Abstract
Objective
To investigate the magnitude of postural sway induced by different balance tasks in adolescents with concussion and to examine the associations of postural sway with concussion symptoms.
Design
Cross-sectional study.
Patients or Other Participants
Fifty-six adolescents (20 girls, 36 boys) between 13 and 17 years of age who sustained a concussion within the past 44 days and were still symptomatic.
Main Outcome Measure(s)
Anterior-posterior postural sway was measured using an accelerometer attached to the participant's lower back while he or she performed 6 static-balance tasks that varied the visual input, type of surface, and foot stance. Participants self-reported symptoms that occurred at the time of the concussion (eg, dizziness, confusion, amnesia) as well as at the time of balance testing (eg, eye and head movement–induced dizziness).
Results
The normalized path length of postural sway during the different balance tasks was greater with the eyes closed (mean = 19.3 mG/s) compared with the eyes open (mean = 12.4 mG/s; P < .001). Furthermore, sway while standing with the feet together on a foam surface (mean = 17.9 mG/s) or while tandem standing on a firm surface (mean = 19.4 mG/s) was greater than sway while standing with the feet together on a firm surface (mean = 10.3 mG/s; P < .001). Greater sway was associated with dizziness and confusion reported at the time of injury (P < .05). Dizziness and headache symptoms at rest were positively correlated with sway (P < .05).
Conclusions
Using accelerometers to measure postural sway during different challenging balance conditions in adolescents with concussion may provide an objective means of quantifying balance impairments in clinical environments. Furthermore, the association of these measurements with symptoms suggests a need to account for symptom severity at the time of testing.
Keywords: balance, vestibular system, dizziness
Key Points
Modifying the visual input, type of surface, and foot stance elicited differences in postural sway among adolescents with concussion.
Greater anterior-posterior sway was related to increased symptoms in adolescents with concussion.
The use of accelerometers to measure postural sway may provide an objective measure for tracking and quantifying balance changes among adolescents with concussion.
Concussion is the most common acquired neurologic disorder among children and young adults.1 It is estimated that 1.6 to 3.8 million sport-related concussions occur annually in the United States.1 Although a majority of concussions resolve within 3 to 4 weeks,2,3 chronic symptoms adversely affect many individuals. Consequently, improving the early identification of patients who might develop chronic impairments remains a challenge for clinicians.
Concussion guidelines4,5 support the measurement of postural stability as an important component of a comprehensive approach to concussion assessment. Deficits in postural instability after sport-related concussion have been reported in many investigations.6–10 In an epidemiologic study, Guskiewicz et al10 found that 28.6% of 888 high school and collegiate football players with concussion had balance impairments as evidenced by positive Romberg tests immediately after injury. Researchers11 have observed that balance deficits noted during a Balance Error Scoring System (BESS) assessment resolved (ie, returned to baseline) within 3 days postconcussion. However, instrumented balance tests that use accelerometers or force platforms appear to detect more subtle balance impairments for a longer period of time after concussion compared with noninstrumented tests such as the BESS.7 They may also better distinguish between individuals with concussion (5 ± 3.3 months postinjury) and healthy controls,12 although the findings of another study8 conflicted with this premise in individuals with concussion tested earlier than 2 weeks after injury.
Maintaining upright standing control requires the integration of the somatosensory, visual, and vestibular systems. Most balance-assessment tools, including the BESS, incorporate multiple conditions to determine how sensory feedback and biomechanical constraints affect balance.13,14 Somatosensory feedback can be altered by using compliant surfaces.14 Information from the visual system can be eliminated when balance is tested with eyes closed (EC).14,15 The size of the base of support is often manipulated and serves as a modifying factor in balance-assessment tools.16 Because concussion is believed to affect the central vestibular pathways, it is important to assess higher-level vestibular function using sensory-integration tasks that disrupt somatosensory and visual feedback.
