Abstract
Objective
Pediatric loss-of-control (LOC) eating is associated with, and predictive of, gains in adiposity and adverse metabolic outcomes. In addition, some preliminary data suggest that anxiety may exacerbate the relationship of LOC eating with weight and metabolic syndrome (MetS)-related measures. We therefore examined whether anxiety moderated the relationship between LOC eating and body mass index z (BMIz), adiposity, and MetS-related measures in youth.
Methods
A convenience sample of non-treatment-seeking boys and girls of varying weight strata were interviewed to determine the presence of LOC eating and completed a questionnaire assessing trait anxiety. BMIz and MetS-related measures (blood pressure, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, glucose, and insulin) were measured after an overnight fast. Adiposity was assessed by air displacement plethysmography or dual-energy x-ray absorptiometry. Analyses adjusted for age, sex, race, height, fat mass, and depressive symptoms, as appropriate.
Results
In all, 379 youths (13.0 ± 2.8 years; 53% female; BMIz = 0.8 ± 1.1; 22% with LOC eating) were studied. Anxiety was not significantly related to BMIz, adiposity, or MetS-related measures. However, anxiety and LOC eating interacted such that only among youth with LOC eating, anxiety was positively associated with fasting insulin (p = .02) and insulin resistance (p = .01). The interaction of anxiety and LOC eating was not significantly related to BMIz, adiposity, or any other MetS-related measure (ps = ns).
Conclusions
Only among non-treatment-seeking youth with LOC eating, anxiety may be associated with increased insulin secretion and insulin resistance. Longitudinal studies are required to confirm these findings and explore mechanisms for these relationships.
Keywords: anxiety, loss of control eating, metabolic syndrome, obesity, pediatrics
Obesity is considered a primary contributor to metabolic syndrome (MetS), defined as a cluster of physiological abnormalities including abdominal obesity, elevated blood pressure, high triglyceride concentrations, low high-density lipoprotein cholesterol (HDL-C), and elevated glucose that increases risk for heart disease and type 2 diabetes (T2D; Zimmet et al., 2007). Reported loss-of-control (LOC) eating, a hallmark feature of binge eating disorder, is reported by children and adolescents of all weight strata (Schlüter, Schmidt, Kittel, Tetzlaff, & Hilbert, 2016; Tanofsky-Kraff, Marcus, Yanovski, & Yanovski, 2008), with rates of roughly 23%–31% (He, Cai, & Fan, 2017; Schlüter et al., 2016), and is predictive of adverse health outcomes, such as excess gain in body mass index (kg/m2, BMI) (Tanofsky-Kraff et al., 2009) and worsening metabolic health (Tanofsky-Kraff et al., 2012). Although obesity is a primary contributor to MetS, studies of adult samples suggest binge eating may play a unique role in metabolic dysfunction (Epel et al., 2004; Hudson et al., 2010) above and beyond body weight. Thus, LOC eating may be a modifiable risk factor that places individuals at risk for metabolic dysfunction. Given LOC eating does not persist in all youth (Hilbert, Hartmann, Czaja, & Schoebi, 2013; Tanofsky-Kraff et al., 2011), determining moderating factors may help to explain the relationship between LOC eating and adverse weight-related outcomes.
Affect theory proposes that LOC eating develops as a result of maladaptive coping with negative emotions to deal with uncomfortable affective states (Agras & Telch, 1998; Heatherton & Baumeister, 1991; Kenardy, Arnow, & Agras, 1996; Stice & Agras, 1998). Research supports a link between negative mood and LOC eating cross-sectionally (Goldschmidt et al., 2008; Shomaker, Tanofsky-Kraff, Elliott, et al., 2010), prospectively (Stice, 2002), and in the feeding laboratory (Ranzenhofer et al., 2013). These data suggest that negative affect may play a role in overeating and subsequent excess weight gain among those with LOC eating. Yet, not all studies have found this relationship (Hilbert, Rief, Tuschen-Caffier, de Zwaan, & Czaja, 2009). Failure to observe this link may be owing to a lack of distinction between different affective states. Shank and colleagues (2017) studied several affective states, including anger, confusion, depression, fatigue, and anxiety, and found that, among adolescent girls with reported LOC eating, only pre-meal state anxiety predicted food intake during a test meal designed to simulate an LOC episode. Given the high comorbidity between anxiety and depression (Seligman & Ollendick, 1998), the salience of anxiety alone among these states is notable. This supports the notion that anxiety may be of particular importance in the onset and maintenance of LOC eating within the affect theory model. Further supporting the importance of anxiety in particular for LOC eating, one program that targeted youth with LOC eating was found to be effective for body mass index z (BMIz) and fat mass outcomes only among youth with high baseline anxiety symptoms, whereas depressive symptoms did not moderate the outcome (Tanofsky-Kraff et al., 2017). Notable, the comorbidity between disordered eating and anxiety is well-established and does not appear to be explained by weight status (Aspen et al., 2014; Pallister & Waller, 2008). Moreover, in both non-treatment-seeking samples (Goossens, Braet, Van Vlierberghe, & Mels, 2009) and those pursuing weight management (Glasofer et al., 2007; Goossens et al., 2009), LOC eating is associated with (Glasofer et al., 2007; Shomaker, Tanofsky-Kraff, Elliott, et al., 2010) and predictive of greater anxiety symptoms in youth (Micali et al., 2015; Tanofsky-Kraff et al., 2011). The relationship between anxiety and LOC is further supported by a neuroimaging study showing that neural responsivity to a social anxiety paradigm in healthy girls with LOC eating, who were not selected for anxiety (Jarcho et al., 2015), paralleled responsivity of youth meeting clinical criteria for anxiety disorders (Guyer et al., 2014).
