, because Pol III activity is known to be closely associated with the immune response in various cell types (15,16), the present study considered the possibility that MAF1 may serve an important role in the immune microenvironment. Immune checkpoint inhibitors are increasingly being used in the clinic, and have been reported to be very effective for the treatment of MSI high tumors, regardless of histology (18). This is because MSI tumors activate the immune system through producing a large number of neoantigens (18,26 29). Notably, the present subgroup anal ysis using a CRC TCGA database revealed that the prognostic impact of MAF1 was more evident in MSI high CRC, thus suggesting that MAF1 may exert its malignant potential more powerfully under immune activated conditions. In this context, transcriptome based gene enrichment analysis demonstrated that Treg infiltration was more abundant in tumors with high expression levels of MAF1. Tregs are known to suppress the immune response against tumor antigens in several types of tissue (30), thus indicating a close association between MAF1 expression in cancer and immune suppression in CRC tissues. However, further investigation is required to fully understand the effects of MAF1 expression on tumor immunity.
In conclusion, the present study revealed that MAF1 expression may be considered a useful prognostic indicator in patients with CRC, particularly in MSI positive cases. These results may help to understand the complex gene regulatory network, including both Pol II and III dependent transcription, and may provide novel medical information that may lead to breakthroughs in the treatment of CRC.
Acknowledgments
The authors would like to thank Ms. Asuka Yasueda and Dr Satoshi Ishikawa (Departments of Gastroenterological Surgery, Osaka University Graduate School of Medicine) for helpful arrangement of the experiments, as well as Dr Hirofumi Yamamoto (Division of Health Sciences, Osaka University) for fruitful discussion.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research (C), JSPS KAKENHI (grant nos. 18K07970, 17K106310, 17K106320 and 16K086210) from the Ministry of Education, Culture, Sports, Science and Technology; and partial support was received from Takeda Science Foundation and Senri Life Science Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contribution
KH initiated this project. KH, NN, NM, HT, NH, TH, CM and TM designed experiments and wrote the manuscript. KH and NN performed in vitro experiments and bioinformatics analysis. CM, TM, YD and MM provided clinical samples and designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Osaka University (approval ID: 08226) and written informed consent was obtained from all patients included in this study.
Patient consent for publication
Consent for publication was obtained from all patients included in this study.
Competing interests
NN received research grants from Chugai Co. Ltd, Yakult Honsha Co. Ltd. and Ono Pharmaceutical Co., Ltd. These funders had no role in supplying expenses, study design, data analysis, decision to publish or preparation of the article.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Colvin H, Mizushima T, Eguchi H, Takiguchi S, Doki Y, Mori M. Gastroenterological surgery in Japan: The past, the present and the future. Ann Gastroenterol Surg. 2017;1:5–10. doi: 10.1002/ags3.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradhan A, Hammerquist AM, Khanna A, Curran SP. The C box rgion of MAF1 regulates transcriptional activity and protein stability. J Mol Biol. 2017;429:192–207. doi: 10.1016/j.jmb.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vannini A, Cramer P. Conservation between the RNA poly merase I, II, and III transcription initiation machineries. Mol Cell. 2012;45:439–446. doi: 10.1016/j.molcel.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 5.White RJ. RNA polymerases I and III, non coding RNAs and cancer. Trends Genet. 2008;24:622–629. doi: 10.1016/j.tig.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Khanna A, Pradhan A, Curran SP. Emerging roles for MAF1 beyond the regulation of RNA polymerase III activity HHS Public Access. J Mol Biol. 2015;427:2577–2585. doi: 10.1016/j.jmb.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson SS, Zhang C, Fromm J, Willis IM, Johnson DL. Mammalian Maf1 is a negative regulator of transcription by all three nuclear RNA polymerases. Mol Cell. 2007;26:367, 379. doi: 10.1016/j.molcel.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Palian BM, Rohira AD, Johnson SAS, He L, Zheng N, Dubeau L, Stiles BL, Johnson DL. Maf1 is a novel target of PTEN and PI3K signaling that negatively regulates oncogenesis and lipid metabolism. PLoS Genet. 2014;10:e1004789. doi: 10.1371/journal.pgen.1004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna A, Pradhan A, Curran SP. Emerging roles for Maf1 beyond the regulation of RNA polymerase III activity. J Mol Biol. 2015;427:2577–2585. doi: 10.1016/j.jmb.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Tsang CK, Wang S, Li XX, Yang Y, Fu L, Huang W, Li M, Wang HY, Zheng XF. MAF1 suppresses AKT mTOR signaling and liver cancer through activation of PTEN tran scription. Hepatology. 2016;63:1928–1942. doi: 10.1002/hep.28507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et al. Japanese Society for Cancer of the Colon and Rectum: Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1–29. doi: 10.1007/s10147-011-0315-2. [DOI] [PubMed] [Google Scholar]
- 12.Sugimura K, Fujiwara Y, Omori T, Motoori M, Miyoshi N, Akita H, Gotoh K, Kobayashi S, Takahashi H, Noura S, et al. Clinical importance of a transcription reverse transcription concerted (TRC) diagnosis using peritoneal lavage fluids obtained pre and post lymphadenectomy from gastric cancer patients. Surg Today. 2016;46:654–660. doi: 10.1007/s00595-015-1235-y. [DOI] [PubMed] [Google Scholar]
- 13.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graczyk D, White RJ, Ryan KM. Involvement of RNA poly merase III in immune responses. Mol Cell Biol. 2015;35:1848–1859. doi: 10.1128/MCB.00990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiu YH, Macmillan JB, Chen ZJ. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG I pathway. Cell. 2009;138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CG, Ahn JB, Jung M, Beom SH, Kim C, Kim JH, Heo SJ, Park HS, Kim JH, Kim NK, et al. Effects of microsatellite insta bility on recurrence patterns and outcomes in colorectal cancers. Br J Cancer. 2016;115:25–33. doi: 10.1038/bjc.2016.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD 1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 20.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senft D, Ronai ZA. Adaptive stress responses during tumor metastasis and dormancy. Trends Cancer. 2016;2:429–442. doi: 10.1016/j.trecan.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 22.McLean KJ, Jacobs Lorena M. Plasmodium falciparum Maf1 confers survival upon amino acid starvation. MBio. 2017;8:e02317 e16. doi: 10.1128/mBio.02317-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orioli A, Praz V, Lhôte P, Hernandez N. Human MAF1 targets and represses active RNA polymerase III genes by preventing recruitment rather than inducing long term transcriptional arrest. Genome Res. 2016;26:624–635. doi: 10.1101/gr.201400.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteside TL. Immune responses to cancer: Are they potential biomarkers of prognosis? Front Oncol. 2013;3:107. doi: 10.3389/fonc.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galon J, Costes A, Sanchez Cabo F, Kirilovsky A, Mlecnik B, Lagorce Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predictclinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 26.Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent Puig P, Gryfe R, Shepherd LE, et al. Tumor microsatellite instability status as a predictor of benefit from fluorouracil-based adjuvant chemo therapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 29.Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, et al. NCI CPTAC Proteogenomic characterization of human colon and rectal cancer. Nature. 2014;513:382–387. doi: 10.1038/nature13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.