Self-reported symptoms are widely used in the assessment of concussion. However, symptom self-report may not be the best method for determining return to activity. Underreporting of symptoms among athletes with concussion has been demonstrated in several studies.17–19 Approximately 40% of high school athletes did not report a concussion because they did not want to leave the game.19 In addition, when compared with matched controls, high school and collegiate athletes with concussion indicated resolution of symptoms as measured using the Post-Concussion Symptom Scale while cognitive impairments were still being manifested.18 Broglio et al17 reported that 38% of collegiate athletes with concussion had impaired cognitive performance that lasted beyond the resolution of self-reported symptoms. Therefore, although symptom self-report is an important tool in managing concussion, additional assessment methods, such as balance performance, may be useful for improving concussion management. Additionally, multivariable assessment tools that include balance function are being developed to predict the risk of persistent postconcussion symptoms that endure for more than 28 days.20 Furthermore, instrumented balance assessment may provide an additional benefit beyond observational balance assessment for concussion management.12,21
The purpose of our study was to investigate the effects of different balance conditions on the magnitude of postural sway during static standing in adolescents with concussion and to determine if the magnitude of sway was associated with overt signs and symptoms reported at the time of injury. Also, we explored the relationship between the amount of postural sway and the different balance conditions with provocative symptom assessment. We hypothesized that (1) the magnitude of normalized path length (NPL) of sway would increase as the participants closed their eyes, stood on an unstable surface, and stood in tandem stance; (2) the magnitude of NPL of sway would be greater in individuals who had positive overt signs and symptoms of concussion at the time of the injury; and (3) the magnitude of NPL of sway in the different balance conditions would be greater in individuals who had symptoms at rest and during provocative testing.
METHODS
Participants
Fifty-six symptomatic adolescents between 13 and 17 years of age (20 girls and 36 boys, age = 15 ± 1.4 years) diagnosed with a sport-related concussion were recruited prospectively from an outpatient concussion-assessment clinic after their initial visit with one of the neuropsychologists who served as the clinic's primary care providers for individuals with sport-related concussion (Table 1). Fifty-three participants (95%) identified their race as white, 2 as African American (4%), and 1 as white and African American (2%). The sports practiced at the time of the injury were football (n = 20), soccer (n = 9), lacrosse (n = 7), baseball (n = 3), hockey (n = 3), swimming (n = 2), and softball (n = 2); the remaining 10 participants were involved in different sport activities. Patients were enrolled in the study by referral from the neuropsychologists in the concussion clinic. The neuropsychologists diagnosed a concussion after an extensive history, interview, survey of symptoms, and computerized neurocognitive testing examination were performed. Exclusion criteria were a history of a more severe traumatic brain injury (Glasgow Coma Scale <13) or brain surgery; a history of a psychiatric condition such as depression, bipolar disorder, or schizophrenic disorder; substance abuse within the last year; or a history of any neurologic disorder, such as epilepsy or seizures. Approval for this study was granted by the institutional review board. Written informed assent and consent were obtained from the participant and a parent or guardian, respectively.
Table 1.
Participants' Characteristics (N = 56, Including 20 Girls)
| Characteristic |
Value |
| Mean ± SD (range) |
|
| Demographic | |
| Age, y | 15 ± 1 (13–17) |
| Height, m | 1.70 ± 0.11 (1.45–1.88) |
| Mass, kg | 68 ± 18 (39–122) |
| Time since concussion, d | 13 ± 11 (1–44) |
| No. (%) |
|
| History | |
| Concussion within the last year | 17 (30) |
| Motion sickness or space discomfort | 12 (21) |
| Migraines | 10 (18) |
| Attention-deficit or attention-deficit hyperactivity disorder | 7 (13) |
| Learning disorder | 2 (4) |
| Overt signs and symptoms at time of injury | |
| Dizziness | 42 (75) |
| Confusion | 36 (64) |
| Anterograde amnesia | 20 (36) |
| Retrograde amnesia | 12 (21) |
| Loss of consciousness | 4 (7) |
Procedures
Procedures were performed in the following order. Recruits and their parent or guardian were met by one of the investigators to explain the study and its purpose. If the recruit and the parent or guardian were interested in participating in the study, they provided informed assent and consent, respectively.
Participants reported their age, sex, race, height, weight, history of concussions, and overt concussion signs and symptoms at the time of their most recent concussion. Symptoms included loss of consciousness, dizziness, retrograde amnesia, anterograde amnesia, and confusion. Medical history was recorded, including any history of learning disorder, attention-deficit hyperactivity disorder or attention-deficit disorder, or migraine headaches.