Anxiety may be an important factor to consider for identifying youth with LOC eating who are at high risk for metabolic complications. In line with the framework of affect theory, anxiety may exacerbate LOC eating and therefore intensify its relationship with weight-related adverse health outcomes. Examining anxiety as a potential moderating factor might prove of importance if it helps to identify who might be at greatest risk for adverse health consequences, and thus might be targeted for early intervention.
Given the salience of anxiety in relation to LOC eating (Aspen et al., 2014; Shank et al., 2017), and reported links between pediatric LOC eating and MetS-related markers of health (Radin et al., 2015; Tanofsky-Kraff et al., 2012), we hypothesized that anxiety might moderate the relationship between LOC eating and weight-related comorbidities among a sample of non-treatment-seeking youths of all weight strata. Specifically, we expected anxiety and LOC eating would be independently related to components of MetS, and that these relationships would be most robust among youth with high anxiety who report LOC eating. Given there have been mixed findings in the relationship between anxiety and adiposity among treatment-seeking and non-treatment-seeking groups (Burke & Storch, 2015), we explored whether the relationship would be observed in our sample.
Method
Participants and Recruitment
A convenience sample was assembled from participants in two nontreatment studies that were conducted at the National Institutes of Health (NIH; ClinicalTrials.gov ID: NCT02390765; ClinicalTrials.gov ID: NCT00320177). Both studies examined eating behaviors that promote pediatric overweight and obesity and associated comorbidities. These protocols were approved by the institutional review boards at the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the Uniformed Services University of the Health Sciences (USUHS). Healthy children of age 8–17 years were recruited through physicians’ offices, local newspaper advertisements, and mailings to families in the greater Washington, DC, metropolitan area. Participants were excluded if they had any major medical illness, current psychiatric condition, or prescription of psychiatric medication likely to impede participation (determined on a case-by-case basis by the investigators).
Procedures
Written informed consent from parents and assent from children and adolescents were obtained. Participants completed psychological assessments and measurements of body composition and MetS-related variables following an overnight fast.
Psychological Assessments
Eating Disorder Examination
The Eating Disorder Examination (EDE) adult (Fairburn & Cooper, 1993) or child version (Bryant-Waugh, Cooper, Taylor, & Lask, 1996) was administered to categorize participants by those who endorsed LOC eating and those who did not in the previous 28 days. Consistent with the literature (Hilbert et al., 2013; Tanofsky-Kraff et al., 2011), LOC eating was examined categorically as opposed to continuously. The adult and child versions collect the same information about LOC eating episodes and differ only in that the child EDE has been edited to make the language more accessible to 8–14-year-olds. The child and adult EDE have been effectively combined for analysis in previous studies (Tanofsky-Kraff et al., 2007). The EDE has demonstrated excellent interrater reliability and good convergent and discriminant validity in samples including children as young as 6 years (Tanofsky-Kraff et al., 2004). In the current study, interrater reliability, assessed with 38 (10%) randomly selected interviews, was good (Cohen’s κ = 0.79). Extensive training and ongoing supervision, as previously described (Tanofsky-Kraff et al., 2004), is required for administration of the EDE. Graduate students and research associates attended 15–20 hr of training workshops involving in-depth review of each interview question, listening to audiotape sample interviews, conducting practice interviews, and watching the trainer conduct interviews. In addition, the trainer observed and co-rated an interview with each trainee. Training observation continued until at least 95% concordance between the trainer and trainee ratings occurred. All interviews were audio-recorded and reviewed at weekly team meetings during data collection to ensure administration quality.
State-Trait Anxiety Inventory for Children
Trait anxiety was measured with the 20-item trait subscale of the State-Trait Anxiety Inventory for Children (STAIC; Spielberger, 1973), which captures individual differences in the likelihood of experiencing anxiety across different situations (Spielberger, 1972). Scores range from 20 to 60 and assess continuous dimensions of trait anxiety, with higher scores indicating higher trait anxiety. The STAIC has demonstrated good reliability and construct validity in children as young as 8 years (Papay & Hedl, 1978). Cronbach’s α was .89.
Children’s Depression Inventory
Depression was measured with the Children’s Depression Inventory (Kovacs & Beck, 1977). This measure consists of 27 items and the total sum score was used in analyses. Depressive symptoms were included as a covariate in the models of BMIz and MetS-related measures owing to the well-established links between depressive symptoms, BMIz, and insulin resistance (Goodman & Whitaker, 2002; Shomaker, Tanofsky-Kraff, Young-Hyman, et al., 2010). The Children’s Depression Inventory has good internal consistency and convergent and discriminant validity (Stiensmeier-Pelster, Schürmann, & Duda, 2000). Cronbach’s α was .87.
Physical Measures
BMI and Fat Mass
Height was measured in triplicate by a stadiometer, and fasting weight was measured by a scale calibrated to the nearest 0.1 kg to calculate BMI (kg/m2). BMIz scores, normed for age and sex, were computed according to the Centers of Disease Control and Prevention growth standards (Kuczmarski et al., 2002). Fat mass (kg) was assessed by air displacement plethysmography (Bod Pod; Life Measurement Inc., Concord, CA) or dual-energy x-ray absorptiometry (DXA; iDXA system, GE Healthcare, Madison WI), and analyzed with GE Encore 15, SP 2 software. Fat mass was adjusted to ensure equivalence between techniques by multiplying girls’ Bod Pod fat percentage by 1.03 (Nicholson et al., 2001).
