Abstract
Side population (SP) cells are a small subpopulation of cells found in many mammalian tissues and organs, identified by their capacity to efflux Hoechst 33342 dye. They are enriched for stem/progenitor cell activity. SP cells isolated from the adult mouse lung can be separated into a CD45+ subset (bone marrow-derived) and a CD45− subset that can be subdivided into CD31− and CD31+ subpopulations. CD45−/CD31− lung SP (LSP) cells are known to be mesenchymal stem cells. However, CD45−/CD31+ LSP cells are not fully characterized. In the present study, it was found that CD45−/CD31+ LSP cells were able to form colonies. Based on the expression of vascular endothelial growth factor receptor 2 (VEGFR2), these cells were separated into VEGFR2− and VEGFR2+ cells. The CD45−/CD31+/VEGFR2− LSP cells expressed genes characteristic of smooth muscle and endothelial progenitors, and were able to differentiate into smooth muscle and endothelial cells in vitro. The CD45−/CD31+/VEGFR2+ LSP cells expressed genes characteristic of endothelial progenitors and gave rise to endothelial cells, although not smooth muscle, in vitro. The data demonstrate that CD45−/CD31+/VEGFR2− LSP cells differentiated into CD45−/CD31+/VEGFR2+ LSP cells and then endothelial cells, indicating that CD45−/CD31+/VEGFR2+ LSP cells are likely to be derived from CD45−/CD31+/VEGFR2− LSP cells. Taken together, the results suggest that CD45−/CD31+ LSP cells can be separated into CD45−/CD31+/VEGFR2− LSP cells, which may be progenitors of endothelial and smooth muscle, whereas CD45−/CD31+/VEGFR2+ LSP cells may serve as late commitment endothelial progenitors in the adult mouse lung.
Keywords: side population cells, lung, endothelial cells, smooth muscle cells, stem cells
Introduction
Chronic pulmonary diseases, including pulmonary hypertension, chronic obstructive pulmonary disease and interstitial pulmonary fibrosis, constitute major contributors to morbidity and mortality rates (1,2). The use of stem cells for cell-based therapy in chronic pulmonary diseases has been under investigation (3–8). In previous years, different populations of lung stem- or progenitor-cells have been reported to reside within the adult mouse lung. They are thought to be involved in tissue maintenance and repair, however, they may also contribute to pulmonary diseases (4,5,8). Using different markers and methods, these cells have been variously described as: i) Airway clara cells (9), ii) alveolar type II cells (10), iii) lung c-kit positive cells (11,12), and iv) lung side population (LSP) cells (13–16). SP cells are identified by their capacity to efflux Hoechst 33342 dye, allowing the weakly-labeled SP cells to be distinguished from the intensely-labeled main population (MP) of cells (17–19). This unique property is mediated through the action of the p-glycoprotein multidrug/ATP-binding cassette transporter protein, ATP-binding cassette super-family G member 2 (ABCG2) on the surface of SP cells, which pumps Hoechst dye out of the cell (20,21). SP cells have been found in numerous adult mammalian tissues and organs, including bone marrow, the heart, liver, skeletal muscle, brain, kidney and lung (14,19,21–23). These cells are rare and they are enriched for stem/progenitor cell activity.
Several studies have suggested that LSP cells are capable of differentiation into epithelial cells, endothelial cells, smooth muscle cells and mature hematopoietic cells (13–16,24). LSP cells are known to be heterogeneous (13,14,24,25). Based on their expression of CD45, LSP cells can be separated into CD45+ and CD45− subpopulations. CD45+ LSP cells possess hematopoietic progenitor and stem cell activity, and are most likely bone marrow-derived (14,24). By contrast, CD45− LSP cells can be further divided into CD45−/CD31− and CD45−/CD31+ subpopulations (15,24). The CD45−/CD31− subpopulation has been shown to comprise resident mesenchymal progenitor cells, capable of differentiating in vitro into cartilage, bone and adipose cells (15). However, the nature and differentiation potential of CD45−/CD31+ LSP cells remain to be fully elucidated. Our previous study demonstrated that CD45− LSP cells may be progenitors of endothelial and smooth muscle cells in the embryonic mouse lung (14,24). In addition, it has been suggested that mouse heart and lung tissues, including blood vessel cells, may share a novel population of progenitor cells (26). In the adult mouse heart, the stem cell antigen-1 (SCA1)+/CD31+ subpopulation of cardiac SP (CSP) cells have been reported to be endothelial progenitors (27,28). Therefore, it was hypothesized that CD45−/CD31+ LSP cells may serve as progenitors of endothelial and smooth muscle cells in the adult mouse lung. Previous studies have indicated that CD45−/VEGFR2+ LSP cells may be endothelial progenitor cells in the adult mouse lung. The association between CD45−/CD31+ and CD45−/VEGFR2+ LSP cells remains to be elucidated. The present study aimed to characterize CD45−/CD31+ LSP cells in the adult mouse lung, to clarify their differentiation potential, and to examine the association between these two types of LSP cells in vitro.
Materials and methods
Animals
Female wild-type C57BL/6J mice (aged 8–10 weeks, weighting 22–25 g) were purchased from Beijing HFK-Biotechnology (Beijing, China). The mice were group-housed in individually-ventilated cages under pathogen-free conditions. The mice were maintained under a 12 h light/dark photoperiod, at 20±1°C with 60±10% relative humidity and food and water were provided ad libitum. The general health status of the animals was monitored daily. Animal care, handling and all animal experiments described herein were conducted strictly according to ethical standards approved by the Animal Ethical Committee of Jinzhou Medical University (Jinzhou, China; approval ID: LY2014D001).
CD45−/CD31+ LSP cell isolation, fluorescence-activated cell sorting (FACS) and analysis
The LSP cells were isolated as previously described (14,15,28,29). Briefly, the mice were sacrificed by isoflurane anesthesia followed by cervical dislocation. Prior to lung extraction, animals were bled by transecting the abdominal aorta. Subsequently, in an attempt to remove circulating blood cells, perfusion of the pulmonary vasculature was performed using ice-cold saline until the lungs were bleached white. The lung extracts were then digested by finely mincing tissue with a razor blade in the presence of 0.1% collagenase (Roche Diagnostics, Indianapolis, IN, USA), 2.4 U/ml dispase (Roche Diagnostics), and 2.5 mM CaCl2 at 37°C for 1 h. Removal of nonspecific debris was accomplished by sequential filtration through 70– and 40–µm filters, and the cells were resuspended at a density of 1×106 cells/ml. Cell staining was performed by incubating with Hoechst 33342 (5 µg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 90 min in the presence or absence of verapamil (50 mM; Sigma-Aldrich; Merck KGaA) (21). The suspension was subsequently incubated with one or more fluorescently-conjugated monoclonal rat anti-mouse antibodies, including anti-CD45-PerCP-Cy5.5 (1:100; cat. no. 550994; BD Biosciences, Franklin Lakes, NJ, USA), anti-CD31-PE (1:100; cat. no. 553373; BD Biosciences), anti-VEGFR2-APC (1:100; cat. no. 561993; BD Biosciences), anti-SCA1-FITC (1:100; cat. no. 557405; BD Biosciences) and anti-c-kit-FITC (1:100; cat. no. 561680; BD Biosciences). Isotype control antibodies were used in parallel as negative controls and to establish gating parameters for positively stained cells. All incubations were performed for 30 min at 4°C in the dark.