Activity-Provoked Dizziness
Activity-provoked dizziness was assessed by asking the participants to rate their dizziness during the past week in 8 situations using a 0 to 6 Likert scale (0 = none, 1 or 2 = mild, 3 or 4 = moderate, and 5 or 6 = severe). They were asked if they had experienced the following during the past week: (1) dizziness; (2) dizziness when looking up; (3) dizziness walking down aisles, hallways, etc; (4) dizziness when turning over, when getting out of bed, or while lying down; (5) dizziness while reading; (6) dizziness during quick head movements; (7) dizziness when bending over; and (8) dizziness in wide-open spaces (Appendix 1). The scale was developed as a precursor to the Vestibular/Ocular Motor Screening assessment.22
Eye-Head Movement–Provoked Symptoms
Eye-head movement–provoked symptoms were assessed by asking the participants to rate their symptoms using the same 0 to 6 Likert scale. The rater instructed each participant to follow commands in performing 6 eye- and head-movement exercises. After each exercise, the rater asked the participant to rate his or her symptoms, which could include dizziness, headache, nausea, or fogginess. The 6 eye and head movements were (1) horizontal eye movements between 2 fixed visual targets (horizontal saccades), (2) vertical eye movements between 2 fixed visual targets (vertical saccades), (3) horizontal head movements while looking at a fixed visual target (horizontal-gaze stability), (4) vertical head movements while looking at a fixed visual target (vertical-gaze stability), (5) horizontal head movements while following a moving visual target (vestibulo-ocular reflex cancellation), and (6) convergence (Appendix 2). The scale was developed as a precursor to the Vestibular/Ocular Motor Screening assessment.22
Symptoms at Rest Before Balance Testing
Symptoms including dizziness, headache, and nausea were assessed with the participants seated, immediately before the experimental balance testing. Participants were asked to rate each symptom using a 0 to 6 Likert scale with 2 verbal anchors (0 = no symptoms and 6 = the worst symptoms imaginable).
Accelerometer-Based Balance Testing
Standing balance measurements were based on the Balance Accelerometry Measure.23 Sock-clad participants stood with their arms across their chests in 6 conditions, including 3 stance conditions: feet together on a firm surface (FT-firm), tandem feet on a firm surface (tandem-firm), and feet together on a foam surface (FT-foam; Airex Balance Pad; Specialty Foams, Sins, Switzerland).24 Each stance was performed with eyes open (EO) and EC. The order of the 6 conditions was FT-firm–EO, FT-firm–EC, tandem-firm–EO, tandem-firm–EC, FT-foam–EO, and FT-foam–EC. During each condition, participants were asked to stand as still as possible for 30 seconds. They were allowed 2 attempts to perform the balance task without moving out of position. If they did not perform the balance task properly (eg, opened eyes in the EC conditions, stumbled or took a step, or moved their arms), it was considered a fail. If the participant was able to maintain balance for at least 15 seconds, the available sway data were included in the analysis.
An accelerometer (model ADXL213AE; Analog Devices, Inc, Norwood, MA)23 was attached to the participant's lower back at the level of the iliac crest. The accelerometer recorded the center-of-mass (COM) acceleration in the anterior-posterior (AP) direction at a sampling rate of 50 Hz. The data were low-pass filtered (fourth-order zero-phase Butterworth filter with a cutoff frequency of 2 Hz) and processed to calculate the NPL in the AP direction. The measurement unit for the NPL is mG/s (1 mG = 0.00981 m/s2 or 1/1000 of the gravitational acceleration constant). The NPL was calculated using the following formula:
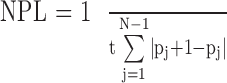 |
where t is time duration, N is the number of time samples, and pj is the acceleration data at time sample j in the AP direction.
Based on a similar tool, Marchetti et al23 reported the AP-NPL test-retest reliability of the COM sway acceleration in 84 healthy adults aged 19 to 85 years was good, with an intraclass correlation coefficient (ICC) of 0.74 or above in all conditions except the tandem-firm–EC condition, which showed poor reliability (ICC = 0.28). In a group of 17 adults with impaired balance, the reliability of the AP-NPL of the COM sway acceleration was good, with an ICC of 0.67 or above in all conditions except the tandem-firm–EC and the FT-foam–EC conditions.