Metabolic Function
Systolic and diastolic blood pressure were measured at the right brachial artery using an automated blood pressure monitor after a 5-min rest. Triglycerides, HDL-C, low-density lipoprotein cholesterol (LDL-C), and glucose were measured from fasting blood samples collected by trained phlebotomists using a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN) by the NIH Clinical Center Department of Laboratory Medicine. Insulin was measured using a Cobas 6000 (Roche Diagnostics) chemiluminescent method by the NIH Clinical Center Department of Laboratory Medicine. For insulin, the analytical sensitivity was 0.2 uU/mL, cross-reactivity with proinsulin was 0.05%, average intraassay coefficient of variation (CV) was 1.1%, and average interassay CV was 4.3%. A Cobas FARA analyzer was used to directly measure HDL-C using reagents from Sigma Chemical (St. Louis, MO). LDL-C was calculated using the formula: Total cholesterol − HDL − (triglycerides/5). Homeostatic model assessment of insulin resistance (HOMA-IR), a measure capturing insulin resistance, was calculated by multiplying fasting insulin (mIU/mL) by fasting glucose (mg/dL) and dividing by 405.
Statistical Analyses
Statistical analyses were conducted using IBM SPSS Statistics 24. Data were screened for outliers and normality. Extreme outliers were recoded to fall within 1.5 times the interquartile range below or above the 25th or 75th percentile (Behrens, 1997). No more than 1% of the data comprising any variable was considered an outlier. Fat mass (kg), triglycerides (mg/dL), insulin (mIU/mL), and HOMA-IR did not achieve normality and were thus log-transformed. Participants (n = 25) with missing data were excluded from the analyses.
A multiple analysis of covariance was used with MetS-related measures as dependent variables and LOC eating, trait anxiety, and the interaction term of trait anxiety by LOC eating status as independent variables. Covariates included fat mass, age, sex, height, race, and depressive symptoms. Owing to data showing that puberty is associated with changes in affect and is associated with a temporary increase in insulin resistance (Goran & Gower, 2001; Moran et al., 1999), and given the wide age range of the sample, follow-up exploratory analyses were conducted examining the two-way interactions of age with anxiety and LOC eating in the MetS model. The interaction terms were nonsignificant and were thus removed from the model.
Two analyses of covariance were conducted to examine the effects of LOC eating and anxiety on anthropomorphic measurements, with BMIz and fat mass as dependent variables. Independent variables were LOC eating presence/absence, trait anxiety, and the interaction of trait anxiety by LOC eating. Partial eta-squared (ηp2) effect sizes are reported. For the BMIz model, the interaction of LOC eating by anxiety was tested, adjusting for race (0 = White, 1 = Other) and depressive symptoms. For the model of fat mass, covariates were age, sex, height, race, and depressive symptoms. Given data suggesting that the link between weight and anxiety varies by sex (Burke & Storch, 2015), a sex by anxiety interaction was examined for BMI and fat mass, but removed because neither term was significant.
Results
Participants were 379 youth (M = 13.0, SD = 2.8 years old; 53% female; 52% non-Hispanic White; 33% non-Hispanic Black; 6% Hispanic/Latino; 5% Asian; 8% Other/multiple races; 22%, n = 85, with LOC eating). Median socioeconomic status (SES), measured by the Hollingshead Index (Hollingshead, 1975), was 2. The percentage of participants with overweight but not obesity (BMIz between 1 and 1.64) was 20.4% (Kuczmarski et al., 2002), and the percentage of participants with obesity (BMIz >1.64) was 28.6%. Average trait anxiety was 30.4, with a range of scores from 20 to 50. Trait anxiety was normally distributed in both the LOC group and the No LOC group. Children with LOC eating had greater BMIz, fat mass, depressive symptoms, and trait anxiety than those without LOC (Table I). A total of 43 youths did not qualify for the studies. For ClinicalTrials.gov ID NCT02390765 (24 excluded), there were no demographic differences between those excluded and included on age, sex, race, or SES. For ClinicalTrials.gov ID NCT00320177 (19 excluded), children who did not complete the study were significantly younger (13.6 ± 2.6 compared with 12.1 ± 3.1 years; p < .01) and of a lower SES (median: 2 compared with 3; p = .01); the two groups did not differ with regard to sex or race.
Table I.
Sample Characteristics for LOC Eating Versus No LOC Eating Groups
| Characteristicsa | Total | LOC eating | No LOC |
|---|---|---|---|
| (n = 379) | (n = 85) | (n = 294) | |
| Depressive symptoms | 5.5 ± 4.8 | 7.8 ± 5.5* | 4.5 ± 4.0 |
| Trait anxiety | 30.4 ± 6.8 | 34.8 ± 7.7* | 29.3 ± 6.4 |
| BMIz score | 0.8 ± 1.1 | 1.2 ± 1.1* | 0.7 ± 1.1 |
| Fat mass (kg) | 18.5 ± 15.4 | 25.0 ± 18.0* | 17.2 ± 14.3 |
| Systolic blood pressure (mm Hg) | 112.2 ± 12.7 | 113.8 ± 13.4 | 111.8 ± 12.6 |
| Diastolic blood pressure (mm Hg) | 63.6 ± 7.9 | 64.6 ± 7.9 | 63.2 ± 7.8 |
| Triglycerides (mg/dL) | 69.2 ± 33.0 | 74.7 ± 37.5 | 68.0 ± 31.8 |
| HDL-C (mg/dL) | 53.2 ± 12.7 | 52.7 ± 12.4 | 53.1 ± 12.7 |
| LDL-C (mg/dL) | 93.3 ± 24.3 | 97.2 ± 26.7 | 92.5 ± 23.7 |
| Glucose (mg/dL) | 88.2 ± 6.6 | 87.4 ± 7.1 | 88.4 ± 6.5 |
| Insulin (mIU/mL) | 11.9 ± 9.5 | 13.4 ± 9.9 | 11.6 ± 9.5 |
| HOMA-IR | 2.7 ± 2.2 | 2.9 ± 2.2 | 2.6 ± 2.2 |
LOC = loss-of-control; BMIz = body mass index z score; HDL-C = high-density-lipoprotein cholesterol; LDL-C = low-density-lipoprotein cholesterol; HOMA-IR = homeostatic model assessment–insulin resistance.
