Abstract
Hyperlipidemia is associated with metastasis in patients with gastric cancer (GC). 25-Hydroxycholesterol (25-HC) is a type of oxysterol which is synthesized from cholesterol and is involved in a number of processes, including inflammation, immune responses and cancer development. However, the role of 25-HC in gastric cancer remains unknown. In the present study, we demonstrated that 25-HC had no effects on GC cell proliferation and apoptosis, whereas it decreased the sensitivity of GC cells to 5-fluorouracil (5-FU), as demonstrated by the increased cell proliferation and the decreased cell apoptosis. On the other hand, exposure to 2.5-10 µM of 25-HC significantly promoted GC invasion, both in vitro (using AGS and MGC-803 GC cell lines) and in vivo (in an animal model), accompanied by the upregulation of the expression levels of matrix metalloproteinases (MMPs). Further investigations revealed that the promotion of GC invasion was, at least in part due to the activation of Toll-like receptor 2 (TLR2)/nuclear factor (NF)-κB signaling. Our results demonstrated that 25-HC promoted GC cells invasion by upregulating TLR2/NF-κB-mediated MMP expression. Thus, on the whole, the findings of this study suggest a novel mechanism of hyperlipidemia-induced GC progression.
Keywords: 25-hydroxycholesterol, gastric cancer, 5-fluorouracil, invasion, Toll-like receptor 2, nuclear factor-κB
Introduction
Oxysterols are oxygenated derivatives of cholesterol formed in the human body or ingested in the diet. Oxysterols are associated with various cardiovascular, metabolic, neurodegenerative and cancerous pathologies (1). Oxysterols have been shown to play pro-cancerous and pro-proliferative roles through the modulation of inflammation or oxysterol-binding proteins in various types of cancer, including breast cancer and lung cancer (2-4). Gastric cancer (GC) is the third leading cause of cancer-related mortality worldwide (5). In China, GC is considered as the second leading cause of cancer-related mortality (6). Dietary patterns, obesity, smoking and chronic infections contribute to the development of GC (7). Hyperlipidemia is associated with lymph node metastasis in patients with GC (8,9). Metformin, a drug used in the treatment of type 2 diabetes for the regulation of glucose and fatty acid metabolism, has been found to inhibit the proliferation and epithelial-mesenchymal transition of AGS GC cells (10,11). In addition, use of statins can reduce the risk of developing GC (12). However, the role of oxysterols in GC and the related mechanisms remain largely unknown.
25-Hydroxycholesterol (25-HC) is a type of oxysterol which is synthesized from cholesterol (13). Johnson et al found that 25-HC was upregulated in serum following the ingestion of a meal rich in oxysterols and following a dietary cholesterol challenge (14). In addition, the levels of 25-HC have been shown to be higher in hypercholesterolemic serum compared to those in normocholesterolemic serum (15). 25-HC has also been found to be involved in the progression of breast and ovarian tumors by activating the estrogen receptor (ER) α-mediated signaling pathway (16) and promoting resistance to anti-hormone treatment in ER-positive breast cancer (17). More recently, 25-HC has been reported to promote the migration and invasion of lung adenocarcinoma cells (18). Increased cholesterol levels are often associated with obesity (19), which has been found to be a risk factor for the development of GC (20). Thus, we hypothesized that 25-HC may play a role in the development of GC. To date, at least to the best of our knowledge, the mechanisms of oxysterol-induced GC progression remain largely unknown.
Therefore, in the present study, we evaluated the role of 25-HC in GC both in vitro and in vivo. Our data revealed that 25-HC had no effects on GC cell proliferation and apoptosis, whereas it decreased the sensitivity of the cells to 5-fluorouracil (5-FU). Moreover, 25-HC promoted GC cell invasion and the underlying mechanisms partly involved the upregulation of Toll-like receptor 2 (TLR2)/nuclear factor (NF)-κB signaling-dependent matrix metalloproteinase (MMP) expression.
Materials and methods
Cells and reagents
The human GC cell lines, AGS and MGC-803, were obtained from the Cell Bank of the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). The AGS cells were cultured in F-12K medium and the MGC-803 cells were cultured in RPMI-1640 medium complemented with 10% heat-inactivated fetal bovine serum (FBS) and maintained at 37˚C with 5% CO2 in a humidified tissue culture incubator. All cell culture reagents were purchased from Invitrogen (Shanghai, China).
25-HC was purchased from Sigma-Aldrich (Shanghai, China) and dissolved in ethanol as a stock solution. Rabbit anti-MMP1 polyclonal antibody (pAb, #10371-2-AP), rabbit anti-MMP2 pAb (#10373-2-AP), rabbit anti-MMP3 pAb (#17873-1-AP), rabbit anti-MMP9 pAb (#10375-2-AP) and rabbit anti-MMP13 pAb (#18165-1-AP) were obtained from Proteintech (Wuhan, China). Rabbit anti-Bcl-2 monoclonal antibody (mAb, #4223), rabbit anti-Bax mAb (#5023), rabbit anti-phospho-PI3K p85/ p55 mAb (#4228), rabbit anti-PI3K p85 mAb (#4292), rabbit anti-phospho-AKT mAb (#4060), rabbit anti-AKT mAb (#4691), rabbit anti-phosphosignal transducer and activator of transcription 3 (STAT3) mAb (#9145), rabbit anti-STAT3 mAb (#4904), rabbit anti-phospho-p38 mAb (#9211), rabbit anti-p38 mAb (#9212), rabbit anti-phospho-extracellular signal-regulated protein kinase (Erk)1/2 mAb (#4370), rabbit anti-Erk1/2 mAb (#4695), rabbit anti-phospho-stress-activated protein kinase (SAPK)/c-Jun NH2-terminal kinase (JNK) mAb (#4668), rabbit anti-SAPK/JNK pAb (#9252), rabbit anti-phospho-NF-κB p65 mAb (#3039), rabbit anti-NF-κB p65 mAb (#8242) and rabbit anti-β-actin mAb (#8457) were purchased from Cell Signaling Technologies (CST; Danvers, MA, USA). Rabbit anti-TLR2 pAb (#DF7002), rabbit anti-TLR3 pAb (#DF6415), rabbit anti-TLR4 pAb (#AF7017), rabbit anti-TLR9 pAb (#DF2970) were purchased from Affinity Biosciences (Jiangsu, China). HRP-linked anti-rabbit IgG Ab (#70-GAR007) was obtained from MultiSciences (Lianke) Biotech Co., Ltd. (Hangzhou, China). FITC anti-human CD44 antibody (#103021) was purchased from BioLegend (San Diego, CA, USA).
Measurement of cell viability
Cell viability was detected by Cell Counting kit-8 assay (Beyotime, Jiangsu, China) according to the manufacturer's instructions. The cells were seeded in 96-well plate at a density of 1×103 cells/well and allowed to grow for 24 h before the medium was replaced with either ethanol as the vehicle or various concentrations of 25-HC (2.5, 5 and 10 µM) in a total volume of 100 µl. Following culture for 24, 48 and 72 h, 10 µl of CCK-8 solution reagent were added to each well and cultured for a further 1 h. The absorbance at 450 nm was measured using an ultra-microplate reader (Emax, Molecular Devices, CA, USA).
To investigate the role of 25-HC in the chemosensitivity to 5-FU, the AGS or MGC-803 cells seeded in 96-well plates were treated with various concentrations of 25-HC with or with 5-FU (5 µM) for 24 or 48 h before cell viability was determined by CCK-8 assay as described above.
Annexin V cell apoptosis assay
Cells in a 6-well plate were treated with various concentrations of 25-HC with or without 5-FU (5 uM) for 48 h before the cells were trypsinized, washed with ice-cold PBS and resuspended. Cell apoptosis was determined with the Annexin V apoptosis kit (Lianke Biotech, Co., Ltd.) according to the manufacturer's instructions. The stained cells were then analyzed by flow cytometry (BD FACScan; BD Biosciences, San Jose, CA, USA).