Data Analysis
We used SPSS Statistics for Macintosh (version 22; IBM Corp, Armonk, NY) for statistical analysis. Descriptive data were reported as means, standard deviations, and ranges for normally distributed variables and medians and interquartile ranges for non-normally distributed variables. Because the AP-NPL data were not normally distributed, we transformed the AP-NPL sway data by calculating the log(AP-NPL) that resulted in a normally distributed data set that was used in a repeated-measures analysis of variance (ANOVA). Results of the ANOVA were similar when performed using the log(AP-NPL) and the AP-NPL sway data; thus, the ANOVA was performed using the untransformed AP-NPL sway data. Repeated-measures ANOVA was used to test for the effects of the vision and support condition tasks on the AP-NPL acceleration. The Mann-Whitney U test was used to investigate the effect of overt concussion signs and symptoms at the time of injury (ie, loss of consciousness, amnesia, dizziness, and confusion) on AP-NPL by constructing 2 groups for each sign or symptom: those with and those without the sign or symptom. Spearman rank-order correlation coefficients were used to test for correlations among AP-NPL sway, activity-provoked dizziness symptoms, eye-head movement–provoked symptoms, and symptoms at rest. To control for the possible inflation of type 1 error due to the multiple correlation tests, we applied the false discovery rate method.25 The significance level was set at P = .05.
RESULTS
The mean time interval between the concussion and balance testing was 13 ± 11 days (range = 1–44 days; Table 1). The significant medical histories of the sample included previous concussion (30%), motion sickness (21%), and migraines (18%). The most prevalent overt signs of concussion at the time of injury were dizziness (75%) and confusion (64%). Forty-nine of 56 participants (88%) displayed at least 1 overt sign or symptom at the time of injury.
Instrumented Balance Assessment
For the 5 conditions not including tandem-firm–EC, a loss of balance occurred during 12 out of 3080 trials, which required a second attempt. In 11 of these cases, the participant was able to complete the second attempt, and the remaining one was completed in 15 to 30 seconds. The most challenging condition was the tandem-firm–EC condition, in which 26 participants lost balance during the first attempt: 8 participants failed to maintain their balance for the second attempt, 6 participants completed the task in 15 to 30 seconds, and 12 completed the second attempt. One participant had missing sway data in the FT-firm–EO condition because of a computer program failure. The participant's missing data were not included in the ANOVA to test the effect of the different stances and eye conditions.
Sway data from 47 participants were included in the ANOVA model. Results of the repeated-measures ANOVA are shown in the Figure. A significant main effect of vision was present (F1,46 = 137.4, P < .001), such that AP-NPL sway in the EC conditions was greater than in the EO conditions. A significant main effect of stance condition occurred (F2,92 = 74.5, P < .001). Post hoc testing demonstrated that the AP-NPL sway in the FT-foam and tandem-firm conditions was greater than in the FT-firm condition (P < .001). No difference in AP-NPL sway was found between the FT-foam and tandem-firm conditions (P = .25).
Figure.
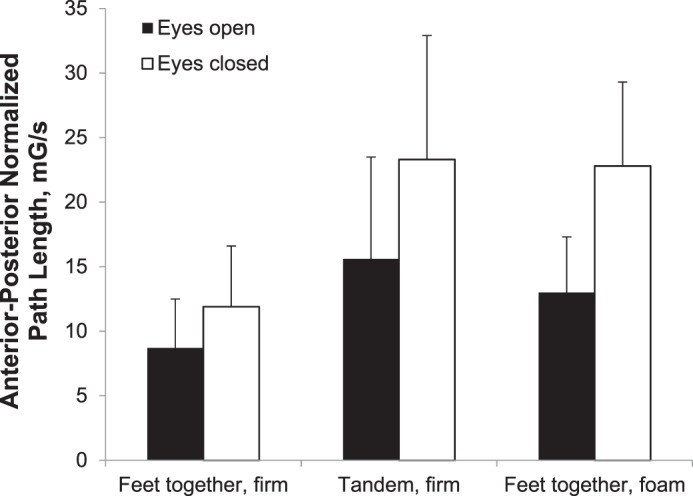
Anterior-posterior normalized path length for vision and stance conditions. Error bars represent +1 standard deviation.
The presence of overt signs or symptoms at the time of injury was associated with increased sway but only for dizziness and confusion symptoms. Participants who reported dizziness at the time of the concussion had greater AP-NPL sway in the FT-firm–EC condition compared with those who did not report initial dizziness (P = .017). Those who described being confused at the time of the concussion had greater AP-NPL sway in the tandem-firm–EO (P = .011) and FT-foam–EO (P = .025) conditions. Anterograde amnesia was not related to any of the sway measures. Furthermore, the length of time since the concussion was not related to the AP-NPL sway in any of the 6 conditions.