FACS was conducted using the MoFlo™ XDP flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) to isolate CD45−/CD31+ LSP cells, CD45−/CD31+/VEGFR2− LSP cells, CD45−/CD31+/VEGFR2+ LSP cells, CD45− lung MP cells and CD45−/CD31+ LMP cells for all experiments. The following six parameters were used to discriminate cells in the experiments: Hoechst 33342 (red), Hoechst 33342 (blue), forward scatter, side scatter, PerCP-Cy5.5-CD45, FITC-SCA1 and PE-CD31 reactivity (green) as previously described (14,27,30). This protocol is illustrated in Fig. 1. The Hoechst dye was excited at 350 nm. A 488-nm argon laser was employed for exciting PerCP-Cy5.5, PE and FITC, and a 633-nm HeNe laser was used for APC. Data analysis was performed using Summit™ V5.2 software (Beckman Coulter, Inc.).
Figure 1.
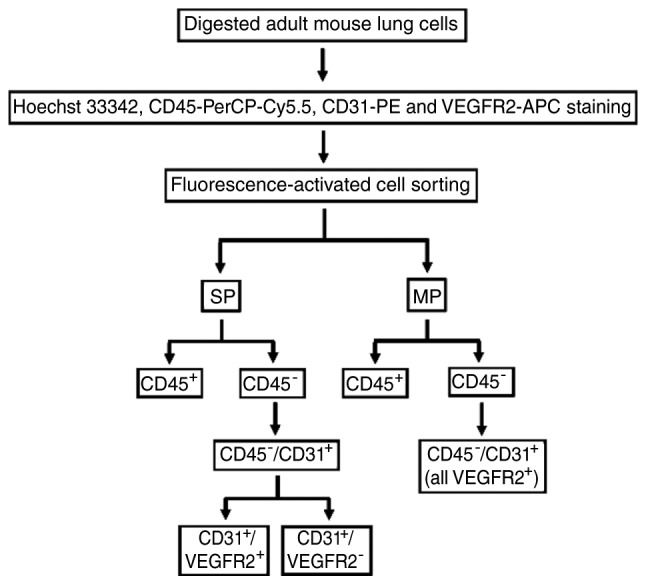
Flow chart illustrating the protocol used in the present study to isolate CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ lung SP cells and CD45−/CD31+ cells from adult mouse lungs. VEGFR2, vascular endothelial growth factor receptor 2; SP, side population; MP, main population.
Primary cell cultures
The murine CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells were cultured on fibronectin-coated 8-well chamber slides (10,000 cells/well; Nunc; Sigma-Aldrich; Merck KGaA) at 37°C under 5% CO2 as previously described (24,28). To promote endothelial cell growth and differentiation, the murine CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells were cultured at 37°C with a defined endothelial differentiation medium (EGM-2) from Cambrex (Baltimore, MA, USA) supplemented with 100 U/ml of penicillin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 µg/ml of streptomycin (Invitrogen; Thermo Fisher Scientitic, Inc.). Following 14 days in culture, the cells were collected for reverse-transcription quantitative polymerase chain reaction (RT-qPCR) and immunostaining analyses, respectively. To examine the association between CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells, the CD45−/CD31+/VEGFR2− LSP cells were cultured in the same conditions mentioned above for 2 days and then collected for RT-qPCR analysis.
To promote smooth muscle growth and differentiation, the CD45−/CD31+/VEGFR2− LSP cells were cultured with a defined smooth muscle differentiation medium (SmGm-2) from Cambrex supplemented with 100 U/ml of penicillin (Invitrogen; Thermo Fisher Scientific, Inc.), and 100 µg/ml of streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). The culture medium was replaced every 2 days. Following 14 days in culture, the cells were collected for RT-qPCR and immunostaining analyses. To determine whether the CD45−/CD31+/VEGFR2+ LSP cells were able to differentiate into smooth muscle cells, they were cultured in the medium described above for 14 days and were then harvested for RT-qPCR analysis.
Cell line culture
The HepG2 human liver cancer cell line, obtained from American Type Culture Collection (Manassas, VA, USA), was cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% GIBCO® fetal calf serum, 100 U/ml of penicillin, and 100 µg/ml of streptomycin (all from Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a 5% CO2 incubator.
Cellular uptake of Dil-labeled acetylated low-density lipoprotein (Dil-Ac-LDL)
The CD45−/CD31+ LSP cells at a density of 7,000 cells/well were incubated with EGM-2 containing 10 mg/ml of Dil-Ac-LDL (Molecular Probes; Thermo Fisher Scientific, Inc.) at 37°C for 8 h as previously described (28). Following washing with PBS, the cells were fixed and nuclei were counterstained with 4-6-diamidino-2-phenylindol-dihydrochloride (DAPI). The slides were examined using an Olympus-DP70 microscope and images were captured with a digital camera (BX51; Olympus Corporation, Tokyo, Japan). HepG2 cells were included to provide a negative control for Ac-LDL uptake.
Methylcellulose assay (colony-forming unit assay)
The CD45−/CD31+ LSP and LMP cells (7,000 cells/ml) were cultured in Methocult GF M3534 medium according to the manufacturer's protocol (StemCell Technologies, Inc., Vancouver, Canada) as described previously (17,19,27,28). Cell colonies, consisting of >30 cells, were scored after 14 days in culture. The cell colonies were fixed with 4% paraformaldehyde and stained with crystal violet based on the manufacturer's protocol (Sigma-Aldrich; Merck KGaA). Photomicrographs were obtained, from which the colonies were counted using an Olympus-DP70 microscope and images were captured with a digital camera (BX51). In order to determine whether the cells retained their CD45−/CD31+ LSP phenotype, Methoult medium was cut into sections and these were incubated with DMEM at 37°C with agitation for 30 min. When the Methocult medium had dissolved in the DMEM, the cells were collected, labeled with the CD45-PerCP-Cy5.5 (1:100), CD31-PE (1:100) and SCA1-FITC (1:100) antibodies, and analyzed by FACS.
RT-qPCR analysis
Total RNA was extracted from the freshly isolated distinct LSP, LMP and cultured cells using an RNAeasy-Mini kit, following the manufacturer's protocol (Qiagen GmbH, Hilden, Germany). Complementary DNA was generated using an M-MLV MicroRNA Reverse Transcription kit (Promega Corporation, Madison, WI, USA). RT-qPCR analysis was performed as previously described using a Rotor-gene 3000 machine (Corbett; Qiagen GmbH) with a SYBR-Green qPCR-SuperMix-UDG kit (Thermo Fisher Scientific, Inc.) following the manufacturer's protocol. Commercial primer sets were obtained from Qiagen GmbH, as follows: ATP-binding cassette super-family G member 2 (ABCG2; cat. no. QT00173138), CD133 (cat. no. QT01065162), VEGFR2 (cat. no. QT00097020), von Willebrand factor (vWF; cat. no. QT00116795), α-smooth muscle tropomyosin (αSMT; cat. no. QT00137354), α-smooth muscle actin (αSMA; cat. no. QT0006746), CD44 (cat. no. QT00173404), myosin heavy chain (cat. no. QT01558130) and β-actin (cat. no. QT01136772). qPCR was performed in a 20-µl reaction system, which contained 10 µl SYBR-Green qPCR-SuperMix-UDG (Thermo Fisher Scientific, Inc.), 2 µl cDNA, 0.4 µl each primer and 7.2 µl RNase-free H2O. The thermal cycler conditions were performed as follows: Cycle 1 (95°C for 3 min), Cycle 2 (35 cycles of 95°C for 30 sec, 57°C for 30 sec, 72°C for 30 sec), Cycle 3 (72°C for 4 min), which was based on the manufacturer's protocol (31). All samples were amplified in triplicate. Amplification data were analyzed with Corbett Rotor-gene 3000 software (Qiagen GmbH) as described previously (27,28). The relative mRNA expression levels of the genes mentioned above were normalized against the expression of housekeeper gene β-actin and calculated using the 2−ΔΔCq method as previously described (28,31,32). Gene expression data are presented in relative units.