Western blot analysis
Cells or tissues from the lungs of mice were washed with ice-cold PBS 3 times and lysed with RIPA buffer (CST, Danvers, MA) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) (CST) and protease inhibitor cocktail. Equal amounts of proteins were separated by 10% SDS-polyacrylamide gel electrophoresis and blotted onto PVDF membranes. The membranes were blocked with PBS containing 5% non-fat milk for 1.5 h at room temperature, followed by incubation with primary antibodies against MMP1, MMP2, MMP3, MMP9, MMP13, Bcl-2, Bax, p-PI3K p85/p55, PI3K p85, p-AKT, AKT p-STAT3, STAT3, p-p38, p38, p-Erk1/2, Erk1/2, p-SAPK/JNK, SAPK/JNK p-NF-κB p65, NF-κB p65, TLR2, TLR3, TLR4, TLR9 and β-actin (all diluted 1:1,000) overnight at 4°C. Following incubation with the HRP-linked goat anti-rabbit secondary antibody (dilution 1:5,000) for 1 h at room temperature, immunoreactive proteins were detected with FluorChem E System (ProteinSimple, Santa Clara, CA, USA).
For the inhibition assay, cells in 6-well plates were pre-treated with the NF-κB-specific inhibitor, Bay 11-7082 at 2 µg/ml (Beyotime Institute of Biotechnology, Shanghai, China) for 1 h and medium were replaced with the complete medium supplemented with 25-HC.
Animal experiments
All the experiments using animals were approved by the Animal Care and Use Committee of Zhejiang University. Five to six-week-old female BALB/c nude mice (90 in total), weighing between 18-20 g were purchased from Shanghai Laboratory Animal Company (SLAC; Shanghai, China) and maintained in the animal facility at Zhejiang University. Mice were provided with water and food (SLAC) ad libitum and kept under standard conditions (temperature 24±2°C, humidity, 50-70%, 12-h light/dark cycle).
For tumor growth assays, 5×106 AGS cells were subcutaneously injected into the right flanks of the nude mice. When the volumes of the xenograft tumors reached an average of 100 mm3, the mice were randomly divided into 4 groups as follows: The PBS and 25-HC groups (with 5 mice in each group), and the PBS + 5-FU and 25-HC + 5-FU groups (with 10 mice in each group). The mice in the PBS + 5-FU and 25-HC + 5-FU groups received 5-FU or/and 25-HC via intraperitoneal injection with 5-FU (25 mg/kg) or/and 25-HC (10 mg/kg) every 3 days for 3 weeks. After 3 weeks, the mice were sacrificed, and the tumors were harvested and weighed, and embedded in paraffin for use in further analyses. Tumor volume was calculated using the following formulae: V = ½× (length × width2). This experiment was repeated under the same setting 3 times (once with 10 mice in total, and another 2 times with 20 mice each time).
For lung metastasis assay, the mice were injected with 1×106 of AGS cells through the tail vein and randomly divided into 2 groups (PBS and 25-HC group) with 8 mice in each group. Mice in the 25-HC group were intraperitoneally injected with 25-HC (10 mg/kg) every 3 days for 3 weeks. This experiment was repeated twice (with 20 mice being prepared each time). After 3 weeks, the mice were sacrificed, and the lungs were removed and weighted. The lung metastatic tumors on the surface were calculated and H&E staining was performed on the lung tissues or part of the lung tissues were extracted for protein extraction for use in western blot analysis. H&E staining was performed by Google Biotechnology Co., Ltd. (Wuhan, China).
Cell cycle assay
The cell cycle was analyzed with the Cell Cycle Staining kit (Lianke Biotech, Co., Ltd.) according to the manufacturer's instructions. Cells in a 6-well plate were treated with various concentrations of 25-HC with or without 5-FU (5 µM) for 48 h before the cells were trypsinized, washed with ice-cold PBS and fixed with 75% ethanol at −20°C overnight. The cells were then stained with PI solution for 30 min in the dark. Cell cycles were analyzed by flow cytometry (BD FACScan; BD Biosciences).
Wound healing assay
The AGS cells were seeded and allowed to grow to 80% confluence in complete medium before cells were wounded by a 200-µl pipette. The cells were then washed with PBS twice and replenished with fresh culture medium with 5% FBS containing the indicated concentrations of 25-HC. Images of the wound morphology were acquired under a light microscope (CKX41; Olympus, Shanghai, China).
Cell invasion assay
Cell invasion assay was carried out using 24-well cell culture chambers with an 8-µm pore filter (Corning Costar Corp., Cambridge, MA, USA). Before the cells were seeded, the upper surfaces of the membranes were coated with 50 µl Matrigel (BD Biosciences) for 6 h at 37°C. The cells were then seeded at a density of 1×105 cells/well in 100 µl serum-free F-12K medium added onto the top chamber of each Transwell with various concentrations of 25-HC. The cells were allowed to invade for 36 h at 37°C. After removing the cells that had remained in the upper side of the membrane with a cotton swab, cells in the lower side of the membrane were fixed with ice-cold methanol, stained with crystal violet (Hushi, Shanghai, China) in 20% ethanol overnight and then counted in 5 pre-determined fields under a light microscope (CKX41; Olympus).
For the inhibition assay, cells in the upper chamber were pre-treated with NF-κB inhibitor Bay 11-7082 at 2 µg/ml (Beyotime) for 1 h and the medium was replaced with FBS-free medium supplemented with 25-HC or not.
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
The AGS cells were exposed to 25-HC at various concentrations for 24 h and total RNA was extracted using the Ultrapure RNA kit (Cwbiotech, Beijing, China). Reverse transcriptase PCR was performed with the HiFiScript cDNA Synthesis kit (Cwbiotech). Subsequently, quantitative PCR (qPCR) was performed using the iTaq Universal SYBR-Green Supermix on a CFX96 Touch Real-Time PCR Detection System (both from Bio-Rad, Hercules, CA, USA). The primer sequences used are listed in Table I. The results of qPCR were analyzed with the relative quantification Delta delta CT (2−ΔΔCq) strategy (21). The calculated threshold cycle was normalized to the value of internal β-actin amplified from the same cDNA and the fold-change was calculated as referenced to control.
Table I.
Sequences of primers used for RT-qPCR.
| Gene | Forward | Reverse |
|---|---|---|
| β-actin | GTATCCTGACCCTGAAGTACC | TGAAGGTCTCAAACATGATCT |
| TLR2 | CTCTTCAGCAAACGCTGTTCT | GGCGTCTCCCTCTATTGTATTG |
| TLR3 | GTGAGATACAACGTAGCTGACTG | TCCTGCATCCAAGATAGCAAGT |
| TLR4 | GCCTTTCAGGGAATTAAGCTCC | GATCAACCGATGGACGTGTAAA |
| TLR9 | ACAACTCTGACTTCGTCCACC | TCTGGGCTCAATGGTCATGTG |
Luciferase reporter assay
The AGS cells were seeded in 96-well plate at a density 1×104 per well 1 day prior to transfection. The cells were transiently transfected with NF-κB luciferase reporter plasmid with Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's instructions. At 24 h post-transfection, the cells were stimulated with the indicated concentrations of 25-HC. After 24 h, the cells were collected, and the luciferase activities were determined by using the Bright-Glo luciferase assay system (Promega Corp., Madison, WI, USA).
siRNA transfection
siRNA targeting human TLR2 (5'-GGA GUCUCUGUCAUGUGAUdTdT-3') and scramble siRNA (siNC) (5'-UUCUCCGAACGUGUCACGUTTdTdT-3') were purchased from GenePharma Technologies (Shanghai, China). The AGS cells were transfected with the siRNAs using Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific) following the manufacturer's instructions. Following transfection for 24 h, the cells were prepared for use in further experiments.
Flow cytometry for the determination of CD44 expression
The AGS cells in 6-well plate were treated with various concentrations of 25-HC for 48 h before the cells were trypsinized, washed with ice-cold PBS, resuspended and counted prior to staining for flow cytometric analysis. In 100 µl of buffer, 1×106 cells were incubated with the anti-CD44-FITC on ice for 30 min. The cells were then washed with buffer and fixed in 1% paraformaldehyde for 30 min on ice. Following fixation, the cells were washed, and the fluorescence intensity was assessed by flow cytometry (BD FACScan; BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data are presented as the means ± SEM. Statistical significance was determined by a Student's t-test or one-way analysis of variance (ANOVA) followed by Dunnett's test or Tukey's test. A P-value <0.05 was considered to indicate a statistically significant difference.