Values presented are M ± SD, unless otherwise noted.
Analyses examining differences between LOC vs. No LOC groups are significant at p < .01.
Main Effects
After adjusting for covariates and including interaction terms in the model, there were no significant main effects of LOC eating on BMIz (p = .64) or fat mass (p = .58; Table II). There were also no main effects of LOC eating on MetS-related measures of systolic blood pressure (p = .62), diastolic blood pressure (p = .99), triglycerides (p = .20), HDL-C (p = .42), LDL-C (p = .80), or glucose (p = .20); however, youth with LOC eating had higher insulin (p = .01) and HOMA-IR (p = .01; Table II).
Table II.
LOC Eating, Trait Anxiety, and Interaction Effects on BMIza, Fat Massb, and MetS-Related Characteristicsc
| Characteristics | LOC eating |
Trait anxiety |
LOC eating × Anxiety |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p-value | ηp2 | F | p-value | ηp2 | F | p-value | ηp2 | R2 | ΔR2 | |
| BMIz score | 0.22 | .64 | <0.01 | 1.01 | .32 | <0.01 | 0.09 | .77 | <0.01 | .09 | .06 |
| Fat mass (kg) | 0.31 | .58 | <0.01 | <0.01 | .97 | <0.01 | <0.01 | .93 | <0.01 | .32 | .02 |
| Systolic BP (mmHg) | 0.24 | .62 | <0.01 | <0.01 | .97 | <0.01 | 0.12 | .73 | <0.01 | .31 | .00 |
| Diastolic BP (mmHg) | <0.01 | .99 | <0.01 | 0.27 | .61 | <0.01 | 0.06 | .81 | <0.01 | .1 | .01 |
| Triglycerides (mg/dL) | 1.69 | .20 | 0.01 | 2.31 | .13 | 0.01 | 1.80 | .18 | 0.01 | .17 | .01 |
| HDL-C (mg/dL) | 0.66 | .42 | <0.01 | <0.01 | .98 | <0.01 | 0.30 | .58 | <0.01 | .16 | .02 |
| LDL-C (mg/dL) | 0.07 | .80 | <0.01 | 0.28 | .60 | <0.01 | 0.01 | .92 | <0.01 | .06 | .01 |
| Glucose (mg/dL) | 1.69 | .20 | 0.01 | 0.98 | .32 | 0.01 | 1.28 | .26 | 0.01 | .12 | .03 |
| Insulin (mIU/mL) | 7.19 | .01* | 0.03 | 1.55 | .21 | 0.01 | 5.92 | .02* | 0.03 | .58 | .02 |
| HOMA-IR | 7.48 | .01* | 0.04 | 1.74 | .19 | 0.01 | 6.05 | .01* | 0.03 | .58 | .02 |
LOC = loss-of-control; BMIz = body mass index z; BP = blood pressure; HDL-C = high density lipoprotein cholesterol; LDL-C = low density lipoprotein cholesterol; HOMA-IR = homeostatic model assessment–insulin resistance.
BMIz model covariates included race and depressive symptoms.
Fat mass model covariates included age, sex, height, race, and depressive symptoms. Pubertal status was not significant when added as a covariate.
MetS model covariates included fat mass, age, sex, height, race, and depressive symptoms. Pubertal status was not significant when added as a covariate.
p < .05. R2 for covariates and ΔR2 for each model provided.
Adjusting for covariates, there were no significant main effects of anxiety on BMIz (p = .32), fat mass (p = .97; Table II), or any of the MetS-related measures, including systolic blood pressure (p = .97), diastolic blood pressure (p = .61), triglycerides (p = .13), HDL-C (p = .98), LDL-C (p = .60), glucose (p = .32), insulin (p = .21), or HOMA-IR (p = .19; Table II).