Immunofluorescence staining of isolated and cultured cells
For analysis of the sorted cells, 1,000 CD45−/CD31+/VEGFR2− LSP cells, CD45−/CD31+/VEGFR2+ LSP cells and CD45− LMP cells were collected onto positively charged slides by cyto-centrifugation at 60 × g for 5 min at room temperature prior to air-drying. The slides were then fixed for 10 min in 4% paraformaldehyde/PBS, washed with 1X PBS, air dried and stored at 4°C prior to staining. The cultured monolayer cells were fixed and prepared for staining using the same procedure. Immunofluorescence staining was performed as previously described (14). Autofluorescence was first quenched with 10 mM sodium borohydride for 30 min at room temperature. Nonspecific binding was blocked by incubating with 1% rabbit serum (cat. no. ab7356; Abcam, Cambridge, MA, USA) or goat serum (cat. no. ab7487; Abcam). The primary antibodies used included monoclonal rat anti-mouse ABCG2 (1:100; cat. no. ab24115; Abcam), polyclonal rabbit anti-mouse vWF (1:100; cat. no. AB7356; Chemicon, Temecula, CA, USA), monoclonal anti-mouse αSMA-Cy3 (1:100; cat. no. C-6198; Sigma-Aldrich; Merck KGaA) and polyclonal rabbit anti-mouse αSMT (1:100; cat. no. T3651; Sigma-Aldrich; Merck KGaA). Secondary antibodies included rabbit anti-rat IgG-FITC (1:200; cat. no. ab6730-1; Abcam), goat anti-rabbit IgG-594 (1:200; cat. no. A11012; Invitrogen; Thermo Fisher Scientific, Inc.) and donkey anti-rabbit IgG-FITC (1:200; cat. no. A21206; Invitrogen; Thermo Fisher Scientific, Inc.). All incubations of primary and secondary antibodies were performed for 60 and 30 min at 4°C in the dark, respectively. The nuclei were counterstained with DAPI. Slides were examined using an Olympus-DP70 microscope and images were captured with a digital camera (BX51).
Statistical analysis
Data are presented as the mean ± standard deviation. Student's t-test (two-tailed unpaired) was used to analyze experimental data containing two groups, and one-way analysis of variance with the Bonferroni post hoc test was used to analyze experimental data with ≥3 groups. Statistical analysis was performed using SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference. Each experiment was repeated three times to obtain the means used for statistical analysis (n=3 experiments) as previously described (28).
Results
LSP cell populations Identification, isolation and gene expression
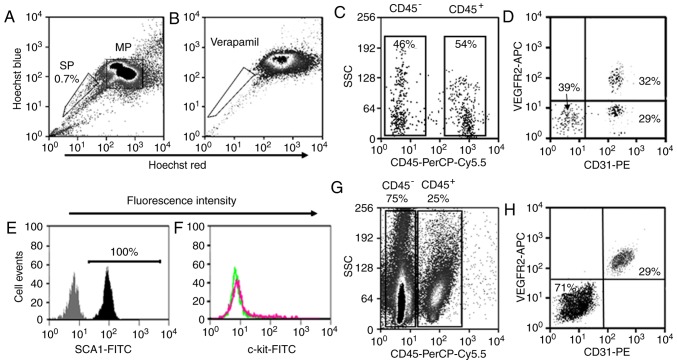
The adult mouse lungs were enzymatically digested into single cell suspensions and subjected to FACS analysis (Fig. 1). The LSP cells were detected on density dot plots based on their capacity to efflux Hoechst 33342 dye. Those cells that were weakly labeled for Hoechst 33342 dye (LSP cells) represented ~0.7% of the total cell population (Fig. 2A). The LSP population disappeared when the ABCG2 transporter was blocked with verapamil (Fig. 2B). The LSP cells were first separated into CD45+ (~54% of total LSP cells) and CD45− (~46% of total LSP cells) subpopulations as previously described (14) (Fig. 2C). Based on the expression of CD31, the CD45− LSP cells were further divided into CD45−/CD31− LSP cells (~39% of total CD45− LSP cells) and CD45−/CD31+ LSP cells (~61%). According to expression of VEGFR2, CD45−/CD31+ LSP cells were further separated into CD45−/CD31+/VEGFR2− (~29% of total CD45− LSP cells) and CD45−/CD31+/VEGFR2+ LSP cells (~32%) (Fig. 2D). All CD45−/CD31+ LSP cells (VEGFR2− and VEGFR2+) expressed SCA1, but none expressed c-kit (Fig. 2E and F). Using the same surface markers, the LMP cells were separated into CD45+ and CD45− subpopulations, which were further divided into CD45−/CD31−/VEGFR2− and CD45−/CD31+/VEGFR2+ LMP cells. Notably, all CD45−/CD31+ LMP cells were VEGFR2+ (Fig. 2G and H). Based on their immune-phenotype, CD45−/CD31+ LSP, CD45−/CD31+/VEGFR2− LSP, CD45−/CD31+/VEGFR2+ LSP, CD45− LMP and CD45−/CD31+ LMP cells were isolated by FACS (Fig. 2).
Figure 2.
Analysis and isolation of LSP and LMP cells from the adult mouse lung by fluorescence-activated cell sorting. (A) Lung SP cells (outlined to lower left) representing ~0.7% of the total heart cell population and MP cells (outlined to right) were identified based upon their intensity of Hoechst staining. (B) SP cells disappeared when cells were labeled in the presence of verapamil pretreatment, which inhibits the transporter (outlined to lower left). (C) Based on the expression of CD45, LSP cells were divided into CD45+ and CD45− representing ~54 and 46% of the total LMP cells, respectively. (D) According to expression of CD31 and VEGFR2, CD45− LSP cells were further separated into CD45−/CD31+/VEGFR2−, CD45−/CD31+/VEGFR2+ and CD45−/CD31−/VEGFR2−, representing ~29, 32 and 39% of the total LSP cells, respectively. CD45−/CD31+ LSP cells (including CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells) were stained with FITC-conjugated (E) SCA1 and (F) c-kit antibody (black and red respectively) or its isotype control (gray and green respectively), showing 100% positive for SCA1 and negative for c-kit. (G) LMP cells were separated into CD45+ (25% of total LMP) and CD45− (75% of total LMP) cells and (H) further divided into CD45−/CD31+/VEGFR2+ and CD45−/CD31−/VEGFR2− LMP cells. SP, side population; MP, main population; LSP, lung SP; LMP, lung MP; VEGFR2, vascular endothelial growth factor receptor 2; SCA1, stem cell antigen-1.