Results
25-HC has no effects on GC cell proliferation and apoptosis in vitro
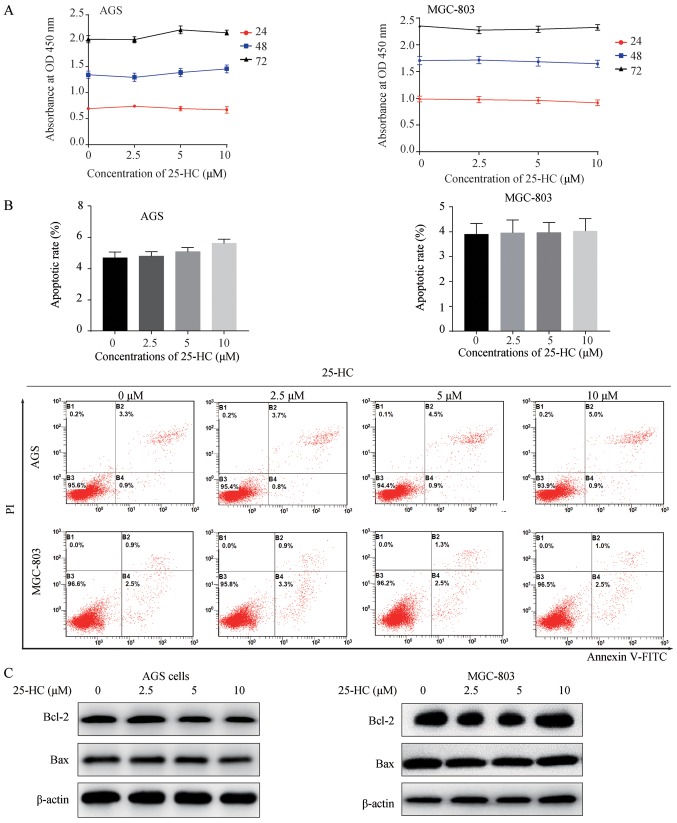
To explore the biological effects of 25-HC on GC cell proliferation, we first evaluated cell proliferation by CCK-8 assay. The AGS or MGC-803 cells were exposed to the indicated concentrations of 25-HC for 24, 48 and 72 h. As shown in Fig. 1A, 25-HC had no effect on GC cell proliferation. The regulation of fatty acid metabolism can decrease GC cell viability and induce cell apoptosis (10). Thus, in this study, we also detected cell apoptosis by flow cytometry, as well as the expression levels of the apoptotic regulators, Bcl-2 and Bax by western blot analysis. Consistent with the results of cell proliferation assay, 25-HC had no marked effects on cell apoptosis (Fig. 1B) or on the expression levels of Bcl-2 and Bax (Fig. 1C). These results demonstrate that 25-HC (2.5-10 µM) has no effects on GC cell proliferation and apoptosis.
Figure 1.
25-HC has no effects on GC cell proliferation and apoptosis in vitro. (A) AGS cells or MGC-803 cells were treated with the indicated concentrations of 25-HC for 24, 48 or 72 h followed by CCK-8 assay. The absorbance at 450 nm was measured. (B) AGS cells or MGC-803 cells were treated with 25-HC at the indicated concentrations for 48 h and apoptosis was examined by FITC-Annexin V/PI staining. The fluorescence intensity of FITC-Annexin V was plotted on the x-axis, and PI was plotted on the y-axis. The statistical analysis of apoptotic GC cells (FITC+) were calculated. (C) Cells were treated with 25-HC for 48 h and total cellular proteins were extracted for western blot analysis to detect the Bcl-2 and Bax expression levels. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. 25-HC, 25-hydroxycholesterol; GC, gastric cancer.
25-HC decreases the sensitivity of GC cells to 5-FU
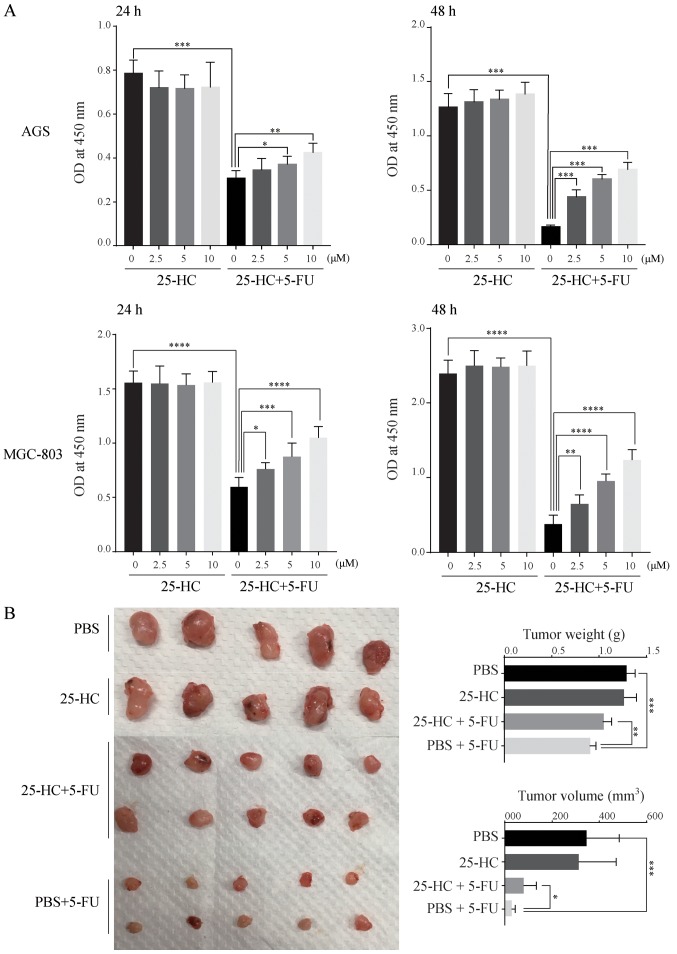
To examine the effects of 25-HC on the chemoresistance of GC cells, the AGS and MGC-803 cells were treated with 25-HC with or without 5-FU and cell proliferation were evaluated. As shown in Fig. 2A, although 25-HC had no effect on cell proliferation directly, it decreased the sensitivity of the GC cells to 5-FU with a significant increase in absorbance at 450 nm compared to 5-FU stimulation alone in a dose-dependent manner. In mouse tumor xenograft experiments, the tumor volume and weight in the 25-HC group exhibited no marked difference compared with the PBS group. However, following 5-FU treatment, tumor growth was enhanced by 25-HC, as evidenced by the greater tumor volume and increased tumor weight compared to the PBS + 5-FU group (Fig. 2B). Thus, these results indicate that 25-HC decreases the sensitivity to 5-FU in AGS cells both in vitro and in vivo.
Figure 2.
25-HC decreases the sensitivity of GC cells to 5-FU. (A) AGS cells and MGC-803 were treated with 25-HC with or without 5-FU (5 µM) for 24 and 48 h and cell proliferation was determined by CCK-8 assay. The absorbance at 450 nm was measured on an ultra-microplate reader. (B) Nude BALB/c mice were injected subcutaneously with AGS cells at 5×106/mouse. When the volumes of tumors reached 100 mm3, the mice were intraperitoneally injected with 5-FU (25 mg/kg) or/and 25-HC (10 mg/kg) every 3 days for 3 weeks. Xenograft tumors were stripped from the nude mice and imaged or tumor weights and tumor volumes were assessed after sacrifice. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance in (A and B) was determined by one-way ANOVA followed by Dunnett's test and Tukey's test, respectively; *P<0.05, **P<0.01 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer.