Symptoms Reported During Testing and Their Relationship to AP-NPL Sway
The median level of symptoms during provocative testing and at rest before balance testing was in the mild range (Table 2). Dizziness that occurred during eye and head movements was rated higher (by median score) than dizziness during activities of daily living in the past week. Of the symptoms reported just before balance testing, headache was the most prevalent.
Table 2.
Descriptive Statistics for Symptoms Reported During Testinga
| Symptom |
Median (Interquartile Range) |
| Activity-provoked dizziness symptoms | 3 (0–14.5) |
| Eye-head movement–provoked symptoms | 7 (0–13) |
| Dizziness self-report | 1 (0–2) |
| Headache self-report | 2 (1–4) |
| Nausea self-report | 0 (0–0.5) |
Activity-provoked symptoms is the sum of the 8 items in Appendix 1, based on a scale from 0 to 6. Eye-head movement–provoked symptoms is the sum of the 6 items in Appendix 2, based on a scale from 0 to 6. Dizziness, headache, and nausea were reported just before balance testing; each was rated on a scale from 0 to 6.
A total of 33 participants reported activity-provoked dizziness symptoms and 39 described eye-head movement–induced symptoms. Among those who reported activity-provoked dizziness, there were no significant correlations with AP-NPL sway magnitude after correcting for the false discovery rate (Table 3). Participants who had greater symptoms provoked by eye-head movements produced greater AP-NPL sway magnitude during the FT-foam–EO condition (ρ = 0.46, P = .003).
Table 3.
Spearman Correlations (ρ) and P Values Between Center-of-Mass Sway Acceleration and Provoked Symptoms and Self-Reported Dizziness and Headache at Rest Just Before Balance Testing for Participants Who Reported Nonzero Ratingsa
| Condition |
Activity-Provoked Dizziness Symptoms |
Eye-Head Movement–Provoked Symptoms |
Dizziness |
Headache |
| FT-firm–EOb | ||||
| ρ | 0.37 | 0.31 | 0.28 | 0.30 |
| P | .037 | .06 | .15 | .035 |
| FT-firm–EC | ||||
| ρ | 0.29 | 0.16 | 0.43 | 0.31 |
| P | .11 | .31 | .019 | .029 |
| Tandem-firm–EOb | ||||
| ρ | 0.17 | 0.25 | 0.51 | 0.38 |
| P | .34 | .12 | .004 | .006 |
| Tandem-firm–ECc | ||||
| ρ | 0.11 | 0.14 | 0.22 | 0.24 |
| P | .58 | .43 | .28 | .12 |
| FT-foam–EO | ||||
| ρ | 0.34 | 0.46 | 0.50 | 0.29 |
| P | .052 | .003 | .006 | .04 |
| FT-foam–EC | ||||
| ρ | 0.14 | 0.09 | 0.32 | 0.34 |
| P | .44 | .57 | .09 | .015 |
Abbreviations: EC, eyes closed; EO, eyes open; FT, feet together.
Statistically significant correlations after adjusting for multiple comparisons within each column, using the Benjamini and Hochberg25 method, are displayed in bold.
n = 55 (1 participant had missing data during the FT-firm–EO task due to computer program error, and 1 participant was not able to perform the tandem-firm–EO task).
n = 48 (8 participants were not able to perform the balance task in 2 attempts).
Twenty-nine participants reported dizziness, 50 noted headache, and 13 depicted nausea at rest. Given the small number of participants who reported nausea, we did not include it in the correlation analysis. Several significant correlations of self-reported symptoms at rest with AP-NPL sway were found (Table 3). Greater dizziness symptoms at rest were significantly associated with greater sway during the FT-firm–EC, tandem-firm–EO, and FT-foam–EO conditions. Greater headache symptoms at rest were significantly associated with greater sway during the tandem-firm–EO, FT-foam–EO, and FT-foam–EC conditions.
DISCUSSION
The vision and stance modifying factors resulted in changes in the amount of AP-NPL sway. In particular, sway increased during the EC compared with the EO condition. In addition, standing on a foam surface or with feet in tandem stance produced more sway than the feet-together stance on a firm surface. Our findings support the previous research8,26–28 on healthy adolescent and young adult populations in which sway increased with the eyes closed and with the feet on a compliant surface.