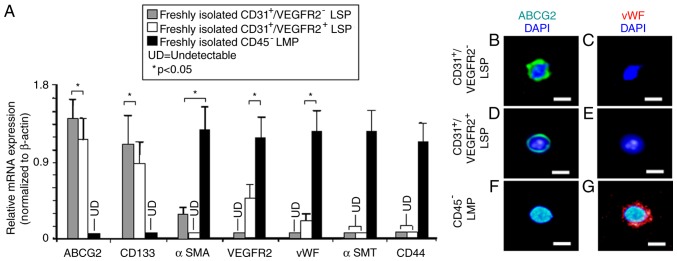
In our previous study, it was found that CD31+ CSP cells are endothelial progenitors of adult mouse heart (27). Therefore, the present study aimed to further characterize CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells by examining the expression of genes specific for stem/progenitor cells and endothelial cells, using RT-qPCR analysis. As expected, the CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells expressed high mRNA levels of the ABCG2 transporter, which is responsible for the efflux of Hoechst 33342 dye (20). Both also expressed CD133, a marker for endothelial progenitor cells (33). By contrast, the CD45− LMP cells expressed neither ABCG2 nor CD133. VEGFR2, a marker of endothelial cells, and vWF, a mature endothelial cell marker, were just detectable in the CD45−/CD31+/VEGFR2+ LSP cells but not in the CD45−/CD31+/VEGFR2− LSP cells (Fig. 3A). CD45− LMP cells, serving as a positive control, expressed high levels of VEGFR2 and vWF (Fig. 3A). The CD45− LMP cells also expressed high levels of mRNAs encoding αSMA, a marker of smooth muscle progenitors (24,34), αSMT, a marker of mature smooth muscle cells (24,34) and CD44, a marker of mesenchymal cells. By contrast, the expression of αSMA was low (but detectable) in the CD45−/CD31+/VEGFR2− LSP cells and completely undetectable in the CD45−/CD31+/VEGFR2+ LSP cells, indicating that CD45−/CD31+/VEGFR2− LSP cells may be smooth muscle progenitors, whereas CD45−/CD31+/VEGFR2+ LSP cells are not. In the CD45−/CD31+/VEGFR2− and the CD45−/CD31+/VEGFR2+ LSP cells, no expression of αSMT and CD44 was detectable (Fig. 3A), suggesting that neither the CD45−/CD31+/VEGFR2− LSP cells nor the CD45−/CD31+/VEGFR2+ LSP cells are mature smooth muscle or mesenchymal cells.
Figure 3.
Gene expression in sorted LSP and LMP cells. Expression of markers for stem cells (ABCG2), endothelial progenitors (CD133), early smooth muscle (αSMA), endothelial cells (VEGFR2), mature endothelial cells (vWF), mature smooth muscle cells (αSMT) and mesenchymal cells (CD44) were compared among sorted populations of cells. (A) Reverse transcription-quantitative polymerase chain reaction analysis compared relative mRNA expression levels in CD45−/CD31+/VEGFR2− (grey bar) and CD45−/CD31+/VEGFR2+ LSP (white bar) cells. CD45− LMP cells (black bar) were used as a control. For immunofluorescent labeling of cells, following sorting, CD45−/CD31+/VEGFR2+ and CD45−/CD31+/VEGFR2− LSP cells and CD45− LMP cells were cytospun onto glass slides. Nuclei (blue) were counterstained with DAPI. CD45−/CD31+/VEGFR2− LSP cells were immunopositive for (B) ABCG2 (green), but not (C) vWF (red). Similarly, CD45−/CD31+/VEGFR2+ LSP cells were positive for (D) ABCG2 (green), but not for (E) vWF (red). By contrast, CD45− LMP cells were not immunopositive for (F) ABCG2 (green) but were for (G) vWF (red). Scale bar, 30 µm. Data are presented as the mean ± standard deviation (n=3 experiments); *P<0.05; UD, undetectable; LSP, lung side population; LMP, lung main population; ABCG2, ATP-binding cassette super-family G member 2; VEGFR2, vascular endothelial growth factor receptor 2; vWF, von Willebrand factor; αSMT, α-smooth muscle tropomyosin; αSMA, α-smooth muscle actin; DAPI, 4-6-diamidino-2-phenylindol-dihydrochloride.
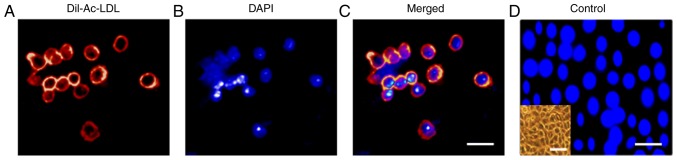
Subsequently, the CD45−/CD31+/VEGFR2− LSP, CD45−/CD31+/VEGFR2+ LSP and CD45− LMP cells were collected onto duplicate glass slides (1,000 cells/slide) and antibody probes were used to examine the expression of proteins. The immunostaining confirmed that the CD45−/CD31+/VEGFR2− LSP and CD45−/CD31+/VEGFR2+ LSP cells expressed the ABCG2 transporter protein, but not the vWF protein (Fig. 3B-E). By contrast, the CD45− LMP cells expressed vWF protein, but not the ABCG2 transporter (Fig. 3F and G). Based on the mRNA analysis, the high expression of CD133, low expression of αSMA, and absence of VEGFR2, vWF, αSMT and CD44, suggested that CD45−/CD31+/VEGFR2− LSP cells may be progenitors for endothelial and smooth muscle cells, but not progenitors for mesenchymal cells. By contrast, the high expression of CD133, low (detectable) expression of VEGFR2 and vWF, and absence of αSMA, αSMT and CD44 indicate that CD45−/CD31+/VEGFR2+ LSP cells may be late-commitment endothelial progenitors. Taken together, these results indicate that CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells may be endothelial progenitor cells. The finding that CD45−/CD31+ LSP cells, including CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells, take up DiI-Ac-LDL (Fig. 4A-D) supports this notion.
Figure 4.
Representative photomicrograph field showing Dil-Ac-LDL uptake by CD45−/CD31+ LSP cells (including CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells). (A) CD45−/CD31+ LSP cells demonstrating cellular uptake of Dil-Ac-LDL. (B) DAPI fluorescence channel, showing nuclei. (C) Merged fluorescence channels. Scale bar, 30 µm. (D) HepG2 cells, which did not take up Dil-Ac-LDL, served as a negative control. Scale bar, 30 µm. The inset on the lower left shows a phase contrast image of the cultured HepG2 cells (scale bar, 60 µm). VEGFR2, vascular endothelial growth factor receptor 2; LSP, lung side population; Dil-Ac-LDL, Dil-labeled acetylated low-density lipoprotein; DAPI, 4-6-diamidino-2-phenylindol-dihydrochloride.