25-HC decreases the pro-apoptotic effects of 5-FU on GC cells
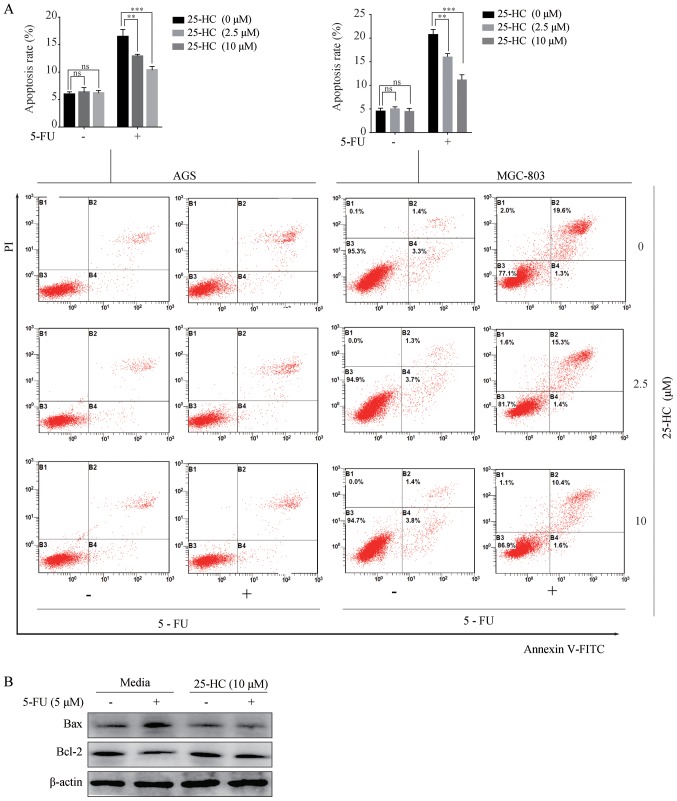
We then investigated the effects of 25-HC on cell apoptosis induced by 5-FU. The cells were treated with 25-HC with or without 5 µM 5-FU for 48 h and the apoptotic cells were determined by Annexin V/PI staining. As shown in Fig. 3A, upon 5-FU treatment, the apoptotic rates of the AGS cells (16.7±0.75%) decreased significantly following 25-HC stimulation (12.9±0.19% at 2.5 µM and 10.4±0.37% at 10 µM; P<0.05). Similar results were obtained with the MGC-803 cells. Moreover, the increased expression of Bcl-2 and the decreased expression of Bax in the AGS cells following treatment with 5-FU were detected (Fig. 3B). Overall, these results indicate that 25-HC decreases the pro-apoptotic effects of 5-FU on GC cells.
Figure 3.
25-HC decreases the apoptotic effects of 5-FU in GC cells. AGS cells or MGC-803 cells were treated with 25-HC with or without 5-FU (5 µM) for 48 h. (A) Cell apoptosis was examined by FITC-Annexin V/PI staining. The statistical analysis of apoptotic AGS cells (FITC+) was performed. Treatment with 25-HC at 2.5 and 10 µM caused a 3.57 and 6.2% decrease in the apoptotic fraction of the AGS cells respectively, and 4.77 and 9.63% decrease of the MGC-803 cells respectively, compared with the control cells. (B) Total cellular proteins in AGS cells treated 25-HC with or without 5-FU (5 µM) were extracted for western blot analysis to detect the Bcl-2 and Bax expression levels. All the values shown are represented as the means ± SEM and statistical significance was determined by one-way ANOVA followed by Dunnett's test; ns, no significance, **P<0.01 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer; 5-FU, 5-fluorouracil.
25-HC arrests the cell cycle of the GC cells at the S-phase following 5-FU treatment
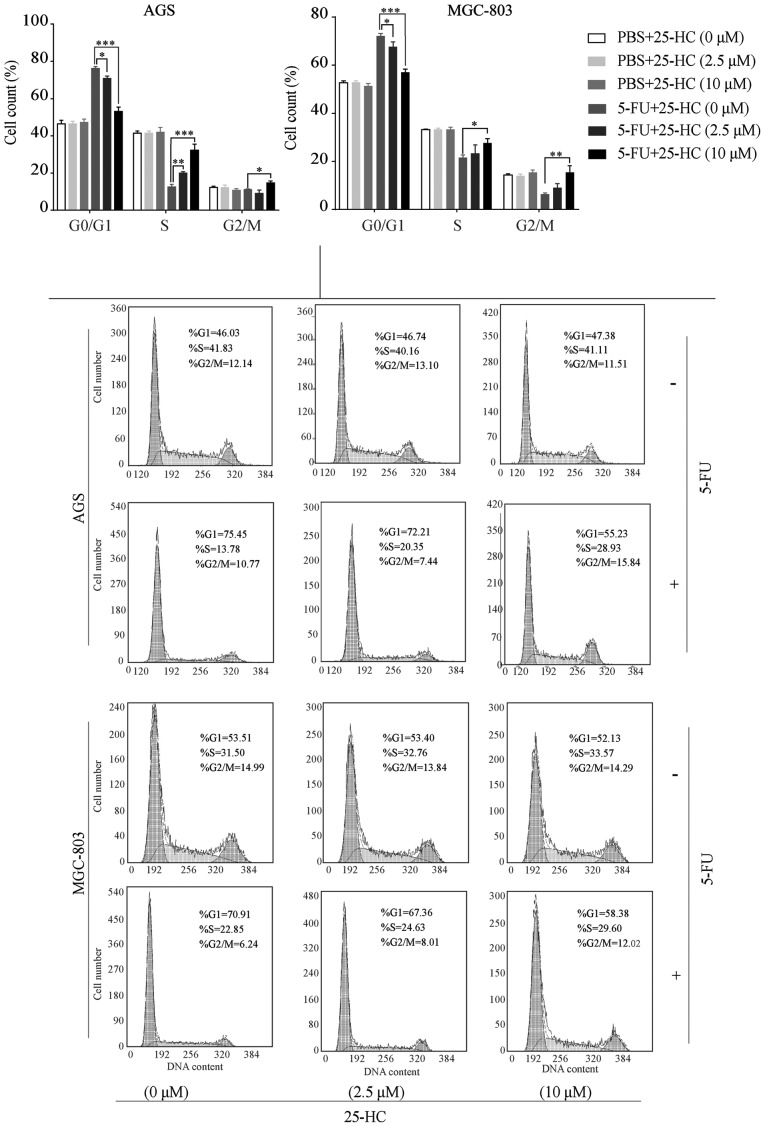
The cell cycle distribution of the 25-HC-exposed GC cells treated with or without 5-FU was analyzed and quantified by flow cytometry. As shown in Fig. 4, 25-HC treatment alone had minimal effects on cell cycle arrest in the AGS and MGC-803 cells, which was consistent with the results of proliferation assay. However, an increase in the population of cells in the S-phase following 5-FU treatment was observed in a dose-dependent manner, ranging from 12.62±0.8% to 20.1±0.4% to 32.24±1.9% in the AGS cells and 21.53±0.7% to 23.33±2.0% to 27.67±0.9% in the MGC-803 cells (25-HC at 0, 2.5 and 10 µM, respectively, P<0.05). Additionally, the numbers of cells in the G0/G1 phase were significantly decreased with the increasing concentrations of 25-HC (P<0.05).
Figure 4.
25-HC arrests the cell cycle of GC cells at S-phase following 5-FU treatment. AGS cells or MGC-803 cells were treated with 25-HC with or without 5-FU (5 µM) for 48 h. Cell cycle distribution was examined by using PI staining and flow cytometry analysis. Representative flow cytometric analysis of cell cycle distribution and the statistical analysis is shown. Results were obtained from 3 independent experiments and are expressed as the means ± SEM and statistical significance was determined by one-way ANOVA followed by Dunnett's test. Representative images are presented. *P<0.05, **P<0.01 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer.
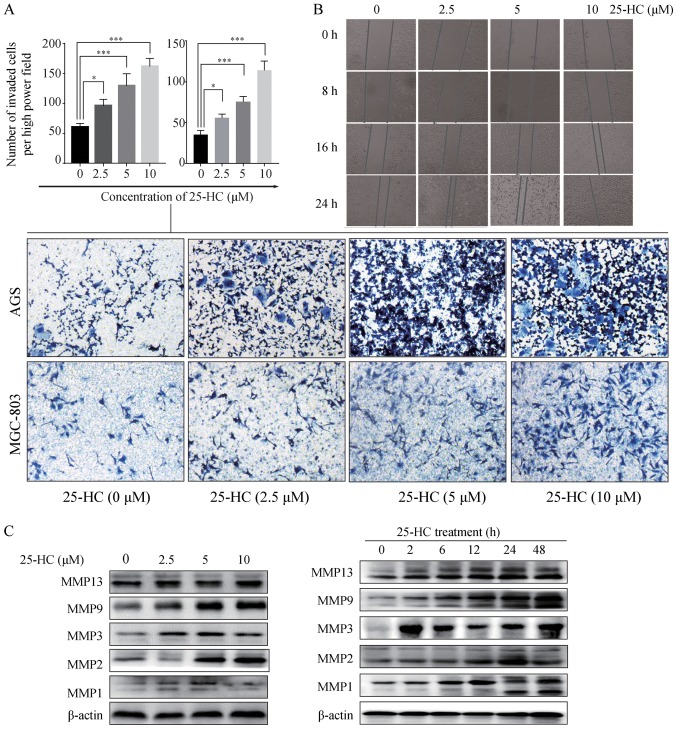
25-HC promotes GC cell migration and invasion in vitro
We then investigated the effects of 25-HC on GC cell migration and invasion in vitro. First, we performed a Transwell invasion assay. Compared to the control group, 25-HC treatment significantly enhanced the AGS cell and MGC-803 invasive ability (P<0.05; Fig. 5A). A wound healing assay was then carried out on the AGS cells. The results revealed that 25-HC treatment induced the more rapid closing of the scratch wounds in the AGS cells (Fig. 5B), indicating that 25-HC efficiently promoted the motility of the AGS cells.