Interaction Effects
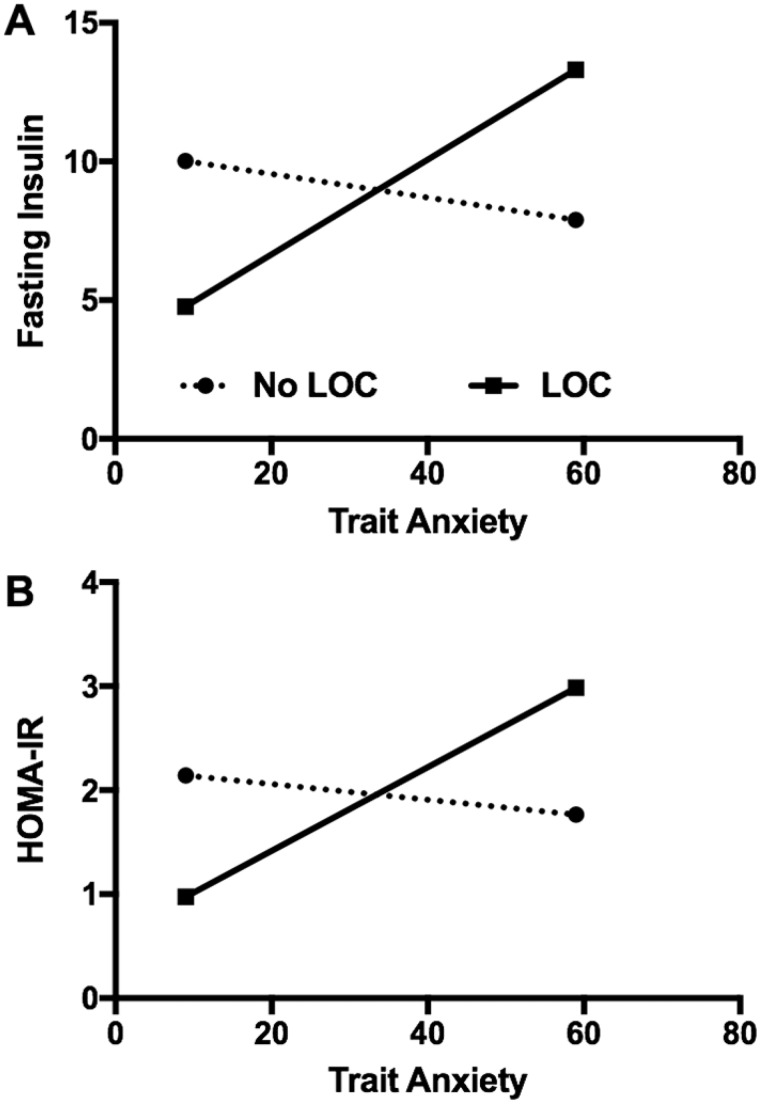
The interaction of LOC and anxiety was significant for fasting insulin (p = .02) and HOMA-IR (p = .01); for youth with LOC eating, higher anxiety was positively associated with higher values (Figure 1). The interaction of LOC and anxiety was not significantly related to any other MetS-related variable (systolic blood pressure, p = .73; diastolic blood pressure, p = .81; triglycerides, p = .18; HDL-C, p = .58; LDL-C, p = .92; glucose, p = .26). The interaction of LOC eating and anxiety was not related to BMIz (p = .77) or fat mass (p = .93).
Figure 1.
Anxiety significantly moderated the link between loss-of-control (LOC) eating and fasting insulin (1A) and homeostatic model assessment of insulin resistance (HOMA-IR; 1B), such that only in youth with LOC eating, anxiety was positively associated with fasting insulin and insulin resistance.
Discussion
In a sample of non-treatment-seeking girls and boys of varying weight strata, the interaction of anxiety and LOC eating was significant such that only in youth with LOC eating, anxiety was positively associated with fasting insulin and insulin resistance. The interaction was significant for only two of the seven MetS-related variables analyzed, and significant results showed small effect sizes. Therefore, findings should be interpreted with caution. However, insulin resistance may be an early indicator of later worsening metabolic dysfunction, and given the healthy, non-treatment-seeking sample, it is possible this finding may explain the lack of significant findings for other MetS-related components. Indeed, it is important to study healthy youth with subthreshold anxiety levels to potentially identify and intervene early with those at increased risk before metabolic characteristics worsen.
Our results showed a lack of a significant relationship between anxiety and adiposity. Although a meta-analysis supported a significant positive relationship between weight status and anxiety in youth, the effect size was very small (r = .08) (Burke & Storch, 2015). Likewise, anxiety was not associated with MetS-related variables. As participants were excluded if they met clinical criteria for an anxiety disorder, it is possible the current sample did not report anxiety levels sufficiently elevated to identify a relationship between anxiety and weight status. Effect sizes may have been larger or additional significant relationships may have emerged if individuals who met criteria for an anxiety disorder were included. Research on youth with elevated or clinical anxiety symptoms is needed to determine whether they are at increased risk for adverse weight-related health outcomes.
In line with the framework of affect theory, anxiety may exacerbate LOC eating and therefore intensify its relationship with insulin and insulin resistance. This finding is notable, as the data are mixed regarding the relationship between pediatric LOC eating and insulin measures. For example, one study found insulin concentrations tended to be higher in girls with LOC eating compared with girls without LOC eating (Lourenço et al., 2008); however, other studies have reported no link (Radin et al., 2015; Tanofsky-Kraff et al., 2012). Children who report both LOC eating and anxiety may experience particularly high levels of distress that can cause chronic hypersecretion of stress hormones such as cortisol (Rosmond, 2005; Taylor & Fishman, 1988) that may result in greater insulin resistance (Charmandari, Kino, Souvatzoglou, & Chrousos, 2003; Chrousos, 2000). Future studies should test if high levels of trait anxiety in conjunction with LOC eating uniquely contribute to increased insulin resistance through elevated cortisol production.
Given the connection between insulin and glucose in relation to excess weight and T2D, it may at first appear surprising that the interaction of LOC eating and anxiety was not significantly related to serum glucose. It may be that pancreatic secretion of excess amounts of insulin is effectively maintaining normal serum glucose for the sample. In the absence of T2D, youth generally compensate for insulin resistance by increasing insulin secretion to maintain normal glucose concentrations (Weiss, Taksali, & Caprio, 2006). However, such pancreatic beta cell compensation may not necessarily last in the long term, and thus will eventually lead to the development of T2D (Weir & Bonner-Weir, 2004). Examining insulin and glucose concentrations in youth with LOC eating and high anxiety longitudinally would be of interest to determine if the combination of LOC eating and anxiety provides an early marker for the risk of diabetes development. Along these same lines, higher insulin resistance is typically associated with worse metabolic characteristics such as high triglycerides and abdominal obesity (Kaur, 2014). Given that insulin resistance is causally linked to the development of worsening components of MetS (Kaur, 2014), longitudinal studies would also clarify if early co-occurrence of LOC eating and anxiety leads to the development of abnormalities in components of MetS that were not detected in this cross-sectional analysis.