In vitro colony-formation by CD45−/CD31+ LSP cells
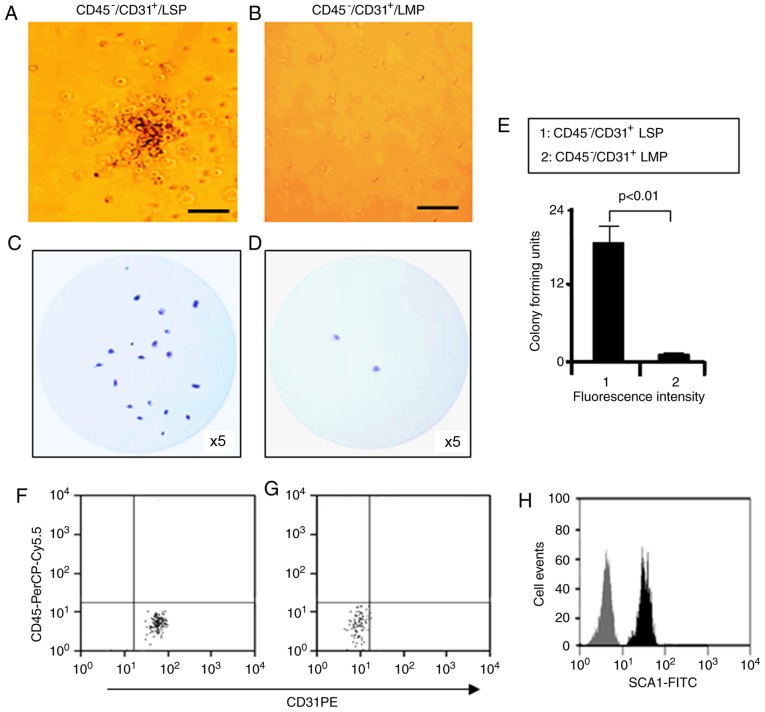
Our previous study showed that CD31+ CSP cells were able to form colonies in vitro (27). Therefore, the present study examined the in vitro colony-forming potential of CD45−/CD31+ LSP cells. LMP and LSP cells freshly isolated from adult murine lungs were plated on methylcellulose media. After 14 days in culture, the number of colonies was counted. A typical colony formed by CD45−/CD31+ LSP cells and a typical field of CD45−/CD31+ LMP cells are shown in Fig. 5A and B, respectively. Compared with the CD45−/CD31+ LMP cells, the CD45−/CD31+ LSP cells produced more colonies (Fig. 5C-E). FACS analysis of the LSP cells that were subsequently isolated from the methylcellulose media revealed surface expression of CD31 (100%) and SCA1 (100%), but not CD45, indicating that the colony forming cells had retained their phenotype following culture (Fig. 5F-H). These findings suggest that CD45−/CD31+ LSP cells possess a substantially greater potential for self-renewal in culture compared with LMP cells.
Figure 5.
Colony formation by CD45−/CD31+ LSP cells. (A) Representative colony formed by CD45−/CD31+ LSP cells in methylcellulose medium, visualized by phase contrast microscopy (scale bar, 50 µm). (B) Representative image of a field of CD45−/CD31+ LMP cells (scale bar, 50 µm). Representative, low magnification images of colony formation by (C) CD45−/CD31+ LSP cells and (D) LMP cells cultured in methylcellulose medium and stained with crystal violet (×5 magnification). (E) Graph showing the ratio of colonies formed by CD45−/CD31+ LSP cells, compared to CD45−/CD31+ LMP cells (P<0.01). (F) Colony-forming cells subsequently isolated from methylcellulose media, labeled with CD45-PerCP-Cy5.5 and CD31-PE, and analyzed by flow cytometry, with the results for the (G) isotype control. (H) Colony-forming cells isolated from methocult media labeled with SCA1-FITC (black) or its isotype antibody (grey) and analyzed by flow cytometry. Data are presented as the mean ± standard deviation (n=3 experiments). LSP, lung side population; LMP, lung main population.
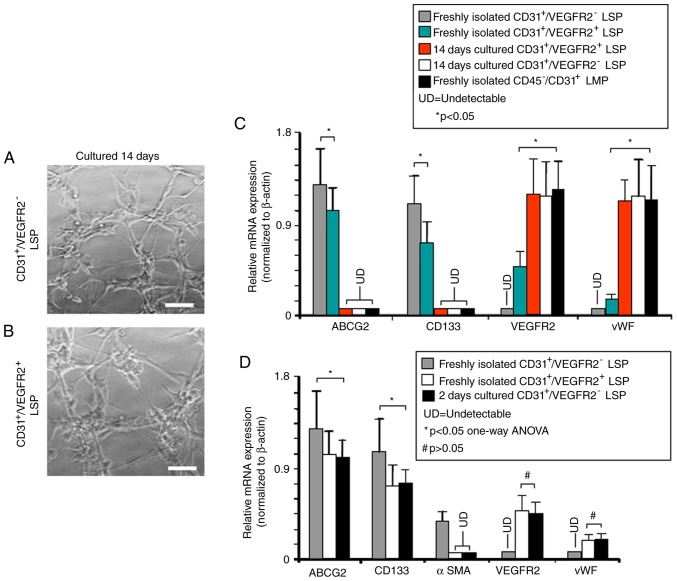
In vitro differentiation of LSP cells
To examine their differentiation potential, CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells were separately isolated and cultured in conditions known to promote the growth and differentiation of either endothelial or smooth muscle cells. Following culture to promote endothelial differentiation for 14 days, the CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells each formed tube-like vascular networks (Fig. 6A and B), suggesting that they had differentiated into endothelial, or endothelial-like, cells. RT-qPCR analysis was then used to examine the expression of stem/progenitor cell genes vs. endothelial cell-associated genes in freshly-isolated and cultured CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells. These were compared with freshly isolated CD45−/CD31+ LMP cells, which represent mature endothelial cells. ABCG2 and CD133 were expressed in the freshly isolated CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells, but not in the cultured cells nor in the freshly isolated CD45−/CD31+ LMP cells (Fig. 6C). Expression levels of the stem/progenitor markers ABCG2 and CD133 were significantly lower in the freshly isolated CD45−/CD31+/VEGFR2+ LSP cells compared with those in the freshly isolated CD45−/CD31+/VEGFR2− LSP cells. No expression of endothelial cell markers VEGFR2 and vWF was detected in the freshly isolated CD45−/CD31+/VEGFR2− LSP cells, and their expression was weak in the CD45−/CD31+/VEGFR2+ LSP cells. However, following culture, the CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells the mRNA expression levels of VEGFR2 and vWF were high, at levels indistinguishable from those in the freshly isolated CD45−/CD31+ LMP cells (Fig. 6C). These results further support the hypothesis that CD45−/CD31+/VEGFR2− LSP cells and CD45−/CD31+/VEGFR2+ LSP cells may be endothelial progenitors, similar to our previous findings with SP cells in the mouse heart (27).
Figure 6.
In vitro endothelial differentiation by CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells. Representative photomicrographs show vascular tube-like networks formed by (A) CD45−/CD31+/VEGFR2− and (B) CD45−/CD31+/VEGFR2+ LSP cells after 2 weeks in culture under endothelial differentiation-inducing conditions (scale bar, 50 µm). (C) RT-qPCR analysis performed to compare relative expression of mRNAs encoding ABCG2, CD133, VEGFR2 and vWF genes in freshly isolated CD45−/CD31+/VEGFR2− LSP cells (grey bars), CD45−/CD31+/VEGFR2+ LSP cells (green bars), in CD45−/CD31+/VEGFR2+ LSP cells after 14 days culture (red bar), and in CD45−/CD31+/VEGFR2− LSP cells after 14 days culture (white bar). Freshly isolated CD45−/CD31+ LMP cells are included as a control (black bar). (D) RT-qPCR analysis was performed to compare the relative expression of mRNAs encoding ABCG2, CD133, αSMA, VEGFR2 and vWF genes in freshly isolated CD45−/CD31+/VEGFR2− LSP (grey bars) CD45−/CD31+/VEGFR2+ LSP (white bars), and in CD45−/CD31+/VEGFR2− LSP after 2 days culture (black bars). Data are presented as the mean ± standard deviation (n=3 experiments; *P<0.05; #P>0.05; LSP, lung side population; LMP, lung main population; UD, undetectable; ABCG2, ATP-binding cassette super-family G member 2; VEGFR2, vascular endothelial growth factor receptor 2; vWF, von Willebrand factor; αSMA, α-smooth muscle actin.