Figure 5.
25-HC promotes GC cell migration and invasion in vitro. (A) Invasion assays of AGS cells or MGC-803 cells treated with various concentrations of 25-HC were performed using cell culture chambers with 8-µm pore filter. After 36 h, the cells invaded to the underside of the membrane were quantitated by cell counting in 5 pre-determined fields. (B) The migratory ability of the AGS cells was determined by wound healing assay. Monolayers of AGS cells were wounded by a 200-µl plastic tip and then incubated for a further 24 h in 5% FBS medium with different concentrations of 25-HC. (C) AGS cells were treated with the indicated concentrations of 25-HC for 24 h or were treated with 5 µM 25-HC for the indicated times before total cellular proteins were extracted for western blot analysis to detect the MMPs expressions. Results were obtained from 3 independent experiments and are expressed as the means ± SEM and statistical significance was determined by one-way ANOVA followed by Dunnett's test. Representative images are presented. *P<0.05 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer.
Metastasis involves tumor cell adhesion to the extracellular matrix (ECM), proteolytic cleavage or destruction of the ECM, and leads to cell migration through the resultant defect (22,23). MMPs are a group of enzymes that can degrade proteins in the ECM by the endopeptidase activity (24). Thus, in this study, we detected the MMP1, MMP2, MMP3, MMP9 and MMP13 expression levels which have been found to be overexpressed in human GC (25,26). As shown in Fig. 5C, 25-HC increased the expression levels of the detected MMPs in a dose- and time-dependent manner.
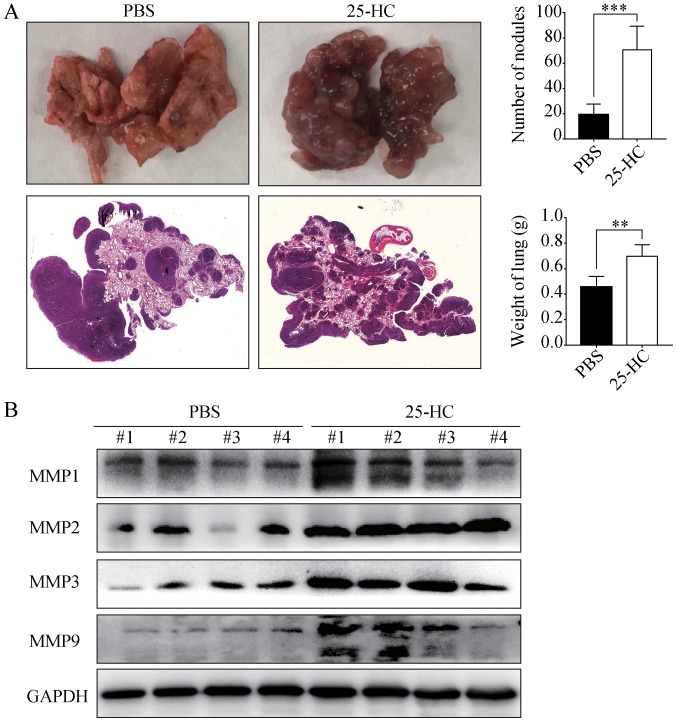
25-HC promotes the distant lung metastasis of AGS cells in vivo
To determine whether the promoting effects of 25-HC on GC cell invasion in vitro can be reproduced in vivo, the AGS cells were injected into the tail veins of BALB/c nude mice pre-treated with PBS or 25-HC. As shown in Fig. 6A, following 25-HC treatment, the number of metastatic nodules in lungs was significantly increased and the lung weight was also increased (P<0.01). In addition, the protein expression levels of MMP1, MMP2, MMP3 and MMP9 in the lung tissues were determined by western blot analysis. As shown in Fig. 6B, the expression levels of all the detected MMPs were much higher in the mice treated with 25-HC. Taken together, these data demonstrate that 25-HC strongly promotes the in vivo lung metastatic potential of GC cells.
Figure 6.
25-HC promotes lung metastasis in vivo. (A) Nude BALB/c mice were injected with AGS cells through the tail vein and randomly divided into the PBS and 25-HC groups. Mice in the 25-HC group were intraperitoneally injected with 25-HC (10 mg/kg) every 3 days. After 3 weeks, the mice were sacrificed, and lungs were removed and weighed. The lung metastatic tumors on the surface were calculated and H&E staining was performed. (B) Proteins were extracted from the lung tissues for western blot analysis to determine the MMP expression levels. Results were obtained from 2 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by Student's t-test. Representative images are presented. **P<0.01 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer; MMP, matrix metalloproteinase.
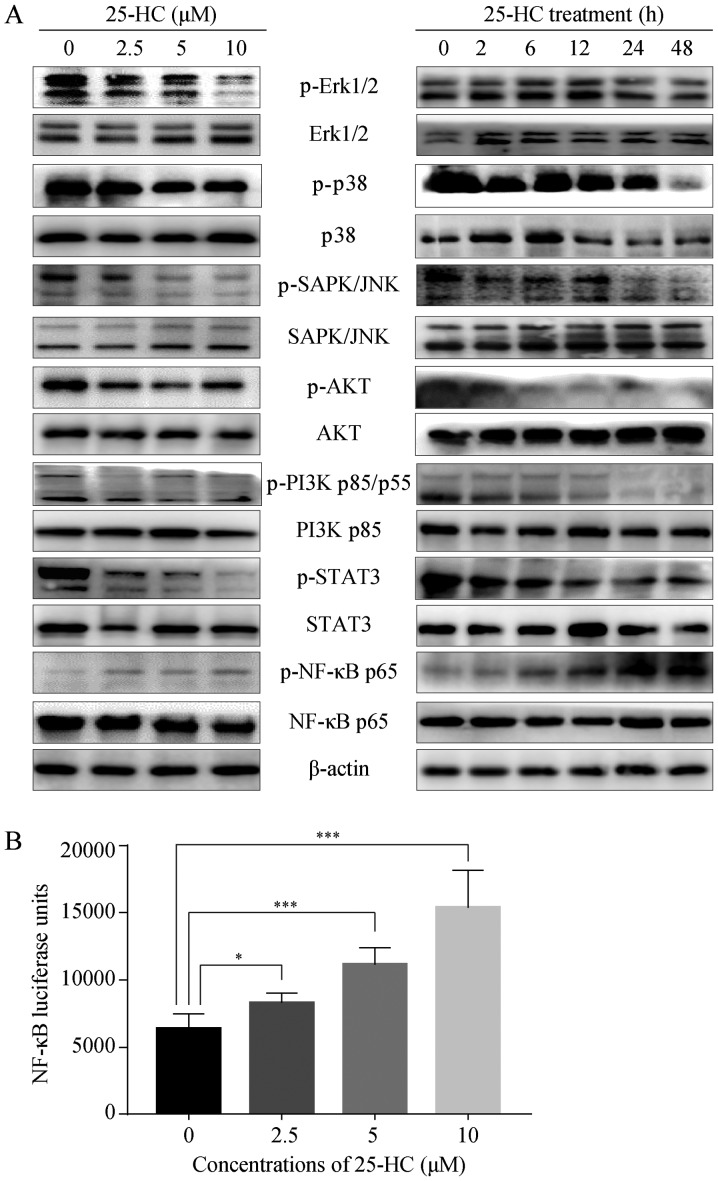
25-HC regulates multiple signaling pathways
To identify the 25-HC-associated signaling pathways, western blot analysis was performed. The mitogen-activated protein kinase (MAPKs, including JNK, Erk and p38), NF-κB, PI3K/ AKT and STAT3 signaling pathways have been associated with GC cell migration and invasion (27,28). The suppression of these signaling pathways can inhibit the migration and invasion of AGS cells, and the activation of these pathways promotes cell invasion and metastasis (29-31). The results of this study demonstrated that 25-HC upregulated the phosphorylation of NF-κB p65 in a dose- and time-dependent manner, while it decreased the phosphorylation levels of all the other proteins detected (Fig. 7A). We also assessed NF-κB activation using a luciferase reporter assay. Following exposure to increasing concentrations of 25-HC, NF-κB activity was significantly induced in a dose-dependent manner (Fig. 7B). These results indicate that NF-κB may be responsible for the induction of the migration and invasion of AGS cells.