Earlier authors8 demonstrated the effects of vision, surface, and stance conditions on postural sway in individuals with concussion and healthy controls. That investigation, which showed greater sway with eyes closed, on the foam surface, and in tandem stance, was conducted among a population with more chronic symptoms, tested an average of 118 days after concussion. An interesting pattern is apparent when comparing the results for the different test conditions. Whereas healthy participants had greater sway when standing with their feet together on a foam surface with EC compared with tandem stance on a firm surface with EC,8,23 individuals with concussion had greater sway in tandem stance.8 However, this finding may depend on the acute nature of the concussion. King et al21 reported that the root mean square measure of mediolateral sway during stance with the EC and feet together best separated individuals with concussion in the past 4 days from healthy controls. Conversely, participants who were tested later in the injury course (and presumably had a more prolonged time to recovery) have shown greater sway in the tandem-stance condition,8 although our data did not demonstrate a relationship between time since concussion and sway magnitude. These different patterns of responses emphasize the need to test multiple balance conditions. Although previous authors have suggested that healthy participants have increased sway or errors when performing tandem stance, potentially limiting this condition's utility, the relative magnitude of sway between the tandem stance on a firm surface and the feet together on a foam surface should be examined in a larger sample.
One goal of our study was to examine the relationship between the immediate signs of concussion at the time of injury and postural sway at the time of testing, 1 to 44 days after injury. Concussion signs and symptoms at the time of injury, including dizziness and confusion, were significantly associated with sway at an average of 13 days after concussion. However, Guskiewicz et al29 reported that amnesia was not associated with deficits or recovery on measures of postural stability, including the Sensory Organization Test and the BESS, tested within 5 days of concussion. Consequently, it is possible that the presence of overt signs and symptoms at the time of concussion is related more to prolonged deficits in postural control than to short-term balance performance. In further support of our findings, others30 found a significant relationship between dizziness at the time of the concussion and a protracted recovery from the injury.
The final purpose of our study was to investigate the relationship between postural sway and self-reported concussion symptoms. Headache, dizziness, and other symptoms provoked by activities and specific eye and head movements are common manifestations of postconcussion syndrome,22,31,32 so we examined the correlation between a structured assessment of these problems and postural sway. After excluding participants who did not report any symptoms, the measures of dizziness and headache at rest had the most significant associations with AP-NPL sway magnitude. These findings suggest that baseline symptoms should be considered potential confounders in standing balance performance when balance is assessed clinically and may also have implications for timing the performance of balance exercises at home. Given the cross-sectional design of this study, future investigation is warranted to confirm this association.
Despite the presence of significant correlations between the sway demonstrated during different conditions and symptoms provoked by activities of daily living and movement, the amount of variance explained indicates that the measures did not overlap and were thus complementary. Therefore, balance impairment may not universally affect all patients with concussion and may instead reflect specific concussion clinical profiles or trajectories involving vestibular dysfunction broadly and vestibulospinal dysfunction more specifically. Furthermore, each assessment domain (eg, symptom provocation during eye and head movements, quantitative balance assessment) may provide significant and unique contributions to a comprehensive concussion assessment and should therefore be measured directly.30 Recent researchers33,34 have proposed that comprehensive assessment should lead to targeted interventions for more effective management of concussion.
A limitation of this study was the variability in time since concussion, which may have affected the magnitude of sway or symptom self-report. In addition, because the study was cross sectional, we were not able to assess how participants' performance compared with their preconcussion postural control. Another important limitation was that we did not measure sway in both the AP and medial-lateral directions; medial-lateral measurements may capture more sway, especially in the tandem-stance condition. Also, we do not know if our participants were a representative sample of all adolescents with concussion.
CONCLUSIONS
Modifying the visual input, type of surface, and foot stance elicited differences in AP-NPL sway among adolescents with concussion. Anterior-posterior NPL sway was significantly related to self-reported symptoms, as well as to immediate signs and symptoms of concussion. Accelerometers are relatively inexpensive, several commercially available devices are on the market, and many smartphone applications are being developed to take advantage of the embedded accelerometers in the phones. In a short period, advances in mobile technology and reduced time requirements to perform such assessments will allow accelerometer-based balance assessment to become a suitable sideline tool. Accelerometers that measure sway may provide an objective way of tracking and quantifying balance changes in adolescents with concussion that augments current approaches to assessment. Consideration of the potential mediating effects of baseline symptom magnitude on balance performance is recommended.
ACKNOWLEDGMENTS
The study was supported in part by grants from the Albert B. Ferguson Jr MD Orthopaedic Fund of the Pittsburgh Foundation and from the National Institute on Deafness and Other Communication Disorders (1K01DC012332-01A1) to Dr Kontos. We thank the Deanship of Scientific Research at Majmaah University for supporting this work under Project No 1440-19.