The detection of the expression of VEGFR2 and vWF, and relatively low levels of ABCG2, CD133 suggested that CD45−/CD31+/VEGFR2+ LSP cells may be late-commitment endothelial progenitors. To investigate whether CD45−/CD31+/VEGFR2+ LSP cells may be derived from CD45−/CD31+/VEGFR2− LSP cells, freshly isolated CD45−/CD31+/VEGFR2− LSP cells were cultured for 2 days under conditions to promote endothelial differentiation. During this culture, the CD45−/CD31+/VEGFR2− LSP cells showed significant reductions in the mRNA expression levels of ABCG2 and CD133, with levels becoming similar to those found in freshly isolated CD45−/CD31+/VEGFR2+ LSP cells (Fig. 6D). As expected, no expression of endothelial markers VEGFR2 and vWF was detected in the freshly isolated CD45−/CD31+/VEGFR2− LSP cells. However, following 2 days in culture, mRNA expression of VEGFR2 and vWF was detected in CD45−/CD31+/VEGFR2− LSP cells at levels comparable with those found in freshly isolated CD45−/CD31+/VEGFR2+ LSP cells (Fig. 6D). The freshly isolated CD45−/CD31+/VEGFR2− LSP cells expressed the smooth muscle marker, αSMA, however, the expression of αSMA disappeared following 2 days in culture. Therefore, the CD45−/CD31+/VEGFR2− LSP cells came to resemble CD45−/CD31+/VEGFR2+ cells in their pattern of gene expression following 2 days in culture (Fig. 6D). This suggested that the 2-day-cultured CD45−/CD31+/VEGFR2− LSP cells and freshly isolated CD45−/CD31+/VEGFR2+ LSP cells had lost their ability to differentiate into smooth muscle cells, which was consistent with the hypothesis that CD45−/CD31+/VEGFR2+ LSP cells are late commitment endothelial progenitor cells. The gene expression profile of CD45−/CD31+/VEGFR2− LSP cells became almost identical to that of CD45−/CD31+/VEGFR2+ LSP cells following 2 days in culture, suggesting that CD45−/CD31+/VEGFR2+ LSP cells may be derived from CD45−/CD31+/VEGFR2− LSP cells (Fig. 6D).
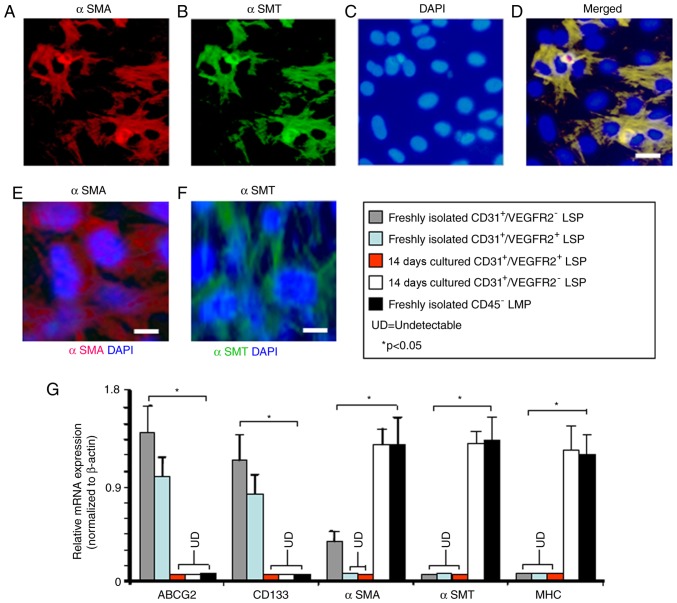
The expression of αSMA by freshly isolated CD45−/CD31+/VEGFR2− LSP cells suggested that they may serve a role as smooth muscle progenitors. To investigate this, the CD45−/CD31+/VEGFR2− LSP cells were cultured in medium previously shown to promote smooth muscle differentiation (24). After 14 days in culture, many of the cells had developed the morphologic characteristics of smooth muscle cells, being large, flat, and stellate in appearance, and showing uniform immunostaining for αSMA and αSMT (Fig. 7A-F). To extend these findings, the gene expression profiles of freshly isolated and cultured CD45−/CD31+/VEGFR2− LSP cells were compared. The mRNA expression of ABCG2 and CD13 was detected in the freshly isolated CD45−/CD31+/VEGFR2− LSP cells, however, the expression of these two genes was no longer detectable following 14 days in smooth muscle medium (Fig. 7G). By contrast, the CD45−/CD31+/VEGFR2− LSP cells showed significantly upregulated expression levels of αSMA following 14 days in smooth muscle media, resulting in levels similar to those in the freshly isolated CD45− LMP cells (Fig. 7G). Following 14 days of culture in smooth muscle differentiation medium, the mature smooth muscle markers, αSMT and myosin heavy chain (35) were expressed by the 14-day-cultured CD45−/CD31+/VEGFR2− LSP cells and freshly isolated CD45− LMP cells, but not by the freshly isolated CD45−/CD31+/VEGFR2− LSP cells (Fig. 7G). By contrast, the CD45−/CD31+/VEGFR2+ LSP cells did not express detectable mRNA levels of αSMA, αSMT or myosin heavy chain either before or after 14 days in smooth muscle differentiation medium (Fig. 7G). Therefore, although CD45−/CD31+/VEGFR2− LSP cells may be smooth muscle progenitor cells, the CD45−/CD31+/VEGFR2+ LSP cells showed no such potential.
Figure 7.
In vitro smooth muscle differentiation potential of CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells. Images (scale bar, 20 µm) show the representative microscopic fields of CD45−/CD31+/VEGFR2− LSP cells double-labeled for αSMA and αSMT after 14 days in culture under smooth muscle differentiation-inducing conditions; numerous cells stained positively with (A) anti-αSMA antibody (red) and (B) anti-αSMT antibody (green). (C) Nuclei were counterstained with DAPI (blue). (D) Merge of the optical channels. Single immunofluorescence labeling with (E) anti-αSMA (red) and (F) anti-αSMT (green) is shown (scale bar, 10 µm). (G) Reverse transcription-quantitative polymerase chain reaction analysis was performed to compare gene expression by freshly isolated CD45−/CD31+/VEGFR2− LSP cells (grey bar), CD45−/CD31+/VEGFR2− LSP cells after 14 days culture (white bar), freshly isolated CD45−/CD31+/VEGFR2+ LSP cells (light blue bar) and CD45−/CD31+/VEGFR2− LSP cells after 14 days culture (red bar). Freshly isolated CD45− LMP cells are included as a control (black bar). Relative expression levels of the ABCG2, CD133, αSMA, αSMT and MHC genes were compared between the three types of cells. Data are presented as the mean ± standard deviation (n=3 experiments). *P<0.05; LSP, lung side population; LMP, lung main population; UD, undetectable; ABCG2, ATP-binding cassette super-family G member 2; VEGFR2, vascular endothelial growth factor receptor 2; vWF, von Willebrand factor; αSMT, α-smooth muscle tropomyosin; αSMA, α-smooth muscle actin; MHC, myosin heavy chain; DAPI, 4-6-diamidino-2-phenylindol-dihydrochloride.