Figure 7.
25-HC regulates multiple signaling pathways. (A) AGS cells were treated with the indicated concentrations of 25-HC for 24 h or treated with 5 µM 25-HC for the indicated times before total cellular proteins were extracted for western blot analysis to detect the p-Erk1/2, Erk1/2, p-p38, p38, p-SAPK/JNK, SAPK/JNK, p-AKT, AKT, p-PI3K p85/p55, PI3K p85/p55, p-STAT3, STAT3, p-NF-κB p65, NF-κB p65 expression levels. Results were representative of 3 independent experiments. (B) AGS cells were transfected with NF-κB luciferase reporter plasmid before the cells were treated with 25-HC. After 24 h, the luciferase activities were determined by using the Bright-Glo luciferase assay system. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Statistical significance was determined by one-way ANOVA followed by Dunnett's test. Representative images are presented. *P<0.05 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer.
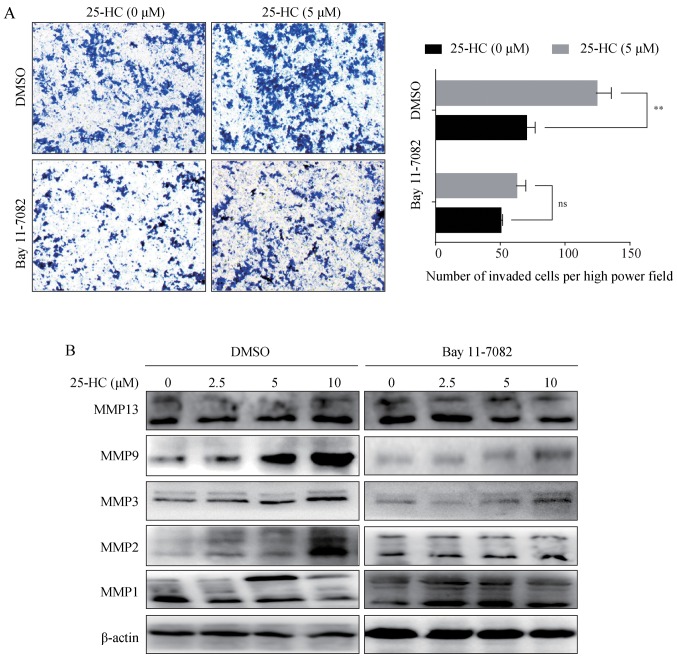
25-HC promotes AGS cell invasion and MMP expression levels through the upregulation of the NF-κB pathway
Subsequently, the AGS cells were treated with the NF-κB specific inhibitor, Bay 11-7082, prior to 25-HC treatment, and the invasive ability and MMP expression levels were analyzed. The results demonstrated that Bay 11-7082 treatment reversed the invasion induced by 25-HC (Fig. 8A) and abolished the upregulation of MMP expression levels (Fig. 8B). Taken together, these results demonstrated that 25-HC-promoted the invasion of and MMP expression levels in AGS cells by upregulating the NF-κB signaling pathway.
Figure 8.
25-HC promotes AGS cell invasion and MMP expression through the upregulation of the NF-κB pathway. (A) AGS cells in the upper chamber were pre-treated with Bay 11-7082 (2 µg/ml) for 1 h and the medium were replaced with FBS-free medium supplemented with 25-HC or not. After 36 h, the cells invaded to the underside of the membrane were quantitated by cell counting in 5 pre-determined fields. (B) AGS cells were pre-treated with Bay 11-7082 for 1 h followed by the treatment with the indicated concentrations of 25-HC for 24 h. Total cellular proteins were extracted for western blot analysis to detect the MMP expression levels. Results were obtained from 3 independent experiments and expressed as the means ± SEM. Statistical significance was determined by a Student's t-test. Representative images are presented; ns, no significance, **P<0.01. 25-HC, 25-hydroxycholesterol; GC, gastric cancer; MMP, matrix metalloproteinase.
The promoting effects of 25-HC on AGS cell invasion and MMP expression levels are partly dependent on TLR2
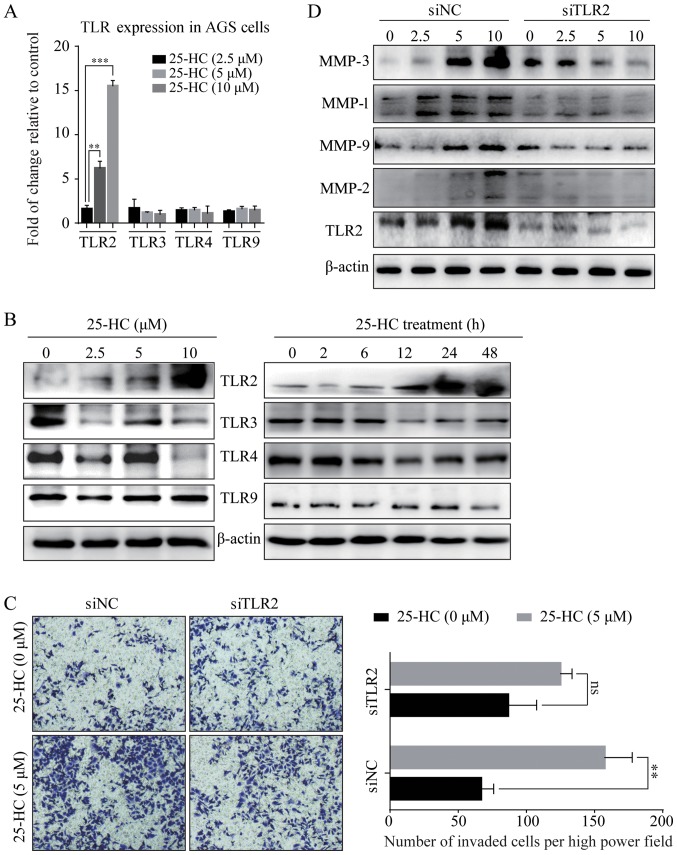
In this study, to investigate whether TLRs are involved in the promoting effects of 25-HC on AGS cell invasion, we first detected the expression levels of TLRs by RT-qPCR (Fig. 9A) and western blot analysis (Fig. 9B). The results revealed that 25-HC significantly induced TLR2 expression in the AGS cells in a time- and dose-dependent manner, while it had no effects on the TLR3, 4 and 9 expression levels. We then knocked down TLR2 in the AGS cells by siRNA transfection and the knock down efficiency was shown in Fig. 9D. As shown in Fig. 9C, the knockdown of TLR2 partly abolished the 25-HC-induced cell invasion. In addition, the expression levels of MMPs were also decreased by the knockdown of TLR2 (Fig. 9D). We also noted that the knockdown of TLR2 only partly decreased the 25-HC-induced AGS cell migration and MMP expressionlevels. Thus, we speculated that TLR2 is not the only signaling pathway responsible for 25-HC-promoted AGS cells invasion and MMPs expressions.
Figure 9.
25-HC-promotes AGS cell invasion and MMP expression and these effects are partly dependent on TLR2. (A) AGS cells were treated with the indicated concentrations of 25-HC for 24 h before the cells were collected, RNA was extracted for RT-qPCR to determine the mRNA expression levels of TLR2, TLR3, TLR4 and TLR9. Statistical significance was determined by one-way ANOVA followed by Dunnett's test. (B) AGS cells were treated with the indicated concentrations of 25-HC for 24 h or treated with 5 µM 25-HC for the indicated times before total cellular proteins were extracted for western blot analysis to detect the TLR2, TLR3, TLR4 and TLR9 expression levels. AGS cells were transfected with siNC or siTLR2 for 24 h. (C) Invasion assay was carried out following stimulation with 5 µM 25-HC. Statistical significance was determined by a Student's t-test. (D) Cells were treated with various concentrations of 25-HC for 24 h before total cellular proteins were extracted for western blot analysis to detect the MMP and TLR2 expression levels. Results were obtained from 3 independent experiments and are expressed as the means ± SEM. Representative images are presented; ns, no significance; **P<0.01 and ***P<0.001. 25-HC, 25-hydroxycholesterol; GC, gastric cancer; MMP, matrix metalloproteinase; TLR, Toll-like receptor.