Appendix 1.
Activity-Provoked Dizziness Symptoms Assessment
|
During the past week, have you experienced any of the following: |
None |
Mild |
Moderate |
Severe |
|||
| Dizziness? (If none, skip) | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness when looking up? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness walking down aisles, hallways, etc? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness when turning over, getting out of bed, or while lying down? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness while reading? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness during quick head movements? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness when bending over? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Dizziness in wide-open spaces? | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Activity score = | |||||||
Appendix 2.
Eye-Head Movement–Provoked Symptoms Assessment
| Rate your symptoms after each of the following exercises: |
None |
Mild |
Moderate |
Severe |
|||
| Horizontal saccades | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Vertical saccades | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Horizontal gaze stability | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Vertical gaze stability | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Vestibulo-ocular reflex cancellation | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Convergence—moving target | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Eye and head movement score = | |||||||
REFERENCES
- 1.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Henry LC, Elbin RJ, Collins MW, Marchetti G, Kontos AP. Examining recovery trajectories after sport-related concussion with a multimodal clinical assessment approach. Neurosurgery. 2016;78(2):232–241. doi: 10.1227/NEU.0000000000001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins M, Lovell MR, Iverson GL, Ide T, Maroon J. Examining concussion rates and return to play in high school football players wearing newer helmet technology: a three-year prospective cohort study. Neurosurgery. 2006;58(2):275–286. doi: 10.1227/01.NEU.0000200441.92742.46. [DOI] [PubMed] [Google Scholar]
- 4.Harmon KG, Drezner JA, Gammons M, et al. American Medical Society for Sports Medicine position statement: concussion in sport. Br J Sports Med. 2013;47(1):15–26. doi: 10.1136/bjsports-2012-091941. [DOI] [PubMed] [Google Scholar]
- 5.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport: the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. doi: 10.1136/bjsports-2017-097699. [DOI] [PubMed] [Google Scholar]
- 6.McCrea M, Barr WB, Guskiewicz KM, et al. Standard regression-based methods for measuring recovery after sport-related concussion. J Int Neuropsychol Soc. 2005;11(1):58–69. doi: 10.1017/S1355617705050083. [DOI] [PubMed] [Google Scholar]
- 7.Peterson CL, Ferrara MS, Mrazik M, Piland S, Elliott R. Evaluation of neuropsychological domain scores and postural stability following cerebral concussion in sports. Clin J Sport Med. 2003;13(4):230–237. doi: 10.1097/00042752-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Furman GR, Lin CC, Bellanca JL, Marchetti GF, Collins MW, Whitney SL. Comparison of the balance accelerometer measure and balance error scoring system in adolescent concussions in sports. Am J Sports Med. 2013;41(6):1404–1410. doi: 10.1177/0363546513484446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guskiewicz KM, Perrin DH, Gansneder BM. Effect of mild head injury on postural stability in athletes. J Athl Train. 1996;31(4):300–306. [PMC free article] [PubMed] [Google Scholar]
- 10.Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643–650. doi: 10.1177/03635465000280050401. [DOI] [PubMed] [Google Scholar]
- 11.McCrea M, Guskiewicz KM, Marshall SW, et al. Acute effects and recovery time following concussion in collegiate football players: the NCAA Concussion Study. JAMA. 2003;290(19):2556–2563. doi: 10.1001/jama.290.19.2556. [DOI] [PubMed] [Google Scholar]
- 12.King LA, Horak FB, Mancini M, et al. Instrumenting the Balance Error Scoring System for use with patients reporting persistent balance problems after mild traumatic brain injury. Arch Phys Med Rehabil. 2014;95(2):353–359. doi: 10.1016/j.apmr.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riemann BL, Guskiewicz KM. Effects of mild head injury on postural stability as measured through clinical balance testing. J Athl Train. 2000;35(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- 14.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction on balance. Suggestion from the field. Phys Ther. 1986;66(10):1548–1550. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 15.Chiari L, Rocchi L, Cappello A. Stabilometric parameters are affected by anthropometry and foot placement. Clin Biomech (Bristol, Avon) 2002;17(9–10):666–677. doi: 10.1016/s0268-0033(02)00107-9. [DOI] [PubMed] [Google Scholar]