Discussion
There have been several reports of the existence of endogenous stem cell-like cell populations in the mouse lung (14,25). In particular, the in vitro differentiation of CD45−/CD31− LSP cells was demonstrated by Summer et al (15). However, little is known about CD45−/CD31+ LSP cells. The present study provides new data showing that CD45−/CD31+ LSP cells can be divided into CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cell subpopulations. To the best of our knowledge, this is the first detailed in vitro investigation of the ability of CD45−/CD31+ LSP cells from the adult mouse lung to form cell colonies, differentiate into endothelial and smooth muscle cells and vascularize. The results suggest that CD45−/CD31+/VEGFR2+ LSP cells differentiate into endothelial cells, whereas CD45−/CD31+/VEGFR2− LSP cells can differentiate into endothelial and smooth muscle cells.
The expression of CD31 in CD45−/CD31+ LSP cells suggests that CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells may be progenitors of lung endothelial cells. This was confirmed by their gene expression profiles. The CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells expressed ABCG2 and CD133 at high levels. The endothelial cell marker vWF was undetectable in freshly isolated CD45−/CD31+/VEGFR2− LSP cells. The CD45−/CD31+/VEGFR2+ LSP cells expressed relatively low mRNA levels of vWF, and no vWF protein was detected. This phenotype is consistent with these SP cells being endothelial stem/progenitor cells (27,36). Of note, the CD45−/CD31+ LSP cells were capable of DiI-Ac-LDL uptake, suggesting that they were endothelial progenitors rather than hematopoietic progenitors. The expression levels of ABCG2 and CD133 were significantly lower in the CD45−/CD31+/VEGFR2+ LSP cells compared with those in the CD45−/CD31+/VEGFR2− LSP cells. In addition, the CD45−/CD31+/VEGFR2− LSP cells expressed αSMA, suggesting that these cells may serve as progenitors for endothelial and smooth muscle cells. This possibility is consistent with previous studies showing that vascular smooth muscle cells are derived from endothelial progenitor cells during vasculo-genesis (27,37). By contrast, the CD45−/CD31+/VEGFR2+ LSP cells expressed detectable levels of vWF and VEGFR2, but no αSMA, indicating that these cells may be relative late commitment endothelial progenitor cells.
The results of the present study showed that CD45−/CD31+ LSP cells possessed a higher colony-forming potential than CD45−/CD31+ LMP cells. This finding is consistent with previous studies that reported SP cells isolated from different tissues have higher colony-forming capability than non-SP cells (19,27,38). A previous study showed that a small number of cells isolated from the CD31+ population from the adult mouse lung were endothelial progenitor cells (39). This group of endothelial progenitor cells may be CD45−/CD31+ LSP cells. However, the data obtained in the present study do not rule out the possibility that other populations of CD31+ cells function as endothelial progenitor cells.
In a previous study, Irwin et al (16) showed that CD45−/VEGFR2+ LSP cells of the mouse lung were able to differentiate into endothelial cells. However, whether these cells expressed CD31 was unclear. The present study found that it was possible to divide CD45−/CD31+ LSP cells into CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ sub-populations. Both the CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells were capable of differentiation into mature endothelial cells, which formed vascular tube-like structures in vitro. However, only the CD45−/CD31+/VEGFR2− LSP cells expressed αSMA and were capable of differentiation into mature smooth muscle cells in vitro (CD45−/CD31+/VEGFR2+ LSP cells did not). When the CD45−/CD31+/VEGFR2− LSP cells were cultured under endothelial differentiation conditions for 2 days, the expression of αSMA was lost. They also started to express VEGFR2 and vWF. The gene expression profiles of those CD45−/CD31+/VEGFR2− LSP cells that had been cultured under endothelial differentiation conditions for 2 days became similar to that of CD45−/CD31+/VEGFR2+ LSP cells (Fig. 6C). Taken together, these findings indicate that the CD45−/CD31+/VEGFR2− LSP cells were capable of differentiation into smooth muscle cells, endothelial cells and CD45−/CD31+/VEGFR2+ LSP cells in vitro. Therefore, CD45−/CD31+/VEGFR2+ LSP cells may be late commitment endothelial progenitors that are capable of differentiating into endothelial cells, but not smooth muscle cells, in vitro. However, the association between CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells requires further elucidation. Several studies have shown that SP cells from different tissues and organs have potential for repairing damaged parts within a tissue or organ (40–42). The role of CD45−/CD31+ LSP cells in repairing injured lung tissue is to be the subject of a future investigation.
Our previous studies demonstrated that CD31+/SCA1+ CSP cells served as endothelial progenitor cells in the adult mouse heart (27,28). Of note, SCA1+/CD31+ CSP cells share several features with CD45−/CD31+ LSP cells (CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2−), including surface expression of SCA1, similarity in gene expression profiles, capacity for colony formation and potential for endothelial differentiation (27,28). Our findings support the hypothesis that a novel population of progenitor cells may contribute to heart and lung tissues, including their blood vessels (26). Our previous study also demonstrated that vascular smooth muscle and airway smooth muscle share a common progenitor cell in the lung. Further investigation is required to determine whether CD45−/CD31+/VEGFR2− LSP cells differentiate into airway smooth muscle cells. Previous studies have found that c-kit+ cells in the adult mouse lung may serve as endothelial progenitor cells (11). However, the expression of c-kit was undetectable in CD45−/CD31+ LSP cells. Therefore, the association between lung c-kit cells and CD45−/CD31+ LSP cells also requires further investigation.
In conclusion, the present study isolated and characterized CD45−/CD31+ SP cells from the adult mouse lung. They were found to consist of CD45−/CD31+/VEGFR2− and CD45−/CD31+/VEGFR2+ LSP cells. Based on their surface protein expression, their gene expression profile, and capacity to form colonies and differentiate into endothelial and smooth muscle cells in vitro, the results suggest that CD45−/CD31+/VEGFR2− LSP cells may serve as a source of endothelial and smooth muscle progenitor cells in the adult mouse lung in vivo. The data also suggest that CD45−/CD31+/VEGFR2+ LSP cells may be derived from CD45−/CD31+/VEGFR2− LSP cells and that this differentiation may involve a lineage commitment to become endothelial progenitor cells. Understanding the function and identifying ways to promote the proliferation and differentiation of these populations of cells may offer potential for the development of novel therapeutic strategies to enhance vessel regeneration in lung injury and diseases.
Acknowledgments
Not applicable.
Abbreviations
- SP
side population
- LSP
lung side population
- MP
main population
- LMP
lung main population
- VEGFR2
vascular endothelial growth factor receptor 2
- CSP
cardiac side population
- FACS
fluorescence-activated cell sorting
- DMEM
Dulbecco's modified Eagle's medium
- Dil-Ac-LDL
Dil-labeled acetylated low-density lipoprotein
- DAPI
4-6-diamidino-2-phenylindol-dihydrochloride
- vWF
von Willebrand factor
- αSMT
α-smooth muscle tropomyosin
- αSMA
α-smooth muscle actin
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81370619 to SXL).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YX, PS and JYW performed experiments and wrote the manuscript; ZZL and RLG performed experiments; XZW performed analysis and interpretation of data; WDP analyzed data and edited the manuscript; SXL designed and organized the entire research, analyzed the data, and edited the manuscript. All the authors have read and approved the final, submitted version of manuscript.