Discussion
25-HC is one of the major cytotoxins in oxidized low-density lipoprotein (oxLDL) and has been demonstrated to exert dose-dependent effects on cell proliferation (32) and the induction of cell apoptosis (33-35). However, little is known about the role of 25-HC in GC. In this study, we demonstrated that 25-HC significantly decreased the sensitivity of AGS and MGC-803 cells to 5-FU, whereas it had no direct effects on cell proliferation and apoptosis. Moreover, 25-HC treatment led to the enhanced invasion of GC cells, accompanied by the increased expression levels of MMPs. Further investigations revealed that 25-HC promoted cells invasion via the TLR2-mediated activation of the NF-κB signaling pathway. These results provide new insight into the roles of 25-HC in GC progression.
The results of the present study are inconsistent with prior studies, since we reported that 25-HC had no direct effects on cell proliferation and apoptosis. We speculate that there are two reasons for this. One reason is that the effector cells are different since other studies were performed on non-tumor cells, including macrophages, vascular cells and differentiated PG12 cells (33-35) and perhaps these cells are more sensitive to 25-HC treatment. The other reason may be the different concentrations of 25-HC used in this study. Chen et al also reported 25-HC promoted A549 and NCL-H1975 lung adenocarcinoma and cell migration and invasion at the concentration of 0.1 µM without affecting cell proliferation, while displaying the inhibitory effect on cell proliferation from 1.0-25 µM (18), indicating that 25-HC plays differential roles at various concentrations. In this study, we reported that 25-HC promoted the migration and invasion of GC cells at the concentrations from 2.5-10 µM without affecting cell proliferation.
NF-κB is constitutively activated in many types of cancer and exerts a variety of pro-tumorigenic effects (36,37). A previous study reported that 25-HC amplified inflammatory signaling by mediating the recruitment of the AP-1 components FBJ osteosarcoma oncogene (FOS) and jun proto-oncogene (JUN) to the promoters of a subset of Toll-like receptor-responsive genes, resulting in the alteration of the inflammatory response (38). 25-HC can enhance the NF-κB DNA binding activity and the translocation of phosphorylated c-Jun into the nucleus (39). The activation of NF-κB is involved in the induction of the MMPs which are associated with the invasion and metastasis of tumor cells. Thus, the activated NF-κB signaling and the upregulated MMP expression levels following treatment with 25-HC in this study could be expected. It has been proven that the activation of NF-κB promotes GC cell proliferation (40). As shown in Figs. 7 and 8, the promotion of the invasion of GC cells by 25-HC was dependent on the NF-κB signaling pathway. It is noteworthy that 25-HC had no effects on cell proliferation, as shown in Fig. 1. Thus, we detected stem cell marker expression in AGS cells treated with 25-HC (Fig. S1), and the results revealed that 25-HC had no effects on the expression levels of CD44, ALDH1, Sox2 and KLF4. We assumed that the probable reason for this was that the effects of 25-HC are complex. Reboldi et al found that 25-HC decreased inflammasome activation in macrophages and consequently decreased the expression of IL-1β and caspase-1 activation (41) and Tricarico et al reported that 25-HC reduced inflammation, but was ineffective in restoring the autophagic flux and decreasing the apoptotic levels (42). All these controversial findings suggest that the effects of 25-HC are complex. Thus, we have reasons to assume that 25-HC may exert inhibitory effects on the activation of other signaling pathways, such as the Wnt or Hedgehog pathways (43) which could affect cell proliferation and apoptosis.
Oxysterols, including 7β-hydroxycholesterol (7β-OHC) has been reported to enhance the sensitivity of tumor cell lines, such as HepG2, U937 and K562 to adriamycin, VP-16, 5-FU and bleomycin (44). In this study, having determined that 25-HC had no direct effects on AGS and MGC-803 cell proliferation (Fig. 1), we thus detected whether 25-HC affects the sensitivity of GC cells to 5-FU. As shown in Fig. 3, 25-HC decreased the sensitivity of GC cells to 5-FU. To the best of our knowledge, this is a novel finding of this study.
To the best of our knowledge, there is limited research available on the association between 25-HC and TLRs. Erridge et al reported that in THP-1 cells, 25-HC stimulated inflammatory signaling via the lipid-recognizing TLRs 1, 2, 4 and 6, while independent of TLR signaling (45). In addition, TLR4 agonist can induce the synthesis of 25-HC in macrophages (46). In this study, we detected the expression levels of TLR2, TLR3, TLR4 and TLR9, which have been reported to be expressed in GC cells (47-50). The results of this study revealed that 25-HC upregulated TLR2 expression; however, the exact mechanisms involved are not yet clear and want further investigation. To the best of our knowledge, this is the first study to report the regulation of TLRs by 25-HC.
In conclusion, in this study, we identified a novel role of 25-HC in GC, in that 25-HC promotes GC cell migration and invasion by upregulating TLR2-NF-κB-mediated MMP expression. Therefore, the targeting of 25-HC may prove to be a potential therapeutic strategy for the treatment of GC.
Supplementary Data
Acknowledgments
Not applicable.
Funding
The present study was supported by a grant from the Key Project of Natural Science Foundation of Zhejiang Province (LZ16H160003 to WC).
Availability of data and materials
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.
Authors' contributions
SW and WC participated in the conception and design of the study. SW, YY, CR and GZ performed the statistical analysis and were involved in the preparation of the figures. SW and YY reviewed the results and participated in the discussion of the data. SW, GZ and WC prepared the manuscript and revised it. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All the experiments using animals were approved by the Animal Care and Use Committee of Zhejiang University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kloudova A, Guengerich FP, Soucek P. The role of oxysterols in human cancer. Trends Endocrinol Metab. 2017;28:485–496. doi: 10.1016/j.tem.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson ER, Chang CY, McDonnell DP. Cholesterol and breast cancer pathophysiology. Trends Endocrinol Metab. 2014;25:649–655. doi: 10.1016/j.tem.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang KA, Chae S, Lee KH, Park MT, Lee SJ, Lee YS, Hyun JW. Cytotoxic effect of 7beta-hydroxycholesterol on human NCI-H460 lung cancer cells. Biol Pharm Bull. 2005;28:1377–1380. doi: 10.1248/bpb.28.1377. [DOI] [PubMed] [Google Scholar]
- 4.Nagano K, Imai S, Zhao X, Yamashita T, Yoshioka Y, Abe Y, Mukai Y, Kamada H, Nakagawa S, Tsutsumi Y, et al. Identification and evaluation of metastasis-related proteins, oxysterol binding protein-like 5 and calumenin, in lung tumors. Int J Oncol. 2015;47:195–203. doi: 10.3892/ijo.2015.3000. [DOI] [PubMed] [Google Scholar]
- 5.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitayama J, Hatano K, Kaisaki S, Suzuki H, Fujii S, Nagawa H. Hyperlipidaemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br J Surg. 2004;91:191–198. doi: 10.1002/bjs.4391. [DOI] [PubMed] [Google Scholar]
- 9.Guo E, Chen L, Xie Q, Chen J, Tang Z, Wu Y. Serum HDL-C as a potential biomarker for nodal stages in gastric cancer. Ann Surg Oncol. 2007;14:2528–2534. doi: 10.1245/s10434-007-9401-0. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, Feng W, Zhang S, Bian K, Yang Y, Fang C, Chen M, Yang J, Zou X. Metformin inhibits gastric cancer via the inhibition of HIF1α/PKM2 signaling. Am J Cancer Res. 2015;5:1423–1434. [PMC free article] [PubMed] [Google Scholar]
- 11.Valaee S, Yaghoobi MM, Shamsara M. Metformin inhibits gastric cancer cells metastatic traits through suppression of epithelial-mesenchymal transition in a glucose-independent manner. PLoS One. 2017;12:e0174486. doi: 10.1371/journal.pone.0174486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: A systematic review and meta-analysis. Ann Oncol. 2013;24:1721–1730. doi: 10.1093/annonc/mdt150. [DOI] [PubMed] [Google Scholar]