- 16.Pan CS, Chiou S, Kau TY, Bhattacharya A, Ammons D. Effects of foot placement on postural stability of construction workers on stilts. Appl Ergon. 2009;40(4):781–789. doi: 10.1016/j.apergo.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Broglio SP, Macciocchi SN, Ferrara MS. Neurocognitive performance of concussed athletes when symptom free. J Athl Train. 2007;42(4):504–508. [PMC free article] [PubMed] [Google Scholar]
- 18.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 19.McCrea M, Hammeke T, Olsen G, Leo P, Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med. 2004;14(1):13–17. doi: 10.1097/00042752-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Zemek R, Barrowman N, Freedman SB, et al. Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA. 2016;315(10):1014–1025. doi: 10.1001/jama.2016.1203. [DOI] [PubMed] [Google Scholar]
- 21.King LA, Mancini M, Fino PC, et al. Sensor-based balance measures outperform modified Balance Error Scoring System in identifying acute concussion. Ann Biomed Eng. 2017;45(9):2135–2145. doi: 10.1007/s10439-017-1856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mucha A, Collins MW, Elbin RJ, et al. A brief vestibular/ocular motor screening (VOMS) assessment to evaluate concussions: preliminary findings. Am J Sports Med. 2014;42(10):2479–2486. doi: 10.1177/0363546514543775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchetti GF, Bellanca J, Whitney SL, et al. The development of an accelerometer-based measure of human upright static anterior-posterior postural sway under various sensory conditions: test-retest reliability, scoring and preliminary validity of the Balance Accelerometry Measure (BAM) J Vestib Res. 2013;23(4–5):227–235. doi: 10.3233/VES-130490. [DOI] [PubMed] [Google Scholar]
- 24.Whitney SL, Roche JL, Marchetti GF, et al. A comparison of accelerometry and center of pressure measures during computerized dynamic posturography: a measure of balance. Gait Posture. 2011;33(4):594–599. doi: 10.1016/j.gaitpost.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 26.Barozzi S, Socci M, Soi D, et al. Reliability of postural control measures in children and young adolescents. Eur Arch Otorhinolaryngol. 2014;271(7):2069–2077. doi: 10.1007/s00405-014-2930-9. [DOI] [PubMed] [Google Scholar]
- 27.Ferber-Viart C, Ionescu E, Morlet T, Froehlich P, Dubreuil C. Balance in healthy individuals assessed with Equitest: maturation and normative data for children and young adults. Int J Pediatr Otorhinolaryngol. 2007;71(7):1041–1046. doi: 10.1016/j.ijporl.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Błaszczyk JW, Beck M, Sadowska D. Assessment of postural stability in young healthy subjects based on directional features of posturographic data: vision and gender effects. Acta Neurobiol Exp (Wars) 2014;74(4):433–442. doi: 10.55782/ane-2014-2006. [DOI] [PubMed] [Google Scholar]
- 29.Guskiewicz KM, Ross SE, Marshall SW. Postural stability and neuropsychological deficits after concussion in collegiate athletes. J Athl Train. 2001;36(3):263–273. [PMC free article] [PubMed] [Google Scholar]
- 30.Lau BC, Kontos AP, Collins MW, Mucha A, Lovell MR. Which on-field signs/symptoms predict protracted recovery from sport-related concussion among high school football players? Am J Sports Med. 2011;39(11):2311–2318. doi: 10.1177/0363546511410655. [DOI] [PubMed] [Google Scholar]
- 31.Ellis MJ, Cordingley D, Vis S, Reimer K, Leiter J, Russell K. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J Neurosurg Pediatr. 2015;16(3):248–255. doi: 10.3171/2015.1.PEDS14524. [DOI] [PubMed] [Google Scholar]
- 32.Heitger MH, Jones RD, Macleod AD, Snell DL, Frampton CM, Anderson TJ. Impaired eye movements in post-concussion syndrome indicate suboptimal brain function beyond the influence of depression, malingering or intellectual ability. Brain. 2009;132(pt 10):2850–2870. doi: 10.1093/brain/awp181. [DOI] [PubMed] [Google Scholar]
- 33.Broglio SP, Collins MW, Williams RM, Mucha A, Kontos AP. Current and emerging rehabilitation for concussion: a review of the evidence. Clin Sports Med. 2015;34(2):213–231. doi: 10.1016/j.csm.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22(2):235–246. doi: 10.1007/s00167-013-2791-6. [DOI] [PubMed] [Google Scholar]