Ethics approval and consent to participate
All animal experiments were conducted strictly according to ethical standards approved by the Animal Ethical Committee of Jinzhou Medical University (approval ID: LY2014D001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Schatz M, Kazzi AA, Brenner B, Camargo CA, Jr, Corbridge T, Krishnan JA, Nowak R, Rachelefsky G. Joint task force report: Supplemental recommendations for the management and follow-up of asthma exacerbations. Introduction. J Allergy Clin Immunol. 2009;124(2 Suppl):S1–S4. doi: 10.1016/j.jaci.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Mosier JM, Hypes C, Joshi R, Whitmore S, Parthasarathy S, Cairns CB. Ventilator strategies and rescue therapies for management of acute respiratory failure in the emergency department. Ann Emerg Med. 2015;66:529–541. doi: 10.1016/j.annemergmed.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Yan Z, Kui Z, Ping Z. Reviews and prospectives of signaling pathway analysis in idiopathic pulmonary fibrosis. Autoimmun Rev. 2014;13:1020–1025. doi: 10.1016/j.autrev.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, Fong GH, Sakmar TP, Rafii S, Ding BS. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22:154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther. 2015;151:107–120. doi: 10.1016/j.pharmthera.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Marriott S, Baskir RS, Gaskill C, Menon S, Carrier EJ, Williams J, Talati M, Helm K, Alford CE, Kropski JA, et al. ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am J Physiol Cell Physiol. 2014;307:C684–C698. doi: 10.1152/ajpcell.00114.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med. 2013;188:820–830. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 10.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, Huang X, Zhang H, Tian X, He L, Yang R, Yan Y, Wang QD, Gillich A, Zhou B. c-kit(+) cells adopt vascular endothelial but not epithelial cell fates during lung maintenance and repair. Nat Med. 2015;21:866–868. doi: 10.1038/nm.3888. [DOI] [PubMed] [Google Scholar]
- 12.Kajstura J, Rota M, Hall SR, Hosoda T, D'Amario D, Sanada F, Zheng H, Ogorek B, Rondon-Clavo C, Ferreira-Martins J, et al. Evidence for human lung stem cells. N Engl J Med. 2011;364:1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L624–L630. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 14.Liang SX, Summer R, Sun X, Fine A. Gene expression profiling and localization of Hoechst-effluxing CD45− and CD45+ cells in the embryonic mouse lung. Physiol Genomics. 2005;23:172–181. doi: 10.1152/physiolgenomics.00059.2005. [DOI] [PubMed] [Google Scholar]
- 15.Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. Am J Respir Cell Mol Biol. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin D, Helm K, Campbell N, Imamura M, Fagan K, Harral J, Carr M, Young KA, Klemm D, Gebb S, et al. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol. 2007;293:L941–L951. doi: 10.1152/ajplung.00054.2007. [DOI] [PubMed] [Google Scholar]
- 17.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/S0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 18.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 20.Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 21.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asakura A, Rudnicki MA. Side population cells from diverse adult tissues are capable of in vitro hematopoietic differentiation. Exp Hematol. 2002;30:1339–1345. doi: 10.1016/S0301-472X(02)00954-2. [DOI] [PubMed] [Google Scholar]
- 23.Liang SX, Phillips WD. Migration of resident cardiac stem cells in myocardial infarction. Anat Rec. 2013;296:184–191. doi: 10.1002/ar.22633. [DOI] [PubMed] [Google Scholar]
- 24.Summer R, Kotton DN, Liang S, Fitzsimmons K, Sun X, Fine A. Embryonic lung side population cells are hematopoietic and vascular precursors. Am J Respir Cell Mol Biol. 2005;33:32–40. doi: 10.1165/rcmb.2005-0024OC. [DOI] [PubMed] [Google Scholar]
- 25.Summer R, Kotton DN, Sun X, Ma B, Fitzsimmons K, Fine A. Side population cells and Bcrp1 expression in lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L97–L104. doi: 10.1152/ajplung.00009.2003. [DOI] [PubMed] [Google Scholar]
- 26.Peng T, Tian Y, Boogerd CJ, Lu MM, Kadzik RS, Stewart KM, Evans SM, Morrisey EE. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500:589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang SX, Khachigian LM, Ahmadi Z, Yang M, Liu S, Chong BH. In vitro and in vivo proliferation, differentiation and migration of cardiac endothelial progenitor cells (SCA1+/CD31+ side-population cells) J Thromb Haemost. 2011;9:1628–1637. doi: 10.1111/j.1538-7836.2011.04375.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang XZ, Gao RL, Sun P, Liu S, Xu Y, Liang DZ, Yin LM, Phillips WD, Liang SX. Proliferation, differentiation and migration of SCA1−/CD31− cardiac side population cells in vitro and in vivo. Int J Cardiol. 2017;227:378–386. doi: 10.1016/j.ijcard.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 29.Liang SX, Tan TY, Gaudry L, Chong B. Differentiation and migration of Sca1+/CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol. 2010;138:40–49. doi: 10.1016/j.ijcard.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Sun P, Wu G, Gao RL, Liu S, Phillips WD, Liang SX. Rifampicin-dependent antibodies target glycoprotein IIb/IIIa and cause clearance of human platelets in NOD/SCID mice. Br J Haematol. 2016;172:137–140. doi: 10.1111/bjh.13470. [DOI] [PubMed] [Google Scholar]
- 31.Qiagen QuantiTect Primer Assay Handbook. https://www.qiagen.com/sg/resources/resourcedetail?id=882a8baa-29df-4182-a2a5-17083f4dbe11&lang=en. Accessed September 18, 2013.
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 34.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 35.Low RB, White SL. Lung smooth muscle differentiation. Int J Biochem Cell Biol. 1998;30:869–883. doi: 10.1016/S1357-2725(98)00049-1. [DOI] [PubMed] [Google Scholar]
- 36.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 37.Ema M, Faloon P, Zhang WJ, Hirashima M, Reid T, Stanford WL, Orkin S, Choi K, Rossant J. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 2003;17:380–393. doi: 10.1101/gad.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct 'side population' of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101:14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schniedermann J, Rennecke M, Buttler K, Richter G, Stadtler AM, Norgall S, Badar M, Barleon B, May T, Wilting J, et al. Mouse lung contains endothelial progenitors with high capacity to form blood and lymphatic vessels. BMC Cell Biol. 2010;11:1471–2121. doi: 10.1186/1471-2121-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge G, Zhang H, Li R, Liu H. The function of SDF-1CXCR4 axis in sp cells-mediated protective role for renal ischemia/reper-fusion injury by SHH/GLI1-ABCG2 Pathway. Shock. 2017;47:251–259. doi: 10.1097/SHK.0000000000000694. [DOI] [PubMed] [Google Scholar]
- 41.He DN, Qin H, Liao L, Li N, Zhu WM, Yu BJ, Wu X, Zhao RC, Li JS. Small intestinal organoid-derived SP cells contribute to repair of irradiation-induced skin injury. Stem Cells Dev. 2005;14:285–291. doi: 10.1089/scd.2005.14.285. [DOI] [PubMed] [Google Scholar]
- 42.Ooka H, Kanda S, Okazaki H, Suzuki H, Mishima K, Saito I, Yagi M, Tomoda K, Nishiyama T. Characterization of side population (SP) cells in murine cochlear nucleus. Acta Otolaryngol. 2012;132:693–701. doi: 10.3109/00016489.2012.657358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.