- 13.Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 14.Johnson KA, Morrow CJ, Knight GD, Scallen TJ. In vivo formation of 25-hydroxycholesterol from endogenous cholesterol after a single meal, dietary cholesterol challenge. J Lipid Res. 1994;35:2241–2253. [PubMed] [Google Scholar]
- 15.Hodis HN, Chauhan A, Hashimoto S, Crawford DW, Sevanian A. Probucol reduces plasma and aortic wall oxysterol levels in cholesterol fed rabbits independently of its plasma cholesterol lowering effect. Atherosclerosis. 1992;96:125–134. doi: 10.1016/0021-9150(92)90059-P. [DOI] [PubMed] [Google Scholar]
- 16.Lappano R, Recchia AG, De Francesco EM, Angelone T, Cerra MC, Picard D, Maggiolini M. The cholesterol metabolite 25-hydroxycholesterol activates estrogen receptor α-mediated signaling in cancer cells and in cardiomyocytes. PLoS One. 2011;6:e16631. doi: 10.1371/journal.pone.0016631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simigdala N, Gao Q, Pancholi S, Roberg-Larsen H, Zvelebil M, Ribas R, Folkerd E, Thompson A, Bhamra A, Dowsett M, et al. Cholesterol biosynthesis pathway as a novel mechanism of resistance to estrogen deprivation in estrogen receptor-positive breast cancer. Breast Cancer Res. 2016;18:58. doi: 10.1186/s13058-016-0713-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Zhang L, Xian G, Lv Y, Lin Y, Wang Y. 25-Hydroxycholesterol promotes migration and invasion of lung adenocarcinoma cells. Biochem Biophys Res Commun. 2017;484:857–863. doi: 10.1016/j.bbrc.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Burkard I, von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis. 2007;194:71–78. doi: 10.1016/j.atherosclerosis.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 20.Yang P, Zhou Y, Chen B, Wan HW, Jia GQ, Bai HL, Wu XT. Overweight, obesity and gastric cancer risk: Results from a meta-analysis of cohort studies. Eur J Cancer. 2009;45:2867–2873. doi: 10.1016/j.ejca.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Høye AM, Erler JT. Structural ECM components in the premetastatic and metastatic niche. Am J Physiol Cell Physiol. 2016;310:C955–C967. doi: 10.1152/ajpcell.00326.2015. [DOI] [PubMed] [Google Scholar]
- 24.Yadav L, Puri N, Rastogi V, Satpute P, Ahmad R, Kaur G. Matrix metalloproteinases and cancer - roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15:1085–1091. doi: 10.7314/APJCP.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 25.Murray GI, Duncan ME, Arbuckle E, Melvin WT, Fothergill JE. Matrix metalloproteinases and their inhibitors in gastric cancer. Gut. 1998;43:791–797. doi: 10.1136/gut.43.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elnemr A, Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Tochiori S, Endou Y, Sasaki T. Expression of collagenase-3 (matrix metalloproteinase-13) in human gastric cancer. Gastric Cancer. 2003;6:30–38. doi: 10.1007/s101200300004. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:11673–11679. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 29.Yang MD, Lai KC, Lai TY, Hsu SC, Kuo CL, Yu CS, Lin ML, Yang JS, Kuo HM, Wu SH, et al. Phenethyl isothiocyanate inhibits migration and invasion of human gastric cancer AGS cells through suppressing MAPK and NF-kappaB signal pathways. Anticancer Res. 2010;30:2135–2143. [PubMed] [Google Scholar]
- 30.Yakata Y, Nakayama T, Yoshizaki A, Kusaba T, Inoue K, Sekine I. Expression of p-STAT3 in human gastric carcinoma: Significant correlation in tumour invasion and prognosis. Int J Oncol. 2007;30:437–442. [PubMed] [Google Scholar]
- 31.Duan H, Qu L, Shou C. Activation of EGFR-PI3K-AKT signaling is required for Mycoplasma hyorhinis-promoted gastric cancer cell migration. Cancer Cell Int. 2014;14:135. doi: 10.1186/s12935-014-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velázquez E, Santos A, Montes A, Blázquez E, Ruiz-Albusac JM. 25-Hydroxycholesterol has a dual effect on the proliferation of cultured rat astrocytes. Neuropharmacology. 2006;51:229–237. doi: 10.1016/j.neuropharm.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 33.Travert C, Carreau S, Le Goff D. Induction of apoptosis by 25-hydroxycholesterol in adult rat Leydig cells: Protective effect of 17beta-estradiol. Reprod Toxicol. 2006;22:564–570. doi: 10.1016/j.reprotox.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Choi YK, Kim YS, Choi IY, Kim SW, Kim WK. 25-hydroxy-cholesterol induces mitochondria-dependent apoptosis via activation of glycogen synthase kinase-3beta in PC12 cells. Free Radic Res. 2008;42:544–553. doi: 10.1080/10715760802146062. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Sinensky MS. 25-Hydroxycholesterol activates a cytochrome c release-mediated caspase cascade. Biochem Biophys Res Commun. 2000;278:557–563. doi: 10.1006/bbrc.2000.3855. [DOI] [PubMed] [Google Scholar]
- 36.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiDonato JA, Mercurio F, Karin M. NF-κB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 38.Gold ES, Diercks AH, Podolsky I, Podyminogin RL, Askovich PS, Treuting PM, Aderem A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc Natl Acad Sci USA. 2014;111:10666–10671. doi: 10.1073/pnas.1404271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koarai A, Yanagisawa S, Sugiura H, Ichikawa T, Kikuchi T, Furukawa K, Akamatsu K, Hirano T, Nakanishi M, Matsunaga K, et al. 25-Hydroxycholesterol enhances cytokine release and Toll-like receptor 3 response in airway epithelial cells. Respir Res. 2012;13:63. doi: 10.1186/1465-9921-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, Byun DS, Chae KS, Lee BH, Chun HS, et al. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135:2030–2042. 2042.e1–3. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tricarico PM, Gratton R, Braga L, Celsi F, Crovella S. 25-Hydroxycholesterol and inflammation in Lovastatin-deregulated mevalonate pathway. Int J Biochem Cell Biol. 2017;92:26–33. doi: 10.1016/j.biocel.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 43.de Weille J, Fabre C, Bakalara N. Oxysterols in cancer cell proliferation and death. Biochem Pharmacol. 2013;86:154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 44.Hyun JW, Holl V, Weltin D, Dufour P, Luu B, Bischoff P. Effects of combinations of 7beta-hydroxycholesterol and anticancer drugs or ionizing radiation on the proliferation of cultured tumor cells. Anticancer Res. 2002;22:943–948. [PubMed] [Google Scholar]
- 45.Erridge C, Webb DJ, Spickett CM. 25-Hydroxycholesterol, 7beta-hydroxycholesterol and 7-ketocholesterol upregulate interleukin-8 expression independently of Toll-like receptor 1, 2, 4 or 6 signalling in human macrophages. Free Radic Res. 2007;41:260–266. doi: 10.1080/10715760601070091. [DOI] [PubMed] [Google Scholar]
- 46.Bauman DR, Bitmansour AD, McDonald JG, Thompson BM, Liang G, Russell DW. 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci USA. 2009;106:16764–16769. doi: 10.1073/pnas.0909142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Li Y, Li Y, Li R, Ma Y, Wang H, Wang Y. Chloroquine inhibits MGC803 gastric cancer cell migration via the Toll-like receptor 9/nuclear factor kappa B signaling pathway. Mol Med Rep. 2015;11:1366–1371. doi: 10.3892/mmr.2014.2839. [DOI] [PubMed] [Google Scholar]
- 48.Yue Y, Zhou T, Gao Y, Zhang Z, Li L, Liu L, Shi W, Su L, Cheng B. High mobility group box 1/toll-like receptor 4/myeloid differentiation factor 88 signaling promotes progression of gastric cancer. Tumour Biol. 2017 Mar 28; doi: 10.1177/1010428317694312. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Garcia B, Eiró N, González-Reyes S, González L, Aguirre A, González LO, Del Casar JM, García-Muñiz JL, Vizoso FJ. Clinical significance of toll-like receptor 3, 4, and 9 in gastric cancer. J Immunother. 2014;37:77–83. doi: 10.1097/CJI.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 50.West AC, Tang K, Tye H, Yu L, Deng N, Najdovska M, Lin SJ, Balic JJ, Okochi-Takada E, McGuirk P, et al. Identification of a TLR2-regulated gene signature associated with tumor cell growth in gastric cancer. Oncogene. 2017;36:5134–5144. doi: 10.1038/onc.2017.121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article or are available from the corresponding author on reasonable request.