Roughly half of youth stop reporting LOC eating over time and those whose LOC eating remits experience few negative consequences (Hilbert et al., 2013; Tanofsky-Kraff et al., 2011). As a result, the current study’s analysis of anxiety as a potential moderating factor might prove of importance if it helps to identify those who might be at greatest risk for adverse health consequences, and thus might be targeted for early intervention. Studies are needed to identify potential mechanisms linking anxiety, LOC eating, and insulin resistance. Longitudinal, prospective designs will clarify directionality in the relationship between LOC, anxiety, weight gain, and adverse health-related outcomes. If prospective studies support the impact of anxiety on adverse health outcomes in youth with LOC eating, anxiety may be an important screening target to identify risk for persistent LOC eating and associated chronic diseases.
Study strengths include a large sample of primarily non-Hispanic Black and White boys and girls across a wide weight range. Importantly, and consistent with a biopsychosocial model, we collected objective measures of metabolic function and body composition and used a clinical interview method to assess LOC eating. Limitations include the use of cross-sectional data that preclude reaching causal conclusions from the findings and the use of a self-report questionnaire measure of anxiety. Also, waist circumference, a component of MetS, was not captured. Measurement invariance by age has not yet been demonstrated for the EDE, and thus should be considered a study limitation. It should also be noted that effect sizes were small and, as would be expected in a non-treatment-seeking sample, were below clinically relevant values. Thus, clinical importance of findings should be interpreted with caution. Finally, although expected, youth in the LOC eating group had higher BMIz and fat mass and consisted of more girls than youth in the group without LOC eating. Although we adjusted for these variables in the primary analyses, sex- and weight-matched groups of youth with and without LOC eating may provide for a more robust analysis if we had recruited more children with reported LOC eating.
In conclusion, we found that anxiety, studied as an independent factor, did not appear to relate to weight or metabolic function in youth. However, the interaction of anxiety and LOC eating was associated with increased fasting insulin and insulin resistance in this study. Despite the lack of significant findings for BMIz and adiposity, given that children can develop T2D at any weight, it is clinically important to study healthy children of all weight statuses to identify early indicators of disease. Given the small effect sizes of this interaction and the lack of variance, anxiety may have minimal impact on disease status in those with LOC eating, especially considering the lack of significant relationship with adiposity. The current findings offer a potential early intervention target to track in future longitudinal studies. If supported by prospective studies, anxiety may be a modifiable factor that could help identify youth with LOC eating at high risk for metabolic complications.
Funding
This work was supported by the Uniformed Services University of the Health Sciences grant R072IC (to M. T. K.); Intramural Research Program, National Institutes of Health, grant 1ZIAHD000641 from the National Institute of Child Health and Human Development with supplemental funding from National Institute on Minority Health and Health Disparities; the National Institutes of Health Clinical Center Bench to Bedside Program; and the Office of Behavioral and Social Sciences Research (OBSSR) of the NIH (to J. A. Y.)
Disclosures: J. A. Yanovski is a Commissioned Officer in the United States Public Health Service (PHS). J.A. Yanovski reports support from Rhythm Pharmaceuticals and Soleno Therapeutics for clinical trials for the treatment of rare syndromes causing obesity that are unrelated to this submission.
Disclaimer: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS, USUHS, or the United States Department of Defense.
Conflicts of interest: None declared.
References
- Agras W. S., Telch C. F. (1998). The effects of caloric deprivation and negative affect on binge eating in obese binge-eating disordered women. Behavior Therapy, 29, 491–503. [Google Scholar]
- Aspen V., Weisman H., Vannucci A., Nafiz N., Gredysa D., Kass A. E., Trockel M., Jacobi C., Wilfley D. E., Taylor C. B. (2014). Psychiatric co-morbidity in women presenting across the continuum of disordered eating. Eating Behaviors, 15, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J. T. (1997). Principles and procedures of exploratory data analysis. Psychological Methods, 2, 131. [Google Scholar]
- Bryant-Waugh R. J., Cooper P. J., Taylor C. L., Lask B. D. (1996). The use of the Eating Disorder Examination with children: A pilot study. International Journal of Eating Disorders, 19, 391–397. [DOI] [PubMed] [Google Scholar]
- Burke N. L., Storch E. A. (2015). A meta-analysis of weight status and anxiety in children and adolescents. Journal of Developmental & Behavioral Pediatrics, 36, 133–145. [DOI] [PubMed] [Google Scholar]
- Charmandari E., Kino T., Souvatzoglou E., Chrousos G. P. (2003). Pediatric stress: Hormonal mediators and human development. Hormone Research in Paediatrics, 59, 161–179. [DOI] [PubMed] [Google Scholar]
- Chrousos G. (2000). The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: Neuro-endocrine and target tissue-related causes. International Journal of Obesity and Related Metabolic Disorders, 24, [DOI] [PubMed] [Google Scholar]
- Epel E., Jimenez S., Brownell K., Stroud L., Stoney C., Niaura R. (2004). Are stress eaters at risk for the metabolic syndrome? Annals of the New York Academy of Sciences, 1032, 208–210. [DOI] [PubMed] [Google Scholar]
- Fairburn C. G., Cooper Z. (1993). The Eating Disorder Examination (12th edition) In Fairburn C. G., Wilson G. T. (Eds.), Binge eating: Nature, assessment, and treatment (pp. 317–360). New York, NY: Guilford Press. [Google Scholar]
- Glasofer D. R., Tanofsky-Kraff M., Eddy K. T., Yanovski S. Z., Theim K. R., Mirch M. C., Ghorbani S., Ranzenhofer L. M., Haaga D., Yanovski J. A. (2007). Binge eating in overweight treatment-seeking adolescents. Journal of Pediatric Psychology, 32, 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt A. B., Jones M., Manwaring J. L., Luce K. H., Osborne M. I., Cunning D., Taylor K. L, Doyle A. C., Wilfley D. E., Taylor C. B. (2008). The clinical significance of loss of control over eating in overweight adolescents. International Journal of Eating Disorders, 41, 153–158. [DOI] [PubMed] [Google Scholar]
- Goodman E., Whitaker R. C. (2002). A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics, 110, 497–504. [DOI] [PubMed] [Google Scholar]
- Goossens L., Braet C., Van Vlierberghe L., Mels S. (2009). Loss of control over eating in overweight youngsters: The role of anxiety, depression and emotional eating. European Eating Disorders Review, 17, 68–78. [DOI] [PubMed] [Google Scholar]
- Goran M. I., Gower B. A. (2001). Longitudinal study on pubertal insulin resistance. Diabetes, 50, 2444–2450. [DOI] [PubMed] [Google Scholar]
- Guyer A. E., Benson B., Choate V. R., Bar-Haim Y., Perez-Edgar K., Jarcho J. M., Pine D. S., Ernst M., Fox N. A, Nelson E. E. (2014). Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology, 26, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Cai Z., Fan X. (2017). Prevalence of binge and loss of control eating among children and adolescents with overweight and obesity: An exploratory meta-analysis. International Journal of Eating Disorders, 50, 91–103. [DOI] [PubMed] [Google Scholar]
- Heatherton T. F., Baumeister R. F. (1991). Binge eating as escape from self-awareness. Psychological Bulletin, 110, 86.. [DOI] [PubMed] [Google Scholar]
- Hilbert A., Hartmann A. S., Czaja J., Schoebi D. (2013). Natural course of preadolescent loss of control eating. Journal of Abnormal Psychology, 122, 684.. [DOI] [PubMed] [Google Scholar]
- Hilbert A., Rief W., Tuschen-Caffier B., de Zwaan M., Czaja J. (2009). Loss of control eating and psychological maintenance in children: An ecological momentary assessment study. Behaviour Research and Therapy, 47, 26–33. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. B. (1975). Four factor index of social status. New Haven, CT: Yale University. [Google Scholar]
- Hudson J. I., Lalonde J. K., Coit C. E., Tsuang M. T., McElroy S. L., Crow S. J., Bulik C. M., Hudson M. S., Yanovski J. A., Rosenthal N. R., Pope H. G. (2010). Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder–. The American Journal of Clinical Nutrition, 91, 1568–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho J. M., Tanofsky-Kraff M., Nelson E. E., Engel S. G., Vannucci A., Field S. E., Romer A. L., Hannallah L., Brady S. M., Demidowich A. P., Shomaker L. B., Courville A. B., Pine D. S., Yanovski J. A. (2015). Neural activation during anticipated peer evaluation and laboratory meal intake in overweight girls with and without loss of control eating. Neuroimage, 108, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J. (2014). A comprehensive review on metabolic syndrome. Cardiology Research and Practice, 2014, 943162.. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kenardy J., Arnow B., Agras W. S. (1996). The aversiveness of specific emotional states associated with binge-eating in obese subjects. Australian and New Zealand Journal of Psychiatry, 30, 839–844. [DOI] [PubMed] [Google Scholar]
- Kovacs M., Beck A. T. (1977). An empirical-clinical approach toward a definition of childhood depression In J. G. Schulterbrandt & A. Raskin (Eds.), Depression in childhood: Diagnosis, treatment, and conceptual models (pp. 1–25). New York: Raven Press. [Google Scholar]
- Kuczmarski R. J., Ogden C. L., Guo S. S., Grummer-Strawn L. M., Flegal K. M., Mei Z., Wei R., Curtin L. R., Roche A. F., Johnson C. L. (2002). 2000 CDC Growth Charts for the United States: Methods and development. Vital and Health Statistics 11, 246, 1–190, [PubMed] [Google Scholar]
- Lourenço B. H., Arthur T., Rodrigues M. D. B., Guazzelli I., Frazzatto E., Deram S., Nicolau C. Y., Halpern A., Villares S. M. F. (2008). Binge eating symptoms, diet composition and metabolic characteristics of obese children and adolescents. Appetite, 50, 223–230. [DOI] [PubMed] [Google Scholar]
- Micali N., Solmi F., Horton N. J., Crosby R. D., Eddy K. T., Calzo J. P., Sonneville K. R., Swanson S. A., Field A. E. (2015). Adolescent eating disorders predict psychiatric, high-risk behaviors and weight outcomes in young adulthood. Journal of the American Academy of Child and Adolescent Psychiatry, 54, 652–659.e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran A., Jacobs D. R., Steinberger J., Hong C.-P., Prineas R., Luepker R., Sinaiko A. R. (1999). Insulin resistance during puberty: Results from clamp studies in 357 children. Diabetes, 48, 2039–2044. [DOI] [PubMed] [Google Scholar]
- Nicholson J. C., McDuffie J. R., Bonat S. H., Russell D. L., Boyce K. A., McCann S., Michael M., Sebring N. G., Reynolds J. C., Yanovski J. A. (2001). Estimation of body fatness by air displacement plethysmography in African American and white children. Pediatric Research, 50, 467–473. [DOI] [PubMed] [Google Scholar]
- Pallister E., Waller G. (2008). Anxiety in the eating disorders: Understanding the overlap. Clinical Psychology Review, 28, 366–386. [DOI] [PubMed] [Google Scholar]
- Papay J. P., Hedl J. J. (1978). Psychometric characteristics and norms for disadvantaged third and fourth grade children on the State-Trait Anxiety Inventory for Children. Journal of Abnormal Child Psychology, 6, 115–120. [DOI] [PubMed] [Google Scholar]
- Radin R. M., Tanofsky-Kraff M., Shomaker L. B., Kelly N. R., Pickworth C. K., Shank L. M., Altschul A. M., Brady S. M., Demidowich A. P., Yanovski S. Z., Hubbard V. S., Yanovski J. A. (2015). Metabolic characteristics of youth with loss of control eating. Eating Behaviors, 19, 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzenhofer L. M., Hannallah L., Field S. E., Shomaker L. B., Stephens M., Sbrocco T., Kozlosky M., Reynolds J., Yanovski J. A., Tanofsky-Kraff M. (2013). Pre-meal affective state and laboratory test meal intake in adolescent girls with loss of control eating. Appetite, 68, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmond R. (2005). Role of stress in the pathogenesis of the metabolic syndrome. Psychoneuroendocrinology, 30, 1–10. [DOI] [PubMed] [Google Scholar]
- Schlüter N., Schmidt R., Kittel R., Tetzlaff A., Hilbert A. (2016). Loss of control eating in adolescents from the community. International Journal of Eating Disorders, 49, 413–420. [DOI] [PubMed] [Google Scholar]
- Seligman L. D., Ollendick T. H. (1998). Comorbidity of anxiety and depression in children and adolescents: An integrative review. Clinical Child and Family Psychology Review, 1, 125–144. [DOI] [PubMed] [Google Scholar]
- Shank L. M., Crosby R. D., Grammer A. C., Shomaker L. B., Vannucci A., Burke N. L., Stojek M., Brady S. M., Kozlosky M., Reynolds J. C., Yanovski J. A., Tanofsky-Kraff M. (2017). Examination of the interpersonal model of loss of control eating in the laboratory. Comprehensive Psychiatry, 76, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker L. B., Tanofsky-Kraff M., Elliott C., Wolkoff L. E., Columbo K. M., Ranzenhofer L. M., Roza C. A., Yanovski S. Z., Yanovski J. A. (2010). Salience of loss of control for pediatric binge episodes: Does size really matter? International Journal of Eating Disorders, 43, 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomaker L. B., Tanofsky-Kraff M., Young-Hyman D., Han J. C., Yanoff L. B., Brady S. M., Yanovski S. Z., Yanovski J. A. (2010). Psychological symptoms and insulin sensitivity in adolescents. Pediatric Diabetes, 11, 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. D. (1972). Anxiety as an emotional state In Spielberger C. D. (Ed.), Anxiety: Current trends in theory and research (pp. 22–49). San Diego, CA: Academic Press. [Google Scholar]
- Spielberger C. D. (1973). Manual for the State-Trait Anxiety Inventory for Children. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stice E. (2002). Risk and maintenance factors for eating pathology: A meta-analytic review. Psychological Bulletin, 128, 825.. [DOI] [PubMed] [Google Scholar]
- Stice E., Agras W. S. (1998). Predicting onset and cessation of bulimic behaviors during adolescence: A longitudinal grouping analysis. Behavior Therapy, 29, 257–276. [Google Scholar]
- Stiensmeier-Pelster J., Schürmann M., Duda K. (2000). Depressions-Inventar für Kinder und Jugendliche:(DIKJ). Hogrefe: Verlag für Psychologie. [Google Scholar]
- Tanofsky-Kraff M., Goossens L., Eddy K. T., Ringham R., Goldschmidt A., Yanovski S. Z., Braet C., Marcus M. D., Wilfley D. E., Olsen C., Yanovski J. A. (2007). A multisite investigation of binge eating behaviors in children and adolescents. Journal of Consulting and Clinical Psychology, 75, 901.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M., Marcus M. D., Yanovski S. Z., Yanovski J. A. (2008). Loss of control eating disorder in children age 12 years and younger: Proposed research criteria. Eating Behaviors, 9, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M., Shomaker L. B., Olsen C., Roza C. A., Wolkoff L. E., Columbo K. M., Raciti G., Zocca J. M., Wilfley D. E., Yanovski S. Z., Yanovski J. A. (2011). A prospective study of pediatric loss of control eating and psychological outcomes. Journal of Abnormal Psychology, 120, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M., Shomaker L. B., Stern E. A., Miller R., Sebring N., DellaValle D., Yanovski S. Z., Hubbard V. S., Yanovski J. A. (2012). Children's binge eating and development of metabolic syndrome. International Journal of Obesity, 36, 956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M., Shomaker L. B., Wilfley D. E., Young J. F., Sbrocco T., Stephens M., Brady S. M., Galescu O., Demidowich A., Olsen C. H., Kozlosky M., Reynolds J. C., Yanovski J. A. (2017). Excess weight gain prevention in adolescents: Three-year outcome following a randomized controlled trial. Journal of Consulting and Clinical Psychology, 85, 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M., Yanovski S. Z., Schvey N. A., Olsen C. H., Gustafson J., Yanovski J. A. (2009). A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. International Journal of Eating Disorders, 42, 26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M., Yanovski S. Z., Wilfley D. E., Marmarosh C., Morgan C. M., Yanovski J. A. (2004). Eating-disordered behaviors, body fat, and psychopathology in overweight and normal-weight children. Journal of Consulting and Clinical Psychology, 72, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Fishman L. M. (1988). Corticotropin-releasing hormone. New England Journal of Medicine, 319, 213–222. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Bonner-Weir S. (2004). Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes, 53, S16–S21. [DOI] [PubMed] [Google Scholar]
- Weiss R., Taksali S. E., Caprio S. (2006). Development of type 2 diabetes in children and adolescents. Current Diabetes Reports, 6, 182–187. [DOI] [PubMed] [Google Scholar]
- Zimmet P., Alberti K. G. M. M., Kaufman F., Tajima N., Silink M., Arslanian S., Wong G., Bennett P., Shaw J., Caprio S. (2007). The metabolic syndrome in children and adolescents–an IDF consensus report. Pediatric Diabetes, 8, 299–306. [DOI] [PubMed] [Google Scholar]